Introduction
It is well known that tumor angiogenesis, which is
the formation of new blood vessels in tumors, has a critical role
in tumor progression and metastasis (1). The heterogeneity of tumor vessels has
increasingly been demonstrated. It has been reported that tumor
microvascular architecture phenotype (T-MAP), in addition to
various bio-characteristics, exhibit heterogeneity and differ from
the normal blood vessels (2,3). Thus, using normal vascular endothelial
cells (ECs) is inappropriate to investigate tumor angiogenesis or
screen candidate anti-cancer drugs that target tumor vessels. It is
necessary to harvest tumor endothelial cells (TECs) from tumor
tissues. Recently, with the method of magnetic active cell sorting
(MACS) that is based on the immunological magnetic beads
conjugating various antibodies specifically to endothelial markers
such as CD105, CD31 and CD34, many laboratories have made it
possible to isolate and purify TECs from tumor mass (4–7). Of
these markers, studies have shown that CD105 is a good marker for
tumor angiogenesis in endometrial carcinoma, cervical cancer,
breast carcinoma, glioblastoma and esophageal squamous cell
carcinoma (8–11). However, it has been found that CD105
expression levels in tumor vessels vary with cancer development
(12–14).
CD105 is a transmembrane glycoprotein expressed
primarily in ECs and tumor cells (15,16).
Tumoral CD105 has been defined as a novel independent prognostic
marker, whereas the microvessel density labelled by the endothelial
marker CD105 (MVD-CD105) negatively correlates with tumor
development of human hepatocellular carcinoma (HCC) and renal cell
carcinoma (13,17). However, there are conflicting studies
as to whether MVD-CD105 is a biological marker for predicting
prognosis of cancer. A contrary example is that a higher score of
MVD-CD105 appears to correlate with a significantly poorer
prognosis in survival rate (14).
Furthermore, CD105 exhibits a regulatory role in normal human
vascular endothelial cells (HUVECs) (18,19).
The aforementioned experimental findings suggest
that CD105 expression levels in TECs exist in high and low states,
depending on tumor stages. CD31 (PECAM-1), a well-known pan marker
for endothelial cells, has also been used for isolation of TECs
(20). In our laboratory we verified
that CD31 is a reliable endothelial marker by phenotypic and
functional assays (unpublished data). Thus, in the present study,
we applied several approaches to detect CD105 and CD31 expression
throughout human HCC tissues with various differentiation status
and explored the association between CD105 negative expression in
TECs and HCC status using a wide range of samples. CD31+
TECs derived from HCC (termed ECDHCC) were isolated and CD105
expression was analyzed in these cells using flow cytometry and
confocal microscopy.
Materials and methods
Patients and tissue microarray
All procedures of this study involving human
materials were performed according to the ethical standards with
the Helsinki Declaration and the China Ministry of Health's
‘Ethical Review of Human Biomedical Research (Tentative, 2007)’.
The study was approved by the Research Ethics Board of the Tumor
Hospital Affiliated to Nantong University. The written informed
consent was obtained as specified in the ethical approval.
We retrospectively collected formalin-fixed
paraffin-embedded (FFPE) tissues from 90 HCC patients with the
complete clinicopathological data from January 2003 to December
2006. The diagnosis had been done by two pathologists who were
blinded to the clinicopathological data at the Tumor Hospital
Affiliated to Nantong University. Clinical follow-up data were
retrieved from patient records at the Department of Epidemiology in
the Tumor Hospital. All underwent hepatic surgical resection
without postoperative systemic chemotherapy in the Surgery
Department. The main clinical characteristics of the patients are
shown in Table I. 78 patients were
men and 12 were women. Histological grades were classified to well
differentiated (n=38), and poorly differentiated (n=52).
Seventy-three were positive for cirrhosis. We prepared 90 pairs
(tumor vs. peritumoral tissues) of tissue microarray (TMA) from the
aforementioned FFPE tissues. Each patient's specimen was
represented by a single 1 mm core of tissue. The 90 paired TMAs
were used for immunohistochemistry (IHC) staining to detect the
expression of CD105 and CD31.
 | Table I.Clinical characteristics of 90 paired
FFPE and 15 fresh tissues from patients with HCC. |
Table I.
Clinical characteristics of 90 paired
FFPE and 15 fresh tissues from patients with HCC.
| Parameter | FFPE tissues n=90
(%) | Fresh tissues n=15
(%) |
|---|
| Age, years |
|
|
| ≤45 | 33 (36.7) | 5 (33.3) |
|
>45 | 57 (63.3) | 10 (66.7) |
| Sex |
|
|
| Male | 78 (86.7) | 6 (40.0) |
|
Female | 12 (13.3) | 9 (60.0) |
| Tumor
differentiation |
|
|
| Well | 38 (42.2) | 8 (53.3) |
| Poor | 52 (57.8) | 7 (46.7) |
| Tumor size, cm |
|
|
| ≤5 | 51 (56.7) | 8 (53.3) |
|
>5 | 39 (43.3) | 7 (46.7) |
| Capsular
integrity |
|
|
|
Positive | 57 (63.3) | 9 (60.0) |
|
Negative | 33 (36.7) | 6 (40.0) |
| Metastasis |
|
|
|
Positive | 14 (15.6) | 3 (20.0) |
|
Negative | 76 (84.4) | 12 (80.0) |
| Vascular
invasion |
|
|
|
Positive | 67 (74.4) | 7 (46.7) |
|
Negative | 23 (25.6) | 8 (53.3) |
| Liver
cirrhosis |
|
|
|
Positive | 73 (81.1) | 11 (73.3) |
|
Negative | 17 (18.9) | 4 (26.7) |
| AFP, ng/ml |
|
|
|
≤50 | 34 (37.8) | 6 (40.0) |
|
>50 | 56 (62.2) | 9 (60.0) |
In order to detect endothelial maker expression
levels in cells derived from HCC by flow cytometry and confocal
microscopic analysis, we collected additional fresh resections,
which contained less necrosis tissues, from 15 HCC patients at
Tumor Hospital Affiliated to Nantong University from January to
December of 2016 with the written informed consent and ethical
approval by Research Ethics Committee of Tumor Hospital Affiliated
to Nantong University. The clinical characteristics of the patients
are shown in Table I. These fresh
samples were from 6 male and 9 female patients with 35–75 year of
age. Histological grades were classified to well differentiated
(n=8), and poorly differentiated (n=7). The diagnosis had been done
by two pathologists who were blinded to the clinicopathological
data and clinical follow-up data were retrieved from patient
records at the Department of Epidemiology in the aforementioned
hospital. Single cell suspension was prepared for fluorescent
antibody staining. We used these 15 paired samples to check the
markers, particularly CD105 expression in tumors vs. peritumoral
areas. A positive expression vs. a negative expression in the
matched pairs was verified as a convincing result.
IHC staining and analysis
FFPE slices were dewaxed in xylene and rehydrated in
graded alcohol. For blocking of endogenous peroxides, 3% hydrogen
peroxide was used for 15 min. Antigen retrieval was routinely
performed by immersing the slices in a thermostatic bath containing
preheated ethylene diamine tetra acetic acid (EDTA) for 30 min at
98°C and cooling down at room temperature for 20 min. Sections were
incubated with monoclonal antibody against a 1:30 dilution of CD105
(mouse-anti-human, clone SN6 h; DAKO, Glostrup, Denmark) and a 1:50
dilution of CD31 (mouse-anti-human, clone JC70A) (both from DAKO,
Glostrup, Denmark) overnight at 4°C. Visualization of the antibody
complex was achieved with a diaminobenzidine (DAKO) reaction,
resulting in brown staining of EC membranes. TMA and slices were
counterstained by Meyer's hematoxylin.
We used both qualitative and quantitative analysis
for evaluation of IHC results. By qualitative analysis, ‘negative
expression’ was indicated by the average number of positive cells
from 3 hot spots covering <5% of the total cells. For
quantitative analysis, we used average optical density (AOD) to
evaluate the intensity of the IHC reaction in order to compare
expression levels between tumor and peritumoral fields of different
markers. AOD was performed by Image-Proplus 6.0 software. AOD
scores were calculated from the optical density of 5 spots selected
randomly on each slice under the microscope (×400).
Isolation of TECs from HCC
A single cell suspension was firstly prepared from
the fresh surgical specimens of the patients with HCC. Briefly, the
specimens were minced with scissors and digested by incubation in
HANK's medium (containing Ca2+, Mg2+)
supplemented with 0.1% collagenase I and collagenase IV
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and DNase (Cell
Culture Grade; Roche Diagnostics, Basel, Switzerland) at 37°C for
45 min. After being washed in medium plus 10% FBS (Sijiqing Co.,
Ltd., Hangzhou, China), the cells were ready for MACS separation.
We used anti-CD31 antibody coupled to magnetic beads and MACS
system (Miltenyi Biotech, Bergisch Gladbach, Germany) to separate
CD31+ TECs from other cells in the cell suspension,
according to the instruction manual.
Cell lines and culture conditions
Normal vascular endothelial cell line (HUVEC) and
isolated ECDHCC cells were maintained in endothelial cell growth
medium (ECM) supplemented with 5% fetal bovine serum (FBS), 2%
VEGF, 100 U/ml penicillin and 100 U/ml streptomycin in 5%
CO2. All these, including HUVEC cell line were purchased
from ScienCell Research Laboratory (Yuhenfeng Company, Beijing,
China).
Flow cytometric analysis
The first step was the preparation of a single cell
suspension. The cultured HUVEC cells were detached from plates with
a nonenzymatic cell dissociation solution (Sigma-Aldrich; Merck
KGaA) and washed in PBS containing 0.5% BSA. The fresh surgical
specimens were enzymatically dissociated into a single cell
suspension. Cells were then incubated for 15 min at 4°C with the
appropriate antibodies or with a control in PBS containing 0.5%
bovine serum albumin (BSA). Cells were analyzed on Becton Dickinson
FACS Aril II. We used primary murine monoclonal antibodies against
human CD105 conjugated to allophycocyanin (APC) (1:30 dilutions)
and human CD31 conjugated to phycoerythrin (PE) (1:100 dilutions).
Unstained cells were used to distinguish between fluorescent
positive and fluorescent negative populations. 7-AAD was added for
10 min prior to FACS analysis, which allowed for the discrimination
of dead vs. live cells. All antibodies were purchased from Becton
Dickinson (Franklin Lakes, NJ, USA).
Confocal microscopy analysis
Immunofluorescent double staining analysis was
performed on ECDHCC and HUVECs which were seeded on sterile slice
cover slips in six well plates overnight. Following several washes
with PBS, cells were fixed with 4% paraformaldehyde for 30 min at
room temperature and permeabilized with 0.1% Triton X-100 in PBS
for 5 min. The cells were overlaid with 5% BSA for 30 min, rinsed
with PBS and incubated with a mixture of rabbit-anti-human CD105
(1:20 dilutions; Abcam, Cambridge, UK) and mouse-anti-human CD31
(1:40 dilutions; DAKO) or a mixture of rabbit-anti-human CD105
(1:20 dilutions) and mouse-anti-human VEGFR-2 (1:200 dilutions)
(both from Abcam) overnight at 4°C. Cells were washed three times
with PBS and then incubated with Alex-488-conjugated
donkey-anti-rabbit IgG and Dylight-649-conjugated donkey-anti-mouse
IgG (1:200 dilutions, Jackson ImmunoResearch, West Grove, PA, USA)
secondary antibodies. The slices were incubated at 37°C for 45 min,
and nuclear staining was performed with DAPI. Coverslips were
mounted with fluorescent mounting medium onto glass slides, and
examined with confocal microscopy (Leica TCS SP5II; Leica
Microsystems, Wetzlar, Germany). Images were collected from at
least three independent experiments and processed for presentation
in figures using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA,
USA).
Statistical analysis
The stata17 software package was used for all
statistical analyses. Comparisons between paired samples were
determined by Wilcoxon signed-rank test. For the quantitative
analysis of endothelial expression, one way ANOVA with post hoc
test (Student-Newman-Keuls) was used for multiple comparisons of
the merged areas covering CD105 with CD31 or VEGFR2. For clinical
data analysis, a Chi-square test was used. A P-value of <0.05
was considered statistically significant.
Results
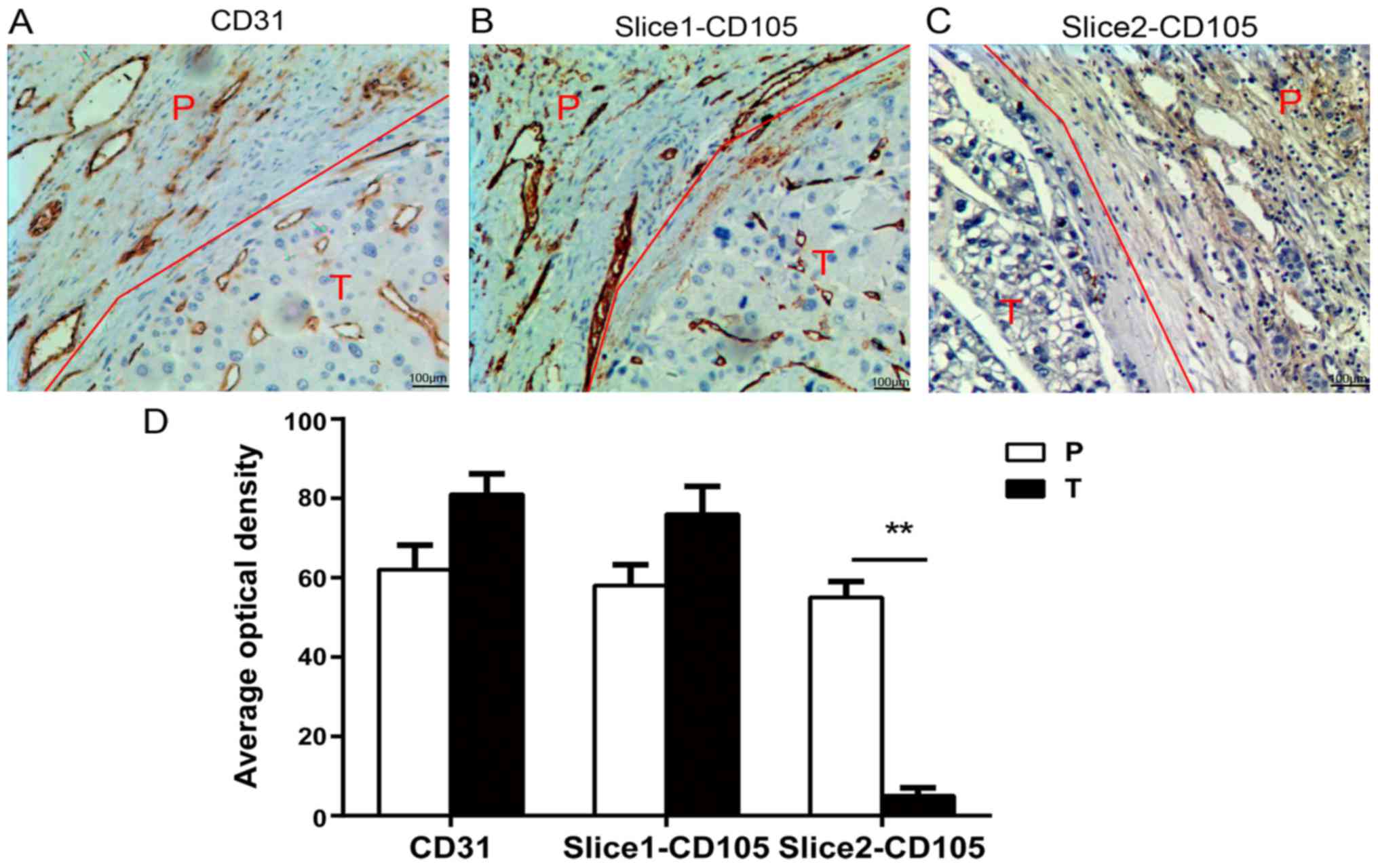
Sections of FFPE tissues derived from
patients with HCC lacked endothelial CD105 expression
As presented in Fig.
1 by IHC detection, CD31 was universally present in vessels of
all tumor and corresponding peritumoral tissues (Fig. 1A). Similarly, CD105 presented in all
peritumoral tissues. However, CD105 in tumor tissues was
differentially expressed: Of 15 patients with HCC, 9 tumor FFPE
tissues presented CD105 abundantly in the vessels and in some tumor
cells (Fig. 1B); whereas 6 tumor
FFPE tissues appeared to exhibit weak or negative CD105 expression
(Fig. 1C). Quantitative analysis
revealed the differences of intensity between CD31 and CD105
expression, as well as between tumor and peritumoral tissues.
Notably, the intensity of CD105 in those 6 tumor FFPE tissues with
little or no CD105 expression (AOD=5±2) was significantly decreased
when compared with corresponding peritumoral tissues (AOD=55±4),
P<0.01 (Fig. 1D).
Furthermore, we qualitatively scored CD31 and CD105
expression in tumor vessels by TMA and found that among 90 patients
with HCC, CD31 was expressed in tumor vessels of all TMA specimens
including tumor and peritumoral areas (Fig. 2A-D and Table II), whereas in 39 out of 90 cases
(43.3%) CD105 expression was absent in tumors in contrast to
corresponding peritumoral tissues in which CD105 was present
(Fig. 2E-L and Table II). In addition, the overall
intensity of the CD105 signal in tumors (AOD=19.2±4.6) was markedly
low, compared with CD31 (AOD=90±4.6) as well as with the intensity
of CD105 in peritumor tissues (AOD=31.3±5.6) (P<0.05) (Fig. 2M). To investigate the clinical
implication of CD105 expression variation, we retrospectively
analyzed the clinicopathological data and found that of 39 CD105
negative cases, only 10 cases (25.6%) were in the
well-differentiated group, and the remaining 29 cases (74.4%) were
in the poor-differentiated group (Table III). Furthermore, the overall
survival in the remaining 29 patients was significantly reduced
(data not shown). In brief, the present results revealed that low
or negative expression of CD105 in tumor vessels may indicate the
deterioration of disease.
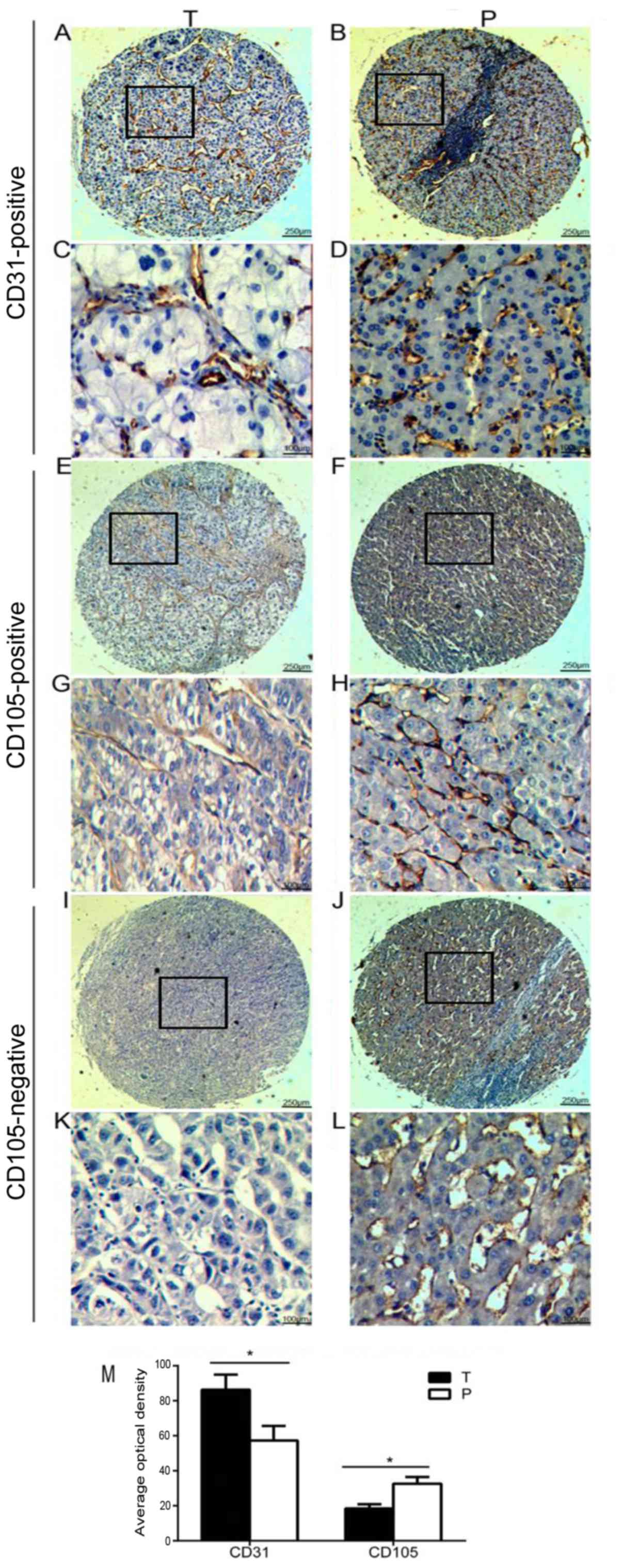 | Figure 2.Tumor vessels with or without CD105
expression on TMA of HCC. (A-D) Representative images of CD31
expression in the T and P tissues (n=90 pairs). (E-H)
Representative images of CD105-positive expression in T and P
tissues (n=51 pairs). (I-L) Representative images of CD105-negative
expression in tumor (I and K) and positive expression of CD105 in
peritumoral (J and L) tissues (n=39 pairs). Magnification ×40 for
A, B, E, F, I, J images, ×100 for C, D, G, H, K, L images of TMA.
(M) Total average optical density of CD31 and CD105 expression in
tumor and peritumoral tissues (n=90 pairs). *P<0.05. T, tumor;
P, peritumoral. |
 | Table II.Overall analysis of HCC blood vessels
with or without CD31 and CD105 expression by TMA with IHC
staining. |
Table II.
Overall analysis of HCC blood vessels
with or without CD31 and CD105 expression by TMA with IHC
staining.
|
|
| Expression (%) |
|
|---|
|
|
|
|
|
|---|
| Marker | No. of cases | Negative | Positive | P-value |
|---|
| CD105 | 90 | 39 (43.3) | 51 (56.7) | <0.01 |
| CD31 | 90 | 0 (0) | 90 (100) |
|
 | Table III.Clinical implication of the 39 cases
with negative expression of CD105. |
Table III.
Clinical implication of the 39 cases
with negative expression of CD105.
| Cases | Well (%) | Poor (%) |
|---|
| Total no. of
cases | 10/90 (11.1) | 29/90 (32.2) |
| No. of
CD105neg cases | 10/39 (25.6) | 29/39 (74.4) |
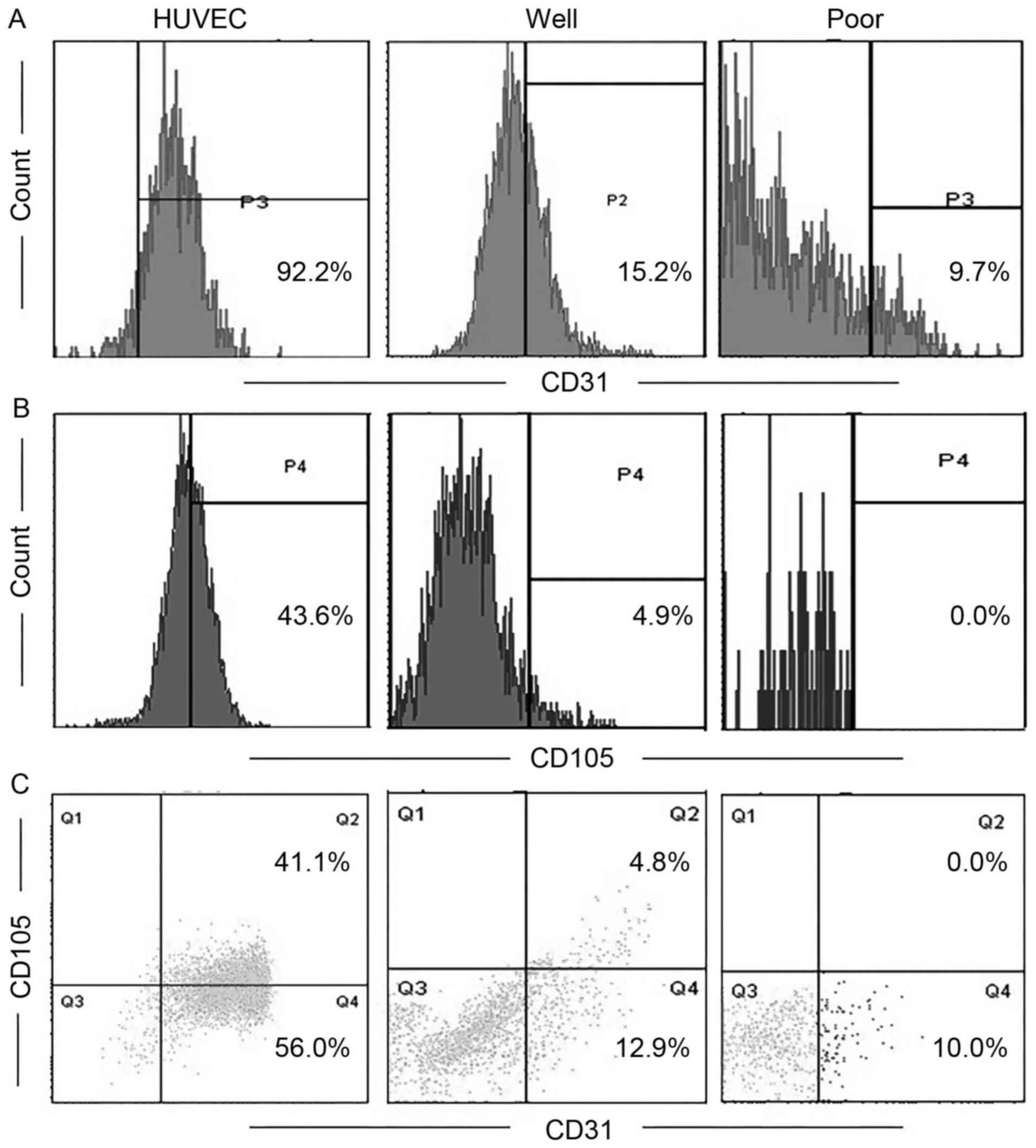
Variation in CD31 and CD105 expression
levels at the cellular level, detected via flow cytometry and
confocal microscopy analysis
In order to effectively conduct these assays to
detect endothelial markers at the cellular level, a TEC single cell
suspension was required. We respectively isolated TECs from 11
fresh resections from patients with HCC that were selected from the
15 aforementioned cases. Representative profiles from the flow
cytometry analysis revealed that the CD31 expression level was
92.2, 15.2, and 9.7% respectively in HUVECs, well- and
poor-differentiated groups (Fig.
3A). CD105 expression was 43.6 and 4.9% in HUVECs and the
well-differentiated group, respectively. A representative sample of
patients with poor-differentiated HCC demonstrated undetectable
levels of CD105 (Fig. 3B).
CD31+CD105+ double positive cells in HCC
tissues decreased compared with in HUVECs (as a positive control)
(Fig. 3C). The result indicated that
the more advanced the stage of HCC, the lower the expression level
of CD105.
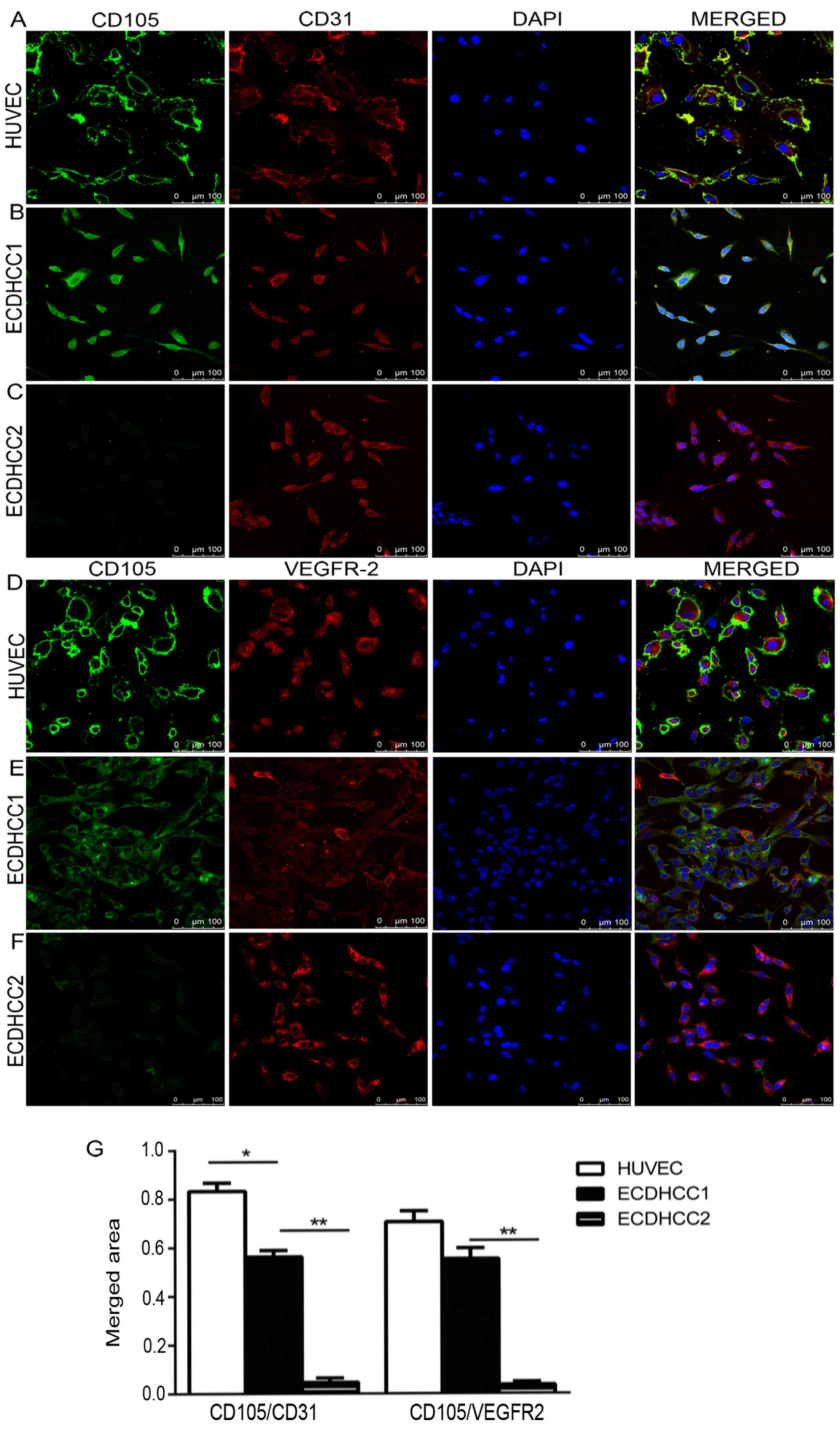
Using ‘double staining’ in cells for confocal
microscopy analysis makes it possible to detect co-localization of
two endothelial makers in a cell. We performed ‘double staining’ in
TEC (ECDHCC) and HUVECs (control) with 3 endothelial markers CD31,
CD105 and VEGFR-2. As shown in Fig.
4, the representative images revealed that CD31 or VEGFR-2 were
expressed abundantly in ECDHCC cells from all tested samples
regardless of the degree of differentiation, in addition to HUVECs
(Fig. 4A-F in the left 2 panels).
However, CD105 positive expression presented in the
well-differentiated cases (n=6/11) only (Fig. 4B and E left panels), and little or no
CD105 was expressed in the poor-differentiated cases (n=5/11)
(Fig. 4C and F left panels). On
investigation of co-expression of CD105 with other endothelial
markers, it was observed that an increased level of co-expression
of CD105 with CD31 or VEGFR-2 appeared in HUVECs with 83.3±5.2 and
70.7±3.9% of cover area (Fig. 4A-D,
right panels and 4G). In the well-differentiated group, the merged
areas of CD105 co-expressed with CD31 or VEGFR-2 were 56.8±2.6 and
55.3±4.8% (Fig. 4B-E, right panels
and 4G). Whereas there was little or no merged area in the
poor-differentiated group (Fig.
4C-F, right panels). The difference of the merged areas of
CD105 with CD31 or VEGFR-2 between the well- and
poor-differentiated group was significant (P<0.01) (Fig. 4G).
Discussion
Antiangiogenic therapies aim to inhibit tumor
angiogenesis by targeting the tumor blood vessels and lead to a
‘starvation effect’ on tumors and are extensively applied in
clinical solid cancer treatment (21,22).
However, it has recently been noted that the efficiency of current
antiangiogenic cancer therapy is limited and the treatment outcomes
are different. This may potentially be due to the complex process
of tumor angiogenesis, that leads to heterogeneity of tumor
microvascular structures, including T-MAP (2), and multiple cell sources of recruitment
for tumor angiogenesis, including cancer stem-like cells (23,24),
bone marrow-derived endothelial and hematopoietic precursor cells
(25). Current drugs targeting tumor
angiogenesis have been developed based on studies using normal ECs.
Thus, it is necessary to isolate endothelial cells from tumor
tissue for further understanding and improved treatment options and
specificity.
Recently, several laboratories have isolated TECs
using a variety of methods, however MACS is a far more specific
method. Typically, endothelial markers including CD31, CD34 and
CD105 are used for making antibodies that are bound with the
magnetic beads, so that the cells expressing these markers can be
positively selected. However, it is a controversial issue as to
which type of endothelial molecule, as a stable marker, can be used
for isolation of TECs. CD31 is known as a pan endothelial marker
(26,27). In our previous study, we
phenotypically and functionally identified CD31+ TECs
from human HCC mass (unpublished data). CD34 has also been used for
isolation of TEC cells (28),
however is primarily expressed in hematopoietic stem/progenitor
cells (29). CD105 not only serves
as an endothelial marker (11,30) but
is also expressed in tumor cells and thus may act as a prognosis
marker for cancer (13,31). These results indicate that the cells
isolated using CD105-MACS contain both endothelial
CD105+ and tumoral CD105+ cells.
The present study demonstrated that all 90 HCC-TMA
cases exhibited CD31 expression in tumor tissue spots, whereas of
these samples 39 HCC-TMA cases exhibited little or no CD105
expression in tumors, however this was not the case in peritumoral
tissue spots. Of the 39 cases with negative CD105 expression, 29
cases (74.4%) were poor-differentiated HCC. Interestingly, we found
that the more advanced the stage of HCC, the lower the expression
level of CD105. These findings were further verified by FACS
analysis of CD31 and CD105 expression levels in a single cell
digested from 11 HCC tissues, and by confocal analysis of TECs
isolated from the same 11 HCC tissues. We found that HCC with poor
differentiation did not express CD105. Similar to our data, it was
demonstrated that CD105 has a lower expression in HCC compared with
tumor free tissues, by MVD-CD105 (13). CD105 was also demonstrated to be
completely negative in 28 cases of 86 HCC sections examined
(17). Conversely, endothelial CD105
has a high expression in a wide range of cancers, including colon,
breast, brain, lung, prostate and cervical (4).
On comparison of CD105 with CD31 in tumor vessels of
HCC, our study demonstrated that there might be a limitation to use
CD105 as an endothelial marker for isolation of TECs, particularly
in poor-differentiated HCC cases, due to lacking CD105 expression.
In addition, there might be a potential risk of a contamination
with CD105+ tumor cells. We believe that CD31, and not
CD105 is a reliable endothelial marker for isolation of TECs.
Further research is required in order to clarify the potential
mechanisms.
Acknowledgements
The authors would like to thank Dr Runhua Luo
(Cancer Research Center Nantong, Tumor Hospital Affiliated to
Nantong University, China) for technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272378), Nantong
Science and Technology Bureau (grant no. MS22015005), Tumor
Hospital Affiliated to Nantong University (grant no. YY201211) and
by the Nantong Medical Young Talents grant (grant no. 2017.33).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HQ and WZ were involved in acquisition and analysis
of confocal and flow cytometry data. HC and SH were involved in the
collection of human tissues. HQ was involved in interpretation of
the data. LY was involved in the conception and design of the
present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by Ethics Committee of Tumor
Hospital Affiliated to Nantong University (no. 2015-0067). Written
informed consent was signed and obtained from all the patients who
were involved in this study.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Holleb AI and Folkman J: Tumor
angiogenesis. CA Cancer J Clin. 22:226–229. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bian XW, Wang QL, Xiao HL and Wang JM:
Tumor microvascular architecture phenotype (T-MAP) as a new concept
for studies of angiogenesis and oncology. J Neurooncol. 80:211–213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohga N, Ishikawa S, Maishi N, Akiyama K,
Hida Y, Kawamoto T, Sadamoto Y, Osawa T, Yamamoto K, Kondoh M, et
al: Heterogeneity of tumor endothelial cells: Comparison between
tumor endothelial cells isolated from high- and low-metastatic
tumors. Am J Pathol. 180:1294–1307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duff SE, Li C, Garland JM and Kumar S:
CD105 is important for angiogenesis: Evidence and potential
applications. FASEB J. 17:984–992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahajan KD, Nabar GM, Xue W, Anghelina M,
Moldovan NI, Chalmers JJ and Winter JO: Mechanotransduction effects
on endothelial cell proliferation via CD31 and VEGFR2: Implications
for immunomagnetic separation. Biotechnol J. 12:2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin M, Guan X, Wang H, Zhang Y, Shen B,
Zhang Q, Dai W, Ma Y and Jiang Y: An effective ex vivo approach for
inducing endothelial progenitor cells from umbilical cord blood
CD34+ cells. Stem Cell Res Ther. 8:252017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang
PY, Zhang JB, Wang L, Wu WZ, Qin LX and Tang ZY: Human
hepatocellular carcinoma tumor-derived endothelial cells manifest
increased angiogenesis capability and drug resistance compared with
normal endothelial cells. Clin Cancer Res. 15:4838–4846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moghaddam Afshar N, Mahsuni P and Taheri
D: Evaluation of endoglin as an angiogenesis marker in
glioblastoma. Iran J Pathol. 10:89–96. 2015.PubMed/NCBI
|
|
9
|
Davidson B, Stavnes HT, Førsund M, Berner
A and Staff AC: CD105 (Endoglin) expression in breast carcinoma
effusions is a marker of poor survival. Breast. 19:493–498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fonsatti E, Nicolay HJ, Altomonte M, Covre
A and Maio M: Targeting cancer vasculature via endoglin/CD105: A
novel antibody-based diagnostic and therapeutic strategy in solid
tumours. Cardiovasc Res. 86:12–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakurai T, Okumura H, Matsumoto M,
Uchikado Y, Owaki T, Kita Y, Setoyama T, Omoto I, Kijima Y,
Ishigami S, et al: Endoglin (CD105) is a useful marker for
evaluating microvessel density and predicting prognosis in
esophageal squamous cell carcinoma. Anticancer Res. 34:3431–3438.
2014.PubMed/NCBI
|
|
12
|
Martinez LM, Labovsky V, Calcagno Mde L,
Davies KM, Rivello HG, Wernicke A, Calvo JC and Chasseing NA:
Comparative prognostic relevance of breast intra-tumoral
microvessel density evaluated by CD105 and CD146: A pilot study of
42 cases. Pathol Res Pract. 212:350–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saroufim A, Messai Y, Hasmim M, Rioux N,
Iacovelli R, Verhoest G, Bensalah K, Patard JJ, Albiges L, Azzarone
B, et al: Tumoral CD105 is a novel independent prognostic marker
for prognosis in clear-cell renal cell carcinoma. Br J Cancer.
110:1778–1784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Y, Pan Y, Chen J, Sun X, Qiu Y and
Ding Y: Endoglin (CD105) expression in angiogenesis of primary
hepatocellular carcinomas: Analysis using tissue microarrays and
comparisons with CD34 and VEGF. Ann Clin Lab Sci. 37:39–48.
2007.PubMed/NCBI
|
|
15
|
Chen CH, Chuang HC, Lin YT, Fang FM, Huang
CC, Chen CM, Lu H and Chien CY: Circulating CD105 shows significant
impact in patients of oral cancer and promotes malignancy of cancer
cells via CCL20. Tumour Biol. 37:1995–2005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nair S, Nayak R, Bhat K, Kotrashetti VS
and Babji D: Immunohistochemical expression of CD105 and TGF-β1 in
oral squamous cell carcinoma and adjacent apparently normal oral
mucosa and its correlation with clinicopathologic features. Appl
Immunohistochem Mol Morphol. 24:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho JW, Poon RT, Sun CK, Xue WC and Fan ST:
Clinicopathological and prognostic implications of endoglin (CD105)
expression in hepatocellular carcinoma and its adjacent
non-tumorous liver. World J Gastroenterol. 11:176–181. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Hampson IN, Hampson L, Kumar P,
Bernabeu C and Kumar S: CD105 antagonizes the inhibitory signaling
of transforming growth factor beta1 on human vascular endothelial
cells. FASEB J. 14:55–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Issa R, Kumar P, Hampson IN,
Lopez-Novoa JM, Bernabeu C and Kumar S: CD105 prevents apoptosis in
hypoxic endothelial cells. J Cell Sci. 116:2677–2685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hewett PW: Isolation and culture of human
endothelial cells from micro- and macro-vessels. Methods Mol Biol.
1430:61–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel AB, Cohen EI, Ocean A, Lehrer D,
Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli
J, et al: Phase II trial evaluating the clinical and biologic
effects of bevacizumab in unresectable hepatocellular carcinoma. J
Clin Oncol. 26:2992–2998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gutierrez M and Giaccone G: Antiangiogenic
therapy in nonsmall cell lung cancer. Curr Opin Oncol. 20:176–182.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ricci-Vitiani L, Pallini R, Biffoni M,
Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G,
Larocca LM, et al: Tumour vascularization via endothelial
differentiation of glioblastoma stem-like cells. Nature.
468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castilho-Fernandes A, de Almeida DC,
Fontes AM, Melo FU, Picanço-Castro V, Freitas MC, Orellana MD,
Palma PV, Hackett PB, Friedman SL, et al: Human hepatic stellate
cell line (LX-2) exhibits characteristics of bone marrow-derived
mesenchymal stem cells. Exp Mol Pathol. 91:664–672. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubatt JM, Darcy KM, Hutson A, Bean SM,
Havrilesky LJ, Grace LA, Berchuck A and Secord AA: Independent
prognostic relevance of microvessel density in advanced epithelial
ovarian cancer and associations between CD31, CD105, p53 status,
and angiogenic marker expression: A Gynecologic Oncology Group
study. Gynecol Oncol. 112:469–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyata Y, Mitsunari K, Asai A, Takehara K,
Mochizuki Y and Sakai H: Pathological significance and prognostic
role of microvessel density, evaluated using CD31, CD34, and CD105
in prostate cancer patients after radical prostatectomy with
neoadjuvant therapy. Prostate. 75:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Bryder D, Adolfsson J, Nygren J,
Månsson R, Sigvardsson M and Jacobsen SE: Identification of
Lin(−)Sca1(+)kit(+)CD34(+)Flt3-short-term hematopoietic stem cells
capable of rapidly reconstituting and rescuing myeloablated
transplant recipients. Blood. 105:2717–2723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Y, Gu H, Xu Y, Li F, Kuang S, Wang Z,
Zhou X, Ma H, Li P, Zheng Y, et al: Targeted antiangiogenesis gene
therapy using targeted cationic microbubbles conjugated with CD105
antibody compared with untargeted cationic and neutral
microbubbles. Theranostics. 5:399–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Zhai Z, Liu D, Zhong X, Meng X, Yang
Q, Liu J and Li H: CD105 promotes hepatocarcinoma cell invasion and
metastasis through VEGF. Tumour Biol. 36:737–745. 2015. View Article : Google Scholar : PubMed/NCBI
|