Introduction
Alveolar echinococcosis (AE) is a lethal hepatic
disease caused by the Echinococcus multilocularis (E.
multilocularis) infection at the larval stage. Humans may get
infected by swallowing the contaminated eggs. The disease is a
prevalent epidemic in the northern hemisphere, especially in
central Europe and western China (1,2). The
larval vesicles of the parasite propagate asexually in liver, the
same as the tumor. Generally, the clinical symptoms could persist
for a long time (up to 10 years), which usually causes liver
dysfunction. At present, most AE patients are diagnosed with
imaging methods, including radiology, magnetic resonance imaging,
ultrasonography, and computed axial tomography (3–6).
However, performing these methods in remote areas is relatively
hard where the resources are limited. Thus, it is of great
significance to diagnose AE patients in remote areas.
Immunodiagnostic has been suggested as a possible method for
screening a large population and as being suitable for diagnosis of
AE (7,8).
To identify and screen human AE, a 2-step procedure
for diagnosis has been recommended by the World Health Organization
(WHO) (9). First, Serologic
screening is firstly conducted. The commonly used techniques
included enzyme-linked immune sorbent assays (ELISAs), radio
allegro sorbent tests (RAST), and indirect hemagglutination (IHA).
Then, the results are further confirmed by the
immunoelectrophoresis (IEP) or immunoblot (IB). Recently, Em18 has
been isolated from the metacestode of the parasites (10), and was demonstrated to have high
sensitivity and specificity for AE diagnosis through ELISA or IB
(8,11,12).
However, these studies only included a relatively small panel of AE
serum samples (approximately 20–30 AE patients). The value of
recombinant Em18 (recEm18) in AE diagnosis with a large number of
serum samples is not reported yet.
Here in this study, we have cloned and expressed the
rEm18 in Escherichia coli (E. coli) as a GST fusion protein.
A large number of serum samples were collected from patients in
Xinjiang. Our aim was to clarify whether recEm18 is of high
sensitivity and specificity in the serodiagnosis of AE, both by
using IB and ELISA.
Materials and methods
Patients and serum samples
Between March 2013 and December 2016, 536 serum
samples (Table I) were enrolled at
the First Affiliated Hospital of Xinjiang Medical University
(Xinjiang, China). All protocols and usage of human sera in the
study were approved by the Ethic Committee of the First Affiliated
Hospital of Xinjiang Medical University. Fifty serum samples were
from AE patients, who were diagnosed by surgery, imaging method,
and serology analysis with a commercially available kit
(Registration number 20153400177, Xinjiang Bestmind Bio Technology
Development Co., Ltd, Urumqi, China) (7), and 222 serum samples were from CE
patients who were confirmed by parasitological examinations after
surgical removal. There were also serum samples collecting from
patients with other unrelated parasitic diseases or nonparasitic
diseases, including cysticercosis (n=9), schistosomiasis (n=7),
paragonimiasis (n=32), clonorchiasis (n=20), cyst (n=6), cancer
(n=8), or other disease (n=76). The remaining samples were
collected from healthy persons (n=106), and 40 sera of them were
used to calculate cut-off value.
 | Table I.Characteristics of patients and
healthy individuals. |
Table I.
Characteristics of patients and
healthy individuals.
| Characteristics | AE (n=50) | CE (n=222) | Other diseases
(n=158) | Healthy individuals
(n=106) |
|---|
| Age at diagnosis
(years), mean ± standard deviation | 44.6±15.7 | 49.6±11.6 | 39.0±16.0 | 46.2±10.7 |
| Sex, n (%) |
|
|
|
|
| Male | 19 (37.5) | 75
(33.7) | 80 (50.6) | 44 (41.6) |
|
Female | 31 (62.5) | 147 (66.3) | 78 (49.4) | 62 (58.3) |
| Source of
drinking |
|
|
|
|
| Water, n (%) |
|
|
|
|
| Well | 25 (50.0) | 115 (51.8) | – | – |
|
Spring | 20 (40.0) | 74
(33.3) | – | – |
|
River | 3 (6.0) | 12 (5.4) | – | – |
|
Tap | 2 (4.0) | 21 (9.5) | – | – |
| Dog in the
family |
|
|
|
|
|
Yes | 23 (46.0) | 77
(34.7) | – | – |
| No | 27 (54.0) | 145 (65.3) | – | – |
| Previous history of
hydatidosis |
|
|
|
|
|
Yes | 14 (28.0) | 55
(24.8) | – | – |
| No | 36 (72.0) | 167 (75.2) | – | – |
Parasites and RNA extraction
Protoscolices (PSCs) were isolated from a
Mongoliangerbils (Meriones unguiculatus) that had E.
Multilocularis infection by intraperitoneal injection
(i.p). After washing with PBS for 10 times, the PSCs were
precipitated and aliquoted. The total RNA was then isolated from
the PSCs with TRIzol reagent (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Reverse transcription PCR and
pGEM-T-Em-18 plasmid construction
The extracted RNAs were reverse transcribed into
cDNA with a reverse transcription kit (SuperScript™
Preamplification System; Promega Corporation, Madison, WI, USA)
per the manufacturer's instructions. To exclude the
potential genomic DNA contamination, parallel reactions without
reverse transcriptase were performed. The total volume for PCR was
50 µl, including 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM
MgCl2, 0.001% (wt/vol) gelatin, 0.1 µM of each primer,
0.2 mM of each deoxynucleoside triphosphate, 2 µl cDNA, and 0.5
units of Taq DNA polymerase (Promega Corporation). The primer
sequences were as follows: Up-stream primer,
5′-CCGGAATTCATGAAGGAGTCTGACTTAGCGGAT-3′ and downstream primer,
5′-CCGCTCGAGTTTGAGGTTGGCCATCTTCGT-3′). The PCR conditions were 94°C
for 5 min followed by 35 cycles of 94°C for 30 sec, 55°C for 30
sec, and 72°C for 2 min. After that, the product was cloned into a
pGEM-T plasmid vector (Promega Corporation) and sequenced.
Sequence analysis
Sequencing data were analyzed using BLAST
(http://www.ncbi.nlm.nih.gov/BLAST/).
The predicted Em18 protein sequences of E. multilocularis
and other parasites were aligned using CLUSTAL (http://www.ebi.ac.uk/clustalw/#). The MEGA6.06
was used to construct the phylogenetic tree (13) and the neighbor-joining method
(14) with 1,000 bootstrap
replications was used.
Subcloning, expression, and
purification of recombinant Em18 (rEm18)
The pET-41a (+)-Em18 plasmid was constructed by
excising Em-18 sequence from thepGEM-T-Em-18 plasmid and ligating
into the EcoRI and XhoI site of the pET-41a (+) expression vector
(Novagen, Inc., Madison, WI, USA). The recombinant pET-41a (+)-Em18
plasmid was transformed into E. coli BL21 (DE3) cells
(Novagen, Inc.). The expression of rEm18-GST fusion protein was
induced with 1.0 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for
3 h at 37°C. The protein was purified using GST binding resin under
native conditions. At last, the purified protein was quantified by
the Bradford assay. Bovine serum albumin was used as a standard
sample.
Immunoblot analysis (IB)
The rEm18-GST fusion protein was separated by 12%
SDS-PAGE gels and then transferred onto a nitrocellulose membrane.
After that, the membrane was cut into strips, with approximately
0.3 µg of rEm18-GST on each strip. The strips were blocked with 5%
skim milk. Then, human serum samples (diluted 1:100) was added and
incubated for 1 h at 37°C. After rinsing with PBST for 3 times, the
secondary antibodies of goat anti-human IgG conjugate with HRP
(Sigma; Merck KGaA, Darmstadt, Germany) were added and incubated
for 2 h at room temperature. Finally, the strips were incubated
with diaminobenzidine (DAB) for 15 min at room temperature for
color development.
ELISA
ELISA were performed using plates coated with rEm18.
Briefly, microtitration plates (Nalge Nunc International, Roskilde,
Danmark) were coated with 100 µl of antigen solution (1 µg/ml) per
well in carbonate/bicarbonate buffer (pH 9.6) at 4°C overnight.
After washing PBST for three times, the plate was incubated with 5%
skim milk for 1 h at 37°C. After washing again, 100 µl of serum
sample (1:100 dilution) was added into each well and incubated at
37°C for 1 h. Then, 100 µl of secondary antibody
(peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG)
(Sigma; Merck KGaA) was added, and the mixture was incubated for 1
h at 37°C. Finally, 100 µl of substrate was added to each well and
incubated for 15 min at 37°C. The optical density at 450 nm
(OD450) was measured with the ELISA plate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Serum samples were
recorded as positive if the OD values were greater than the three
times of the OD450 values for 40 healthy normal controls.
Statistical analysis
The values of sensitivity, specificity, and positive
and negative predictive values were calculated. Fisher's exact test
was used for comparison among groups. A P<0.05 was considered to
be statistically significant.
Results
Molecular cloning and characterization
of Em18
The Em18 gene sequence (GenBank accession no.
AY513691.1), which comprised a 486-bp coding region, was amplified
with the specific primers by PCR from cDNA of E.
multilocularis PSCs. Em18 protein contains 161 amino acid and
has a molecular mass of 18.3 kDa. Based on the sequence homology
analysis, the recEm18 was similar to recEm18 from Sichuan (GenBank
accession no. AAS00619.1) with only three different amino acids.
Meanwhile, comparative analysis between Em18 and Eg18 from the
Xinjiang isolate (GenBank accession no. AY513265.1) revealed an
identical nucleotide sequence. The pET-41a (+)-Em18 plasmid was
constructed and the expression of rEm18-GST was induced with IPTG.
After that, the fusion protein was purified through GST affinity
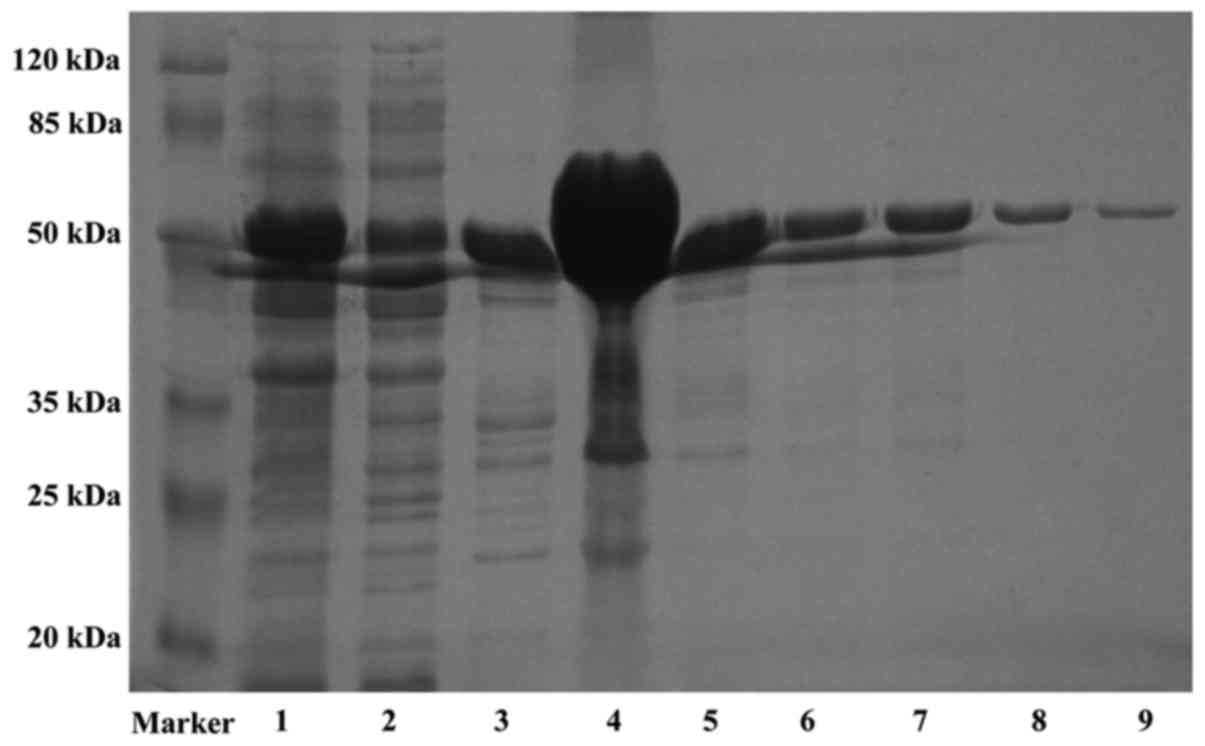
beads, which was approximately 51 kDa (Fig. 1). These results indicate that Em18
from Sichuan and Xinjiang have little difference in the amino acid
sequence.
Phylogenetic comparison of Em18 and
Eg18
The neighbor joining method was used to construct a
phylogenetic tree. As shown in Fig.
2, Em18 was closely related to Eg18, Eg10, EmII/3 as well as
E. granulosus tegument protein, and Moesin/ezrin/radixin
protein. However, the homology of this recombinant antigen with
human and other parasitic moesin family proteins was the lowest.
Thus, the phylogenetic data suggested that AE patients can be
easily distinguished from patients contaminated infected with other
parasites by rEm18.
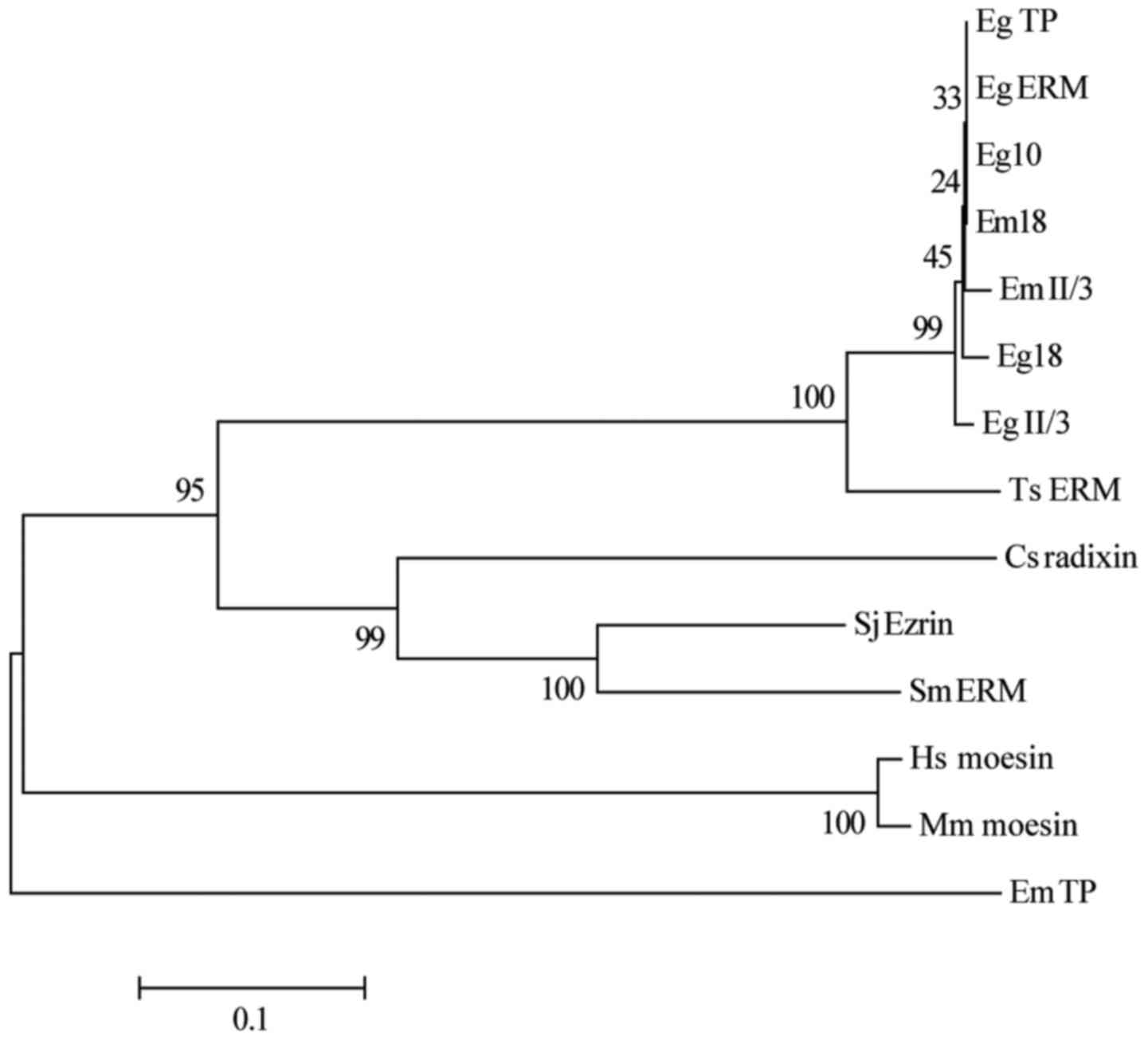 | Figure 2.Phylogenetic tree for consensus of
Em18 sequences and relative reference sequences by Neighbor-joining
method. The phylogenetic tree was constructed using the
neighbor-joining method with MEGA6.06 based on the amino acid
alignment (Clustal W). The bootstrap percentage values of 1,000
replicates are indicated at each branch node. The GenBank accession
numbers of the analyzed sequences are as follows: EmTP, E.
multilocularis tegument protein, AAA29063.1; EmII/3, E.
multilocularis antigen II/3, AAA50580.1; EgII/3, E.
granulosus, antigen II/3, AAA50580.1; Em18, E.
multilocularis, AAR99825.1; Eg18, E. granulosus,
AAS00620.1; Ts ERM, Taenia solium ezrin-radixin-moesin-like
protein, BAI49989.1; Eg10, E. granulosus, CAA82625.1; Sj
Ezrin, Schistosoma japonicum Ezrin, CAX72657.1; Sm ERM,
Schistosoma mansoni merlin/moesin/ezrin/radixin protein,
CCD82162.1; EgTP, E. granulosus tegument protein,
CDS19541.1; Eg ERM, E. granulosus Moesin/ezrin/radixin
protein, EUB64035.1; Cs radixin, Clonorchis sinensis
radixin, GAA42464.2; Hs moesin, Homo sapiens moesin,
NP_002435.1; and Mm moesin, Mus musculus moesin,
NP_034963.2. |
Diagnostic performance of rEm18-GST by
IB and ELISA
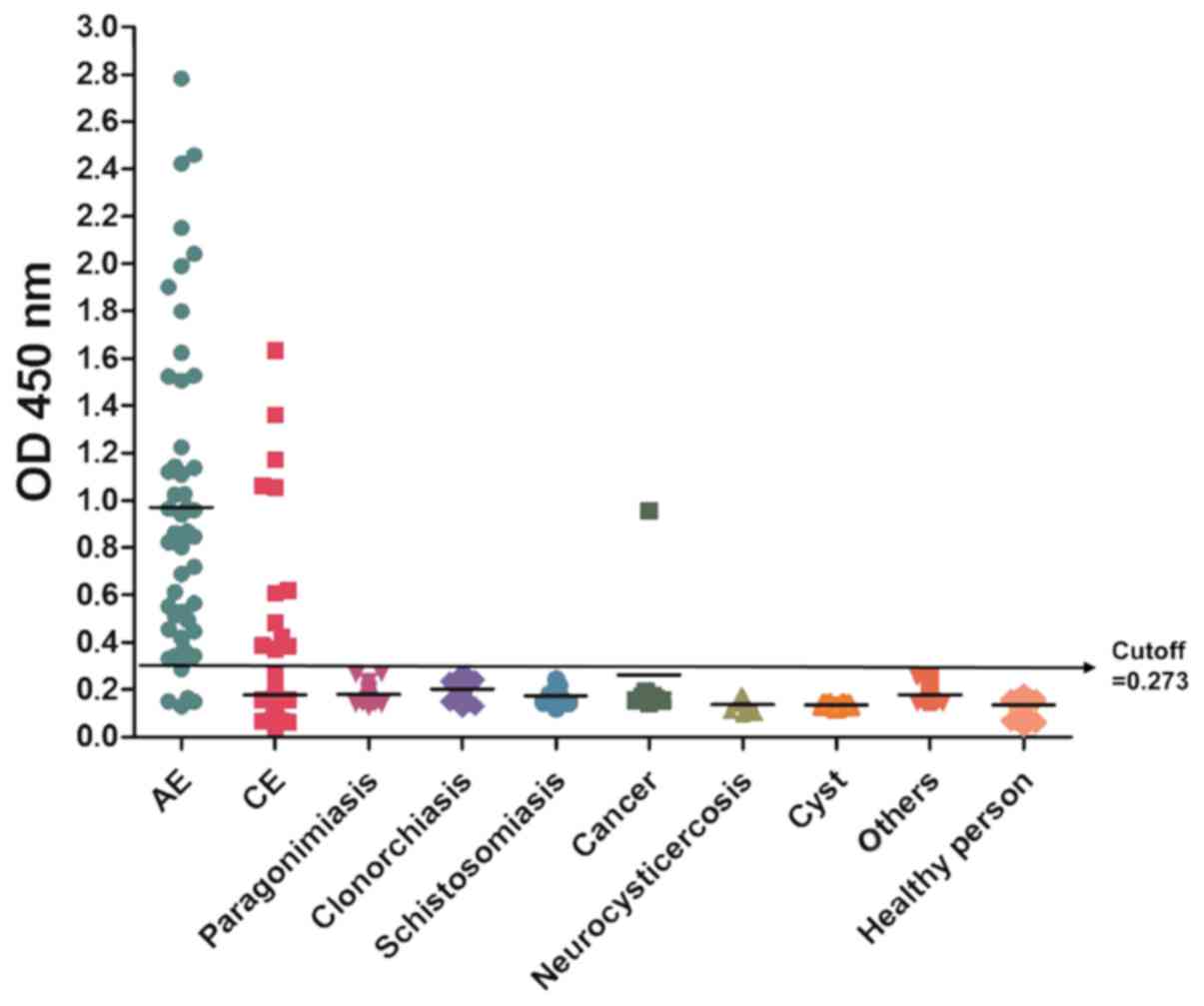
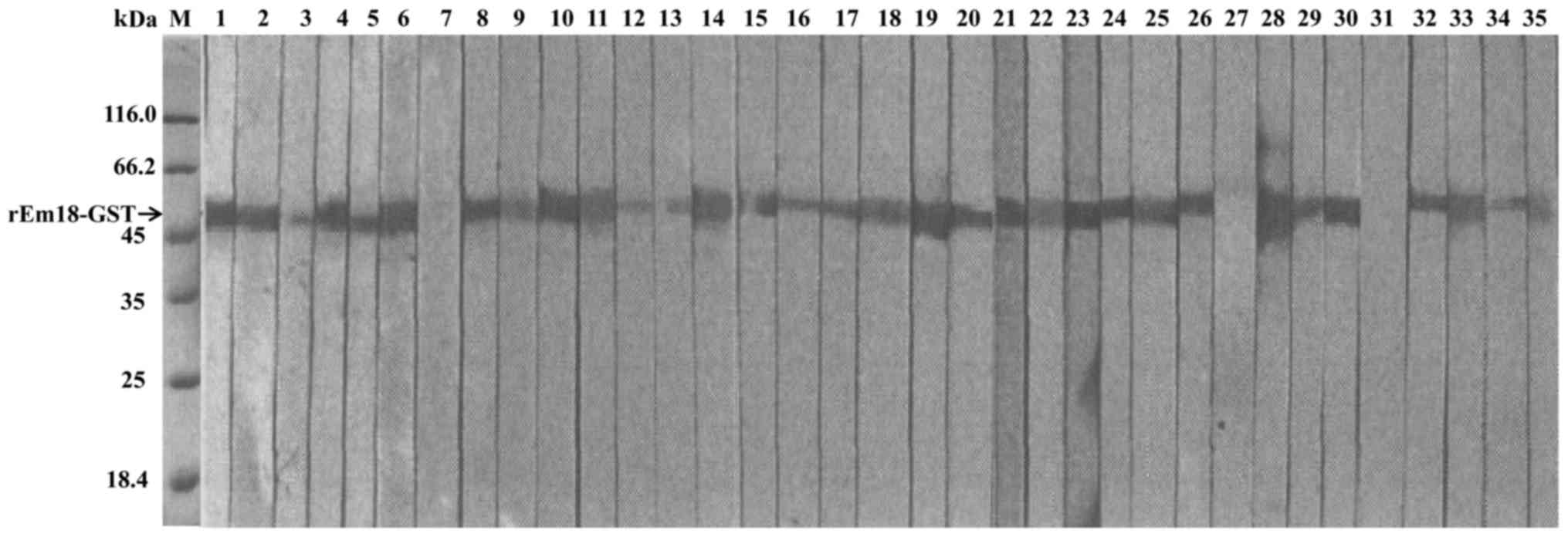
ELISA (Fig. 3) and IB
(Fig. 4) methods were used to
evaluate the diagnostic value of the purified rEm18-GST fusion
protein. The serum samples were collected from AE patients. As
shown in Table II, rEm18-GST showed
positive reactions with 94% (47/50) of serum samples from AE
patients, as revealed by IB testing, and the ratio was 92% (46/50)
by ELISA. For patients who had unrelated diseases, a total of 3.42%
of the serum samples (n=380) had positive reaction with rEm18-GST,
among which, 5.41% (12/222) of serum samples from CE patients
cross-reacted with the recEm18-GST. One sample from lung cancer
patient who have not the particular history, also cross-reacted
with rEm18-GST. However, no positive results were observed with
serum from patients with neurocysticercosis (n=9), patients with
diseases (n=149), or healthy individuals (n=106). The statistical
significance were not observed between IB and ELISA groups
(P>0.05).
 | Table II.Diagnostic performance of rEm18-GST
for human AE by IB and ELISA. |
Table II.
Diagnostic performance of rEm18-GST
for human AE by IB and ELISA.
|
|
| Positive serum
samples, no. (%) |
|---|
|
|
|
|
|---|
| Serum samples | No. | Em18-IB | Em18-ELISA |
|---|
| AE |
|
|
|
|
Surgerya | 31 | 29 (93.55) | 28 (90.32) |
| Image
analysisb plus serology | 19 | 18 (94.74) | 18 (94.74) |
| CE |
|
|
|
|
Surgerya | 106 | 3 (2.83) | 3 (2.83) |
| Image
analysisb plus serology | 116 | 9 (7.76) | 9 (7.76) |
|
Neurocysticercosis | 9 | 0 (0) | 0 (0) |
| Paragonimiasis | 32 | 0 (0) | 0 (0) |
| Clonorchiasis | 20 | 0 (0) | 0 (0) |
|
Schistosomiasis | 7 | 0 (0) | 0 (0) |
| Cancer | 8 | 1 (12.5) | 1 (12.5) |
| Cyst | 6 | 0 (0) | 0 (0) |
| Other | 76 | 0 (0) | 0 (0) |
| Healthy person | 106 | 0 (0) | 0 (0) |
| Total | 536 |
|
|
Discussion
Alveolar echinococcosis (AE) causes high chronic
morbidity and mortality in Central Asia, European countries,
North/Latin America and northwest China (15). One of the clinical features of
tissue-invasive larval cestodiases is slow progression with minimal
symptoms and signs, unless infected parasites provoke acute
symptoms (16). Early diagnosis of
AE can significantly improve the both quality of disease diagnosis
and treatment. The multiple serological studies have been carried
out that serology can be used to detect early (asymptomatic) cases
in populations living in endemic areas (6,17,18).
Immunodiagnostic of AE is doubtlessly one of the most valuable
diagnostic methods in the early detection of AE infection (19). In most cases, these methods are
cheap, relatively easy to use, and are necessary for large-scale
screening in high-risk groups (20).
Here, the fusion protein rEm18-GST was constructed and purified.
The role of rEm18-GST in the early diagnosis of AE was investigated
by IB and ELISA.
Em2 is isolated from the metacestode of E.
multilocularis (21). As a
species-specific natural antigen, Em2 has been widely used for
serodiagnostic studies (22) and has
shown encouraging results in the immunodiagnostic of human AE
(23). However, the sensitivity of
Em2 in ELISA, with range of 77 to 92%, is dependent on the
patient's geographical origin. The works on other recombinant E.
multilocular molecules have also been undertaken for the
diagnostic purposes, and the results are encouraging. The Em2 plus
ELISA, which is a combination of Em2 with a II/3-10recombinant
protein, has increased the sensitivity to 97% (21). However, the Em2 plus assay has also
shown a cross-reaction to CE (in 25.8% of cases), which is higher
than that for individual Em2 (5.6%) or II/3–10 (6.5%),
respectively. To explain why these similar proteins have distinct
immunogenicity in AE and CE patients, Sako et al (10) have speculated that E.
multilocularis may contact invasively and intimately with host
tissues. For this reason, in CE patients, B-cell responses to Em18
might be low. Further studies are needed to analyze the
relationship of the B-cell epitopes and Em18. The serodiagnostic
performance of rEm13 protein (sensitivity and specificity) has also
been analyzed (24,25), however, only a small numbers of serum
samples were tested. Our results here suggest that the diagnostic
performance of rEm18-GST by both IB and ELISA may be better than
other reported reagents, particularly when a large number of serum
samples were tested.
To sum up, our results suggest that rEm18 diagnosis
is both easy to use and cheap. This method may be used to confirm
the clinical findings of AE and a follow-up to surgery or
pharmacological therapy (26). And,
this method has the minimum requirement for equipment and time,
especially during the first screening.
Acknowledgements
Not applicable.
Funding
This work was supported by the Xinjiang Tianshan
Innovation Team Program (grant no. 201705120), The XinjiangYouth
Science and Technology Innovation Talent Training Project-Xinjiang
Outstanding Youth Natural Science Foundation Project (grant no.
QN2016JQ0327) and the National Natural Science Foundation of China
(grant nos. 81560330, 81371838, 81760368 and 81660341).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RYL designed and directed the experiment. XJB, LL,
YW and CSZ performed the experiments. XJB, GDL and HW performed the
statistical analysis. YMS and TA contributed in collecting clinical
samples. JL and WBZ provided Mongolian gerbils infected with Em18
and performed the isolation of protoscolices. XJB and YW wrote the
manuscript. JL, WBZ, HW and RYL reviewed and edited the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was granted from the
Ethic Committee of the First Affiliated Hospital of Xinjiang
Medical University and all patients gave written informed
consent.
Patient consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torgerson PR, Keller K, Magnotta M and
Ragland N: The global burden of alveolar echinococcosis. PLoS Negl
Trop Dis. 4:e7222010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou
X, Wen H and McManus DP: Epidemiology and control of echinococcosis
in central Asia, with particular reference to the People's Republic
of China. Acta Trop. 141:235–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McManus DP, Gray DJ, Zhang W and Yang Y:
Diagnosis, treatment, and management of echinococcosis. BMJ.
344:e38662012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bresson-Hadni S, Delabrousse E,
Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA and
Vuitton DA: Imaging aspects and non-surgical interventional
treatment in human alveolar echinococcosis. Parasitol Int. 55
Suppl:S267–S272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azizi A, Blagosklonov O, Lounis A, Berthet
L, Vuitton DA, Bresson-Hadni S and Delabrousse E: Alveolar
echinococcosis: Correlation between hepatic MRI findings and
FDG-PET/CT metabolic activity. Abdom Imaging. 40:56–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romig T, Kratzer W, Kimmig P, Frosch M,
Gaus W, Flegel WA, Gottstein B, Lucius R, Beckh K and Kern P: An
epidemiologic survey of human alveolar echinococcosis in
southwestern Germany. Römerstein Study Group. Am J Trop Med Hyg.
61:566–573. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng X, Wen H, Zhang Z, Chen X, Ma X,
Zhang J, Qi X, Bradshaw H, Vuitton D and Craig PS: Dot immunogold
filtration assay (DIGFA) with multiple native antigens for rapid
serodiagnosis of human cystic and alveolar echinococcosis. Acta
Trop. 113:114–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knapp J, Sako Y, Grenouillet F,
Bresson-Hadni S, Richou C, Gbaguidi-Haore H, Ito A and Millon L:
Comparison of the serological tests ICT and ELISA for the diagnosis
of alveolar echinococcosis in France. Parasite. 21:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poretti D, Felleisen E, Grimm F, Pfister
M, Teuscher F, Zuercher C, Reichen J and Gottstein B: Differential
immunodiagnosis between cystic hydatid disease and other
cross-reactive pathologies. Am J Trop Med Hyg. 60:193–198. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sako Y, Nakao M, Nakaya K, Yamasaki H,
Gottstein B, Lightowers MW, Schantz PM and Ito A: Alveolar
echinococcosis: Characterization of diagnostic antigen Em18 and
serological evaluation of recombinant Em18. J Clin Microbiol.
40:2760–2765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tappe D, Sako Y, Itoh S, Frosch M, Grüner
B, Kern P and Ito A: Immunoglobulin G subclass responses to
recombinant Em18 in the follow-up of patients with alveolar
echinococcosis in different clinical stages. Clin Vaccine Immunol.
17:944–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito A, Nakao M and Sako Y: Echinococcosis:
Serological detection of patients and molecular identification of
parasites. Future Microbiol. 2:439–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Zheng T, Zheng X, Han N, Chen X and
Zhang D: Molecular characterization of two fatty Acyl-CoA reductase
genes from phenacoccus solenopsis (Hemiptera: Pseudococcidae). J
Insect Sci. 16:pii: 49. 2016. View Article : Google Scholar
|
|
14
|
Saitou N and Nei M: The neighbor-joining
method: A new method for reconstructing phylogenetic trees. Mol
Biol Evol. 4:406–425. 1987.PubMed/NCBI
|
|
15
|
Ahn CS, Bae YA, Kim SH, Kim JG, Yu JR,
Yang HJ, Eom KS, Wang H, Kang I, Yang Y and Kong Y: Spatiotemporal
expression patterns and antibody reactivity of Taeniidae endophilin
B1. J Clin Microbiol. 54:2553–2562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cecconi A, Maroto L, Vilacosta I, Luaces
M, Ortega L, Escribano N, Vivas D, Ferreirós J, Montes L, Vilchez
JP, et al: Acute pericarditis secondary to hydatid cyst rupture:
Diagnosis by multimodality imaging. Circulation. 128:2073–2074.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottstein B, Saucy F, Deplazes P, Reichen
J, Demierre G, Busato A, Zuercher C and Pugin P: Is high prevalence
of Echinococcus multilocularis in wild and domestic animals
associated with disease incidence in humans? Emerg Infect Dis.
7:408–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bresson-Hadni S, Laplante JJ, Lenys D,
Rohmer P, Gottstein B, Jacquier P, Mercet P, Meyer JP, Miguet JP
and Vuitton DA: Seroepidemiologic screening of Echinococcus
multilocularis infection in a European area endemic for alveolar
echinococcosis. Am J Trop Med Hyg. 51:837–846. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McManus DP, Zhang W, Li J and Bartley PB:
Echinococcosis. Lancet. 362:1295–1304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Li J and McManus DP: Concepts in
immunology and diagnosis of hydatid disease. Clin Microbiol Rev.
16:18–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pektaş B, Altintaş N, Akpolat N and
Gottstein B: Evaluation of the diagnostic value of the ELISA tests
developed by using EgHF, Em2 and EmII/3–10 antigens in the
serological diagnosis of alveolar echinococcosis. Mikrobiyol Bul.
48:461–468. 2014.(In Turkish). View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deplazes P and Gottstein B: A monoclonal
antibody against Echinococcus multilocularis Em2 antigen.
Parasitology. 103:41–49. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gottstein B, Deplazes P and Aubert M:
Echinococcus multilocularis: immunological study on the
‘Em2-positive’ laminated layer during in vitro and in vivo
post-oncospheral and larval development. Parasitol Res. 78:291–297.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frosch PM, Geier C, Kaup FJ, Müller A and
Frosch M: Molecular cloning of an echinococcal microtrichal antigen
immunoreactive in Echinococcus multilocularis disease. Mol Biochem
Parasitol. 58:301–310. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi X, Liu Y, Wei W, Huang X and Zuo Y:
Effects of the C-terminal of endostatin on the tumorigenic
potential of H22 cells. Biomed Rep. 1:761–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sako Y, Tappe D, Fukuda K, Kobayashi Y,
Itoh S, Frosch M, Grüner B, Kern P and Ito A: Immunochromatographic
test with recombinant Em18 antigen for the follow-up study of
alveolar echinococcosis. Clin Vaccine Immunol. 18:1302–1305. 2011.
View Article : Google Scholar : PubMed/NCBI
|