Introduction
Although the incidence of gastric cancer has
decreased in recent years (1,2), it
remains the third leading cause of cancer-associated mortality
worldwide, with ~1,000,000 new cases diagnosed each year (3,4).
Improved techniques for the treatment of gastric cancer have been
developed, including novel surgical methods, radiation and
chemotherapy protocols; however, the prognoses of patients with
advanced gastric cancer remain poor, with a 5-year survival of
5–20% (5,6). As such, identifying efficient
chemo-adjuvants from herbal medicines may contribute to improving
treatment outcomes for patients with gastric cancer.
Traditional Chinese herbal medicines have been
studied as potential candidates for cancer treatment and a number
of studies have focused on the identification of new bioactive
compounds (7–9). Tanshinone IIA (Tan IIA), a major
bioactive compound extracted from the root of Salvia
miltiorrhiza, has been reported to exhibit a number of
pharmacological activities in cardiovascular and cerebrovascular
diseases (10–12). A number of studies have reported that
Tan IIA serves as an effective adjunctive reagent in different
types of cancer, including breast, bladder, colorectal and prostate
cancers (13–15). Although a number of studies have
indicated that Tan IIA exerts powerful anticancer effects in
gastric cancer (16,17), the detailed molecular mechanisms
remain to be elucidated.
Signal transducer and activator of transcription 3
(STAT3) is a member of the STAT family of signal-responsive
transcription factors (18). STAT3
has been demonstrated to regulate cell proliferation, survival,
angiogenesis and immunosuppression (19). It has previously been reported that
STAT3 is constitutively activated in gastric cancer and serves a
critical role in tumorigenesis (20,21). The
inactive form of STAT3 is activated by cytokines, growth factors
and oncogenic proteins via the sequential phosphorylation of
tyrosine 705 and serine 727 (22).
There is evidence that constitutive STAT3 activation may contribute
to the progression of gastric cancer (23,24), and
so identifying a novel therapeutic agent that inhibits STAT3
signaling is important for the treatment of human gastric cancer.
The aim of the present study was to investigate the effects of Tan
IIA on gastric cancer proliferation using SNU-638 cells in
vitro and in vivo and to clarify whether the underlying
mechanism is associated with the inhibition of STAT3
activation.
Materials and methods
Cell culture and drug treatment
Human gastric cancer cell lines (SNU-638, MKN1 and
AGS) were obtained from the Cell Resource Center (Chinese Academy
of Sciences, Shanghai, China). SNU-638 and MKN1 cells were cultured
in RPMI 1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and AGS cells were cultured in Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.). Cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. Tan IIA was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany; purity, ≥97%) and
dissolved in dimethyl sulfoxide (DMSO) to make 2.5 µg/ml stock
solutions. Gastric cancer cells were treated with Tan IIA in
different times (0, 12, 24, 48 and 72 h) or concentrations (0, 2.5,
5 and 10 µg/ml) at 37°C in humidified air and 5% CO2
atmosphere.
Cell proliferation assay
A modified MTT assay was used to determine the
proliferation of human gastric cancer cells. Briefly, cells were
seeded in 96-well plates at a density of 1×104
cells/well and treated with Tan IIA. Following 24 h culture at
37°C, 20 µl MTT dye solution (Sigma-Aldrich; Merck KGaA) was added
to each well and incubated for a further 2 h. The medium was
subsequently removed and replaced with Solubilization/Stop solution
(100 µl/well; Sigma-Aldrich; KGaA). Absorbance was measured at 570
nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Apoptosis assay
Apoptosis was determined using an Annexin V-FITC/PI
kit (cat. no. BB-4101-3; BestBio company, Shanghai, China)
according to the manufacturer's protocol. Following treatment with
Tan IIA, SNU-638 cells were harvested and washed with PBS. Cells
were subsequently stained with propidium iodide (PI) (20 µg/ml)
with or without Annexin V-fluorescein isothiocyanate (FITC) for 30
min in the dark at room temperature. The cells were analyzed using
a Beckman Coulter CyAn ADP Flow Cytometer (Beckman Coulter, Inc.,
Brea, CA, USA).
Western blotting
Cells were washed twice with ice-cold PBS and lysed
using RIPA lysis buffer (Thermo Fisher Scientific, Inc.) at room
temperature for 30 min. Protein concentrations were measured using
a BCA Protein Assay Reagent (Pierce; Thermo Fisher Scientific,
Inc.) according to manufacturer's protocol. An equal amount of
protein (40 µg) were separated by 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. The membranes were then
incubated at 4°C overnight with one of the following primary
antibodies: Cleaved caspase-3 (1:1,000; cat. no. 9664),
phosphorylated (p)-STAT3 (1:1,000; cat. no. 9145) and STAT3 (1:500;
cat. no. 12640) (both from Cell Signaling Technology Inc., Danvers,
MA, USA). B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no. sc-56015) and
Bcl-2-associated X protein (Bax; 1:1,000; cat. no. sc-4239; both
Santa Cruz Biotechnology Inc., Dallas, TX, USA). Membranes were
washed with TBST and incubated with a horseradish
peroxidase-conjugated anti-rabbit Immunoglobulin G (1:2,000; cat.
no. 7074; Cell Signaling Technology, Inc.) for 60 min at room
temperature. Blots were developed with enhanced chemiluminescence
(cat. no. 34580; Pierce; Thermo Fisher Scientific, Inc.) and
detected using the Las 4000 imager (GE Healthcare, Chicago, IL,
USA). β-actin (dilution 1:5,000; cat. no. SAB2100037;
Sigma-Aldrich; Merck KGaA) was used as an internal control for
normalization. The relative density of each band was analyzed using
Image J software (version 4.0; National Institutes of Health,
Bethesda, MD, USA).
STAT3 reporter plasmid transfection
and luciferase assays
SNU-638 cells were transiently co-transfected with
0.4 µg of STAT3 reporter plasmid (pSTAT3-LUC; Sangon Biotech Co.,
Ltd., Shanghai, China) and 0.4 µg of control plasmid phRL-TK
(Promega Corp., Madison, WI, USA) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 24 h following transfection,
the luciferase activity of STAT3 was measured using a
dual-luciferase reporter assay system (Promega Corp.) according to
manufacturer's protocol. Data are expressed as relative fold
activation to that of non-stimulated (−) sets.
Xenograft tumor model of human gastric
cancer
BALB/c nude mice (male, 6–8 weeks old; weight, 18–24
g) were purchased from the laboratory Animal Center of Southern
Medical University (Guangzhou, China). Mice were allowed free
access to food and water under standard conditions (temperature,
20–25°C; relative humidity, 50–60%), and exposed to a 12 h
light/dark cycle. A total of 24 BALB/c nude mice were randomly
assigned to four groups (each, n=6). SNU-638 cells were injected
into nude mice subcutaneously. At 14 days following tumor cell
injection, each mouse was injected intraperitoneally with Tan IIA
(12.5, 25 or 50 mg/kg) 3 times a week for 28 days. The control
group received an equal volume of saline. Following 28 days, mice
were sacrificed. All animal experimental procedures were approved
by the Institutional Animal Care and Use Committee of the Southern
Medical University (Guangzhou China).
Statistical analysis
Data are presented as the mean + standard error of
the mean. Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Statistical comparisons were
made using one way analysis of variance followed by Bonferroni's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tan IIA treatment inhibits the
proliferation of human gastric cancer cells
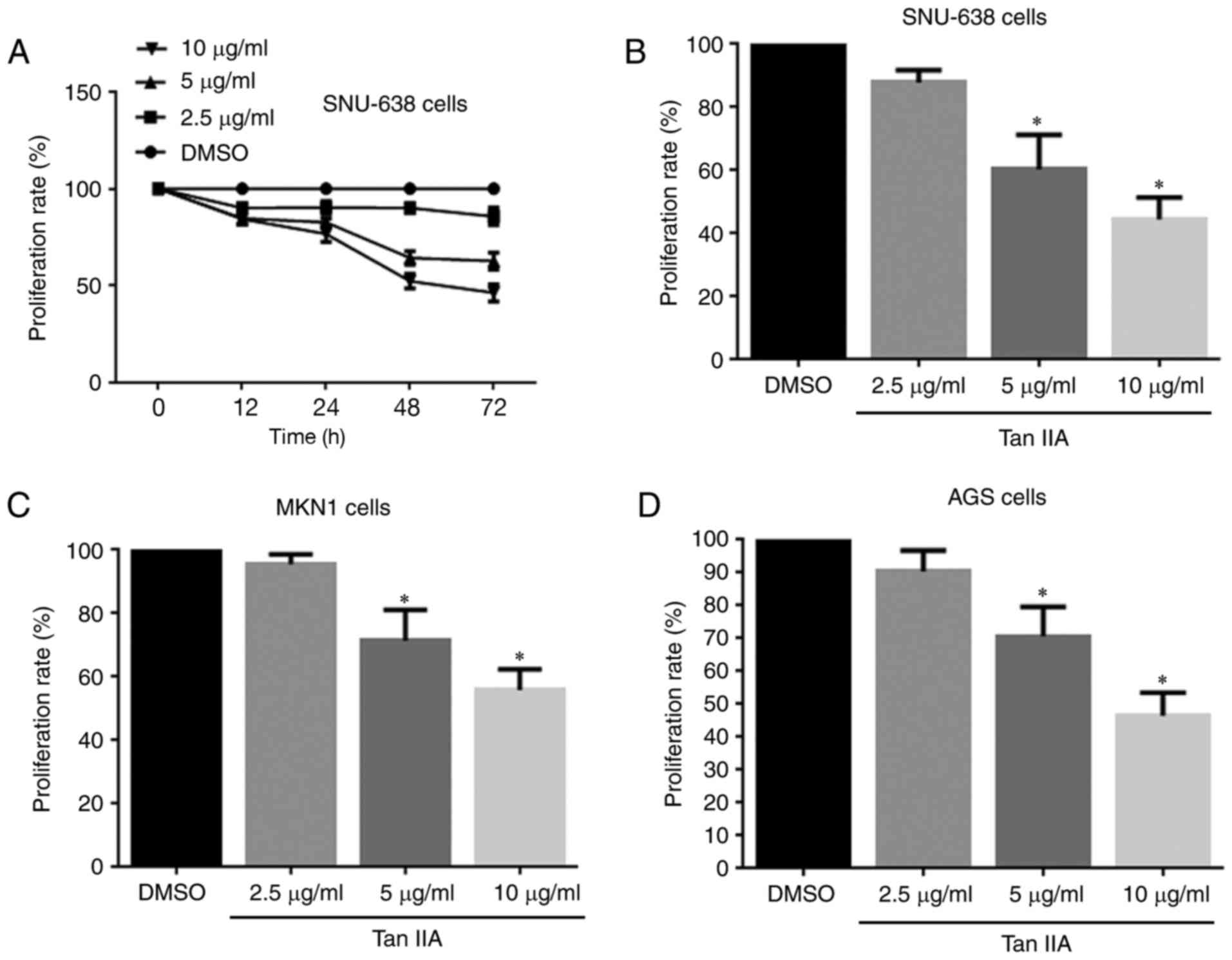
An MTT assay was performed to evaluate the
inhibitory effect of Tan IIA on gastric cancer cell proliferation.
The results revealed that Tan IIA significantly inhibited the
proliferation of SNU-638 cells in a time- and dose-dependent manner
(Fig. 1A and B). A dosage of 10
µg/ml Tan IIA was most effective, and so this dosage was used for
subsequent experiments. Similar results were observed in MKN1 AGS
cells (Fig. 1C and D). These results
suggest that Tan IIA inhibits the proliferation of human gastric
cancer cells.
Tan IIA treatment induces apoptosis in
human gastric cancer cells
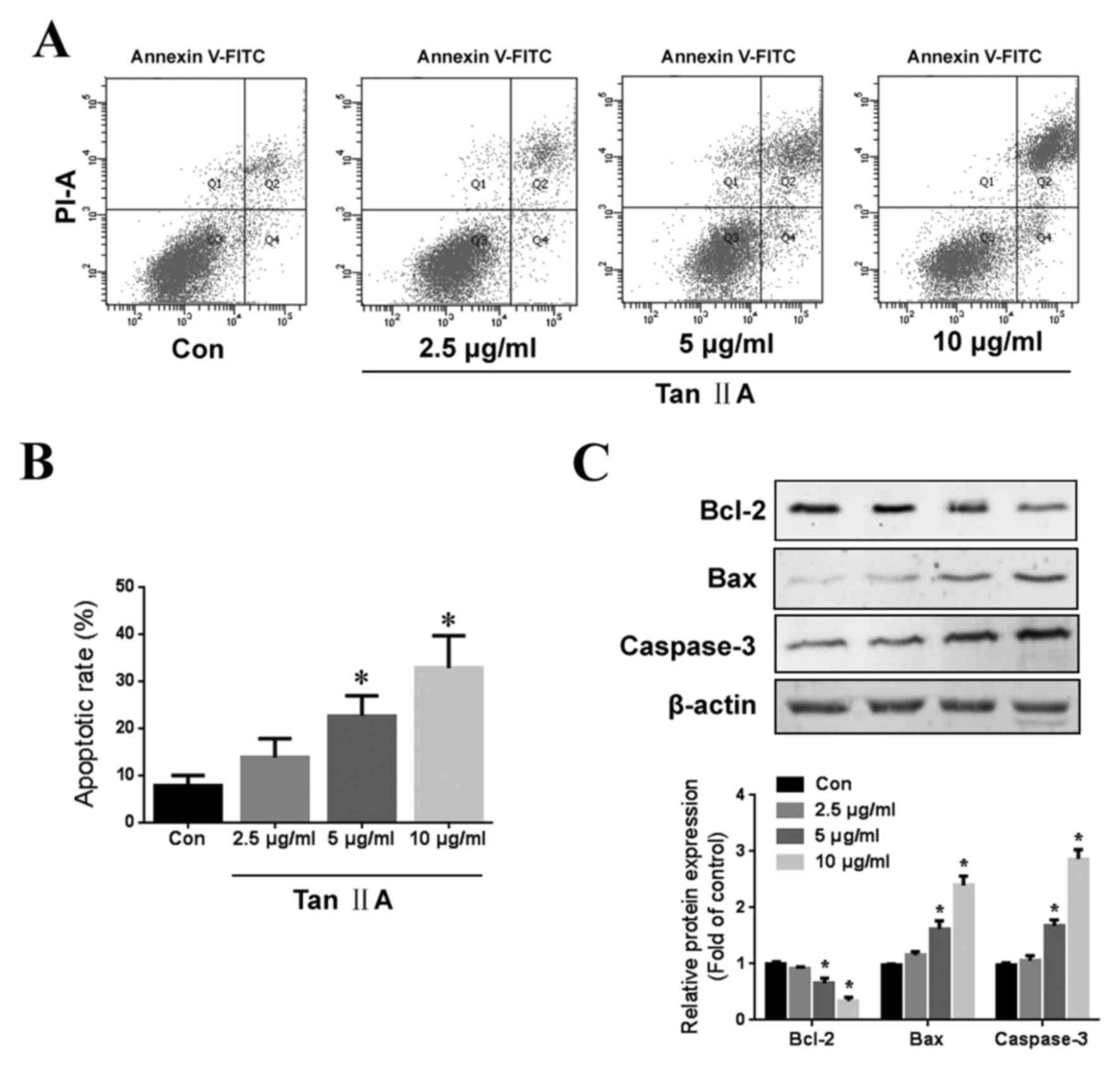
The apoptotic effects of Tan IIA were assessed using
Annexin V-FITC and PI apoptosis double staining. Tan IIA treatment
induced a significant dose-dependent increase in apoptosis
(Fig. 2A and B). Furthermore, Tan
IIA treatment increased the expression of Bax and cleaved
caspase-3, as well as decreasing the expression of Bcl-2 (Fig. 2C). These results suggest that Tan IIA
treatment induces gastric cancer cell apoptosis.
Potential role of STAT3 as a target
for Tan IIA-induced inhibition of human gastric cancer cell
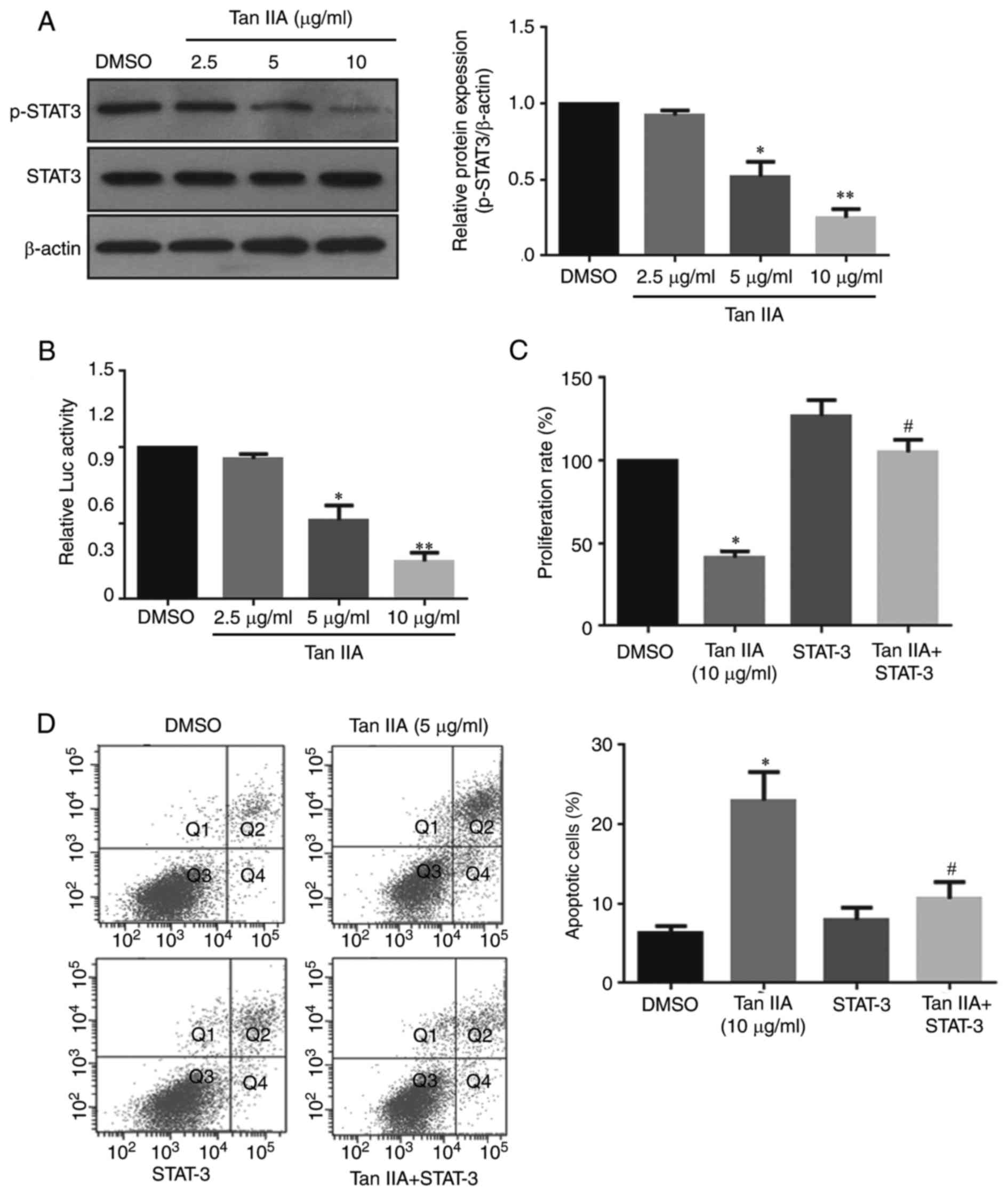
To examine whether Tan IIA inhibits gastric cancer
cell proliferation via the inhibition of STAT3 signaling. Treatment
with 5 or 10 µg/ml Tan IIA significantly inhibited STAT3
phosphorylation at Tyr705 by ~54 and ~77%, respectively (Fig. 3A). Furthermore, Tan IIA treatment
significantly reduced STAT3 trans-activity (Fig. 3B). To further confirm whether STAT3
signaling is associated with the anti-proliferative effect of Tan
IIA, SNU-638 cells were co-transfected with a STAT3 reporter
plasmid (pSTAT3-LUC). The results demonstrated that STAT3
overexpression significantly reversed the Tan IIA-induced
inhibition of cell growth and apoptosis, suggesting that STAT3 is
required for Tan IIA-induced cell growth inhibition in gastric
cancer cells.
Tan IIA suppresses tumor growth in a
xenograft model of human gastric cancer
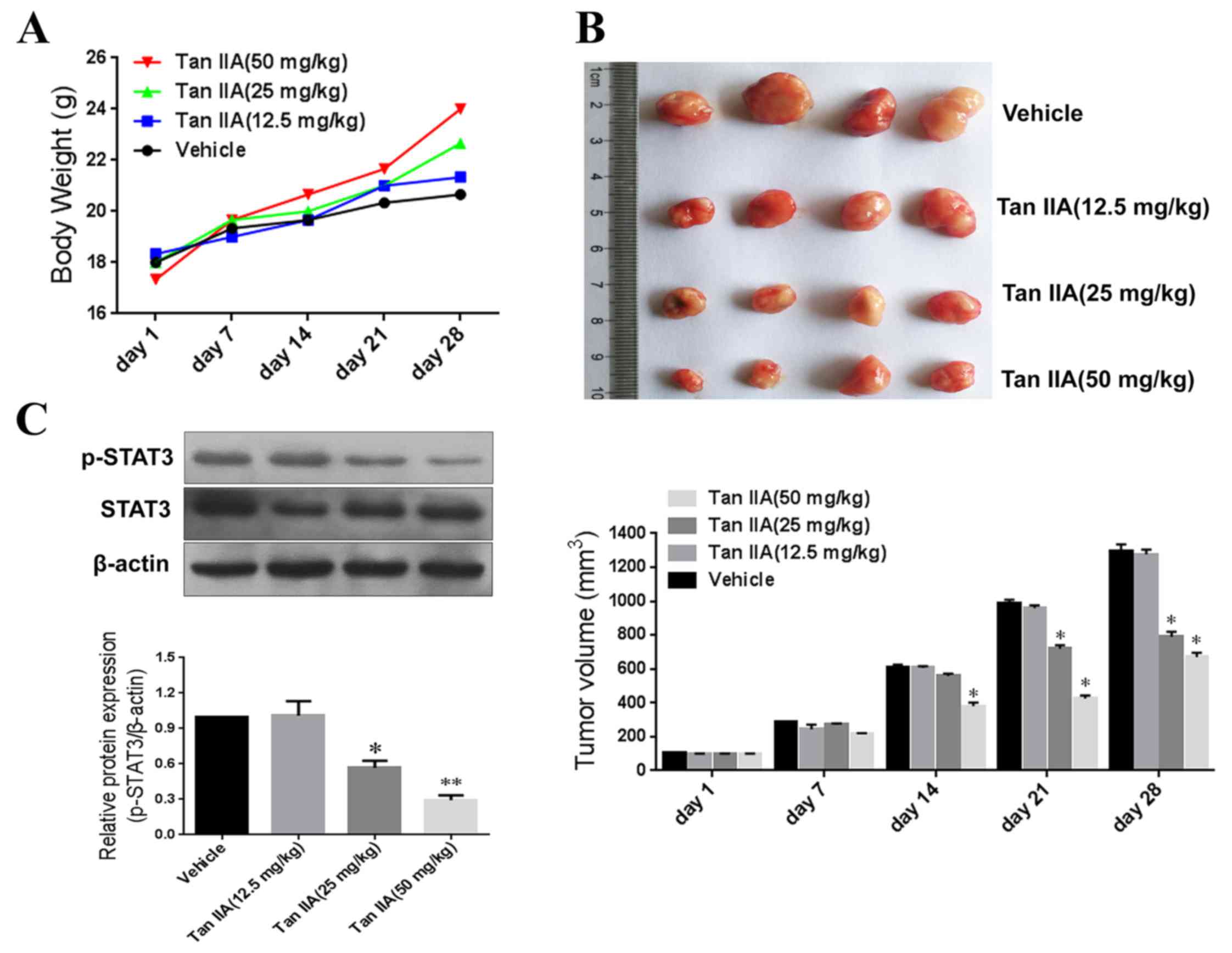
To further examine the anti-tumor efficacy of Tan
IIA in vivo, a xenograft model of human gastric cancer was
constructed using mice. No significant differences in body weight
were observed between the treatment groups (Fig. 4A); however, tumor volumes were
significantly smaller in the Tan IIA-treated groups (25 and 50
mg/kg) compared with the control after 21 days of treatment
(Fig. 4B). In addition, Tan IIA
treatment significantly inhibited STAT3 phosphorylation (Fig. 4C). These results suggest that Tan
IIA-mediated inhibition of tumor growth occurs via the suppression
of STAT3 signaling.
Discussion
The results of the present study demonstrate that
Tan IIA, a bioactive component isolated from traditional Chinese
medicine S. miltiorrhiza, exerts its anti-cancer effects in
3 human gastric cancer cell lines in vitro and a xenograft
tumor model in vivo. Tan IIA exhibited significantly
anti-proliferative and pro-apoptotic effects against gastric
cancer, inducing apoptosis and inhibiting STAT3 phosphorylation at
Tyr705. The anticancer effect of Tan IIA could be inhibited by
co-transfection with STAT3 plasmid to induce STAT3 overexpression.
These results suggest that Tan IIA exerts its anti-proliferative
effects in gastric cancer cells via downregulating STAT3
activation.
Gastric cancer is the third leading cause of
cancer-associated mortality worldwide (25). Although great advances have been made
in the diagnosis and treatment of gastric cancer, the outcome for
patients with gastric cancer is generally poor (26,27). The
side effects associated with chemotherapy reduce patients' quality
of life and may also lead to life-threatening complications
(28). Identifying effective
chemo-preventive agents derived from herbal medicines are therefore
of great importance for improving treatment regimens for gastric
cancer (29).
Tan IIA is a herbal medicine extracted from the root
or rhizomes of S. miltiorrhiza Bunge (30) and has been reported to have potential
chemo-preventative effects relevant to various human cancers
(31). Xu et al (32) reported that Tan IIA reverses the
malignant phenotype of SGC7901 gastric cancer cells, which
indicates that it may be promising therapeutic agent. The results
of the present study indicate that Tan IIA effectively inhibits the
proliferation of gastric cancer cells in three cell lines. In
addition, a xenograft tumor model was used to demonstrate that Tan
IIA reduces the volume of tumors derived from SNU-638 cells. These
observations, combined with the effects observed in vitro,
suggest a direct anticancer effect of Tan IIA.
As a multi-target drug, the molecular targets of Tan
IIA include apoptotic-regulating proteins, transcription factors
and inflammatory mediators (17,33). In
the present study, Tan IIA treatment induced apoptosis, increased
the expression of Bax and cleaved caspase-3 and decreased the
expression of Bcl-2. In future studies, the effect of Tan IIA on
the expression of apoptosis-associated proteins in the xenograft
tissues of SNU-638 cells should be investigated. It was also
demonstrated that Tan IIA significantly prevents the
phosphorylation of STAT3. Previous studies have reported that
constitutive activation of STAT3 signaling is important for cancer
initiation, development and progression (21,34). To
further investigate the mechanisms underlying the anticancer
effects of Tan IIA in gastric cancer cells, STAT3 phosphorylation
was examined. The results revealed that STAT3 phosphorylation was
inhibited by Tan IIA in a dose-dependent manner and similar results
were observed in the xenograft model. However, when STAT3
overexpression was induced in SNU-638 cells, Tan IIA failed to
inhibited cell proliferation. It has been reported that direct
STAT3 suppression induces apoptosis in prostate cancer cells
(35). The results of the present
study demonstrate that STAT3 overexpression abrogate Tan
IIA-induced apoptosis in human cancer SNU-638 cells. However, one
limitation of the present study was that normal gastric cells were
not included, thus it cannot be determined whether the effects of
Tan IIA are cancer-specific.
In summary, the present study demonstrates that Tan
IIA exerts significant anticancer effects in human gastric cancer
cells and this is mediated at least in part by STAT3 inhibition.
However, Tan IIA may also act via other or additional nonspecific
mechanisms and this requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YJZ, SG and JF designed the present study. YJZ
performed assays, analyzed and interpreted the data and wrote the
manuscript. BP, YZ and TC made substantial contributions to the
experimental design and revised the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Southern
Medical University Animal Policy and Welfare Committee (Guangzhou,
China) and complied with the National Institutes of Health
Guidelines (Guide for the Care and Use of Laboratory Animals).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Tan IIA
|
Tanshinone IIA
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Lazăr DC, Tăban S, Cornianu M, Faur A and
Goldiş A: New advances in targeted gastric cancer treatment. World
J Gastroenterol. 22:6776–6799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu T, Chen X, Lin T, Liu J, Li M, Zhang W,
Xu X, Zhao W, Liu M, Napier DL, et al: KLF4 deletion alters gastric
cell lineage and induces MUC2 expression. Cell Death Dis.
7:e22552016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apicella M, Corso S and Giordano S:
Targeted therapies for gastric cancer: Failures and hopes from
clinical trials. Oncotarget. 8:57654–57669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina-Castro S, Pereira-Marques J,
Figueiredo C, Machado JC and Varon C: Gastric cancer: Basic
aspects. Helicobacter. 22 Suppl 1:2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Wu J, Lu J, Ma R, Sun D and Tang
J: Regulation of the cell cycle and PI3K/Akt/mTOR signaling pathway
by tanshinone I in human breast cancer cell lines. Mol Med Rep.
11:931–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutheil WG, Reed G, Ray A, Anant S and
Dhar A: Crocetin: An agent derived from saffron for prevention and
therapy for cancer. Curr Pharm Biotechnol. 13:173–179. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao H, Lamusta J, Zhang WF, Salmonsen R,
Liu Y, O'Connell E, Evans JE, Burstein S and Chen JJ: Tumor cell
selective cytotoxicity and apoptosis induction by an herbal
preparation from brucea javanica. N Am J Med Sci (Boston). 4:62–66.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei B, You MG, Ling JJ, Wei LL, Wang K, Li
WW, Chen T, Du QM and Ji H: Regulation of antioxidant system,
lipids and fatty acid β-oxidation contributes to the
cardioprotective effect of sodium tanshinone IIA sulphonate in
isoproterenol-induced myocardial infarction in rats.
Atherosclerosis. 230:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang J, Little PJ and Xu S:
Atheroprotective effects and molecular targets of tanshinones
derived from herbal medicine danshen. Med Res Rev. 38:201–228.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X,
Chen Y, Cui H and Gao N: Tanshinone IIA inhibits HIF-1α and VEGF
expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1
signaling pathway. PLoS One. 10:e01174402015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SY, Chang SF, Liao KF and Chiu SC:
Tanshinone IIA inhibits epithelial-mesenchymal transition in
bladder cancer cells via modulation of STAT3-CCL2 signaling. Int J
Mol Sci. 18:pii: E1616. 2017. View Article : Google Scholar
|
|
15
|
Zhang Y, Jiang P, Ye M, Kim SH, Jiang C
and Lü J: Tanshinones: Sources, pharmacokinetics and anti-cancer
activities. Int J Mol Sci. 13:13621–13666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su CC: Tanshinone IIA inhibits human
gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl1 and
BclxL and increasing Bax and CHOP protein expression. Int J Mol
Med. 34:1661–1668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Wang X, Li Y and Tang B: Tanshinone
IIA suppresses gastric cancer cell proliferation and migration by
downregulation of FOXM1. Oncol Rep. 37:1394–1400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park E, Park J, Han SW, Im SA, Kim TY, Oh
DY and Bang YJ: NVP-BKM120, a novel PI3K inhibitor, shows synergism
with a STAT3 inhibitor in human gastric cancer cells harboring KRAS
mutations. Int J Oncol. 40:1259–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo C, Su J, Li Z, Xiao R, Wen J, Li Y,
Zhang M, Zhang X, Yu D, Huang W, et al: The G-protein-coupled bile
acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell
proliferation and migration through antagonizing STAT3 signaling
pathway. Oncotarget. 6:34402–34413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Chatterjee-Kishore M, Staugaitis
SM, Nguyen H, Schlessinger K, Levy DE and Stark GR: Novel roles of
unphosphorylated STAT3 in oncogenesis and transcriptional
regulation. Cancer Res. 65:939–947. 2005.PubMed/NCBI
|
|
23
|
Chen J, Wang J, Lin L, He L, Wu Y, Zhang
L, Yi Z, Chen Y, Pang X and Liu M: Inhibition of STAT3 signaling
pathway by nitidine chloride suppressed the angiogenesis and growth
of human gastric cancer. Mol Cancer Ther. 11:277–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong H, Du W, Wang JL, Wang YC, Tang JT,
Hong J and Fang JY: Constitutive activation of STAT3 is predictive
of poor prognosis in human gastric cancer. J Mol Med (Berl).
90:1037–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mastoraki A, Benetou C, Mastoraki S,
Papanikolaou IS, Danias N, Smyrniotis V and Arkadopoulos N: The
role of surgery in the therapeutic approach of gastric cancer liver
metastases. Indian J Gastroenterol. 35:331–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang X, Wang W, Yang Y, Du L, Yang X,
Wang L, Zheng G, Duan W, Wang R, Zhang X, et al: Identification of
circulating microRNA signatures as potential noninvasive biomarkers
for prediction and prognosis of lymph node metastasis in gastric
cancer. Oncotarget. 8:65132–65142. 2017.PubMed/NCBI
|
|
27
|
Kagawa S, Shigeyasu K, Ishida M, Watanabe
M, Tazawa H, Nagasaka T, Shirakawa Y and Fujiwara T: Molecular
diagnosis and therapy for occult peritoneal metastasis in gastric
cancer patients. World J Gastroenterol. 20:17796–17803. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohelnikova-Duchonova B, Melichar B and
Soucek P: FOLFOX/FOLFIRI pharmacogenetics: The call for a
personalized approach in colorectal cancer therapy. World J
Gastroenterol. 20:10316–10330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang F, Zhang D, Mao J, Ke XX, Zhang R,
Yin C, Gao N and Cui H: Morusin inhibits cell proliferation and
tumor growth by down-regulating c-Myc in human gastric cancer.
Oncotarget. 8:57187–57200. 2017.PubMed/NCBI
|
|
30
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong Y, Morris-Natschke SL and Lee KH:
Biosynthesis, total syntheses, and antitumor activity of
tanshinones and their analogs as potential therapeutic agents. Nat
Prod Rep. 28:529–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JY, Ke YM, Lai JS and Ho TF:
Tanshinone IIA enhances the effects of TRAIL by downregulating
survivin in human ovarian carcinoma cells. Phytomedicine.
22:929–938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee H, Herrmann A, Deng JH, Kujawski M,
Niu G, Li Z, Forman S, Jove R, Pardoll DM and Yu H: Persistently
activated Stat3 maintains constitutive NF-kappaB activity in
tumors. Cancer Cell. 15:283–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mora LB, Buettner R, Seigne J, Diaz J,
Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, et
al: Constitutive activation of Stat3 in human prostate tumors and
cell lines: Direct inhibition of Stat3 signaling induces apoptosis
of prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|