Introduction
Gastric cancer (GC) remains the second most common
malignant cancer and gives rise to a number of cancer-associated
fatalities worldwide every year (1).
GC has become a major threat to human life. Notably, the majority
of patients with GC are diagnosed at an advanced stage, which makes
GC treatment difficult due to extensive tumor invasion and
lymphatic metastasis (2,3). The pathogenesis of GC is correlated
with a complexity of factors, including oncogene activation and
tumor-suppressor inactivation (4).
Therefore, in order to provide improved intervention of GC, a
greater understanding of GC pathogenesis and the identification of
novel biomarkers and therapeutic targets is urgently required.
MicroRNAs (miRs) belong to a class of short
non-coding RNAs of ~22 nucleotides in length that have the ability
to regulate gene expression by directing target mRNAs for
degradation (5,6). In past decades, a large number of
studies have indicated that miRs serve as key regulators in various
physiological processes, including cell proliferation, metabolism,
apoptosis, invasion and migration (5,7,8). Aberrant expression of miRs has been
linked to the development and progression of various types of human
cancer (9), including GC (10). Increasing evidence has demonstrated
that miRs are effective biomarkers for the diagnosis and prognosis
of human cancer (11). Also several
reports imply miRs may be promising therapeutic targets for cancer
treatment (12).
A recent study demonstrated that miR-6852
overexpression induces necrosis in cervical cancer cells (13). However, the role of miR-6852 in GC
cells remains largely unknown. The aim of the present study was to
assess the role of miR-6852 in GC cells in order to clarify if the
miR may be a promising therapeutic target for treating GC.
Materials and methods
Patient samples
A total of 56 fresh GC tissue samples and adjacent
normal tissues (male, 30; female, 26; median age, 54±11 years) were
collected at Huazhong University of Science and Technology (Wuhan,
China) between June 2014 and December 2016. Tissues were
immediately snap frozen in liquid nitrogen and stored at −80°C
until total RNA was extracted. Patients were diagnosed with GC
prior to this study. Patients who received radiation- or
chemotherapy prior to surgery were excluded. Written informed
consent was obtained from each patient who participated in the
present research. Furthermore, the present study was approved by
the Ethics Committee of Huazhong University of Science and
Technology.
Cell culture
Human GC cell lines (MGC-803, MKN-1, SGC-7901,
BGC-823 and AGS) and the normal gastric epithelium GES-1 cell line
were acquired from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). All cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS, Invitrogen, Thermo Fisher Scientific, Inc.), 100
U/ml of penicillin and 100 mg/ml of streptomycin (Invitrogen,
Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing
5% CO2.
Reverse transcription-polymerase chain
reaction (RT-qPCR)
Total RNAs were extracted from GC tissues or
cultured cell lines using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using the PrimeScript RT
reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.,
Dalian, China), and 1 µg total RNA was reverse transcribed for each
sample. qPCR was performed with a Taqman MicroRNA Assay kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Thermocycling conditions were as
follows: Denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and elongation at 60°C for 1 min.
Results were normalized to U6 or GAPDH expression. Expression fold
change was determined by the 2−ΔΔCq method (14). Primer sequences were as follows:
miR-6852 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-CCCTGGGGTTCTGAGGACATG-3′; U6 forward, 5′-AACGAGACGACGACAGAC-3′
and reverse, 5′-GCAAATTCGTGAAGCGTTCCATA-3′; forkhead box J1 (FOXJ1)
forward, 5′-CAGAATCGCTGCCTCCTCTC-3′ and reverse,
5′-CAGGGTCCTTTAGCCGGTTT-3′; GAPDH forward,
5′-ATGTTGCAACCGGGAAGGAA-3′ and reverse,
5′-AGGAAAAGCATCACCCGGAG-3′.
Cell Counting Kit (CCK)-8
proliferation assays
Transfected cells were collected at 24 h
post-transfection and seeded into 96-well plates at a density of
3×103 cells per well. Following incubation for 0, 24, 48
and 72 h at 37°C, the CCK-8 assay was performed according to the
manufacturer's instructions. In brief, 10 µl of CCK-8 reagent
(Beyotime Institute of Biotechnology, Shanghai, China) was added to
each well. The cells were incubated at 37°C in an atmosphere
containing 5% CO2 for 2 h. Absorbance was determined at
a wavelength of 450 nm using an ELx808 absorbance reader (BioTek
Instruments, Inc., Winooski, VT, USA). Each assay was performed in
triplicate and repeated three times.
Migration and invasion assays
Migration was measured using 6.5-mm Transwell
inserts with 8.0 µm pore polycarbonate membranes (Costar; Corning
Incorporated, Corning, NY, USA). Cell invasion assays were
performed with 6.5-mm Transwell inserts with 8.0 µm pore
polycarbonate membranes (pre-coated with Matrigel for invasion;
Costar; Corning Incorporated). Briefly, 2×105
transfected and non-transfected cells were suspended and seeded
into the upper chambers of the inserts in 200 µl serum-free DMEM,
while the 600 µl complete DMEM containing 10% FBS was added to the
lower chambers. A total of 24 h following incubation at 37°C, cells
on the upper surface of the membrane were removed but cells in the
lower membrane were fixed with 100% methanol at room temperature
for 20 min and stained with 0.1% crystal violet at room temperature
for 20 min. Cells were observed using an optical microscope
(magnification, ×100; Olympus Corporation, Tokyo, Japan) and
counted in five random fields of view from each well. The mean
number of migrated or invaded cells was calculated.
Luciferase assay
The mutated FOXJ1 3′-untranslated region (UTR)
sequence (in which all predicted sites were mutated) was
synthetized by Sangon Biotech Co., Ltd. (Shanghai, China). The
wild-type 3′-UTR sequence of FOXJ1 or the mutated 3′-UTR sequence
of FOXJ1 was incorporated into the pGL3 control vector (Promega
Corporation, Madison, WI, USA), to obtain the wild-type FOXJ1-3′UTR
or mutant FOXJ1-3′UTR, respectively. GC cells were seeded into
24-well plates the day prior to transfection and then cotransfected
with wild-type or mutant 3′-UTR FOXJ1, along with miR-6852 mimics
or controls using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. At
48 h after co-transfection, the luciferase activity for the
wild-type or mutant FOXJ1 3′-UTR was measured using a dual
luciferase reporter assay (Promega Corporation). Luciferase
activity was normalized to Renilla.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM, SPSS, Inc., Chicago, IL, USA) and GraphPad Prism
(version 6; GraphPad Software, Inc., La Jolla, CA, USA). The
Student's t-test and one-way analysis of variance followed by
Tukey's post hoc test were used to analyze two or multiple groups
for statistical significance, respectively. Pearson correlation
coefficient analysis was used to determine the correlations. The
χ2 test was used to assess the association between
miR-6852 expression and clinicopathological features in patients
with GC. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-6852 is underexpressed in GC
tissues
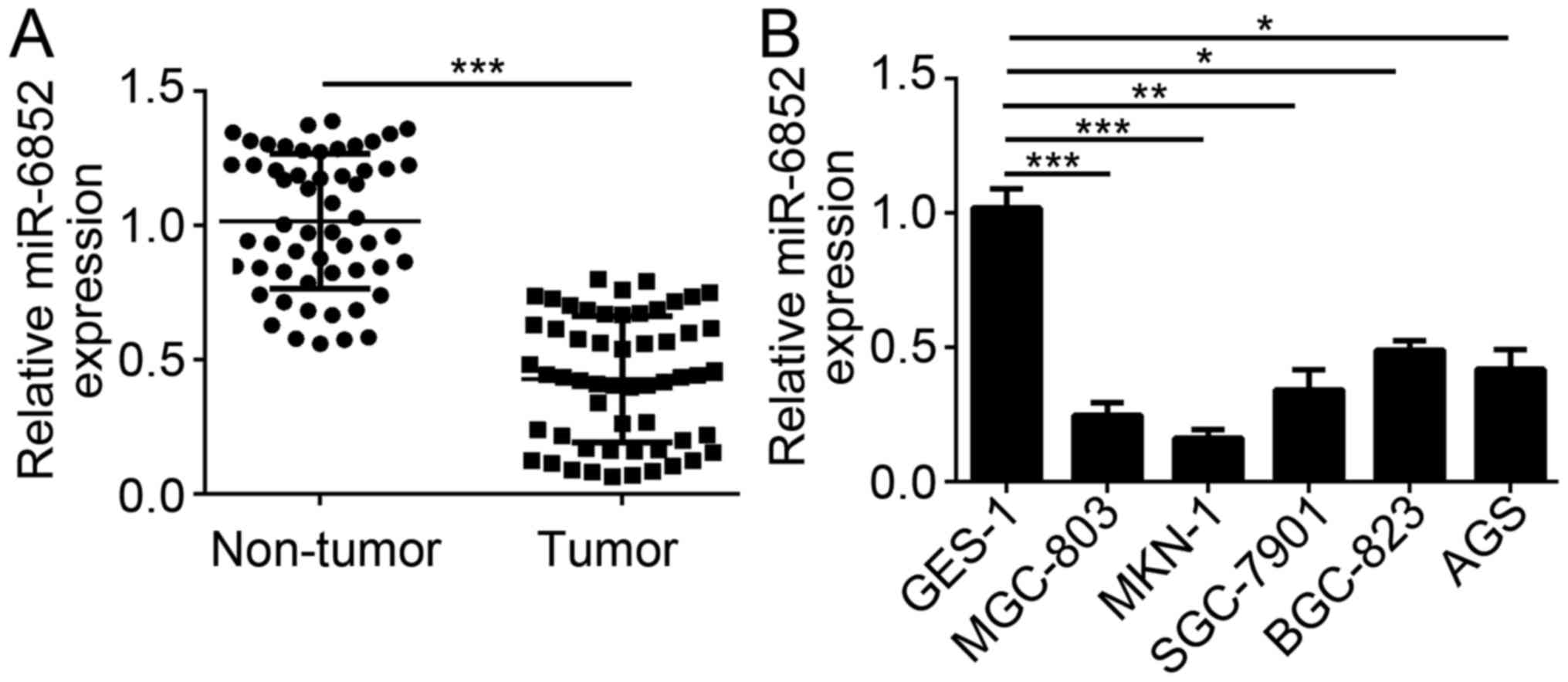
To explore the potential function of miR-6852 in GC
cells, the expression pattern of miR-6852 was assessed by RT-qPCR.
Results indicated that miR-6852 expression was significantly
downregulated in GC tissues compared with adjacent normal tissues
(Fig. 1A). Consistently, RT-qPCR
results also indicated that the expression of miR-6852 was
downregulated in GC tissues compared with the normal gastric
epithelium GES-1 cells (Fig. 1B).
Furthermore, the correlation between miR-6852 expression and
clinicopathological features in patients with GC was determined.
Notably, lower expression of miR-6852 in patients with GC was
associated with increased tumor metastasis, higher TNM stage and
poorer tumor differentiation; however, there was no correlation
between miR-6852 expression level and patient age (Table I). These results demonstrated that
miR-6852 was downregulated in GC tissues and implied that miR-6852
may serve as a tumor suppressor in GC.
 | Table I.Correlation between microRNA-6852
expression and clinicopathological features in patients with
gastric cancer. |
Table I.
Correlation between microRNA-6852
expression and clinicopathological features in patients with
gastric cancer.
| Feature | n=56 | miR-6852 low
(n=31) | miR-6852 high
(n=25) | P-value |
|---|
| Age (years) |
|
|
| 0.559 |
|
<60 | 17 | 8 | 9 |
|
| ≥60 | 39 | 23 | 16 |
|
| Differentiation |
|
|
| 0.418 |
|
Well/moderate | 23 | 11 | 12 |
|
| Poor | 33 | 20 | 13 |
|
| Metastasis |
|
|
| 0.007 |
|
Absent | 26 | 9 | 17 |
|
|
Present | 30 | 22 | 8 |
|
| TNM stage |
|
I–II | 32 | 13 | 19 | 0.015 |
|
III–IV | 24 | 18 | 6 |
|
miR-6852 overexpression suppresses GC
cell proliferation, migration and invasion
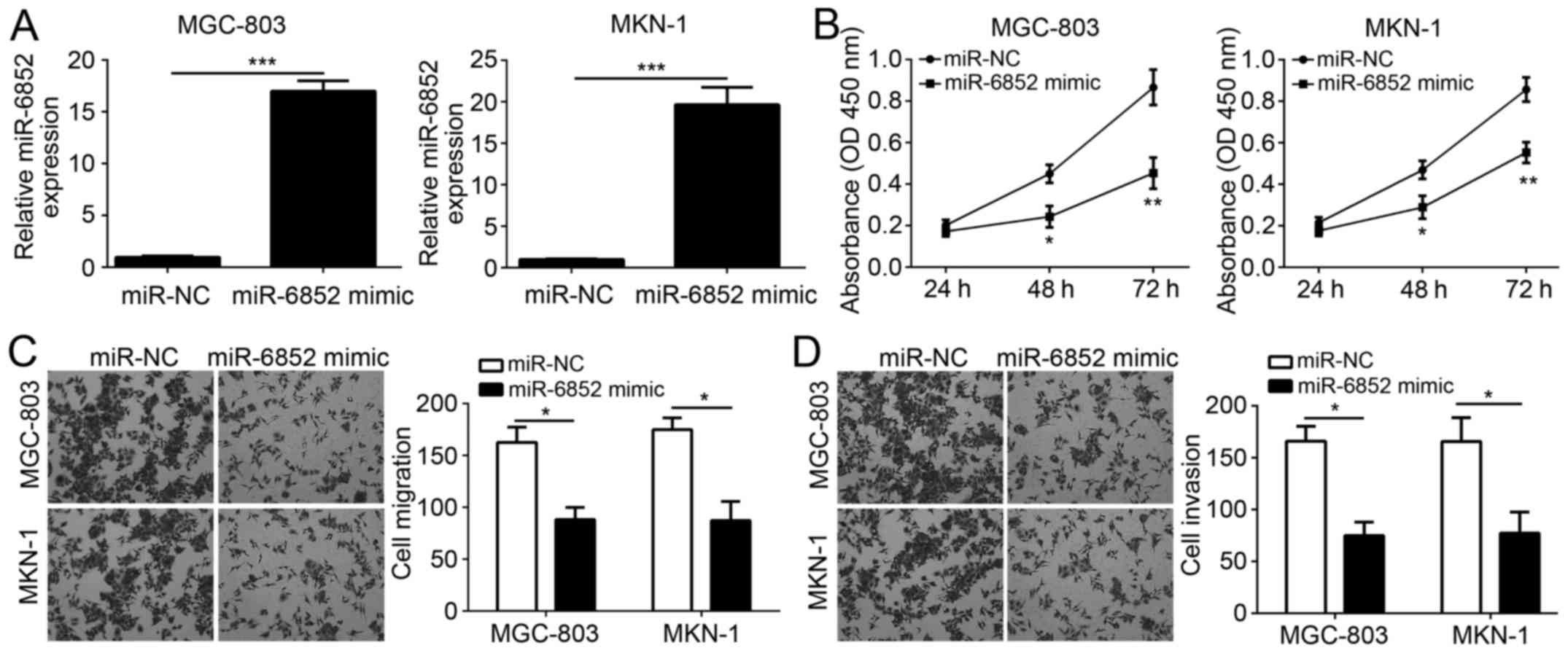
To investigate the role of miR-6852, miR-6852 was
overexpressed in GC cell lines MGC-803 and MKN-1. RT-qPCR analysis
indicated that miR-6852 was effectively overexpressed in MGC-803
and MKN-1 cells following miR-6852 transfection (Fig. 2A). Subsequently, CCK-8 assays were
performed to assess cellular proliferation. Notably, overexpression
of miR-6852 significantly inhibited the proliferation of MGC-803
and MKN-1 cells (Fig. 2B).
Furthermore, the identified correlation between miR-6852 expression
and tumor metastasis was validated using Transwell assays. Results
indicated that overexpression of miR-6852 significantly decreased
the numbers of migrated or invaded MGC-803 and MKN-1 cells
(Fig. 2C and D). Taken together,
these data demonstrated that miR-6852 suppressed the proliferation,
migration and invasion of GC cells.
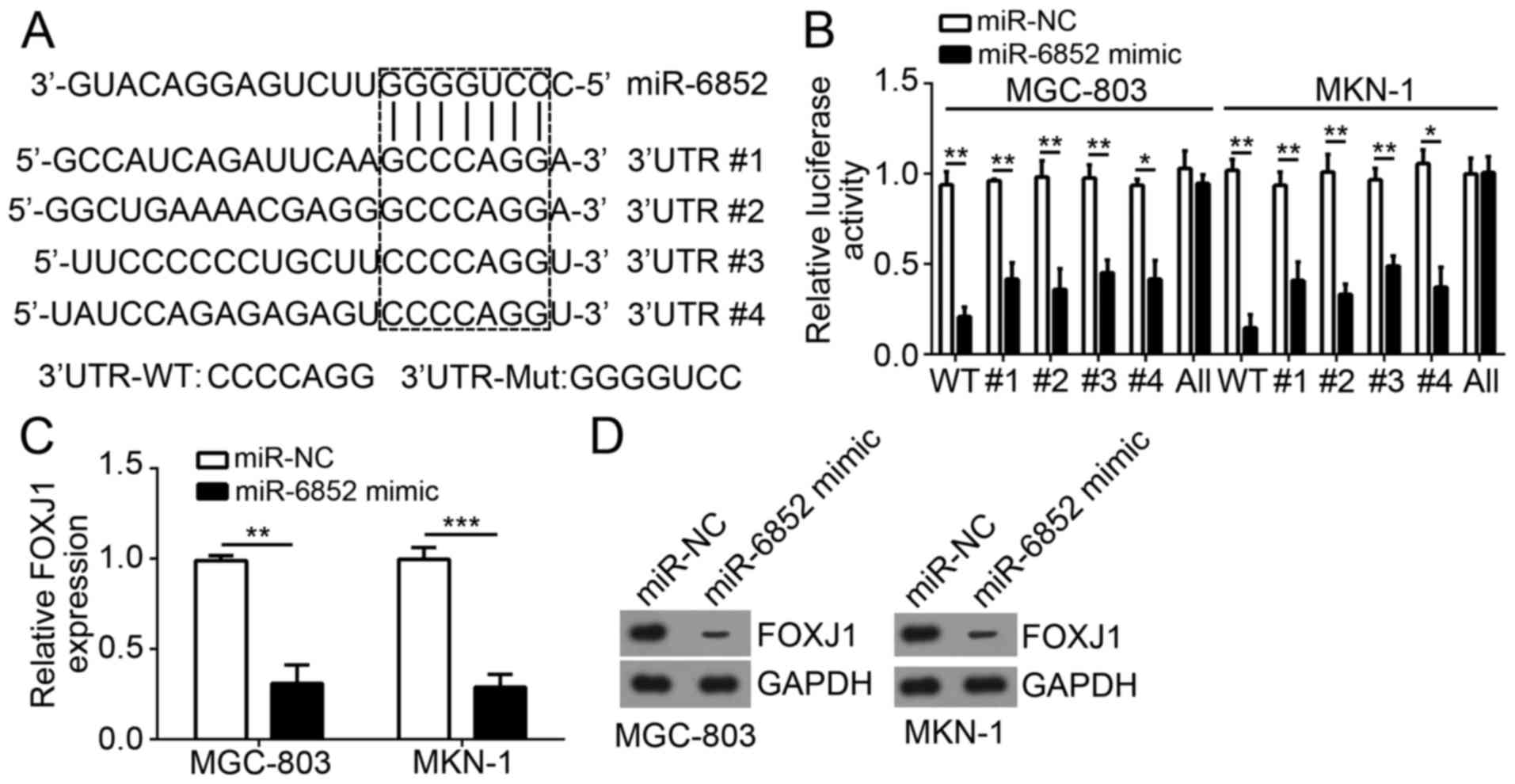
FOXJ1 is a target of miR-6852
miRs have been demonstrated to regulate gene
expression via targeting the 3′-UTR region of mRNAs. To determine
the molecular mechanism of miR-6852 function, the target genes of
miR-6852 in GC cells were investigated. Bioinformatics analysis
determined that FOXJ1 was a potential target of miR-6852. As
indicated in Fig. 3A, there were
four potential binding sites of miR-6852 in the 3′-UTR region of
FOXJ1 mRNA. Subsequently, a luciferase reporter plasmid containing
wild-type or mutant 3′-UTR binding site of FOXJ1 mRNA was
constructed (Fig. 3A). Luciferase
reporter assays indicated that overexpression of miR-6852
significantly inhibited the luciferase activity in MGC-803 and
MKN-1 cells transfected with wild-type FOXJ1 3′-UTR but not with
mutant FOXJ1 3′-UTR (all four sites mutated; Fig. 3B). Notably, mutation of either
predicted recognition site in FOXJ1 3′-UTR could not affect the
inhibitory effect of miR-6852 on luciferase activity (Fig. 3B), suggesting the four sites could be
recognized by miR-6852. Furthermore, overexpression of miR-6852
significantly inhibited the mRNA expression levels of FOXJ1 in
MGC-803 and MKN-1 cells (Fig. 3C).
Similarly, the protein expression levels of FOXJ1 were also
decreased in MGC-803 and MKN-1 cells transfected with miR-6852
mimics (Fig. 3D). The above findings
demonstrated that FOXJ1 was a direct target of miR-6852 in GC
cells.
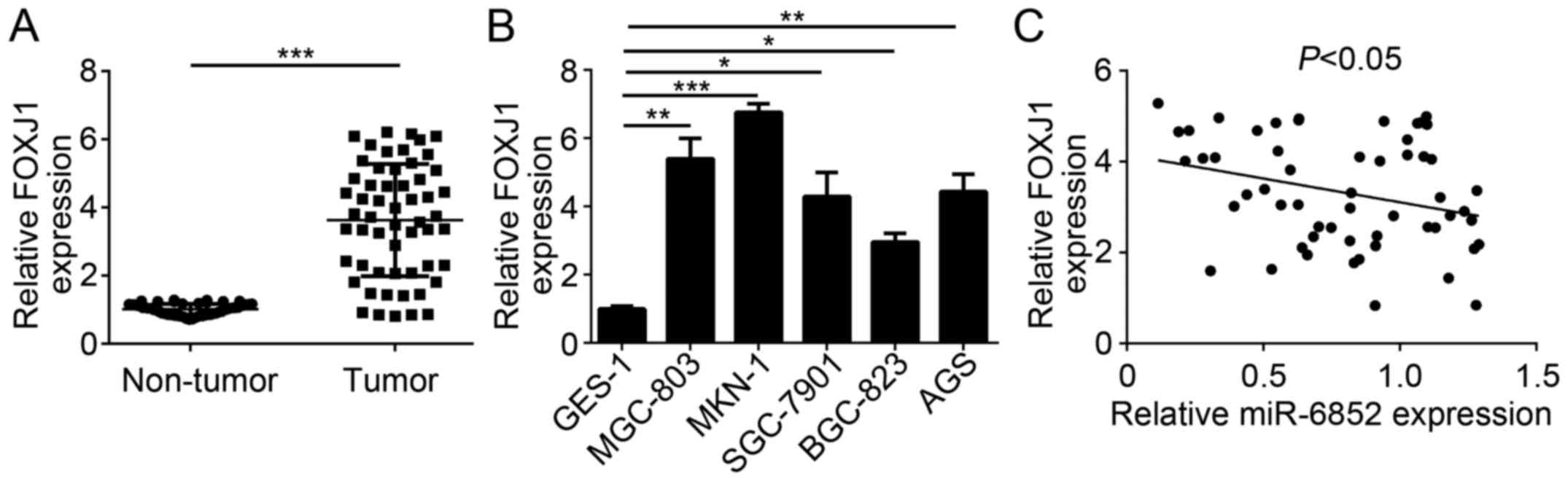
FOXJ1 expression is upregulated and
reversely correlated with miR-6852 expression levels in GC
tissues
To further confirm the previous findings, the
expression patterns of FOXJ1 in GC cells were determined. RT-qPCR
analysis revealed that FOXJ1 was significantly upregulated in GC
tissues compared with adjacent normal tissues (Fig. 4A). Consistently, the expression of
FOXJ1 was significantly upregulated in GC cell lines compared with
GES-1 cells (Fig. 4B). Furthermore,
a reverse correlation between the expression of miR-6852 and FOXJ1
expression levels was indicated in patients with GC (Fig. 4C). These data indicated that FOXJ1
was targeted by miR-6852 and may be involved in GC progression.
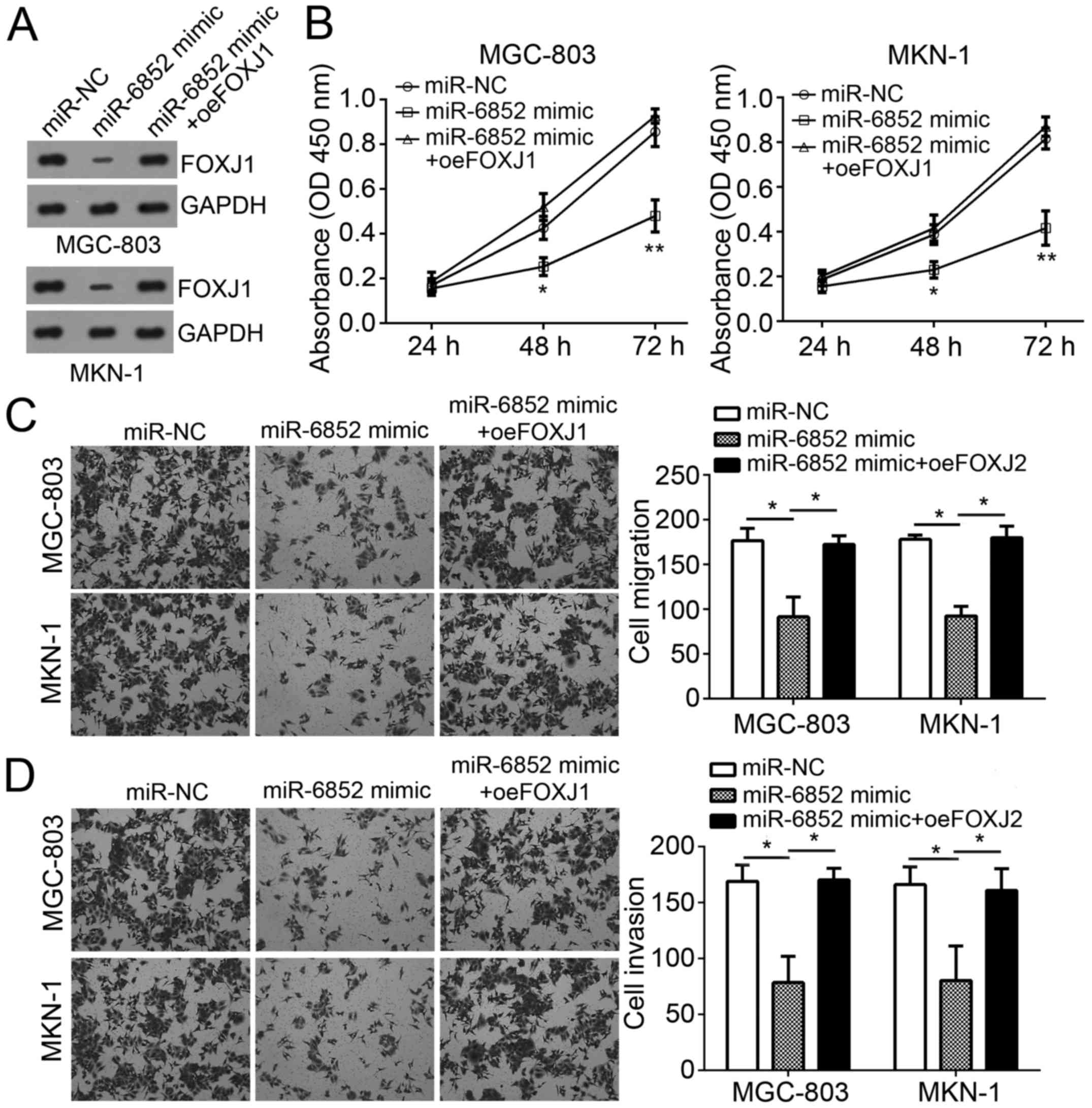
Restoration of FOXJ1 expression
reverses the effects of miR-6852 transfection
To determine the role of FOXJ1 in miR-6852-regulated
GC progression, FOXJ1 expression was restored in MGC-803 and MKN-1
cells transfected with miR-6852 mimics. Western blot analysis
indicated that FOXJ1 was markedly upregulated in GC cells (Fig. 5A). CCK-8 assay analysis revealed that
miR-6852 significantly inhibited cellular proliferation, whereas
overexpression of FOXJ1 abolished this effect in MGC-803 and MKN-1
cells (Fig. 5B). In addition,
Transwell assay results also demonstrated that overexpression of
FOXJ1 abrogated the suppressive effects of miR-6852 on the
migration and invasion of MGC-803 and MKN-1 cells (Fig. 5C and D). Taken together, these
results demonstrated that miR-6852 suppressed the proliferation,
migration and invasion of GC cells by targeting FOXJ1.
Discussion
GC is the second leading cause of cancer-associated
mortality around the world (15).
Patients with GC typically have a very low 5-year overall survival,
which is primarily due to systemic tumor metastasis (16). Thus, it is crucial to determine the
molecular mechanism underlying the development and progression of
GC. In the present study, the function of miR-6852 in GC was
explored and the results revealed that miR-6852 was significantly
downregulated in GC tissues and cell lines. Furthermore, miR-6852
expression was significantly correlated with tumor differentiation,
metastasis and TNM stage, which suggested miR-6852 may be involved
in GC progression. Results from functional experiments indicated
that miR-6852 overexpression inhibited the proliferation, migration
and invasion of GC cells. These data demonstrated that miR-6852
serves as a tumor suppressor in GC and may be a promising
therapeutic target for GC treatment.
miRs have been acknowledged as important players in
tumorigenesis and can regulate tumor cell proliferation, survival
and metastasis (17–19). A large number of studies indicate
that miRs serve as oncogenes or tumor suppressors in almost all
types of human cancer, including breast cancer (20), non-small cell lung cancer (21), prostate cancer (22), osteosarcoma (23), glioma (24) and GC (25). For example, Ding et al
(26) reported that miR-367
regulates cell proliferation and metastasis by targeting
metastasis-associated protein 3 in clear-cell renal cell carcinoma.
He et al (27) indicated that
miR-186 regulates the invasion and metastasis of bladder cancer via
vascular endothelial growth factor C. Notably, a recent study
demonstrated that miR-6852 overexpression induces necrosis in
cervical cancer cells (13).
However, the role of miR-6852 in other tumors remains elusive. In
the present study, miR-6852 was downregulated in GC tissues and
negatively correlated with GC severity. Furthermore, it was
demonstrated that miR-6852 suppressed GC cell proliferation,
migration and invasion according to CCK-8 and Transwell assay
results.
FOXJ1 belongs to the forkhead box gene family, which
is a large group of transcription factors, and widely participates
in various biological processes, including development and
tumorigenesis (28,29). Several reports have demonstrated that
FOXJ1 regulates human cancer development and progression (30,31). For
instance, Liu et al (32)
reported that FOXJ1 is upregulated and promotes colorectal cancer
progression via activating b-catenin signaling. Another study also
indicated that FOXJ1 was downregulated in GC and serves as a
prognostic marker for patients with GC (33). Consistent with a previous report, the
present study indicated that FOXJ1 was a target of miR-6852 and its
expression was upregulated in GC tissues. Furthermore, it was
revealed that FOXJ1 expression was inversely correlated with that
of miR-6852 in GC tissues. Additionally, through a series of
functional experiments, it was indicated that restoration of FOXJ1
reversed the effects of miR-6852 transfection on GC cell
proliferation, migration and invasion.
In conclusion, the present findings demonstrated
that miR-6852 expression was downregulated in GC tissues and
correlated with tumor severity. Furthermore, the results suggested
that miR-6852 may be a tumor suppressor in GC through targeting
FOXJ1. These findings indicate that miR-6852 may be a potential
therapeutic target for GC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL, HY and JZ initiated and designed the present
work and analyzed and interpreted the results. QW, YD, WZ and FL
performed experiments. JL wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China) and all enrolled patients signed a
written informed consent document.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dassen AE, Lemmens VE, van de Poll-Franse
LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Wurff Vd AA, Bosscha K
and Coebergh JW: Trends in incidence, treatment and survival of
gastric adenocarcinoma between 1990 and 2007: A population-based
study in the Netherlands. Eur J Cancer. 46:1101–1110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang H, Wang L, Tang X and Bai W: miR-203a
suppresses cell proliferation by targeting E2F transcription factor
3 in human gastric cancer. Oncol Lett. 14:7687–7690.
2017.PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wienholds E and Plasterk RH: MicroRNA
function in animal development. FEBS Lett. 579:5911–5922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agrawal L, Sahu S, Ghosh S, Shiga T,
Fujita D and Bandyopadhyay A: Inventing atomic resolution scanning
dielectric microscopy to see a single protein complex operation
live at resonance in a neuron without touching or adulterating the
cell. J Integr Neurosci. 15:435–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Ge W, Zou G, Yu L, Zhu Y, Li Q,
Zhang Y, Wang Z and Xu T: MiR-382 targets GOLM1 to inhibit
metastasis of hepatocellular carcinoma and its down-regulation
predicts a poor survival. Am J Cancer Res. 8:120–131.
2018.PubMed/NCBI
|
|
10
|
Kang W, Huang T, Zhou Y, Zhang J, Lung
RWM, Tong JHM, Chan AWH, Zhang B, Wong CC, Wu F, et al: miR-375 is
involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in
gastric carcinogenesis. Cell Death Dis. 9:922018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilmott JS, Zhang XD, Hersey P and Scolyer
RA: The emerging important role of microRNAs in the pathogenesis,
diagnosis and treatment of human cancers. Pathology. 43:657–671.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kashyap D, Tuli HS, Garg VK, Goel N and
Bishayee A: Oncogenic and tumor-suppressive roles of MicroRNAs with
special reference to apoptosis: Molecular mechanisms and
therapeutic potential. Mol Diagn Ther. 22:179–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poudyal D, Herman A, Adelsberger JW, Yang
J, Hu X, Chen Q, Bosche M, Sherman BT and Imamichi T: A novel
microRNA, hsa-miR-6852 differentially regulated by Interleukin-27
induces necrosis in cervical cancer cells by downregulating the
FoxM1 expression. Sci Rep. 8:9002018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: A review. Gastric Cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimagaki T, Yoshizumi T, Harimoto N,
Yoshio S, Naito Y, Yamamoto Y, Ochiya T, Yoshida Y, Kanto T and
Maehara Y: MicroRNA-125b expression and intrahepatic metastasis are
predictors for early recurrence after hepatocellular carcinoma
resection. Hepatol Res. 48:313–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui
J, Zhang W, Zen K, Zhang CY, Hou D, et al: miR-23a/b promote tumor
growth and suppress apoptosis by targeting PDCD4 in gastric cancer.
Cell Death Dis. 8:e30592017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao N, Lin T, Zhao C, Zhao S, Zhou S and
Li Y: MicroRNA-588 is upregulated in human prostate cancer with
prognostic and functional implications. J Cell Biochem. Oct
5–2017.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Shivapurkar N, Vietsch EE, Carney E,
Isaacs C and Wellstein A: Circulating microRNAs in patients with
hormone receptor-positive, metastatic breast cancer treated with
dovitinib. Clin Transl Med. 6:372017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu C, Xie Z and Peng Q: MiRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
22
|
Egan SM, Karasik E, Ellis L and Gollnick
SO: miR-30e* is overexpressed in prostate cancer and promotes
NF-κB-mediated proliferation and tumor growth. Oncotarget.
8:67626–67638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Wu QM, Wang XQ and Zhang CQ: Long
noncoding RNA miR210HG sponges miR-503 to facilitate osteosarcoma
cell invasion and metastasis. DNA Cell Biol. 36:1117–1125. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Y, Lu X, Xu L, Chen Z, Li Q and Yuan
J: MicroRNA-675 promotes glioma cell proliferation and motility by
negatively regulating retinoblastoma 1. Hum Pathol. 69:63–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Liu B, Wen F and Song Y:
MicroRNA-454 inhibits the malignant biological behaviours of
gastric cancer cells by directly targeting mitogen-activated
protein kinase 1. Oncol Rep. 39:1494–1504. 2018.PubMed/NCBI
|
|
26
|
Ding D, Zhang Y, Wen L, Fu J, Bai X, Fan
Y, Lin Y, Dai H, Li Q, Zhang Y and An R: MiR-367 regulates cell
proliferation and metastasis by targeting metastasis-associated
protein 3 (MTA3) in clear-cell renal cell carcinoma. Oncotarget.
8:63084–63095. 2017.PubMed/NCBI
|
|
27
|
He X, Ping J and Wen D: MicroRNA-186
regulates the invasion and metastasis of bladder cancer via
vascular endothelial growth factor C. Exp Ther Med. 14:3253–3258.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Overdier DG, Ye H, Peterson RS, Clevidence
DE and Costa RH: The winged helix transcriptional activator HFH-3
is expressed in the distal tubules of embryonic and adult mouse
kidney. J Biol Chem. 272:13725–13730. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abedalthagafi MS, Wu MP, Merrill PH, Du Z,
Woo T, Sheu SH, Hurwitz S, Ligon KL and Santagata S: Decreased
FOXJ1 expression and its ciliogenesis programme in aggressive
ependymoma and choroid plexus tumours. J Pathol. 238:584–597. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xian S, Shang D, Kong G and Tian Y: FOXJ1
promotes bladder cancer cell growth and regulates Warburg effect.
Biochem Biophys Res Commun. 495:988–994. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lan Y, Hu X, Jiang K, Yuan W, Zheng F and
Chen H: Significance of the detection of TIM-3 and FOXJ1 in
prostate cancer. J BUON. 22:1017–1021. 2017.PubMed/NCBI
|
|
32
|
Liu K, Fan J and Wu J: Forkhead box
protein J1 (FOXJ1) is overexpressed in colorectal cancer and
promotes nuclear translocation of β-catenin in SW620 cells. Med Sci
Monit. 23:856–866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Cai X, Xia L, Zhou J, Xin J, Liu
M, Shang X, Liu J, Li X, Chen Z, et al: Decreased expression of
FOXJ1 is a potential prognostic predictor for progression and poor
survival of gastric cancer. Ann Surg Oncol. 22:685–692. 2015.
View Article : Google Scholar : PubMed/NCBI
|