Introduction
Chronic osteomyelitis is a common surgical disease
that can be divided into three categories: spread of local
infection due to incomplete debridement of an infected open wound,
chronic inflammation developed from acute osteomyelitis, and
iatrogenic infection (1,2). Pathogenic bacteria can develop drug
resistance due to long-term use of antibiotics for serious local
injuries, resulting in delayed healing (3). The treatment of chronic osteomyelitis
is difficult and the procedure is complex. Control of infection and
repair of bone defects are main focuses of the treatment of chronic
osteomyelitis. Inappropriate treatments often lead to recurrent
local inflammation and serious complications such as muscle atrophy
or even amputation (4). Traditional
treatment methods, such as bone graft surgery, are more effective
in patients with small bone defects than in patients with large
bone defects such as femoral and tibia defects due to limited
resources of donor bone (5–7). Skeletal traction is commonly used for
the treatment of large bone defects in clinic. However, the
operation procedure is complicated and multiple complications may
occur (8). Induced membrane
technique is a new and popular treatment method that has been
reported to be commonly used for the treatment of large bone
defects in clinic. Induced membrane technique is a 2-step
procedure: formation of a membrane at the site of bone loss induced
by putting a transitory poly(methyl methacrylate) spacer, and
integration therein of autologous cancellous bone graft collected
from other parts of the patient's body. The procedure is simple and
efficacy is high (9,10). The treatment with induced membrane
technique focuses on two key aspects: control of infection in the
bone defects and reconstruction of the damaged bone (11). A study found that membrane induction
technology in the treatment of chronic osteomyelitis in rabbits can
reduce the incidence of infection and inflammatory cell
concentrations (12). Membrane
induction technology for the treatment of patients with clinical
chronic osteomyelitis can shorten bone healing time and reduce the
incidence of postoperative complications (13). At present, studies on the treatment
of chronic osteomyelitis with induced membrane technique mainly
focus on therapeutic efficacy. There is no study on animal models
which may facilitate an in-depth investigation. In this study, the
induced membrane technique was used to treat rats with chronic
osteomyelitis. Treatment efficacy and changes of clinical
indications were recorded. The application value of membrane
induction technology was evaluated using indexes including serum
leukocyte count, C-reactive protein (CRP) and tumor necrosis
factor-α (TNF-α). Our study provided reference for the treatment of
chronic osteomyelitis with induced membrane technique.
Materials and methods
Animals
A total of 180 healthy male Sprague-Dawley (SD)
rats, weighing 190–220 g, were purchased from Beyotime Institute of
Biotechnology (Shanghai, China) and fed under standard conditions.
The rats were randomly divided into sham operation group (control
group), chronic osteomyelitis model group (model group) and
Masquelet induced membrane therapy + chronic osteomyelitis model
group (observation group); 60 rats in each group. A rat model of
traumatic osteomyelitis was established using a modified blunt
trauma method in model and observation group. All rats in
observation group were treated with membrane induction technology,
while those in control group were not. The present study was
approved by the Ethics Committee of the Cangzhou Central Hospital
(Cangzhou, China).
Establishment of osteomyelitis
model
A rat traumatic osteomyelitis model was established
using a modified rat blunt trauma method (12). SD rats were fed for 1 week under
standard conditions. Rats were fasted for 12 h and subperitoneal
anesthesia was performed with 10% chloral hydrate at a dose of 10
mg/100 g. Rats were then fixed on the operation table. Operation
area was shaved and disinfected and a 7-gauge needle was inserted
into the bone marrow cavity from the anterior middle. Sodium
morrhuate (0.1 ml of 5%), 0.3 ml of Staphylococcus aureus
suspension and 0.3 ml of normal saline were injected through the
needle. After 2 weeks, local swelling and pus were observed. X-ray
scanning was performed to show periosteal proliferation, cortical
thickening and formation of sequestrum in the long bone.
Treatment with induced membrane
technique
Treatment with induced membrane technique was
performed in 2 steps and all rats were under general anesthesia. In
the first step, radical debridement was performed and implantation
of antibiotics and bone cement was performed. Briefly, debridement
was performed to expose normal cortical bone interface. Bone defect
was fixed using intramedullary nails. Vancomycin and PMMA bone
cement was mixed at a ratio of 1:20 and put into bone defect to
connect both ends. Then the incision was closed. If inflammation
occurred within 6–8 weeks the first step was repeated. In step 2,
bone defect was reconstructed by cancellous bone grafting. Briefly,
an incision was made to expose the induced membrane formed around
the defect. Membrane was cut longitudinally and then bone cement
was removed carefully. The defect was filled with morselized
cancellous bone graft. The incision was closed, washed with saline
and disinfected with iodophor.
Observed indicators
After the rat model was established, the following
indicators were observed or measured: the levels of wound swelling
and pus formation, the rat body temperature recorded at 10:00 a.m.
daily from day 1 to day 8 after surgery, the rat body weight
recorded on the day of surgery as well as day 7 and day 14 after
surgery, and the number of bacteria in the wound. Blood was
collected from tail blood to prepare serum. Serum TNF-α was
detected using an ELISA kit (Shanghai Fanke Biotechnology Co.,
Ltd., Shanghai, China). White blood cells were counted using a
blood cell analyzer (MEK-822K) (Νihon Kohden, Tokyo, Japan), and
CRP level was measured using an automatic biochemical analyzer
(Hitachi, Ltd., Tokyo, Japan) (14).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analyses of the data. Data were
presented as mean ± SD. Quantitative data were processed using
analysis of variance or t-test. SNK-q test was used for pairwise
comparison. Spearman's correlation analysis was used to determine
the correlation between body temperature and days after treatment.
All P-values represent bilateral probability, and the level of
significance α is 0.05.
Results
Basic information of rat
The 180 rats were divided into 3 groups and each
group included 10 cases of tibia fracture, femoral fracture,
humerus fracture, ulnar and radial fractures, diaphysis fracture
and metaphyseal fracture, respectively. The average age of rats in
the three groups was 8.20±0.35, 8.25±0.45 and 8.61±0.51 weeks,
respectively (P>0.05), and the average body weight was
210.51±10.62, 209±9.89 and 211.52±11.01 g, respectively
(P>0.05).
Complications of induced membrane
technique
In observation group, 5 (8.33%) rats developed flap
marginal necrosis, and 3 (5.00%) rats developed superficial
infection of patella. In model group, 14 (23.33%) rats had flap
marginal necrosis, and 12 (20.00%) rats showed superficial
infection of patella. Significant differences were found between
the two groups (P<0.05) (Table
I).
 | Table I.Treatment of the complications of
chronic osteomyelitis in rats with induced membrane technique. |
Table I.
Treatment of the complications of
chronic osteomyelitis in rats with induced membrane technique.
| Complication
type | No. of cases (%) |
|---|
| Partial necrosis at
edge of the flap | 5 (8.33) |
| Superficial infection
around the incision site in the ilium | 3 (5.00) |
| No complications | 52 (86.67) |
Outcomes of treatment with induced
membrane technique
Primary bone healing was achieved in 50 (83.33%)
rats with an average healing time of 15±1.56 weeks. Among them, 38
(76%) rats restored weight-bearing function after 20 weeks. Seven
(11.67%) rats experienced infection after surgery, but complete
bone healing was achieved after treatment with induced membrane
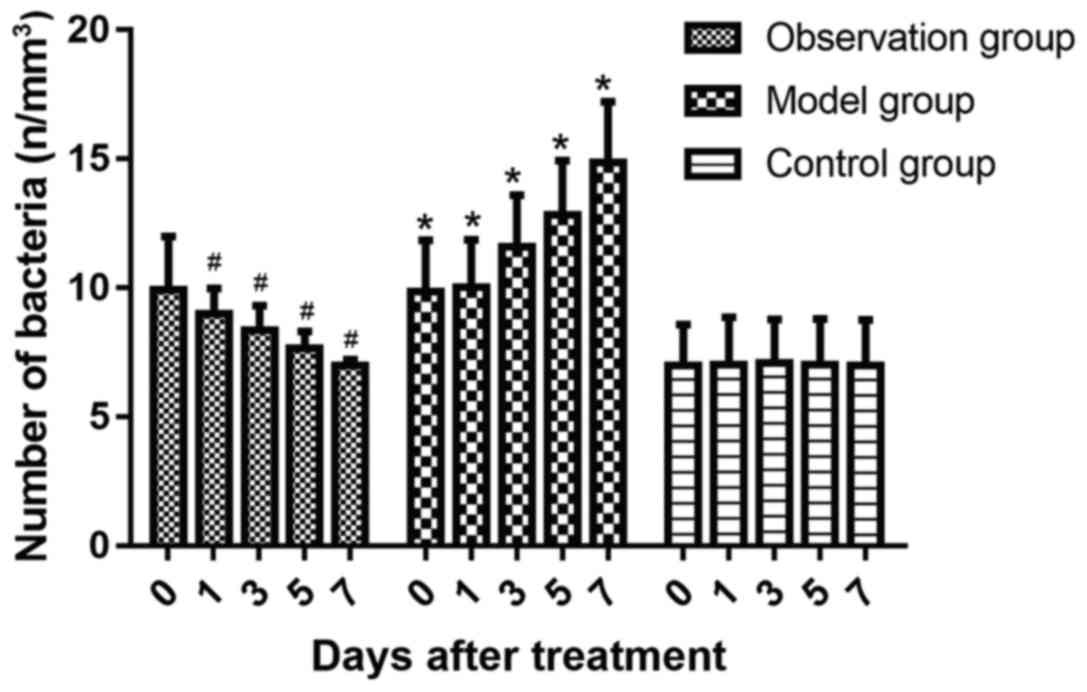
technique again (Table II). Number
of bacteria in the surgical wound before and after treatment with
induced membrane technique was shown in (Table III).
 | Table II.Outcomes of the treatment of chronic
osteomyelitis in rats with induced membrane technique. |
Table II.
Outcomes of the treatment of chronic
osteomyelitis in rats with induced membrane technique.
| Treatment
outcomes | Cases (%) or mean ±
SD |
|---|
| Primary bone
healing | 50 (83.33) |
| Healing time
(weeks) | 15±1.56 |
| Restoration of
weight-bearing function | 38 (63.33) |
| Time for restoration
of weight-bearing function (weeks) | 20±1.80 |
| Postoperative
infection | 7
(11.67) |
 | Table III.Number of bacteria in the surgical
wound before and after treatment with induced membrane technique
(n/mm3, mean ± SD). |
Table III.
Number of bacteria in the surgical
wound before and after treatment with induced membrane technique
(n/mm3, mean ± SD).
|
|
| After treatment |
|---|
|
|
|
|
|---|
| Indicator | Before treatment | Day 1 | Day 3 | Day 5 | Day 7 |
|---|
| Bacteria count | 20,618.71±865.25 | 1,120.63±121.28 | 218.51±30.16 | 50.44±11.92 | 10.28±1.26 |
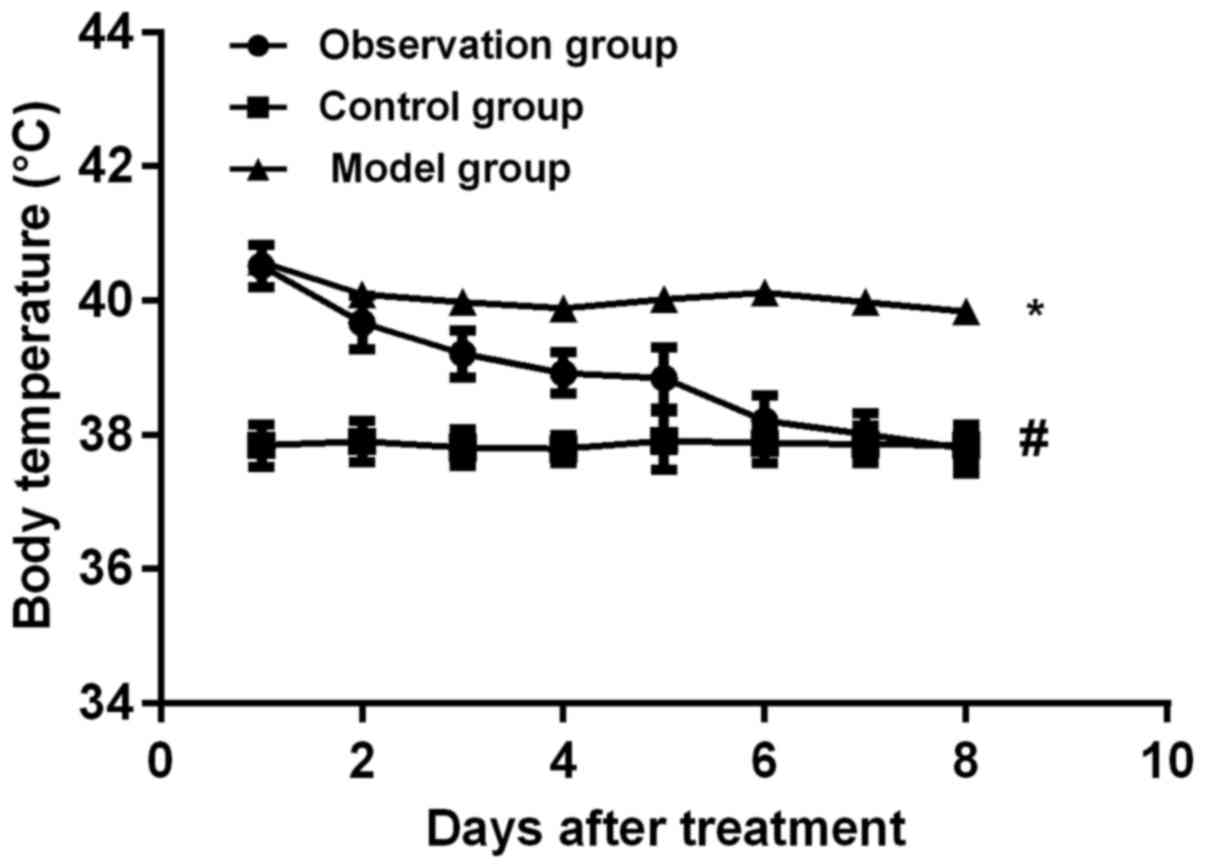
Rat body temperature and weight after treatment with
induced membrane technique. The body temperature of rats from day 1
to day 8 after surgery was compared among the three groups. The
body temperature of rats in the observation group gradually
decreased. The decrease in body temperature was correlated with the
number of days after treatment (r=0.976, P<0.001). With the
increase of treatment time normal body temperature gradually
returned. There was no significant difference in body temperature
and treatment time between the control and model group (r=0.098,
P>0.05). On the 7th day after surgery, the body temperature in
observation group was lower than that in model group (P<0.05)
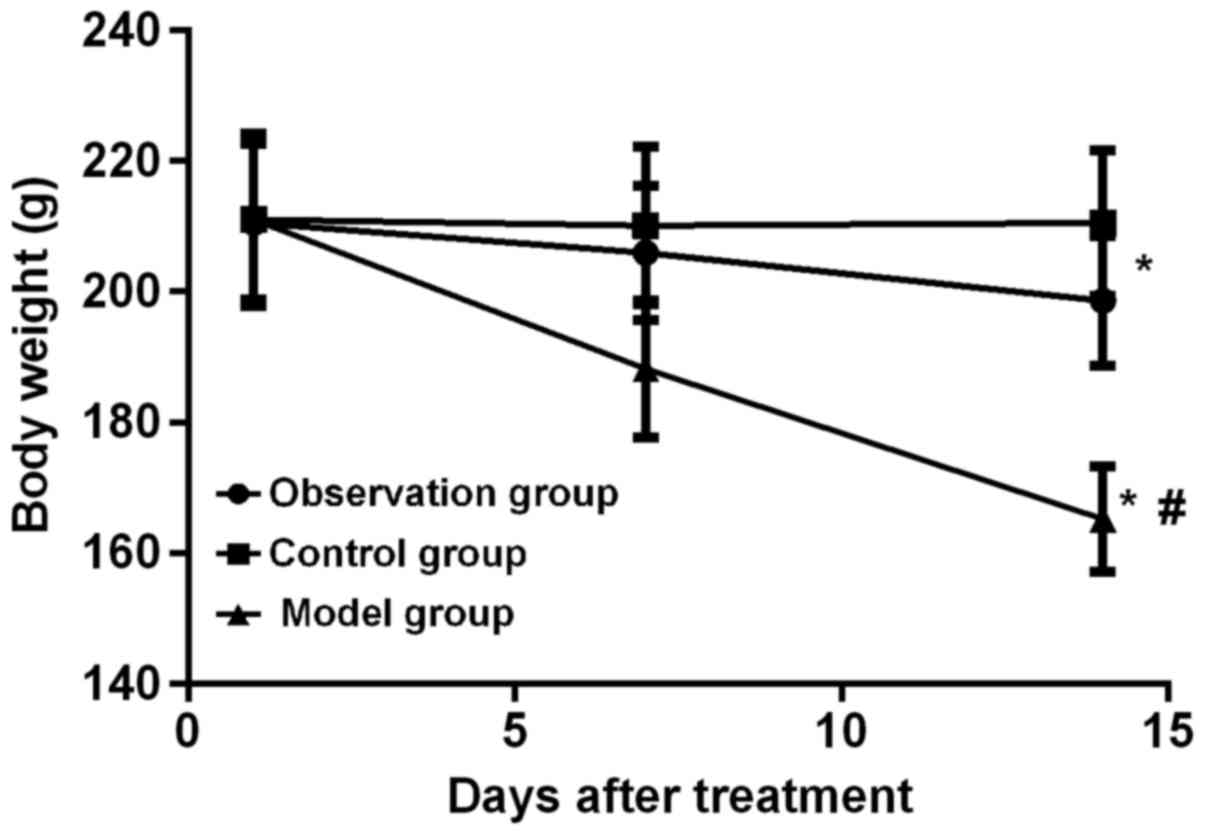
(Fig. 1). On the 1st, 7th and 14th
day after the application of membrane induction technique, the body
weight of rats in the observation and model group gradually
decreased (F=8.916, P=0.005; F=12.021, P<0.001). There was no
significant change in body weight in control group (P>0.05). On
the 14th day, the weight of the rats in the observation group was
higher than that of the rats in the model group (P<0.05), and
the weight of the rats in control group was higher than that in
observation group (P<0.05) (Fig.
2).
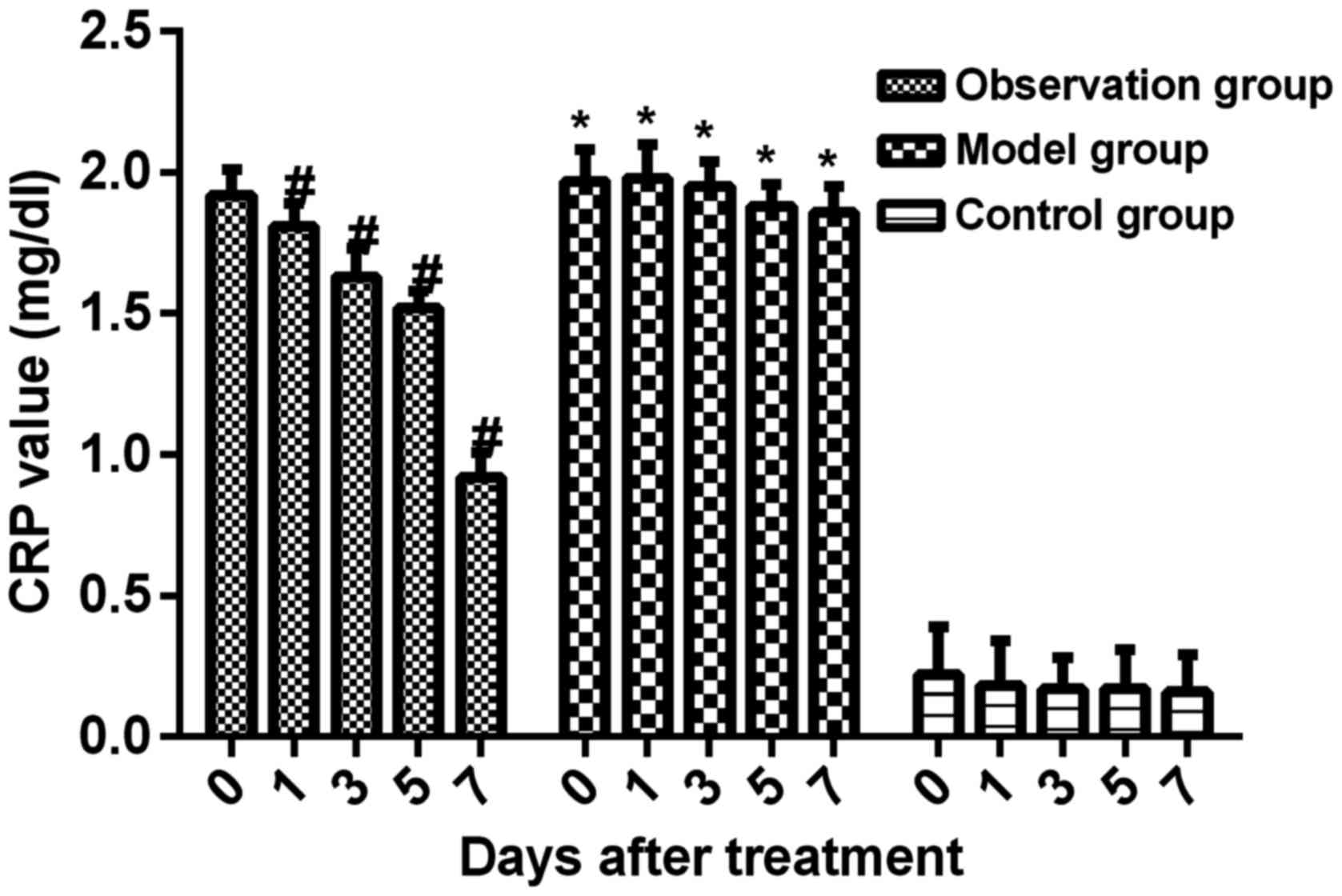
Changes of serum WBC, TNF-α and CRP
levels in rats at different time-points
During the whole course of treatment, serum WBC,
TNF-α and CRP levels in the model group were significantly higher
than those in control group (P<0.05). Compared with those in
model group, WBC, TNF-α and CRP levels in observation group
significantly decreased after treatment (P<0.05) (Figs. 3–5).
Discussion
Chronic osteomyelitis, as a major challenge to
orthopedic surgery, is hard to treat (15). Staphylococcus aureus is the
most common pathogen found in chronic osteomyelitis. Radical
debridement by surgery is a crucial step where particular attention
should be paid because large bone defects are created following
radical debridement (16).
Therefore, reconstruction of bone defects is another key challenge
in treating chronic osteomyelitis. Concentration of antibiotics
under systemic medication is too low to effectively kill bacteria
at chronic osteomyelitis lesion due to poor local blood circulation
(17). Traditional treatment
methods, such as open bone grafting, vacuum sealing drainage and
antibiotic irrigation-perfusion, have a series of issues including
complicated procedures, poor treatment outcomes, resulting in more
complications, prolonged treatment time, and poor patient
acceptance (18–21).
Induced membrane technique was first described by
Masquelet in treatment of chronic osteomyelitis (5). The treatment process consists of two
relatively independent surgical stages: the first step includes
debridement and implantation of antibiotic bone cement in the bone
defect; in the second step, membrane structure formation induction
in the bone defect and bone reconstruction by autologous cancellous
bone grafting are performed. In the first step, antibiotics and
bone cement are mixed. After application, the antibiotics are
gradually released to increase local drug concentration in chronic
osteomyelitis lesions and thereby enhancing the treatment efficacy.
Bone cement protects the damaged bone tissue, thereby reducing the
risk of a bone fracture following radical debridement. In addition,
self-solidification of antibiotic bone cement in the body allows
for high local drug concentration around bone defects. Induced
membrane technique can be used to treat chronic osteomyelitis
resulted from a variety of causes, due to the simple surgical
procedures and satisfactory clinical efficacy. Various studies on
clinical treatment of osteomyelitis using induced membrane
technique have been reported and satisfactory outcomes have been
observed (22,23). Pelissier et al (22) used the induced membrane technique in
rabbits to study the expression of various cytokines in the process
of bone formation. Aho et al (23) performed membrane induction surgery on
patients, and analyzed the components of induced membrane under
different conditions.
In this study, traumatic osteomyelitis rat models
were established and treatment with induced membrane technique was
performed. Results showed that 50 of 60 rats achieved primary bone
healing with a healing time of only 15 weeks, and 38 rats restored
weight-bearing function. Seven rats experienced infection after
surgery probably due to incomplete debridement in the first stage,
but complete bone healing was achieved after treatment with induced
membrane technique for the second time. The induced membrane
technique treatment method is superior to traditional treatment
methods in terms of number of surgeries, treatment efficacy,
hospitalization expenses, complications, and recovery time
(24–26). Masquelet and Begue reported that all
35 patients with bone defects in their study achieved radiographic
healing following treatment with induced membrane technique
(5). Another study also reported
that 90% of patients achieved bone union (27).
It was found in this study that rat weight, body
temperature and number of bacteria in the wound decreased over time
following treatment with induced membrane technique. Weight loss
may be caused by poor appetite resulted from stress during model
establishment and treatment. The decreases in rat body temperature
and number of bacteria in the wound suggested that local high
concentration of antibiotics was achieved around bone defect after
implantation of antibiotic bone cement, which effectively inhibited
the growth of various bacteria and reduced the incidence of
postoperative infection.
In conclusion, through establishment of rat chronic
osteomyelitis models followed by treatment with induced membrane
technique, we observed a high rate of bone union with low incidence
of postoperative complications. In addition, the weight-bearing
function of the affected limb was restored. Our study provided
reference for the use of the induced membrane technique for the
treatment of chronic osteomyelitis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC and JL were major contributors in writing the
manuscript,, they designed the methods and conceived the idea of
this study. TC, JL and PZ were involved in the follow up of
patients. TC, JL and QG were responsible for the collection of the
data. TC and XF participated in the analysis and discussion of the
data. CL was also involved in the conception of the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Cangzhou Central Hospital (Cangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang X, Yu S, Sun D, Fu J, Wang S, Huang K
and Xie Z: Current data on extremities chronic osteomyelitis in
southwest China: Epidemiology, microbiology and therapeutic
consequences. Sci Rep. 7:162512017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrando A, Part J and Baeza J: Treatment
of cavitary bone defects in chronic osteomyelitis: Biogactive glass
S53P4 vs. calcium sulphate antibiotic beads. J Bone Jt Infect.
2:194–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kabore C, Wouters A, Frippiat F and Gillet
P: Management of chronic osteomyelitis by long-term antibiotic
suppression. Rev Med Liege. 72:363–368. 2017.(In French).
PubMed/NCBI
|
|
4
|
Geurts J, Hohnen A, Vranken T and Moh P:
Treatment strategies for chronic osteomyelitis in low- and
middle-income countries: Systematic review. Trop Med Int Health.
22:1054–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masquelet AC and Begue T: The concept of
induced membrane for reconstruction of long bone defects. Orthop
Clin North Am. 41:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rousset M, Walle M, Cambou L, Mansour M,
Samba A, Pereira B, Ghanem I and Canavese F: Chronic infection and
infected non-union of the long bones in paediatric patients:
Preliminary results of bone versus beta-tricalcium phosphate
grafting after induced membrane formation. Int Orthop. 42:385–393.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azi ML, de Almeida Teixeira AA, Cotias RB,
Joeris A and Kfuri Junior M: Bone union with an in situ spacer
after the first stage of the induced membrane technique. Injury. 48
Suppl 4:S17–S20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masquelet AC: Induced membrane technique:
Pearls and pitfalls. J Orthop Trauma. 31 Suppl 5:S36–S38. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Konda SR, Gage M, Fisher N and Egol KA:
Segmental bone defect treated with the induced membrane technique.
J Orthop Trauma. 31 Suppl 3:S21–S22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han W, Shen J, Wu H, Yu S, Fu J and Xie Z:
Induced membrane technique: Advances in the management of bone
defects. Int J Surg. 42:110–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan XJ: Intravenous antibiotic perfusion
in chronic osteomyelitis. Zhonghua Hu Li Za Zhi. 19:337–338.
1984.(In Chinese). PubMed/NCBI
|
|
12
|
Avdeeva EY, Slizovsky GV, Skorokhodova MG,
Fomina TI, Zorkaltsev MA, Zavadovskaya VD, Krasnov EA, Ivanov VV
and Stepanov MY: Experimental simulation of traumatic osteomyelitis
in rats. Bull Exp Biol Med. 161:137–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Argenta LC and Morykwas MJ:
Vacuum-assisted closure: a new method for wound control and
treatment: Clinical experience. Ann Plast Surg. 38:563–576;
discussion 577. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henry SL: Discussion: The efficacy of
perforator flaps in the treatment of chronic osteomyelitis. Plast
Reconstr Surg. 140:189–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kinik H and Karaduman M: Cierny-Mader type
III chronic osteomyelitis: The results of patients treated with
debridement, irrigation, vancomycin beads and systemic antibiotics.
Int Orthop. 32:551–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong JPJ, Goh TLH, Choi DH, Kim JJ and Suh
HS: The efficacy of perforator flaps in the treatment of chronic
osteomyelitis. Plast Reconstr Surg. 140:179–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurup Narayana JK, Singasani R and Mohanty
SP: Rare case of disseminated rhinosporidiosis with chronic
osteomyelitis of the calcaneum treated by a simple technique of
negative pressure wound therapy. BMJ Case Rep 2017.
bcr-2017-221786. 2017.
|
|
18
|
Stanger KM, Albert F, Kneser U, Bogdan C
and Horch RE: Management of chronic osteomyelitis of the tibia with
life-threatening complications under negative pressure wound
therapy and isolation of Helcococcus kunzii. Int Wound J.
12:443–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng B, Song CY, Jin HT, Xiao LW and Tong
PJ: Clinical diagnosis and treatment of chronic osteomyelitis.
Zhongguo Gu Shang. 28:870–873. 2015.(In Chinese). PubMed/NCBI
|
|
20
|
Jiang N, Ma YF, Jiang Y, Zhao XQ, Xie GP,
Hu YJ, Qin CH and Yu B: Clinical characteristics and treatment of
extremity chronic osteomyelitis in southern China: A retrospective
analysis of 394 consecutive patients. Medicine (Baltimore).
94:e18742015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gokalp MA, Guner S, Ceylan MF, Doğan A and
Sebik A: Results of treatment of chronic osteomyelitis by ‘gutter
procedure and muscle flap transposition operation’. Eur J Orthop
Surg Traumatol. 24:415–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelissier P, Masquelet AC, Bareille R,
Pelissier SM and Amedee J: Induced membranes secrete growth factors
including vascular and osteoinductive factors and could stimulate
bone regeneration. J Orthop Res. 22:73–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aho OM, Lehenkari P, Ristiniemi J,
Lehtonen S, Risteli J and Leskelä HV: The mechanism of action of
induced membranes in bone repair. J Bone Joint Surg Am. 95:597–604.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marais LC and Ferreira N: Bone transport
through an induced membrane in the management of tibial bone
defects resulting from chronic osteomyelitis. Strateg Trauma Limb
Reconstr. 10:27–33. 2015. View Article : Google Scholar
|
|
25
|
Marais LC, Ferreira N, Aldous C and Le
Roux TL: The outcome of treatment of chronic osteomyelitis
according to an integrated approach. Strateg Trauma Limb Reconstr.
11:135–142. 2016. View Article : Google Scholar
|
|
26
|
Dzyuba GG, Reznik LB, Erofeev SA and
Odarchenko DI: Efficiency of local cement reinforcing antibacterial
implants in surgical treatment of long bones chronic osteomyelitis.
Khirurgiia (Mosk). 5:31–36. 2016.(In Russian).
|
|
27
|
Karger C, Kishi T, Schneider L, Fitoussi F
and Masquelet AC: French Society of Orthopaedic Surgery and
Traumatology (SoFCOT): Treatment of posttraumatic bone defects by
the induced membrane technique. Orthop Traumatol Surg Res.
98:97–102. 2012. View Article : Google Scholar : PubMed/NCBI
|