Introduction
Ovarian cancer is the most lethal gynecological
malignancy and the fifth leading cause of cancer-associated deathin
women (1,2). Ovarian cancers are responsible for more
than half of the female genital tract cancers caused deaths, with
more than 2 million new cases being diagnosed every year (1,3,4). Despite significant advances in surgery
and chemotherapy, the long-term survival rate of patients with
ovarian cancers is still very low (3). Thus, there is clearly an urgent need to
unravel the molecular mechanisms of ovarian cancer progression for
an improved treatment outcome.
Secreted protein acidic and rich in cysteine-like
protein 1 (SPARCL1) is a member of the SPARC family, which is
associated with various biological behaviors including osteoblast
differentiation and development of cancers. Previous studies have
suggested SPARCL1 as a potential tumor suppressor (5–7). Low
SPARCL1 expression is associated with the development and
progression of cervical cancer (8).
Zhao et al (5) detected that
SPARCL1 was significantly down-regulated in human osteosarcoma cell
lines and clinical tissue samples. Interestingly, a recent study
revealed that SPARCL1 was strongly down-regulated by 6.4-fold in
594 ovarian cancer cases compared with eight normal ovaries and was
suspected to contribute to tumor invasiveness (9). However, the effect of SPARCL1 on
ovarian cancer and its underlying mechanisms remain elusive.
The present study aimed to investigate the role of
SPARCL1 in ovarian cancer. In this study we are the first to
evaluate the expression levels of SPARCL1 in human ovarian cancer
tissues and the specific effects of SPARCL1 on ovarian cancer cell
proliferation and migration, and to explore possible mechanisms of
action.
Materials and methods
Clinical tissues
The present study was approved by the Medical Ethics
Committee of the Third Affiliated Hospital, Xinjiang Medical
University (Urumqi, China). Signed written informed consent was
obtained from all subjects. Twenty ovarian cancer tissues and
paired adjacent normal tissues were collected during surgery from
ovarian cancer patients without prior chemotherapy or radiotherapy.
Disease status had been confirmed in all specimens by pathological
diagnosis.
Immunohistochemistry (IHC)
IHC staining was performed using the standard
immunoperoxidase staining procedure as previously reported
(10–12), to detect SPARCL1 expression in
paraffin-embedded specimens. The slides of 3 µm thickness were
incubated overnight with polyclonal rabbit anti-SPARCL1 antibody
(1:100; Abcam, Cambridge, UK). After a secondary antibody was
applied for 30 min at room temperature, the slides were stained
with diaminobenzidine (DAB) and hematoxylin. The samples were
blinded during the independent assessment of immunostaining
intensity by three senior pathologists. Each section was
photographed and semi-quantitative analyzed using computerized
image analysis (Image J; National Institutes of Health, Bethesda,
MD, USA).
Cell culture
The human ovarian cancer cell line SKOV-3 was
purchased commercially from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The cells were grown in RPMI-1640
medium (Sigma, Shanghai, China) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 50 µg/ml streptomycin and 50 IU/ml penicillin, according to
the instructions of the American Type Culture Collection (ATCC,
Manassas, VA, USA). Cells were cultured at 37°C in a humidified
atmosphere of 5% CO2 and were regenerated every 3 days
when they reached 70–90% confluence.
Lentiviral transfection
A lentiviral short hairpin RNA (shRNA) construct
targeting SPARCL1 (with targeting sequences to SPARCL1 mRNA as
follows: 5′-CCCGACAAATGCAAGATTATT-3′) was obtained from Jikai
Corporation (Jikai, Shanghai, China). The oligonucleotides were
then phosphorylated, annealed, and cloned into the pLKO.1
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) following the
manufacturer's instructions. The overexpression particles of
SPARCL1 were purchased from GenePharma (Shanghai, China).
Transfection of SPARCL1 was performed following the manufacturer's
instructions.
Cell proliferation
The cell proliferation was assessed using a MTT
assay kit (Beyotime Institute of Biotechnology, Haimen, China). To
evaluate the effects of SPARCL1 on human ovarian cells, SKOV-3
cells (5,000 cells/well in 100 µl medium) were seeded into 96-well
plates on days 1, 2, 3, and 4 post-transfection. After 24 h, the
medium was removed, and replaced with 100 µl RPMI-1640 medium
without FBS supplemented with 20 µl MTT (5 mg/ml). After incubation
for 4 h at 37°C, the absorbance at 450 nm was determined using a
microplate reader (iMark; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Scratch wound healing assay
SKOV-3 cells (1×106 cells/well) were
seeded into 6-well plates and cultured to a 100% confluent
monolayer. In each well a wound was induced by scratching a
straight line into the cell layer with a 200 µl pipette tip. After
24 h of incubation with RPMI 1640 medium without FBS, images of the
wound healing areas were captured and measured with a Leica DM2500
image analysis system (Leica, Mannheim, Germany).
Cell migration assay
The cell migration assay was performed to analyze
the effect of SPARCL1 on the migration of SKOV-3 cells. SKOV-3
cells (2.5×104 cells) were suspended in serum-free
medium and seeded into the upper chamber of a transwell plate
(Corning Costar, Rochester, NY, USA). RPMI-1640 medium containing
10% FBS was added in the lower chamber. The cells were allowed to
migrate for 24 h at 37°C. Then, the non-migratory cells that
remained in the upper chamber were removed, while the cells that
had migrated to the lower chamber were fixed and stained with 0.1%
crystal violet. Six randomly selected fields were photographed, and
the cells per field of view were counted under a light microscope
(at ×200 magnification).
Western blot analysis
Following treatment, the SKOV-3 cells were harvested
and lysed in protein lysis buffer supplemented with a protease
inhibitor (Beyotime Institute of Biotechnology). The total protein
concentrations were determined using a bicinchoninic acid (BCA)
protein assay kit (Bio-Rad Laboratories, Inc.). Equal amounts of
protein were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and then transferred to a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA). After blocking in 5% non-fat dry milk for 2 h at room
temperature, the membranes were incubated overnight at 4°C with
primary antibodies specific to GAPDH (1:8,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), SPARCL1 (1:1,000; Abcam),
p-mitogen-activated protein kinase kinase (p-MEK; 1:1,000; Cell
Signaling Technology, Inc.), t-MEK (1:1,000; Cell Signaling
Technology, Inc.), p-extracellular signal-related kinase (p-ERK;
1:1,000; Cell Signaling Technology, Inc.), t-ERK (1:1,000; Cell
Signaling Technology, Inc.) and MMP-2 (1:1,000; Cell Signaling
Technology, Inc.). Then the membrane was incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:1,000) for 2 h
at room temperature. Immunoreactive bands were developed by
enhanced chemiluminescence reagents (Pierce; Thermo Fisher
Scientific, Inc.) and quantified using Bio-Rad Quantity One
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were conducted at least three times.
Data were presented as the mean ± standard deviation. The results
between groups were statistically analyzed by Student's t-test or
one-way analysis of variance with Bonferroni's post hoc test using
SPSS 23.0 software (IBM Corp., Armonk, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
SPARCL1 was downregulated in clinical
ovarian cancer specimens
The expression levels of SPARCL1 in ovarian cancer
tissues and adjacent normal tissues was determined by IHC using
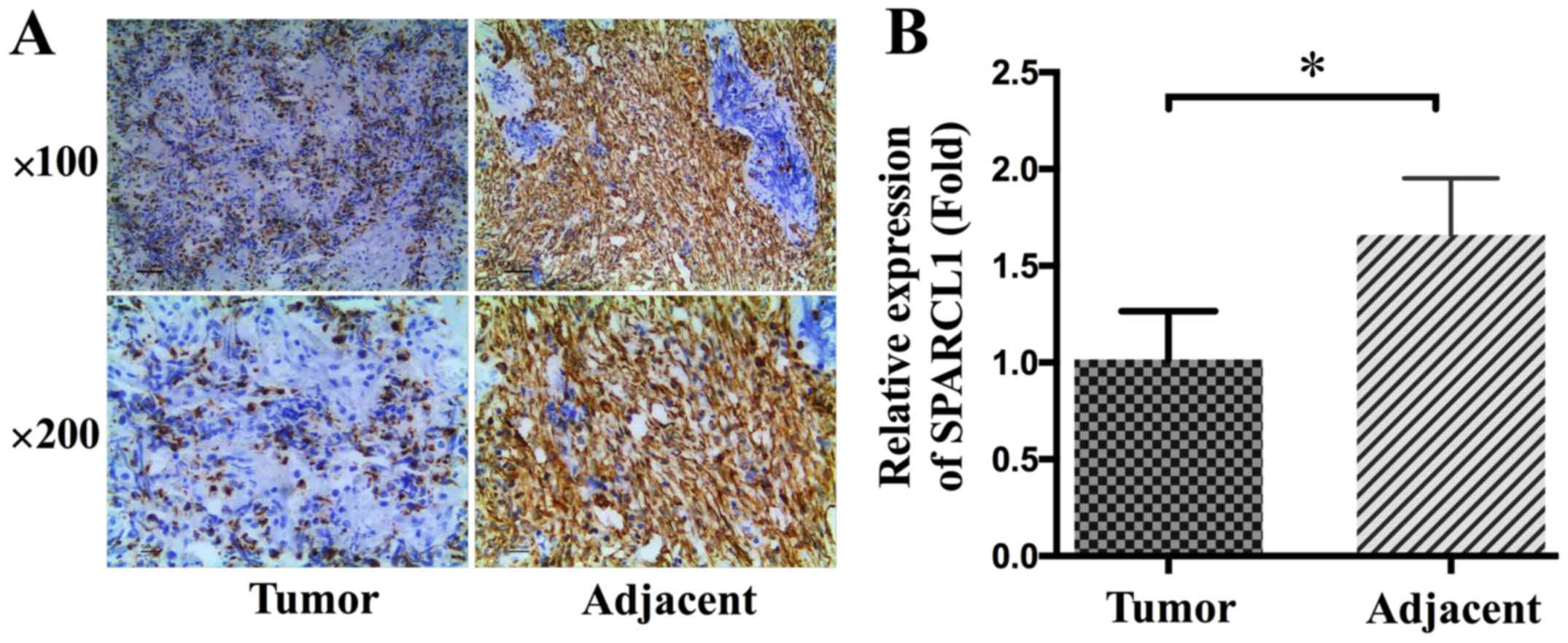
SPARCL1 specific antibodies. As shown in Fig. 1A, SPARCL1 was predominantly localized
to cytoplasm of the adjacent normal tissues, whereas there was none
or low level staining observed in ovarian cancer tissues. After
quantification we found that significantly lower levels of SPARCL1
were detected in ovarian cancer tissues than in adjacent normal
tissues (P<0.05; Fig. 1).
SPARCL1 inhibited SKOV-3 cell
proliferation
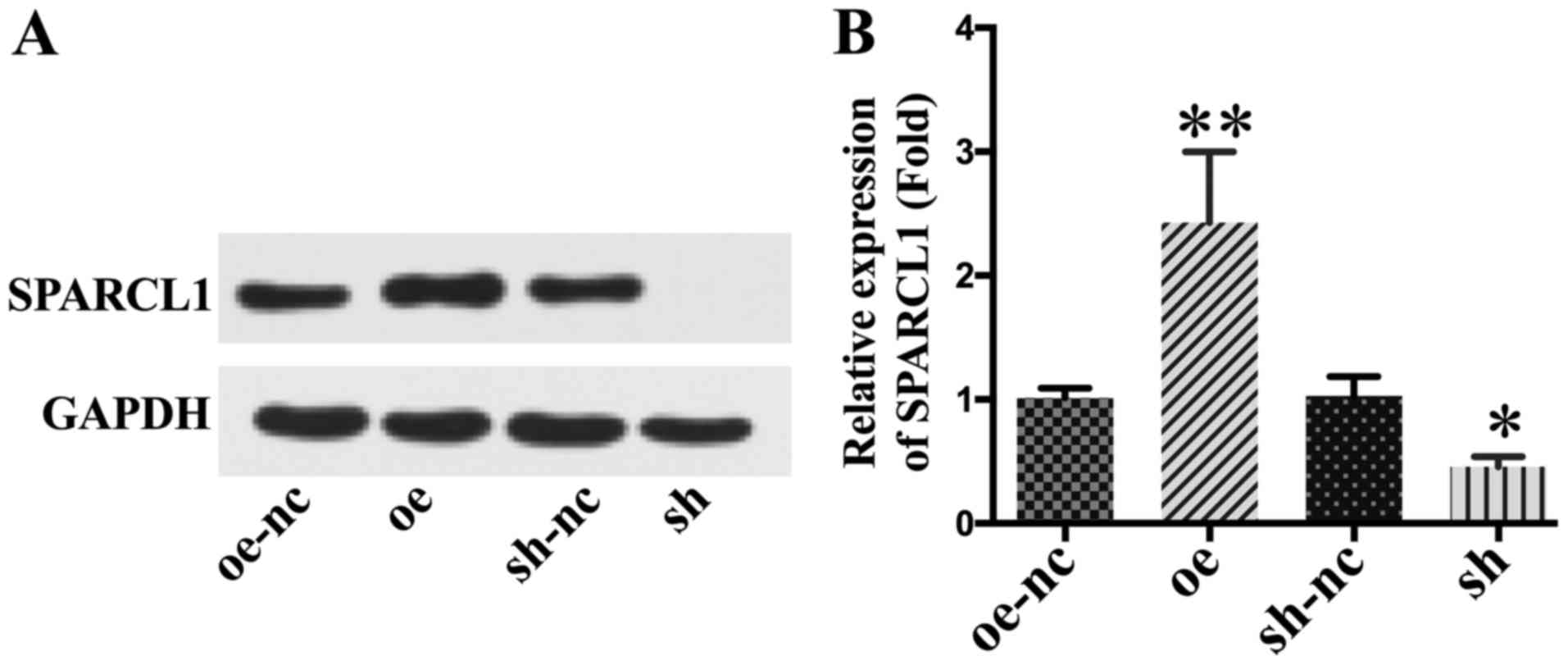
Western blotting analysis verified the successful
up- or down-regulation of SPARCL1 expression levels in SKOV-3 cells
after lentiviral transfection (Fig.
2). The results of the MTT assay revealed a significantly lower
proliferation level in the SPARCL1 overexpression group compared
with the other groups. After knock-down of SPARCL1, the
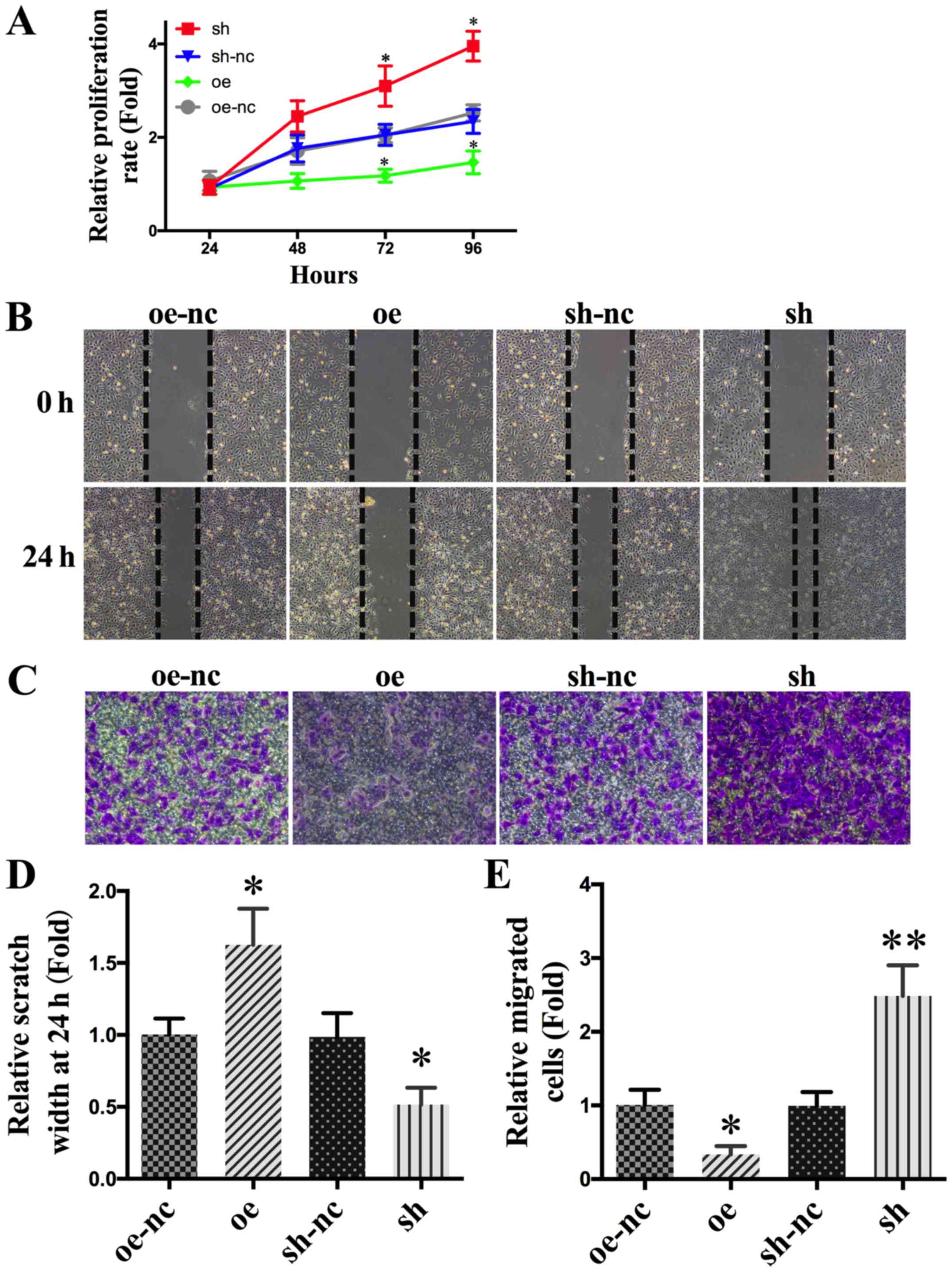
proliferation rate increased significantly (Fig. 3A).
SPARCL1 inhibits migration of SKOV-3
cells
Relevant photographs of wound healing assay
were taken immediately after induction of the wound and 24 h later
on the following day. SPARCL1-overexpressing SKOV-3 cells had
significantly lower levels of wound healing than those of the
control group. In contrast, wound healing was significantly
enhanced after SPARCL1 knockdown (Fig.
3B and D). In order to further examine the effect of SPARCL1 on
human ovarian cancer cell migration, a transwell assay was
performed. SPARCL1-overexpression or knockdown was initiated in
SKOV-3 cells for 24 h. As shown in Fig.
3C and E, the migration of SKOV-3 cells was significantly
inhibited by SPARCL1 overexpression. Furthermore, migration of
SKOV-3 cells was significantly enhanced after SPARCL1 knockdown
(Fig. 3C and E).
SPARCL1 inhibited the MEK/ERK
signaling pathway
To investigate the underlying mechanism of action of
SPARCL1 that affected the proliferation and migration of SKOV-3
cells, we investigated different key players of the MEK/ERK
signaling pathway, because this pathway has a key regulator
function in ovarian cancer cells. As shown in Fig. 4A, western blotting analysis showed
that SPARCL1 overexpression led to a significantly reduced
expression of activated MMP-2, while SPARCL1 knockdown led to
increased expression levels of activated MMP-2. In addition,
SPARCL1 overexpression significantly decreased the levels of p-MEK
and p-ERK. In contrast, the expression of p-MEK and p-ERK were
significantly upregulated in SPARCL1-knockdown cells (Fig. 4A-C).
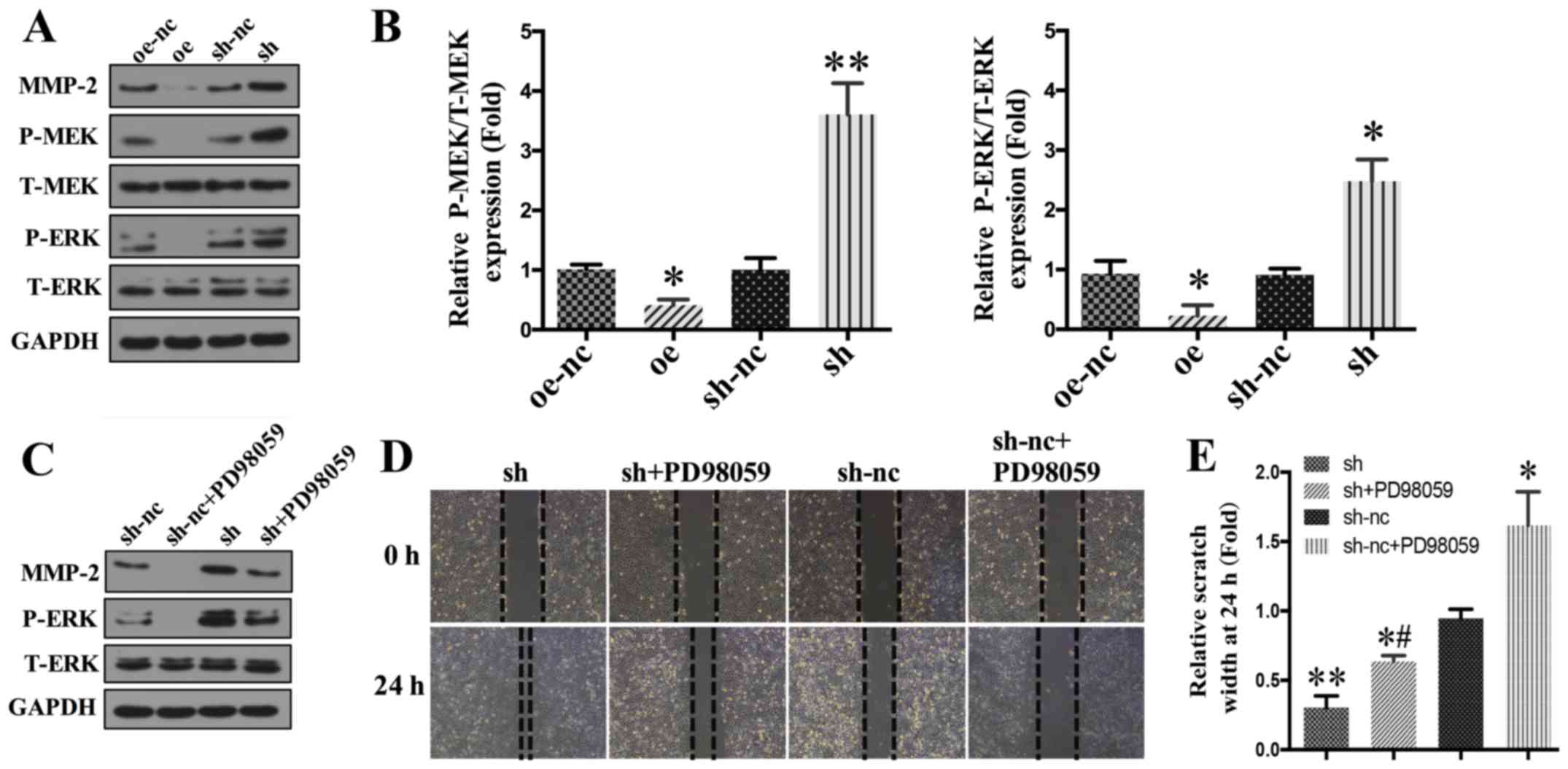 | Figure 4.SPARCL1 inhibits the MEK/ERK signaling
pathway. (A) The protein levels of MMP-2, p-MEK, MEK, p-ERK and ERK
were assessed by western blot analyses. (B) Relative quantitative
comparison of the p-MEK and p-ERK protein expression. *P<0.05
and **P<0.01 vs. oe-nc. (C) The effect of SPARCL1-knockdown on
p-MEK and p-ERK expression level following the application of
PD98059. (D) The effect of SPARCL1-knockdown on SKOV-3 cells
migration following the application of PD98059 (magnification,
×200). (E) Relative quantitative comparison of scratch width of the
wound healing assay. *P<0.05 and **P<0.01 vs. sh-nc group;
#P<0.05 vs. sh group. oe, overexpression; nc, normal
control; sh, short hairpin RNA; SPARCL1, secreted protein acidic
and rich in cysteine-like protein 1; p-, phosphorylated; T-, total;
MEK, mitogen-activated protein kinase kinase; ERK, extracellular
signal-related kinase; MMP, matrix metalloproteinase. |
To examine the mechanism by which SPARCL1 mediated
the inhibition of MEK/ERK, a MEK1 specific inhibitor PD98059 (50
µmol/l), was used to inhibit MEK expression. As shown in Fig. 4C, PD98059 significantly inhibited the
expression of matured MMP-2. In addition, the phosphorylation of
MEK and ERK was blocked by the pretreatment of the cells with
PD98059 (Fig. 4C). Furthermore, the
results of the wound healing assay showed that the increased
migration that was induced by SPARCL1 knockdown were also reduced
after PD98059 exposure (Fig. 4D),
suggesting that the inhibition effect of SPARCL1 on SKOV-3 cells
may be mediated, at least partly, due to the downregulation of
MEK/ERK signaling.
Discussion
Although previous studies have revealed a role of
SPARCL1 in tumor suppression (5–9), it
remains unknown whether SPARCL1 has any effect on ovarian cancer
cell proliferation and migration and by which molecular mechanisms.
The present study investigated the role of SPARCL1 in ovarian
cancer. Our results show that the expression of SPARCL1 in ovarian
cancer tissues was significantly lower than in the adjacent normal
tissues. SPARCL1 overexpression significantly inhibited the
proliferation and migration of SKOV-3 cells. Moreover, the tumor
suppressing effect of SPARCL1 might be mediated by inhibiting the
MEK/ERK pathway and downregulating the expression of MMP-2.
SPARCL1 was found to be downregulated in several
human tumors, particularly in non-small cell lung cancer (NSCLC),
pancreas and colon carcinomas, osteosarcoma, and prostate cancers
(5–7,13–16).
Previous studies have demonstrated SPARCL1 to mediate cell
detachment of cultured cells in vitro and suggested a
putative role of SPARCL1 as tumor suppressor (6,7,16). Bendik et al (17) found that SPARCL1 was significantly
downregulated in nine patients with NSCLC. In another study, Xiang
et al (7) found that SPARCL1
inhibited the migration and invasion in a prostate cancer cell line
both in vitro and in vivo, suggesting a putative role
of SPARCL1 in suppressing prostate cancer metastasis. Naschberger
et al (18) reported that
SPARCL1 is of key importance for the modulation of the tumor
microenvironment in colorectal carcinoma, by promoting stromal cell
plasticity. Moreover, SPARCL1 was more strongly expressed in highly
differentiated tumors than in lowly differentiated ones, which
correlated significantly with patient prognosis (19). Similarly, in our study, IHC revealed
lower SPARCL1 expression in ovarian cancer samples, suggesting that
low SPARCL1 expression levels may be associated with a better
prognosis. Overexpression of SPARCL1 inhibited the proliferation
and migration of human ovarian cancer cells, indicating that
SPARCL1 may inhibit the development and metastasis of ovarian
cancer.
The MEK/ERK pathway is one of the key signaling
pathways involved in cell survival and proliferation that transmits
the signal by phosphorylation of a series of downstream molecules
(20–27). In squamous cell carcinoma cells,
MEK/ERK signaling has been demonstrated to support cell survival by
inducing the anti-apoptotic protein Bcl-2 (28). Previous studies have shown that
alteration of the MEK/ERK pathway is also strongly implicated in
ovarian cancer pathogenesis (29–37).
Al-Ayoubi et al (38)
reported that signaling via the MEK/ERK pathway helps cells escape
anoikis and maintain anchorage-independent growth in several
ovarian cancer cell lines. Moreover, several studies have suggested
that inhibition of the MEK/ERK pathway disrupts functions essential
for ovarian cancer progression (29,35,37).
Dang et al (39) reported
that metformin in combination with cisplatin inhibits cell
viability and induces apoptosis of human ovarian cancer cells by
inactivating ERK. In another study, Chan et al (40) demonstrated that therapies targeting
the ERK signaling pathway reduce the aggressiveness of ovarian
cancer cells. In our study, SPARCL1 was found to inhibit the
expression of p-MEK and p-ERK via the MEK/ERK pathway, which was
then rescued by a MEK inhibitor. In line with previous studies, we
found that inhibition of MEK/ERK was associated with ovarian cancer
cell proliferation and migration. Thus, we conclude that the
inhibition effect of SPARCL1 on SKOV-3 cells may be, at least
partly, due to the downregulation of MEK/ERK signaling.
To our knowledge, this is the first study that
investigates the effect of SPARCL1 on ovarian cancer cells.
However, a key limitation of this study is that is an in
vitro study. Thus, the results of the current study need to be
validated by in vivo experiments in suitable animal models.
Furthermore, there may be additional signal pathways involved apart
from the MEK/ERK signaling. Further studies are required to
determine possible alternative signal pathways that may also play a
role in ovarian cancer progression.
In summary, our results show that SPARCL1 suppresses
ovarian cancer cell proliferation and migration by downregulating
signaling via the MEK/ERK pathway. These findings suggest that
SPARCL1, a possible tumor suppressor, has potential as therapeutic
target for developing novel therapeutic strategies in the
prevention and intervention of ovarian cancers.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Natural Science Foundation of Xinjiang Uygur Autonomous Region
(grant no. 2017D01C409).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL conceived and designed the present study. YM and
YX performed the experiments, analyzed and interpreted the data,
and were also the predominant contributors in the writing of the
manuscript. YM made contributions to the interpretation of data and
critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Third Affiliated Hospital, Xinjiang Medical
University (Xinjiang, China). Signed written informed consent was
obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen C, Wu J, Zhu P, Xu C and Yao L:
Investigating isoquinoline derivatives for inhibition of inhibitor
of apoptosis proteins for ovarian cancer treatment. Drug Des Devel
Ther. 11:2697–2707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Su J, Xu H, Yu S, Liu Y, Zhang Y,
Sun L, Yue Y and Zhou X: Dicumarol inhibits PDK1 and targets
multiple malignant behaviors of ovarian cancer cells. PLoS One.
12:e01796722017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Gao JF and Qi BL: PDCD1 strengthens
the sensitivity of ovarian cancer to cisplatin chemotherapy by
promoting apoptosis. J BUON. 22:746–756. 2017.PubMed/NCBI
|
|
4
|
Zhao Y, Cui L, Pan Y, Shao D, Zheng X,
Zhang F, Zhang H, He K and Chen L: Berberine inhibits the
chemotherapy-induced repopulation by suppressing the arachidonic
acid metabolic pathway and phosphorylation of FAK in ovarian
cancer. Cell Prolif. 50:e123932017. View Article : Google Scholar
|
|
5
|
Zhao SJ, Jiang YQ, Xu NW, Li Q, Zhang Q,
Wang SY, Li J, Wang YH, Zhang YL, Jiang SH, et al: SPARCL1
suppresses osteosarcoma metastasis and recruits macrophages by
activation of canonical WNT/β-catenin signaling through
stabilization of the WNT-receptor complex. Oncogene. 37:1049–1061.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye H, Wang WG, Cao J and Hu XC: SPARCL1
suppresses cell migration and invasion in renal cell carcinoma. Mol
Med Rep. 16:7784–7790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiang Y, Qiu Q, Jiang M, Jin R, Lehmann
BD, Strand DW, Jovanovic B, DeGraff DJ, Zheng Y, Yousif DA, et al:
Yi Y: SPARCL1 suppresses metastasis in prostate cancer. Mol Oncol.
7:1019–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu DM, Shi J, Liu T, Deng SH, Han R and Xu
Y: Integrated analysis reveals down-regulation of SPARCL1 is
correlated with cervical cancer development and progression. Cancer
Biomark. 21:355–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Gao Y, Zhao B, Li X, Lu Y, Zhang J,
Li D, Li L and Yin F: Discovery of microarray-identified genes
associated with ovarian cancer progression. Int J Oncol.
46:2467–2478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang G, Yang D, Wang L, Zhang X, Xu H,
Miao Y, Wang E and Zhang Y: A novel biomarker ARMc8 promotes the
malignant progression of ovarian cancer. Hum Pathol. 46:1471–1479.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Josahkian JA, Saggioro FP, Vidotto T,
Ventura HT, Candido Dos Reis FJ, de Sousa CB, Tiezzi DG, de Andrade
JM, Koti M and Squire JA: Increased STAT1 expression in high grade
serous ovarian cancer is associated with a better outcome. Int J
Gynecol Cancer. 28:459–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei W, Li Y, Lv S, Zhang C and Tian Y:
PARP-1 may be involved in angiogenesis in epithelial ovarian
cancer. Oncol Lett. 12:4561–4567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao F, Wang K, Zhu R, Hu YW, Fang WZ and
Ding HZ: Clinicopathological significance of reduced SPARCL1
expression in human breast cancer. Asian Pac J Cancer Prev.
14:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hurley PJ, Hughes RM, Simons BW, Huang J,
Miller RM, Shinder B, Haffner MC, Esopi D, Kimura Y, Jabbari J, et
al: Androgen-regulated SPARCL1 in the tumor microenvironment
inhibits metastatic progression. Cancer Res. 75:4322–4334. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurley PJ, Marchionni L, Simons BW, Ross
AE, Peskoe SB, Miller RM, Erho N, Vergara IA, Ghadessi M, Huang Z,
et al: Secreted protein, acidic and rich in cysteine-like 1
(SPARCL1) is down regulated in aggressive prostate cancers and is
prognostic for poor clinical outcome. Proc Natl Acad Sci USA.
109:14977–14982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jakharia A, Borkakoty B and Singh S:
Expression of SPARC like protein 1 (SPARCL1), extracellular
matrix-associated protein is down regulated in gastric
adenocarcinoma. J Gastrointest Oncol. 7:278–283. 2016.PubMed/NCBI
|
|
17
|
Bendik I, Schraml P and Ludwig CU:
Characterization of MAST9/Hevin, a SPARC-like protein, that is
down-regulated in non-small cell lung cancer. Cancer Res.
58:626–629. 1998.PubMed/NCBI
|
|
18
|
Naschberger E, Liebl A, Schellerer VS,
Schutz M, Britzen-Laurent N, Kolbel P, Schaal U, Haep L,
Regensburger D, Wittmann T, et al: Matricellular protein SPARCL1
regulates tumor microenvironment-dependent endothelial cell
heterogeneity in colorectal carcinoma. J Clin Invest.
126:4187–4204. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Widegren E, Wang DW and Sun XF:
SPARCL1: A potential molecule associated with tumor diagnosis,
progression and prognosis of colorectal cancer. Tumour Biol.
32:1225–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chi F, Wu R, Jin X, Jiang M and Zhu X:
HER2 induces cell proliferation and invasion of non-small-cell lung
cancer by upregulating COX-2 expression via MEK/ERK signaling
pathway. Onco Targets Ther. 9:2709–2716. 2016.PubMed/NCBI
|
|
21
|
Li K, Guo Q, Yang J, Chen H, Hu K, Zhao J,
Zheng S, Pang X, Zhou S, Dang Y and Li L: FOXD3 is a tumor
suppressor of colon cancer by inhibiting EGFR-Ras-Raf-MEK-ERK
signal pathway. Oncotarget. 8:5048–5056. 2017.PubMed/NCBI
|
|
22
|
Shen MJ, Xu LJ, Yang L, Tsai Y, Keng PC
and Chen Y, Lee SO and Chen Y: Radiation alters PD-L1/NKG2D ligand
levels in lung cancer cells and leads to immune escape from NK cell
cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget.
8:80506–80520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Guo X, Xie C and Jiang J: KIF15
promotes pancreatic cancer proliferation via the MEK-ERK signalling
pathway. Br J Cancer. 117:245–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Xiao H, Wu H, Yao C, He H, Wang C
and Li W: G protein subunit alpha q regulates gastric cancer growth
via the p53/p21 and MEK/ERK pathways. Oncol Rep. 37:1998–2006.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang K, Gao K, Hu G, Wen Y, Lin C and Li
X: CTGF enhances resistance to 5-FU-mediating cell apoptosis
through FAK/MEK/ERK signal pathway in colorectal cancer. Onco
Targets Ther. 9:7285–7295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Shen M, Xu LJ, Yang X, Tsai Y,
Keng PC, Chen Y and Lee SO: Enhancing NK cell-mediated cytotoxicity
to cisplatin-resistant lung cancer cells via MEK/Erk signaling
inhibition. Sci Rep. 7:79582017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Yu P, Zhu L, Zhao Q, Lu X and Bo
S: Blockade of alpha7 nicotinic acetylcholine receptors inhibit
nicotine-induced tumor growth and vimentin expression in non-small
cell lung cancer through MEK/ERK signaling way. Oncol Rep.
38:3309–3318. 2017.PubMed/NCBI
|
|
28
|
Shen X and Kramer RH: Adhesion-mediated
squamous cell carcinoma survival through ligand-independent
activation of epidermal growth factor receptor. Am J Pathol.
165:1315–1329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai RX, Wang WP, Zhao PW and Li CB:
Ghrelin attenuates the growth of HO-8910 ovarian cancer cells
through the ERK pathway. Braz J Med Biol Res. 49:e50432016.
View Article : Google Scholar
|
|
30
|
Bartholomeusz C, Itamochi H, Nitta M, Saya
H, Ginsberg MH and Ueno NT: Antitumor effect of E1A in ovarian
cancer by cytoplasmic sequestration of activated ERK by PEA15.
Oncogene. 25:79–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang MC, Chen CA, Chen PJ, Chiang YC,
Chen YL, Mao TL, Lin HW, Lin Chiang WH and Cheng WF: Mesothelin
enhances invasion of ovarian cancer by inducing MMP-7 through
MAPK/ERK and JNK pathways. Biochem J. 442:293–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujisawa T, Joshi BH and Puri RK: IL-13
regulates cancer invasion and metastasis through IL-13Rα2 via
ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J
Cancer. 131:344–356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohta T, Isobe M, Takahashi T,
Saitoh-Sekiguchi M, Motoyama T and Kurachi H: The Akt and ERK
activation by platinum-based chemotherapy in ovarian cancer is
associated with favorable patient outcome. Anticancer Res.
29:4639–4647. 2009.PubMed/NCBI
|
|
34
|
Song H, Wei M, Liu W, Shen S, Li J and
Wang L: Cisplatin induced apoptosis of ovarian cancer A2780s cells
by activation of ERK/p53/PUMA signals. Histol Histopathol.
33:73–79. 2018.PubMed/NCBI
|
|
35
|
Su S, Lin X, Ding N, Zhang H, Zhang Q,
Ding Y, Hou X and Tian Y: Effects of PARP-1 inhibitor and ERK
inhibitor on epithelial mesenchymal transitions of the ovarian
cancer SKOV3 cells. Pharmacol Rep. 68:1225–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vergara D, Simeone P, Toraldo D, Del
Boccio P, Vergaro V, Leporatti S, Pieragostino D, Tinelli A, De
Domenico S, Alberti S, et al: Resveratrol downregulates Akt/GSK and
ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol
Biosyst. 8:1078–1087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Ayoubi A, Tarcsafalvi A, Zheng H,
Sakati W and Eblen ST: ERK activation and nuclear signaling induced
by the loss of cell/matrix adhesion stimulates
anchorage-independent growth of ovarian cancer cells. J Cell
Biochem. 105:875–884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dang JH, Jin ZJ, Liu XJ, Hu D, Wang J, Luo
Y and Li LL: Metformin in combination with cisplatin inhibits cell
viability and induces apoptosis of human ovarian cancer cells by
inactivating ERK 1/2. Oncol Lett. 14:7557–7564. 2017.PubMed/NCBI
|
|
40
|
Chan DW, Hui WW, Cai PC, Liu MX, Yung MM,
Mak CS, Leung TH, Chan KK and Ngan HY: Targeting GRB7/ERK/FOXM1
signaling pathway impairs aggressiveness of ovarian cancer cells.
PLoS One. 7:e525782012. View Article : Google Scholar : PubMed/NCBI
|