Introduction
Nasopharyngeal carcinoma (NPC), which is considered
the most common malignant epithelial tumor of the head and neck in
Southern China, represents a significant disease burden that
seriously impacts the quality of human life (1–4). Due to
the frequent metastasis and poor prognosis, NPC is considered a
highly malignant tumor (5). The
standard approach for the treatment of NPC is chemoradiotherapy
(6). Although great progress has
been made in the development of therapeutic strategies for treating
NPC, the therapeutic efficacy is still unsatisfactory. Therefore,
it is of great importance to identify novel effective treatment
therapies for NPC.
MicroRNAs (miRs) are conserved short non-coding RNAs
that are ~22 nucleotides in length. miRNAs can negatively regulate
gene expression by binding to the 3′-untranslated region (3′-UTR)
of the targeted mRNA (7). It has
been reported that miRs are involved in various tumor cellular
processes, including proliferation, differentiation, apoptosis and
metastasis (8–11). Various studies have suggested that
miRs participate in the initiation and development of NPC,
including miR-29c, the let-7 family of miRs, miR-10b and miR-92a,
and their exact roles in NPC have been elucidated (12–15).
Thus, miRs may be considered as a potential target for NPC
treatment.
Among the numerous identified miRs, miR-663b has
been identified to be a novel cancer-associated miR. A previous
study indicated that miR-663b exerts its tumor-suppressive function
via targeting insulin-like growth factor 2 in pancreatic cancer
(16). A further study suggested
that circulating miR-663b in plasma may be a potential novel
biomarker for bladder cancer (17).
Notably, Zhao et al (18)
reported the upregulation of miR-663b in osteosarcoma.
Additionally, a previous study demonstrated that miR-663 was
upregulated in the serum of patients with NPC, and its increased
levels were associated with malignant progression and poor
prognosis (19). However, to the
best of our knowledge, the expression and exact role of miR-663b in
NPC remain to be fully elucidated. Thus, in the present study, the
expression of miR-663b in NPC was investigated and its role in NPC
and the mechanisms were further explored.
Materials and methods
Clinical specimens
A total of 30 paired human NPC tissues and matched
adjacent normal tissues were collected from 30 patients (12 women
and 18 men; age range, 32–61 years), who had undergone routine
surgical procedures in the Central Hospital of Wuhan (Wuhan,
China). The tissue samples were collected between January 2014 and
August 2016. All tissue samples were immediately flash-frozen in
liquid nitrogen and stored at −80°C. The present study was approved
by the Ethics Committee of the Central Hospital of Wuhan. Informed
consent was obtained from every patient.
Cell culture
Normal nasopharyngeal epithelial cells NP69 and the
NPC cell line C666-1 were obtained from the Chinese Academy of
Sciences Cell Bank (Shanghai, China) and cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare, Logan, UT, USA) and 1% penicillin-streptomycin solution
at 37°C in a 5% CO2 incubator.
Cell transfection
C666-1 cells (5×104 cells per well) were
transfected with 50 nM miR-663b mimics, 100 nM miR-663b inhibitors
or 50 nM negative control (NC; Genepharm, Inc., Sunnyvale, CA, USA)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Following transfection for 48 h, the subsequent experiments were
performed. All experiments were repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific Inc.)
according to the manufacturer's protocol. Following this, cDNA was
generated from the RNA using a cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). qPCR was performed using Maxima SYBR-Green/ROX
qPCR Master Mix (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The thermocycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles of denaturation at
95°C for 15 sec and annealing/elongation at 60°C for 30 sec. All
reactions were performed in triplicate. U6 and GAPDH were used as
an internal control for miRNA and mRNA expression, respectively.
The 2−ΔΔCq method was applied to determine the relative
expression values (20). The primer
sequences for RT-qPCR were as indicated in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence (5′-3′) |
|---|
| Smad7 | Forward |
TCCTGCTGTGCAAAGTGTTC |
| Smad7 | Reverse |
TTGTTGTCCGAATTGAGCTG |
| E-cadherin | Forward |
TTAAGCACAACAGCAACAG |
| E-cadherin | Reverse |
GCATCAGCATCAGTCACT |
| N-cadherin | Forward |
GACAATGCCCCTCAAGTGTT |
| N-cadherin | Reverse |
CCATTAAGCCGAGTGATGGT |
| Vimentin | Forward |
AGATGGCCCTTGACATTGAG |
| Vimentin | Reverse |
TGGAAGAGGCAGAGAAATTC |
| MMP-9 | Forward |
CCAAAACTACTCGGAAGACTTGC |
| MMP-9 | Reverse |
GCGACACCAAACTGGATGA |
| miR-663b | Forward |
CGCTAACAGTCTCCAGTC |
| miR-663b | Reverse |
GCGACACCAAACTGGATGA |
| U6 | Forward |
GCTTCGGCAGCACATATACTAAAAT |
| U6 | Reverse |
CGCTTCACGAATTTGCGTGTCAT |
| GAPDH | Forward |
CTTTGGTATCGTGGAAGGACTC |
| GAPDH | Reverse |
GTAGAGGCAGGGATGATGTTCT |
Cell Counting kit (CCK-8) assay
C666-1 cells transfected with miR-663b mimics,
miR-663b inhibitors or NC were collected at 24, 48 and 72 h
following transfection. Cells were seeded in a 96-well plate at the
concentration of 5×103 cells per well. Subsequently, 10
µl CCK-8 assay solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well and cells were incubated
for a further 1 h at 37°C. Cell proliferation was assessed by
measuring the absorbance at 450 nm using a microplate reader.
Western blot analysis
A total of 48 h after transfection, C666-1 cells
were harvested using trypsin. Total cellular proteins were
collected using the radioimmunoprecipitation assay lysis buffer
(Auragene Bioscience, Changsha, China). The bicinchoninic acid kit
(Pierce; Thermo Fisher Scientific, Inc.) was performed to determine
the protein concentration. Protein samples (25 µg/lane) were loaded
and separated by 10% SDS-PAGE and then transferred to
nitrocellulose membranes (GE Healthcare Life Sciences, Little
Chalfont, UK). Membranes were blocked with 5% non-fat milk in
phosphate-buffered saline solution at room temperature for 1.5 h
and then incubated overnight at 4°C with the following primary
antibodies: SMAD7 (cat. no. Sc-11392; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) E-cadherin (cat. no. 3195),
N-cadherin (cat. no. 13116), Vimentin (cat. no. 5741), and matrix
metalloproteinase (MMP)-9 (cat. no. 13667; all 1:1,000) and β-actin
(cat. no. 4970; 1:2,000; all Cell Signaling Technology, Inc.,
Danvers, MA, USA). Subsequently, membranes were washed with
Tris-buffered saline with Tween-20 three times and incubated with
horseradish-peroxidase conjugated anti-rabbit immunoglobulin G
secondary antibodies (cat. no. 7074; 1:1,000; Cell Signaling
Technology, Inc.). at room temperature for 1 h. Blots were
visualized with enhanced chemiluminescence reagent (EMD Millipore,
Billerica, MA, USA). Protein bands were analyzed using WinMDI
version 2.5 software (Purdue University Cytometry Laboratories,
West Lafayette, IN, USA).
Dual luciferase reporter assay
TargetScan (http://www.targetscan.org/) was applied to predict the
putative targets of miR-663b. To confirm whether miR-663b directly
targets the 3′-UTRs of SMAD7, the pmiR-RB-Report™ dual
luciferase reporter gene plasmid vector (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) with the wild-type (WT) and mutated (MUT)
3′UTR of SMAD7 mRNA were constructed (SMAD7-3′UTR-WT and
SMAD7-3′UTR-MUT, respectively). Following this, 293T cells were
co-transfected with SMAD7-3′UTR-WT or SMAD7-3′UTR-MUT and miR-663b
or its NC (NC) vector using Lipofectamine 2000 according to the
manufacturer's instructions. Following transfection for 48 h, the
Dual-Luciferase Reporter Assay kit (Promega Corp., Madison, WI,
USA) was performed to detect the luciferase activity according to
the instructions of the manufacturer. Data were normalized to
Renilla luciferase activity.
Statistical analysis
Data were expressed as mean ± standard deviation.
SPSS 17.0 statistical software (IBM Corp., Armonk, NY, USA) was
performed for all statistical analyses. Student's t-test and
one-way analysis of variance followed by Tukey's test were applied
to analyze the association between groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-663b expression levels increase in
NPC tissues and C666-1 cells
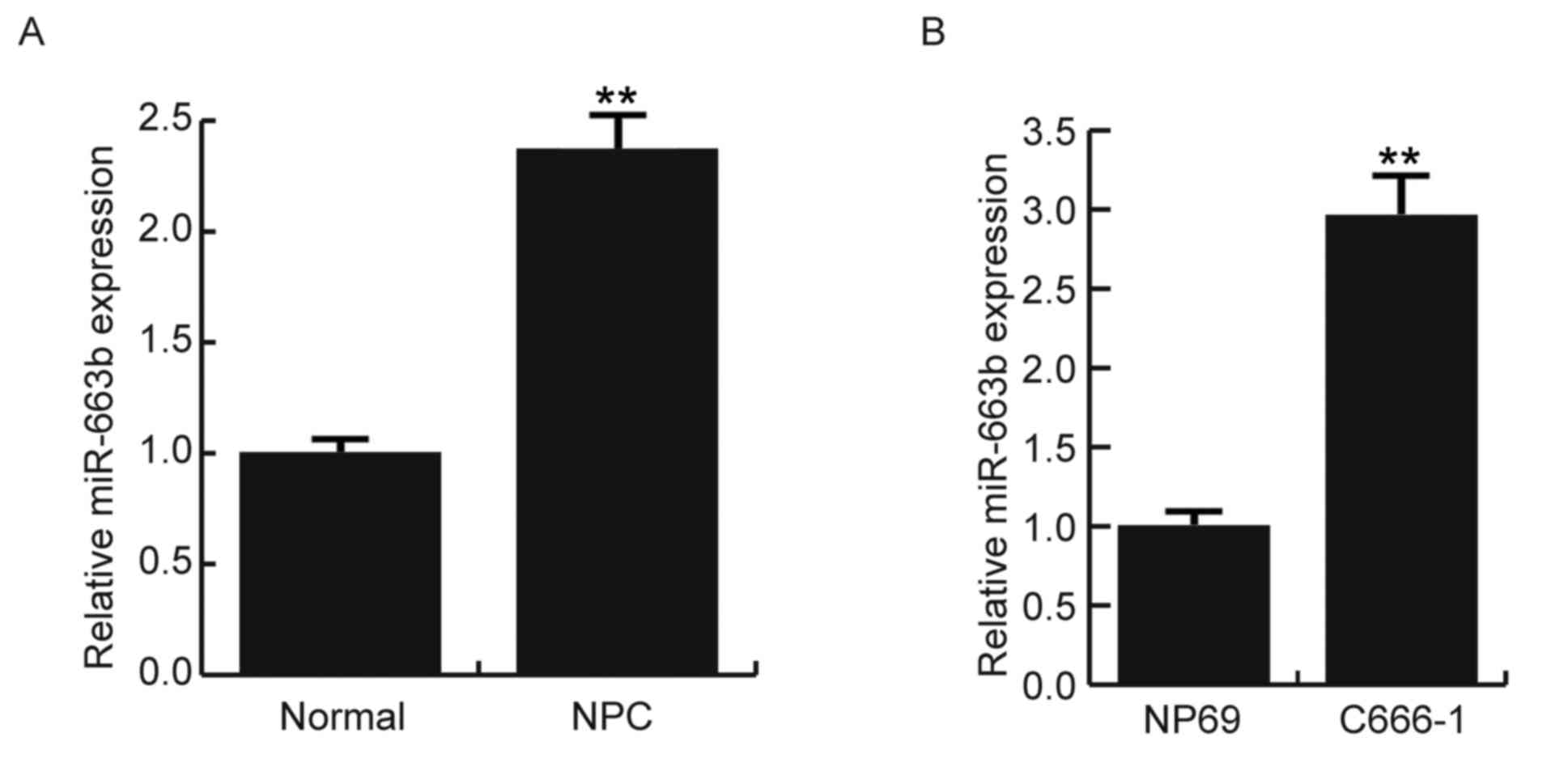
To determine the expression levels of miR-663b in
NPC tissues and NPC cell lines, RT-qPCR was performed. As indicated
in Fig. 1A, miR-663b expression
levels were significantly increased in NPC tissues when compared
with those in normal tissues (P<0.01). Furthermore, the results
also indicated that miR-663b expression levels were significantly
upregulated in the C666-1 cells compared with the NP69 cells
(P<0.01; Fig. 1B).
miR-663b directly targets Smad7
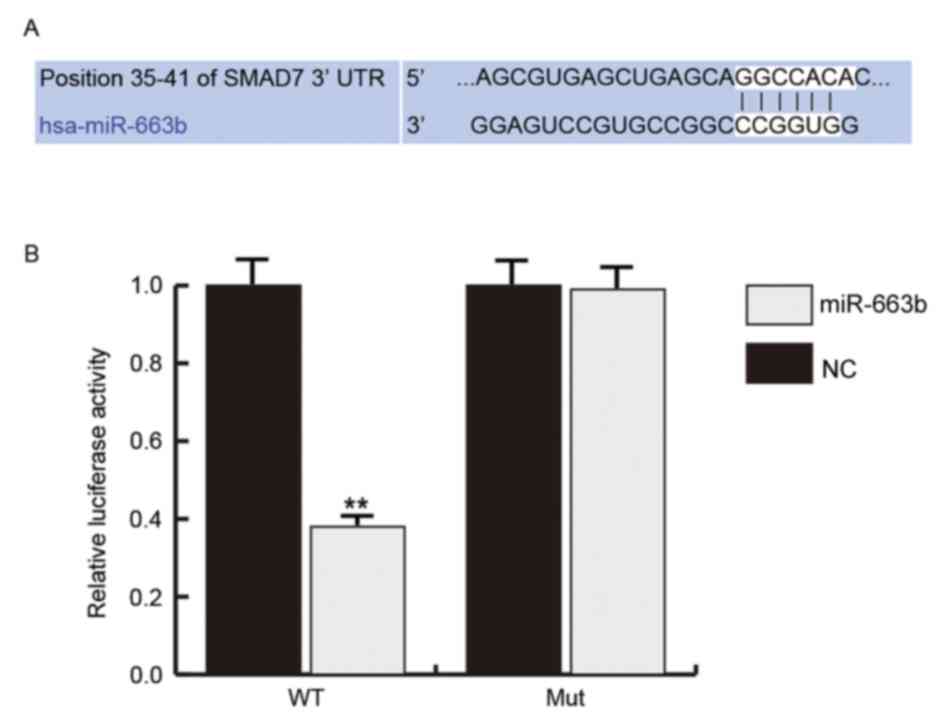
In order to investigate the role of miR-663b in NPC,
the putative targets of miR-663b were predicted using TargetScan
(Fig. 2A). Furthermore, the dual
luciferase reporter assay was performed to verify the prediction.
Results indicated that miR-663b significantly reduced the
luciferase activity in 293T cells transfected with the WT 3′-UTR of
SMAD7 compared with the NC groups (P<0.01), whereas the MUT
SMAD7 3′-UTR abrogated the suppression by miR-663b (Fig. 2B). These findings indicated that
miR-663b targets Smad7.
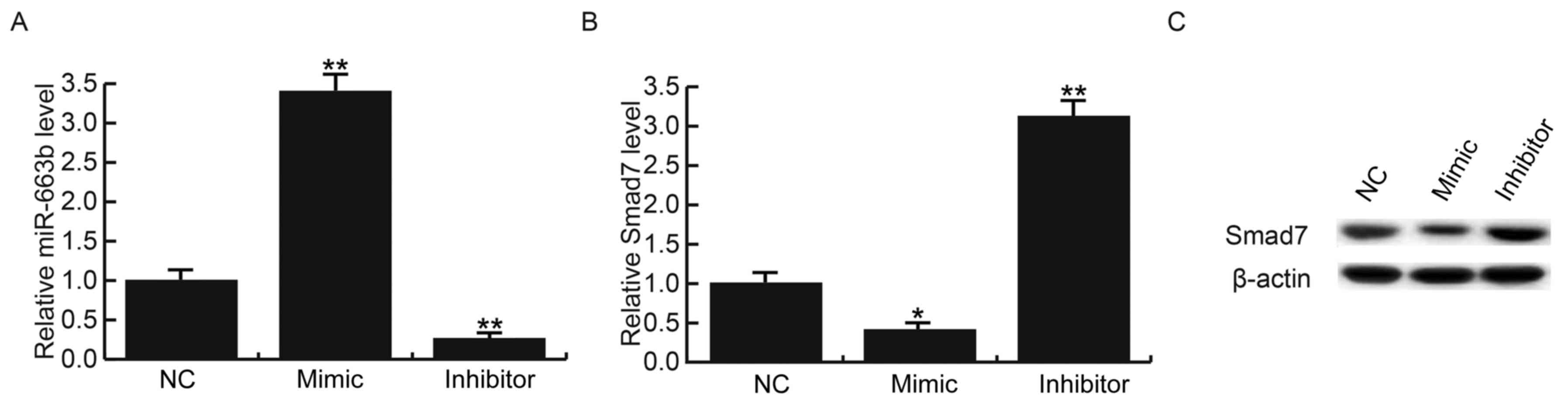
To further investigate the regulation of miR-663b on
Smad7 expression in NPC cells, C666-1 cells were transfected with
miR-663b mimics, miR-663b inhibitors or control, respectively. The
transfection efficiency was confirmed by RT-qPCR (Fig. 3A). The overexpression of miR-663b
significantly inhibited the mRNA expression levels of SMAD7 in
C666-1 cells, whereas the miR-663b inhibitor significantly
increased SMAD7 mRNA expression levels (P<0.05 and P<0.01,
respectively; Fig. 3B). Similar
effects were observed regarding the protein expression levels of
SMAD7 (Fig. 3C). These results
indicated that SMAD7 is a target of miR-663b and that miR-663b may
function in NPC via regulating SMAD7.
miR-663b promotes NPC cell
proliferation
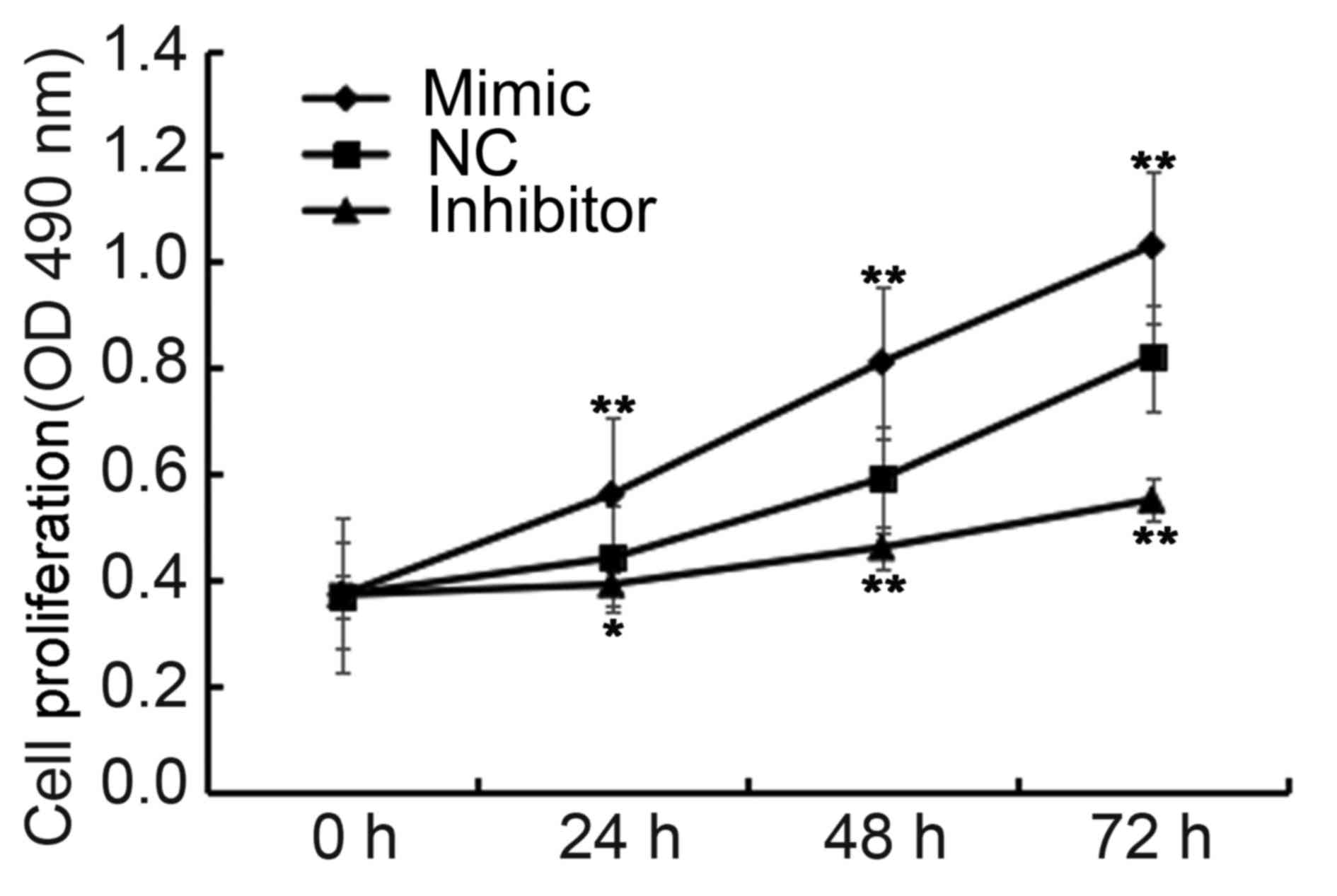
To investigate the effect of miR-663b on NPC cell
proliferation, the CCK-8 assay was performed using a CCK-8 kit. The
findings indicated that, when compared with the NC group the cell
proliferation was significantly increased when C666-1 cells were
transfected with miR-663b mimics, whereas the cell proliferation
was significantly decreased in cells transfected with miR-663b
inhibitors (P<0.05 and P<0.01; Fig. 4). These data suggested that miR-663b
promotes NPC cell proliferation.
miR-663b promotes NPC cell epithelial
mesenchymal transition (EMT)
As Smad7 is an inhibitor of the transforming growth
factor (TGF)-β signaling pathway that participates in the EMT of
tumor cells, the effects of miR-663b on NPC cell EMT were explored
in the present study. The protein expression levels of
EMT-associated proteins E-cadherin, N-cadherin and Vimentin were
determined following transfection with miR-663b mimics or miR-663b
inhibitors in C666-1 cells, respectively. Results indicated that
the protein expression levels of E-cadherin were markedly decreased
in C666-1 cells transfected with miR-663b mimics compared with the
NC group (Fig. 5A). Futhermore, mRNA
expression of E-cadherin was significantly decreased in C666-1
cells transfected with miR-663b mimics compared with the NC group
(P<0.01; Fig. 5B). Notably, the
protein expression levels of N-cadherin, Vimentin and MMP-9 were
markedly increased in C666-1 cells transfected with miR-663b mimics
compared with the control group (Fig.
5A). As expected, the mRNA expression of N-cadherin
(P<0.01), Vimentin (P<0.05) and MMP-9 (P<0.05) were
significantly increased in C666-1 cells transfected with miR-663b
mimics compared with the NC group (Fig.
5C-E). However, transfection of C666-1 cells with miR-663b
inhibitors had the opposite effects. These data suggested that
miR-663b could promote NPC cell EMT.
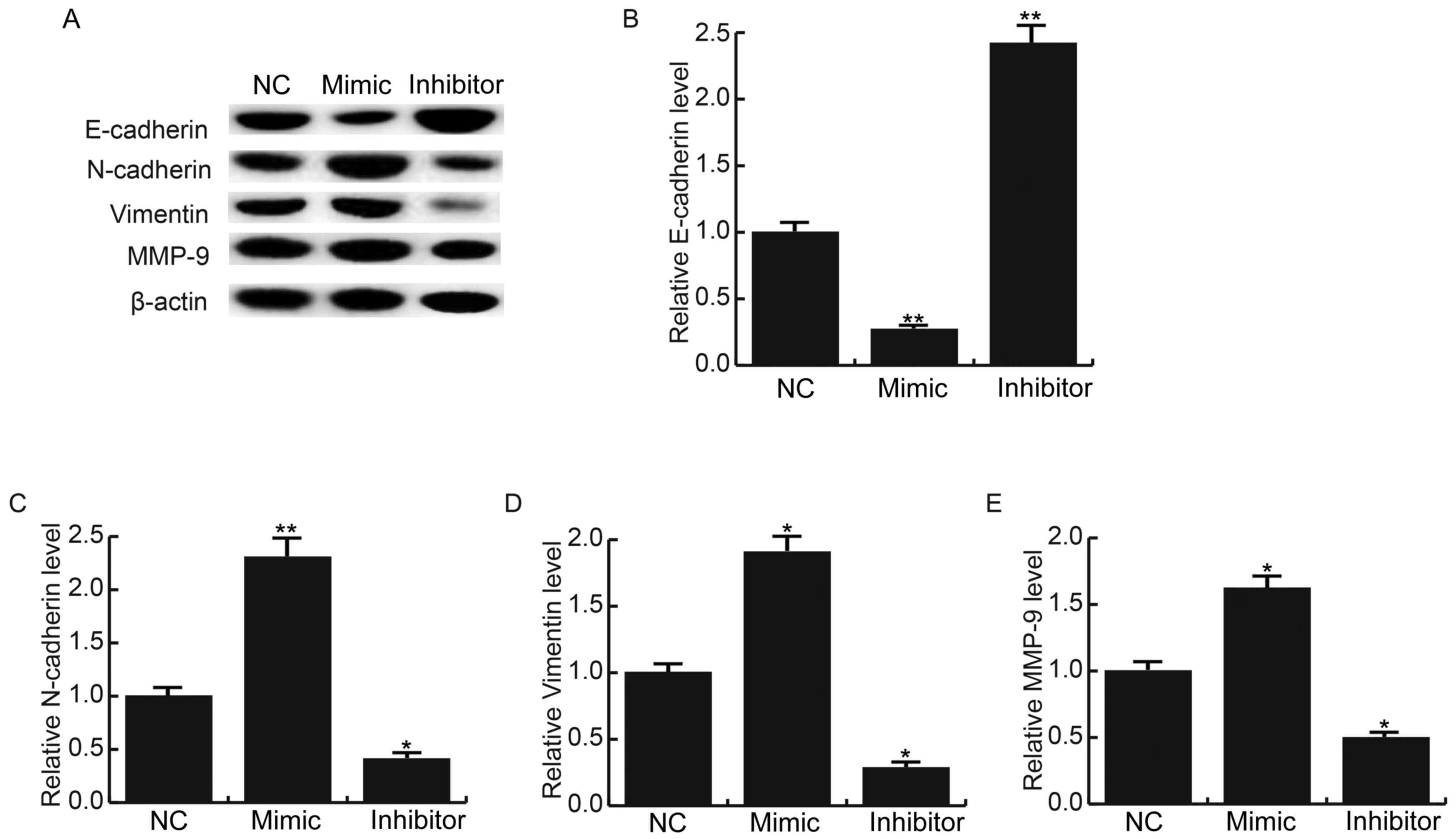 | Figure 5.miR-663b affects the expression of
EMT-associated proteins in C666-1 cells. A total of 48 h following
transfection, the protein and mRNA expression levels of E-cadherin,
N-cadherin, Vimentin and MMP-9 in C666-1 cells were detected by
western blot analysis and reverse transcription-quantitative
polymerase chain reaction, respectively. (A) Protein expression
levels of E-cadherin, N-cadherin, Vimentin and MMP-9. mRNA
expression levels of (B) E-cadherin, (C) N-cadherin, (D) Vimentin
and (E) MMP-9. Data are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01 vs. NC. NC, negative control group; MMP,
matrix metalloproteinase. |
Discussion
miRs are a class of highly conserved non-coding
single-stranded small molecule RNAs. Over 50% of the miRs are
located in the tumor-associated genome region (21). Chromosomal abnormalities directly
lead to changes in the number of miR gene copies, which results in
abnormal expression of miRs in a variety of tumors that serves the
role of oncogene or tumor suppressor (22). At present, miRs have been indicated
to be disordered in NPC tissues and cells, and widely involved in
various processes in NPC cells, including proliferation, invasion
and metastasis (23).
Various studies concerning the role of miRs in NPC
regulation have been conducted (24,25).
Notably, a study reported that miR-216 was downregulated in NPC
tissues and cells and inhibited the proliferation and invasion of
NPC cells (26). A further study
indicated that miR-200a could promote the EMT of NPC cells by
regulating ZEB2 and β-catenin (27).
miR-26a has been reported to be downregulated in NPC and acts as an
inhibitor of the proliferation, invasion and migration of NPC cells
(28,29). It has been acknowledged that miRs are
majorly involved in the development of NPC and therefore have an
immeasurable prospect in the basic and clinical research of
NPC.
To the best of our knowledge, miR-663b, a novel
cancer-associated miR, has rarely been studied in NPC. Thus, in the
present study, the expression and role of miR-663b in NPC
progression was investigated. Results indicated that miR-663b was
significantly upregulated in NPC tissues and NPC cells. To
investigate the role of miR-663b in NPC, the putative targets of
miR-663b were predicted using TargetScan, which identified hundreds
of candidate targets, including cluster of differentiation 99,
TP73, SCAI and Smad7. Smad7 belongs to the I-Smad family and serves
an inhibitory role in the TGF-β signaling pathway (30). The TGF-β signaling pathway is one of
the important signaling pathways in tumor cell EMT, which is an
important cause of distant metastasis of malignant tumor cells
(31). Blocking the TGF-β signaling
pathway may effectively control tumor cell metastasis (32). Notably, Smad7 is one of the target
genes of TGF-β, which can provide feedback to modulate the
TGF-β/Smad signaling pathway and maintain the balance of the
pathway (33). In the present study,
it was hypothesized that miR-663b may affect NPC cell proliferation
and EMT via regulating Smad7. As expected, miR-663b mimics were
demonstrated to negatively regulate Smad7 expression in NPC cells
and resulted in changes of the protein expression levels of
EMT-associated proteins. These findings suggested the promoting
role of miR-663b in NPC cell EMT and indicated that miR-663b
functioned as a tumor promoter in NPC via promoting NPC cell
proliferation and EMT. It was also suggested that miR-663b may
exert its functions though directly targeting SMAD7.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that miR-663b was
upregulated in NPC tissues and NPC cells. Furthermore, it was
indicated that miR-663b could promote the proliferation and EMT of
NPC cells by regulating Smad7 expression. These findings suggest
miR-663b may be a novel therapeutic target for NPC.
Acknowledgements
The authors would like to thank Dr Yadong Zhang of
the Key Laboratory of Molecular Diagnosis of Hubei Province, Tongji
Medical College, Huazhong University of Science and Technology, The
Central Hospital of Wuhan for assistance with the experiments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW contributed to study design, statistical
analysis, data interpretation, manuscript preparation and the
literature search. MJ and KY contributed to study design, data
collection and statistical analysis.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Central Hospital of Wuhan. Informed consent was
obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ou SH, Zell JA, Ziogas A and Anton-Culver
H: Epidemiology of nasopharyngeal carcinoma in the United States:
Improved survival of Chinese patients within the keratinizing
squamous cell carcinoma histology. Ann Oncol. 18:29–35. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX,
Liu WS, Qin HD, Feng QS, Chen LZ, Yao SY and Zeng YX: Traditional
Cantonese diet and nasopharyngeal carcinoma risk: A large-scale
case-control study in Guangdong, China. BMC Cancer. 10:4462010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sze H, Blanchard P, Ng WT, Pignon JP and
Lee AW: Chemotherapy for nasopharyngeal carcinoma-current
recommendation and controversies. Hematol Oncol Clin North Am.
29:1107–1122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong C, Nie Y, Qu S, Liao JY, Cui X, Yao
H, Zeng Y, Su F, Song E and Liu Q: miR-21 induces myofbroblast
differentiation and promotes the malignant progression of breast
phyllodes tumors. Cancer Res. 74:4341–4352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Othman N and Nagoor NH: The role of
microRNAs in the regulation of apoptosis in lung cancer and its
application in cancer treatment. Biomed Res Int. 2014:3180302014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lönnroth C, Andersson M, Asting AG,
Nordgren S and Lundholm K: Preoperative low dose NSAID treatment
influences the genes for stemness, growth, invasion and metastasis
in colorectal cancer. Int J Oncol. 45:2208–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu N, Tang LL, Sun Y, Cui RX, Wang HY,
Huang BJ, He QM, Jiang W and Ma J: MiR-29c suppresses invasion and
metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer
Lett. 329:181–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong TS, Man OY, Tsang CM, Tsao SW, Tsang
RK, Chan JY, Ho WK, Wei WI and To VS: MicroRNA let-7 suppresses
nasopharyngeal carcinoma cells proliferation through downregulating
c-Myc expression. J Cancer Res Clin Oncol. 137:415–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Hong H, Sun X, Jiang H, Ma S,
Zhao S, Zhang M, Wang Z, Jiang C and Liu H: MicroRNA-10b regulates
epithelial-mesenchymal transition by modulating
KLF4/Notch1/E-cadherin in cisplatin-resistant nasopharyngeal
carcinoma cells. Am J Cancer Res. 6:141–156. 2016.PubMed/NCBI
|
|
15
|
Zhang H, Cao H, Xu D and Zhu K:
Microrna-92a promotes metastasis of nasopharyngeal carcinoma by
targeting the PTEN/AKT pathway. Onco Targets Ther. 9:3579–3588.
2016.PubMed/NCBI
|
|
16
|
Cai HH, An Y, Chen X, Sun D, Chen T, Peng
Y, Zhu F, Jiang Y and He X: Epigenetic inhibition of miR-663b by
long non-coding RNA HOTAIR promotes pancreatic cancer cell
proliferation via up-regulation of insulin-like growth factor 2.
Oncotarget. 7:86857–86870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du M, Shi D, Yuan L, Li P, Chu H, Qin C,
Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b in
plasma are potential novel biomarkers for bladder cancer. Sci Rep.
5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated inhuman osteosarcomaand
inhibits osteosarcoma cells proliferation, migrationand invasion,
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang S, Zhang N, Deng Y, Chen L, Zhang Y,
Zheng Z, Luo W, Lv Z, Li S and Xun T: Increased serum level of
MicroRNA-663 is correlated with poor prognosis of patients with
nasopharyngeal carcinoma. Dis Markers. 2016:76482152016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
He ML, Luo MX, Lin MC and Kung HF:
MicroRNAs: Potential diagnostic markers and therapeutic targets for
EBV-associated nasopharyngeal carcinoina. Biochim Biophys Acta.
1825:1–10. 2012.PubMed/NCBI
|
|
24
|
Tan GJ, Tang XW and Tang FQ: The role of
microRNAs in nasopharyngeal carcinoma. Tumour Biol. 36:69–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruce JP and Liu FF: MicroRNAs in
nasopharyngeal carcinoma. Chin J Cancer. 33:539–544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng M, Tang H, Zhou Y, Zhou M, Xiong W,
Zheng Y, Ye Q, Zeng X, Liao Q, Guo X, et al: miR-216b suppresses
tumor growth and invasion by targeting KRAS in nasopharyngeal
carcinoma. J Cell Sci. 124:2997–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia H, Cheung WK, Sze J, Lu G, Jiang S,
Yao H, Bian XW, Poon WS, Kung HF and Lin MC: miR-200a regulates
epithelial-mesenchymal to stem-like transition via ZEB2 and
beta-catenin signaling. J Biol Chem. 285:36995–37004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu L, Lu J, Zhang B, Liu X, Wang L, Li SY,
Peng XH, Xu X, Tian WD and Li XP: miR-26a inhibits invasion and
metastasis of nasopharyngeal cancer by targeting EZH2. Oncol Lett.
5:1223–1228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo L, Li N, Lv N and Huang D: SMAD7: A
timer of tumor progression targeting TGF-β signaling. Tumour Bio.
35:8379–8385. 2014. View Article : Google Scholar
|
|
31
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muraoka RS, Dumont N, Ritter CA, Dugger
TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals
PJ, et al: Blockade of TGF-beta inhibits mammary tumor cell
viability, migration, and metastases. J Clin Invest. 109:1551–1559.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iyengar PV and Eichhorn PJ:
(De)-Ubiquitination in the TGF-β pathway. Cancer Res Ther Oncol.
1:1–6. 2013.
|