Introduction
Primary liver cancer (PLC), particularly
hepatocellular carcinoma (HCC), is one of the common malignant
tumors worldwide; ~748,000 new PLC cases were diagnosed worldwide
in 2008 (1). China has the highest
incidence of liver cancer worldwide, 55% of all liver cancers
incidences are diagnosed in China, and 50% of all cancer associated
mortalities are patients with liver cancer (2).
Treatment methods for HCC include surgical excision,
liver transplantation, interventional therapy, molecular targeted
therapy and radiotherapy. Surgical resection of early HCC can
result in improved clinical results compared with advanced HCC
(3). However, as early symptoms of
HCC are not readily apparent, the majority of liver cancer (88%) is
identified in late stages (4).
Additionally, the presence of early intrahepatic spread or
widespread cirrhosis of the liver allows for surgical removal in
<25% of patients following a liver cancer diagnosis (5). Current clinical practice for patients
that do not qualify for surgery or whose conditions do not allow
for radical resections, prioritizes interventional therapy,
including hepatic artery chemoembolization (6).
High metastasis and recurrence rates of HCC are
major factors affecting prognosis (5,6).
Following surgical screening, patients with vascular infiltration
or metastasis are treated with embolization and effects of
embolization on liver cancer metastasis are further addressed
(7). The effects of embolization on
inhibition or promotion of tumor metastasis and on different stages
of liver cancer remain unknown. In addition, effects of metastasis
on embolization efficacy and methods of improving curative effects
of embolization while inhibiting tumor metastasis remain
unclear.
The majority of clinical staging is determined by
clinical examination, test results and imaging examination
(8). Tumor progression is a complex
process involving cells, growth factors and their receptors,
adhesion and extracellular matrix molecules, tumor blood vessels
and the immune system (9). It is
important to determine the staging of liver cancer accurately, as
different stages require varying treatments and improper staging
can lead to delayed or overtreatment (10).
Green fluorescent protein (GFP) is a low molecular
weight protein that emits green fluorescence at 597 nm when excited
at 488 nm. It can be fused to target proteins without affecting the
spatial conformation or function of the gene products (11–14). For
the study of tumors, GFP marker genes can be used to determine gene
expression levels and to estimate changes in gene quantity, in
order to explore the roles and underlying molecular mechanisms of
specific genes in tumor occurrence and development (15). Expression of GFP in tumor cells can
be used to determine the initiation and progression of tumor
metastasis (16).
The current study was designed to establish an
enhanced (E) GFP vector and for lentivirus-mediated transfection of
McARH7777 cells to produce stable gene expression. Stable
expression of GFP in a rat liver cancer model using
EGFP-overexpressing McA-RH7777 cells was established to evaluate
biological characteristics. This study aims to provide novel ideas
for clinical diagnosis and treatment of tumors.
Materials and methods
Ethics statement
All animals were treated in accordance with the
Guide for the Care and Use of Laboratory Animals of the Zhongshan
Hospital of the Fudan University (Shanghai, China) and all
experiments were approved and performed according to the guidelines
of the Ethics Committee of the Affiliated Zhongshan Hospital of
Fudan University (Shanghai, China). All surgical procedures were
performed under anesthesia and every effort was made to minimize
suffering.
Cell lines and cell culture
Liver cancer cells (McA-RH7777) were purchased from
the American Type Culture Collection (Manassas, VA, USA; CRL-1601)
and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C and 5%
CO2. Medium was replaced every 3 days.
Lentivirus-mediated expression of EGFP
in McA-RH7777
McA-RH7777 cells were seeded into 96-well plates at
3×103 cells/well. For EGFP overexpression, McA-RH7777
cells were transfected with lentivirus (Nanjing SenBeiJia
Biological Technology Co., Ltd., Nanjing, China) harboring
Lv-pGC-FU-EGFP-IRES-puromycin (Shanghai GenePharma Co., Ltd.,
Shanghai, China) containing an EGFP overexpression sequence. All
lentivirus transfections were performed in the presence of 5 µg/ml
polybrene (Nanjing SenBeiJia Biological Technology Co., Ltd.).
Following culturing in DMEM supplemented with 10% FBS at 37°C in a
5% CO2 incubator for 96 h, transfected McA-RH7777 cells
were examined for EGFP expression under an inverted fluorescence
microscope (magnification, ×200). The number of EGFP-positive cells
was used to calculate the transfection efficiency. Successfully
transfected McA-RH7777 cells (McA-RH7777-EGFP) were selected using
puromycin (1 µg/ml) over ٢ weeks at ٣٧°C in a ٥٪ CO2
incubator. EGFP expression was confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. Untransfected McA-RH7777 cells served as
negative control.
Transwell assays
Invasiveness of McA-RH7777 and McA-RH7777-EGFP cells
was studied with Transwell assays (Axygen; Corning, Inc., Corning,
NY, USA). Briefly, prior to addition of cells into Transwell
chambers, membranes of each chamber were coated with membrane
Matrigel (50 mg/l; dilution, 1:8; BD Biosciences Franklin Lakes,
NJ, USA) for 30 min at room temperature prior to following
experiments. Cell suspensions (1×105 cells) prepared in
serum-free medium were added to the upper chambers and the lower
chambers were filled with complete medium supplemented with 10%
FBS. Following incubation for 24 h at 37°C, residual cells in the
upper chambers were wiped off with a cotton swab and cells that
migrated to the lower surface of the membrane were fixed with 4%
formaldehyde for 20 min at room temperature and stained with
crystal violet for 20 min at room temperature. Cells were counted
in five random fields using an inverted microscope (magnification,
×200). Experiments were performed in triplicate. The relative
invasion rate was determined as follows: Number of
McA-RH7777-EGFP/number of migrated McA-RH7777 ×100%.
Comparison of cell viability and
mobility
McA-RH7777 and McA-RH7777-EGFP cells (1 ml) were
seeded into 24-well plates at 1×104/ml and were cultured
at 37°C in 5% CO2. Medium was replaced every 2 days. A
Cell-IQ Analyzer (Chip-Man Technologies, Ltd., Tampere, Finland)
was used to monitor cell activity and mobility every 12 h for 108
days using Cell-IQ-200 Analyzer software (version IQ200; Chip-Man
Technologies, Ltd.).
Flow cytometry
Flow cytometry was used to analyze the cell cycle of
McA-RH7777-EGFP and McA-RH7777 cells. Cells (1×106) were
collected (2,000 × g, 5 min, room temperature) following a 0.25%
trypsin digest, washed with PBS and fixed with 70% ethanol for 12 h
at 4°C. The stationary liquid was removed prior to staining with
RNase A (100 µl) and peridinin chlorophyll protein
complex-cytochrome 5-5A (400 µl; BD Biosciences, Franklin Lakes,
NJ) for 15 min at room temperature in the dark. Binding buffer (400
µl; BD Biosciences) was added to each sample and samples were
analyzed on a flow cytometer and evaluated using FlowJo software
(version 7.6.5; FlowJo LLC, Ashland, OR, USA). Each experiment was
performed in triplicate.
Animal experiments
For tumor growth assays, McA-RH7777 and
McA-RH7777-EGFP cells (2.0×106) were injected
subcutaneously into the right scapula of male Buffalo rats (age, 5
weeks; n=6 per group, Shanghai SIPPR-Bk Lab Animals Co. Ltd.,
Shanghai, China). All rats had free access to food and water and
were housed under controlled conditions (12-h light/dark cycles;
humidity, 60±5%; temperature, 22±3°C). Rats were observed over 35
days for tumor formation, the tumor volume (V) was recorded every 7
days and calculated using the following formula: V=0.5 × length ×
width2. On day 35, rats were anesthetized by
intraperitoneal injection of sodium pentobarbital (30 mg/kg body
weight) prior to sacrificing. Tumors were used for EGFP detection.
Tumor tissues were cut into 5-µm slices and analyzed using a
fluorescence microscope (magnification, ×400).
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation. Statistical significance of multiple groups was
evaluated by one-way analysis of variance followed by Tukey's
multiple comparison test and pairwise comparison by two-sided
Student's t-test using GraphPad Prism software, version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of biological
characteristics of McA-RH7777 and McA-RH7777-EGFP cells
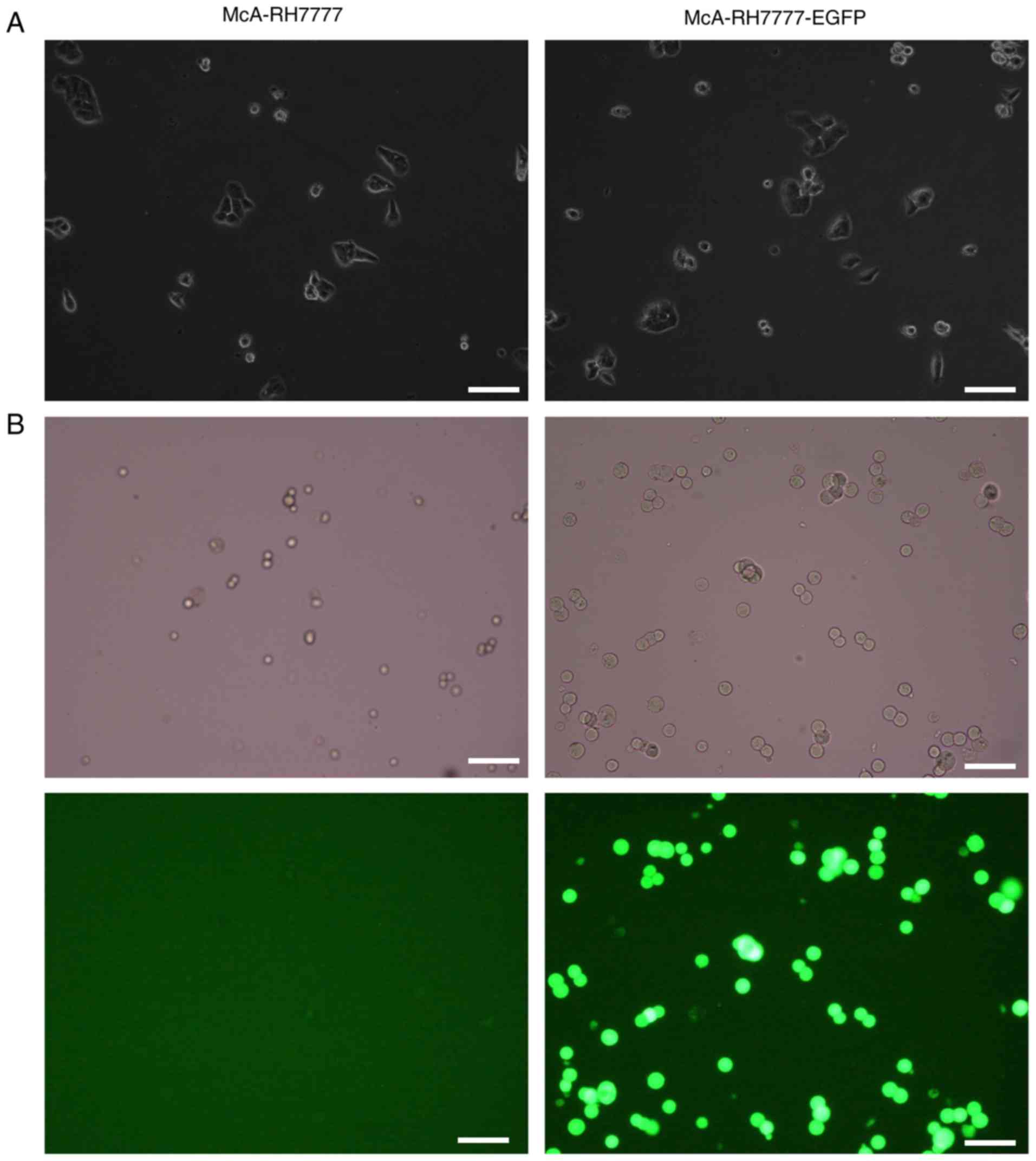
Experimental observations indicate that the
lentiviral vector carrying EGFP was successfully transfected into
McA-RH7777 cells. The established McA-RH7777-EGFP cells exhibited
stable expression of EGFP and puromycin resistance. Cell-IQ live
cell monitoring indicated that McA-RH7777-EGFP and wild type
McA-RH7777 cells exhibited stable adherent growth, fusiform,
polygonal character and no growth inhibition. Lentiviral
transfection had no influence on morphology and growth of
McA-RH7777 cells (Fig. 1A).
Following 108 days in vitro culturing, fluorescence
intensity and expression were stable (Fig. 1B). McA-RH7777-EGFP exhibited stable
expression of green fluorescence in vitro and fluorescence
intensity was not markedly reduced in long-term culturing.
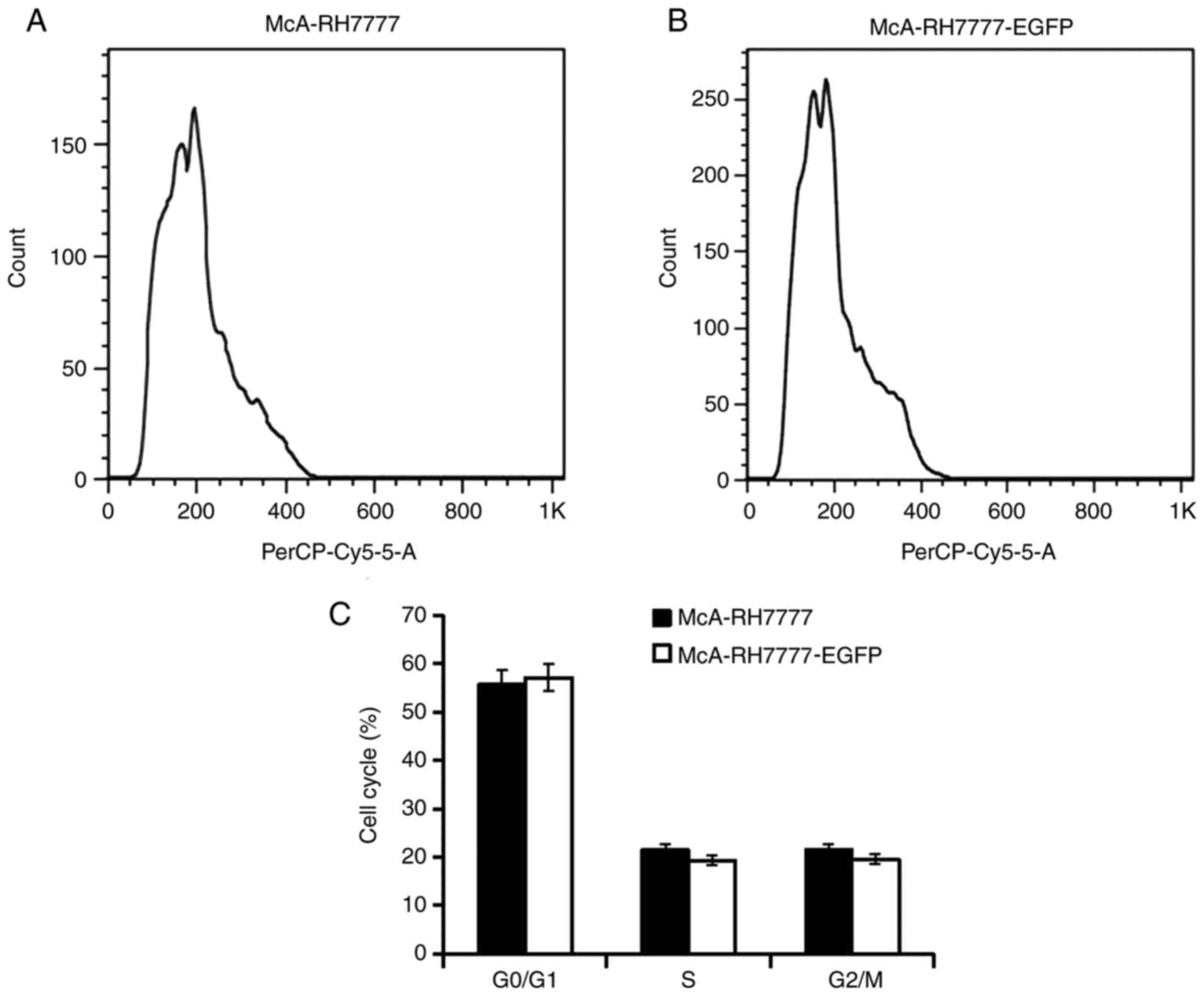
Flow cytometry analysis further indicated no
significant difference between McA-RH7777 and McA-RH7777-EGFP cells
with respect to the cell cycle (Fig.
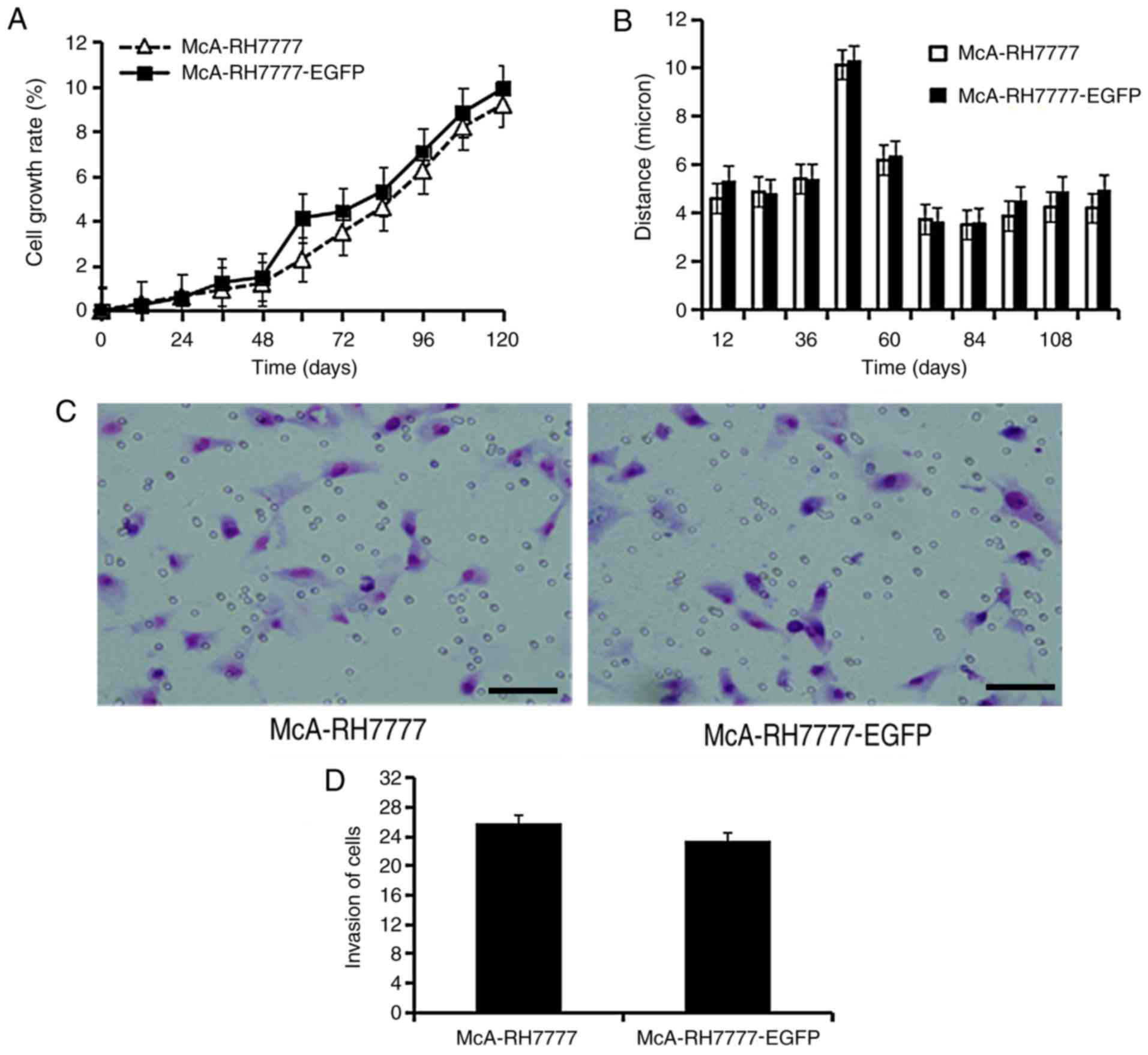
2). Cell-IQ-200 Analyzer software analysis indicated no
significant differences in cell growth (Fig. 3A) and mobility (Fig. 3B) between McA-RH7777 and
McA-RH7777-EGFP cells. Transwell assays indicated that EGFP
overexpression did not affect cell invasiveness (Fig. 3C and D).
Effects of EGFP expression on tumor
growth and maintenance of green fluorescence in vivo
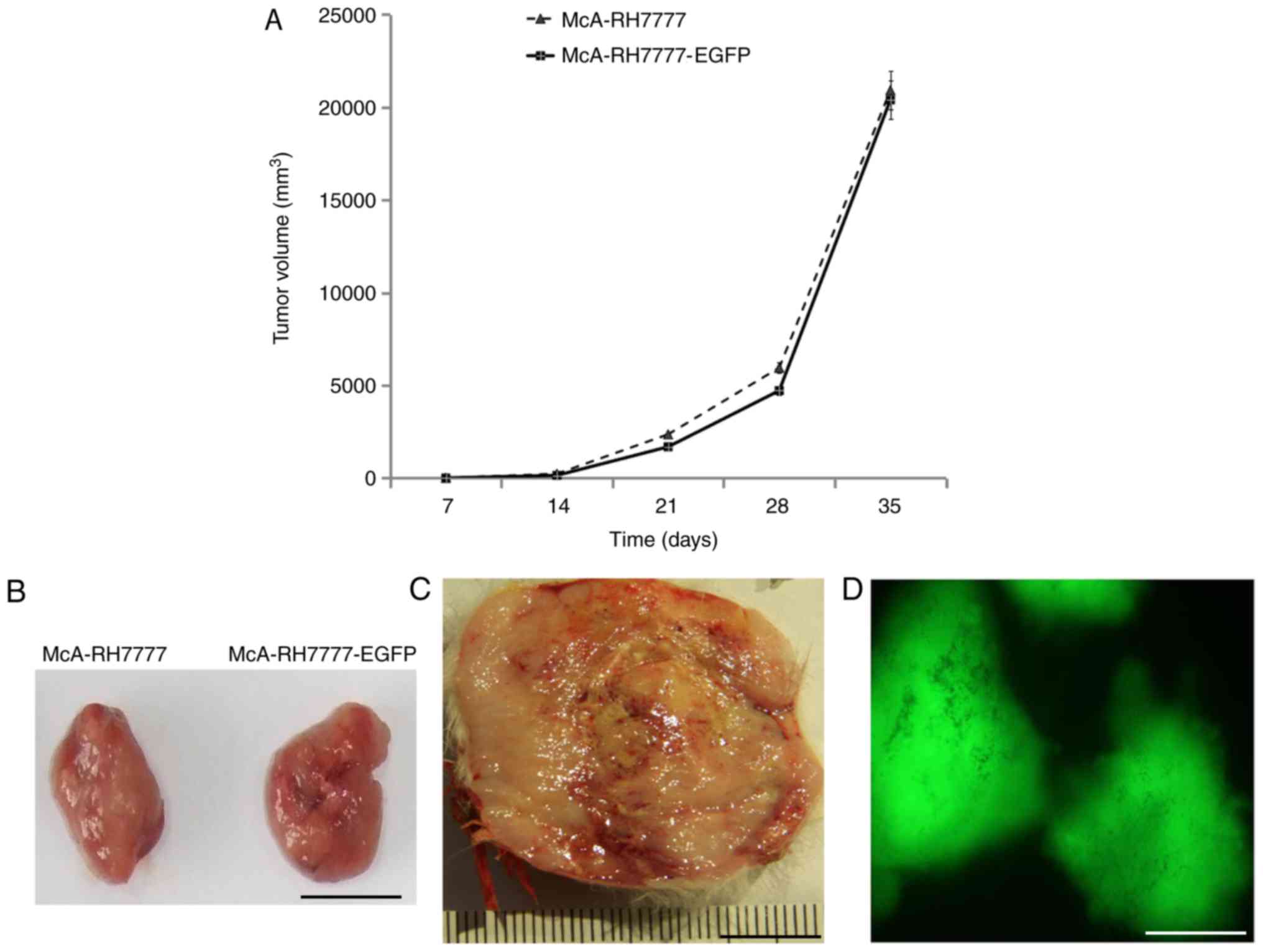
McA-RH7777 and McA-RH7777-EGFP cells were
subcutaneously injected into rats. All animals grew subcutaneous
tumors and the tumor formation rate was 100%. On day 35 of
follow-up, the tumors were collected. Tumor volumes for McA-RH7777
and McA-RH7777-EGFP injected animals were 20,909.5±4,707.46 and
20,392.4±3,506.3 mm3, respectively. Statistical analysis
suggested no significant difference among the groups (Fig. 4A). The growth rate of the tumors was
consistent between the two groups (Fig.
4B), suggesting that McA-RH7777-EGFP had no effect on tumor
growth. Fig. 4C presents an excised
tumor from an animal of the McA-RH7777-EGFP group. Following 35
days follow-up, the tumors were isolated from the sacrificed
animals and tumor slices were analyzed for EGFP expression under a
fluorescence microscope and expression was observed (Fig. 4D). GFP exhibited stable expression
and no influence on tumor growth was suggested.
Discussion
Metastasis and recurrence of HCC begins with the
shedding of single cells from a primary lesion, making it difficult
to accurately trace the path that tumor cells follow (17). Fluorescence detection of tumor cells
may aid understanding of the effects of various interventions on a
tumor in a timely and accurate manner (18). In the field of malignant tumor
metastasis, research progress has been limited due to a lack of
technology to detect the transfer of cells. However, with the
development of suitable optical imaging technology, it is now
possible to detect tumor cells and gene expression accurately
(19–21).
GFP is a low molecular weight protein; its
chromophore is formed by internal amino acid dehydrogenation
cyclization and oxidation, including tyrosine, glycine and serine
(22–24). Under blue light excitation, GFP emits
green fluorescence and the detection is intuitive and accurate.
Using a gene carrier to import the GFP gene into cells allows the
direct observation of these cells under a fluorescence microscope.
GFP reporter genes can be transfected into tumor cells, which then
divide, grow and pass fluorescence on to next generations (25). GFP may be fused to other target
proteins and rarely affects the spatial conformation and function
of the gene products (26). GFP
expression allows for quantitative analysis of gene expression,
accurately reporting the location and quantity of target gene
expression in tumor cells (27).
Researchers have used GFP in applications, including drug
evaluation and studies of tumor mechanisms (16).
In fluorescence-labeling, lentiviral transfection
has a broad spectrum of applications and high transfection
efficiencies. Lentiviral transfection allows for stable fluorescent
protein expression (28,29). Lee et al (30) have observed that retrovirus-mediated
transfection of GFP is able to produce stable expression in target
cells in the mouse bodies for >3 months. Long-term external
observations have revealed that this model retains the biological
characteristics of the original system while stably expressing
fluorescent protein.
In the current study, lentiviral-mediated
transfection was performed to establish McA-RH7777-EGFP cells
expressing EGFP in vitro over 108 days of culturing,
indicating that cells stably expressed EGFP. In vitro
experiments further suggested that tumor characteristics, including
cell invasion and proliferation, were retained in McA-RH7777-EGFP
cells. A rat tumor model established using subcutaneous injection
suggested that the tumor formation rate was 100% and tumor growth
indicated no significant differences between McA-RH7777-EGFP and
McA-RH7777. There was also no significant difference between cells
with respect to proliferation and activity. In addition, on day 35
of growth, the tumor tissue exhibited stable expression of green
fluorescence. In vivo and in vitro experiments
confirmed that biological characteristics of the transfected cells
were not significantly different compared with wild-type cells.
Within the chosen cancer cell line, EGFP expression provided a
simple, intuitive and effective method to evaluate the invasion and
metastasis of tumor cells.
The McA-RH7777-EGFP cell line may be used to
construct liver cancer animal models. The dynamic process of tumor
cell formation and growth in the evaluated system may be stable,
continuous and yielding high efficiency, allowing for accurate
observation using optical imaging. The system may further be used
to evaluate the mechanism and efficacy of various targeted therapy
drugs, to accurately reveal tumor stages and to identify and
evaluate new treatment methods. Owing to the high visibility,
stable EGFP expression provides an effective tool for detecting
tumor cells and performing tumor molecular research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Talent Training
Program of Zhongshan Hospital (grant no. 2015ZSYXGG18).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WZ, SQ, GY and LZ generated and analyzed the data.
BZ, JW, RL, XQ and ZY designed the experiments and drafted the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animals were treated in accordance with the
Guide for the Care and Use of Laboratory Animals and all
experiments were approved and performed according to the guidelines
of the Ethics Committee of the Affiliated Zhongshan Hospital of
Fudan University (Shanghai, China). All surgical procedures were
performed under anesthesia and every effort was made to minimize
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fidler MM, Bray F and Soerjomataram I: The
global cancer burden and human development: A review. Scand J
Public Health. 46:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye JZ, Wang YY, Bai T, Chen J, Xiang BD,
Wu FX and Li LQ: Surgical resection for hepatocellular carcinoma
with portal vein tumor thrombus in the Asia-Pacific region beyond
the Barcelona clinic liver cancer treatment algorithms: A review
and update. Oncotarget. 8:93258–93278. 2017.PubMed/NCBI
|
|
4
|
Jaka H, Mshana SE, Rambau PF, Masalu N,
Chalya PL and Kalluvya SE: Hepatocellular carcinoma:
Clinicopathological profile and challenges of management in a
resource-limited setting. World J Surg Oncol. 12:2462014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geuskens M and Uriel J: Subcellular
immunolocalization of alphafetoprotein (AFP) in cell lines
established from Morris hepatoma 7777 and 8994. Lack of effect of
dexamethasone on the ultrastructural detection of AFP in the 8994
cells. Oncodev Biol Med. 3:291–300. 1982.PubMed/NCBI
|
|
6
|
Belanger L, Commer P and Chiu JF:
Isolation of rat alpha1-fetoprotein messenger RNA from Morris
hepatoma 7777. Cancer Res. 39:2141–2148. 1979.PubMed/NCBI
|
|
7
|
Yen CW, Hsu LS, Chen CW and Lin WH:
Hepatocellular carcinoma with thoracic metastases presenting as
hemothorax: A case report and literature review. Medicine
(Baltimore). 97:e109452018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vieth V, Schulz R, Heindel W, Pfeiffer H,
Buerke B, Schmeling A and Ottow C: Forensic age assessment by 3.0T
MRI of the knee: Proposal of a new MRI classification of
ossification stages. Eur Radiol. 28:3255–3262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khialeeva E, Chou JW, Allen DE, Chiu AM,
Bensinger SJ and Carpenter EM: Reelin deficiency delays mammary
tumor growth and metastatic progression. J Mammary Gland Biol
Neoplasia. 22:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelen SM, Beets GL and Beets-Tan RG:
Role of preoperative local and distant staging in rectal cancer.
Onkologie. 30:141–145. 2007.PubMed/NCBI
|
|
11
|
Miyayama S, Matsui O, Nishida H, Yamamori
S, Minami T, Shinmura R, Kozaka K, Notsumata K, Toya D, Tanaka N,
et al: Transcatheter arterial chemoembolization for unresectable
hepatocellular carcinoma fed by the cystic artery. J Vasc Interv
Radiol. 14:1155–1161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bae SI, Yeon JE, Lee JM, Kim JH, Lee HJ,
Lee SJ, Suh SJ, Yoon EL, Kim HR, Byun KS and Seo TS: A case of
necrotizing pancreatitis subsequent to transcatheter arterial
chemoembolization in a patient with hepatocellular carcinoma. Clin
Mol Hepatol. 18:321–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozcinar B, Guven K, Poyanli A and Ozden I:
Necrotizing pancreatitis after transcatheter arterial
chemoembolization for hepatocellular carcinoma. Diagn Interv
Radiol. 15:36–38. 2009.PubMed/NCBI
|
|
14
|
Kim W, Clark TW, Baum RA and Soulen MC:
Risk factors for liver abscess formation after hepatic
chemoembolization. J Vasc Interv Radiol. 12:965–968. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffman RM and Bouvet M: Imaging the
microenvironment of pancreatic cancer patient-derived orthotopic
xenografts (PDOX) growing in transgenic nude mice expressing GFP,
RFP, or CFP. Cancer Lett. 380:349–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paris S and Sesboue R: Metastasis models:
The green fluorescent revolution? Carcinogenesis. 25:2285–2292.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SL, Luo YY, Chen M, Zhou YP, Lu FR,
Deng DF and Wu YR: A systematic review and meta-analysis comparing
the prognosis of multicentric occurrence and vs. intrahepatic
metastasis in patients with recurrent hepatocellular carcinoma
after hepatectomy. HPB (Oxford). 19:835–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Juratli MA, Siegel ER, Nedosekin DA,
Sarimollaoglu M, Jamshidi-Parsian A, Cai C, Menyaev YA, Suen JY,
Galanzha EI and Zharov VP: In Vivo Long-Term Monitoring of
Circulating Tumor Cells Fluctuation during Medical Interventions.
PLoS One. 10:e01376132015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ntziachristos V, Ripoll J, Wang LV and
Weissleder R: Looking and listening to light: The evolution of
whole-body photonic imaging. Nat Biotechnol. 23:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iyer M, Berenji M, Templeton NS and
Gambhir SS: Noninvasive imaging of cationic lipid-mediated delivery
of optical and PET reporter genes in living mice. Mol Ther.
6:555–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maggi A and Ciana P: Reporter mice and
drug discovery and development. Nat Rev Drug Discov. 4:249–255.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebe T: Green fluorescent protein as a
marker gene expression. Tanpakushitsu Kakusan Koso. 52:1766–1767.
2007.(In Japanese). PubMed/NCBI
|
|
23
|
Bottin A, Larche L, Villalba F, Gaulin E,
Esquerre-Tugaye MT and Rickauer M: Green fluorescent protein (GFP)
as gene expression reporter and vital marker for studying
development and microbe-plant interaction in the tobacco pathogen
Phythphthora parasitica var. nicotianae. FEMS Microbiol Lett.
176:51–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh E, Gustafson K and Boulianne GL: Green
fluorescent protein as a vital marker and reporter of gene
expression in Drosophila. Proc Natl Acad Sci USA. 92:7036–7040.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Apfel J, Reischmann P and Muller O: A new
fluorescence-based reporter gene vector as a tool for analyzing and
fishing cells with activated wnt signaling pathway. ISRN Oncol.
2013:6031292013.PubMed/NCBI
|
|
26
|
Shorter SA, Pettit MW, Dyer PDR, Coakley
Youngs E, Gorringe-Pattrick MAM, El-Daher S and Richardson S: Green
fluorescent protein (GFP): Is seeing believing and is that enough?
J Drug Target. 25:809–817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoffman RM: Application of GFP imaging in
cancer. Lab Invest. 95:432–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doerner A, Rhiel L, Zielonka S and Kolmar
H: Therapeutic antibody engineering by high efficiency cell
screening. FEBS Lett. 588:278–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feki A, Berardi P, Bellingan G, Major A,
Krause KH, Petignat P, Zehra R, Pervaiz S and Irminger-Finger I:
Dissemination of intraperitoneal ovarian cancer: Discussion of
mechanisms and demonstration of lymphatic spreading in ovarian
cancer model. Crit Rev Oncol Hematol. 72:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee K, Majumdar MK, Buyaner D, Hendricks
JK, Pittenger MF and Mosca JD: Human mesenchymal stem cells
maintain transgene expression during expansion and differentiation.
Mol Ther. 3:857–866. 2001. View Article : Google Scholar : PubMed/NCBI
|