Introduction
Tooth development derives from the interaction
between the outer epithelial cell and the mesenchymal cell
(1). The enamel organ is initially
formed, followed by dental papilla and the dental sac. Mesenchymal
stem cells (MSCs) can form other structures of tooth, such as
dentin, dental pulp and the primary periodontal pocket (1). Dental pulp serves a vital role in tooth
tissue regeneration following tooth eruption (1). Furthermore, cells in the dental pulp
will form new odontoblasts in the presence of dental tissue trauma
and irreversible disease damage. In this way, pulp can secrete the
dentin matrix, thus forming the so-called reparative dentin
(1).
Currently, various applications of false teeth
remain the major repair method for missing teeth (2). Tooth regeneration is one of the major
challenges in oral medicine at present. Biological tissue
engineering technology has been applied in organ reconstruction,
tissue regeneration and tooth regeneration for years (2). However, the research status of global
tooth regeneration is far beyond the reach of providing
tissue-engineered teeth for clinical application. Dental pulp stem
cells are important for tooth tissue engineering. Cells that can be
used in tooth tissue engineering include dental pulp stem cells
(DPSCs) and odontogenic mesenchymal cell (3). DPSCs are located in the dental pulp,
which possesses self-renewal ability and can differentiate into
odontoblasts (4). Previous research
has indicated that there are several factors affecting DPSC
proliferation and differentiation (5). They include basic fibroblast growth
factor, transforming growth factor, bone morphogenetic protein,
β-phosphoglycerol and dentin (5).
In recent years, research on non-coding small RNA
has also affected the development of dental pulp regeneration
research (6). microRNAs (miRNAs or
miRs) are a class of endogenous non-coding small RNAs that can bind
with the 3′-untranslated region of target gene mRNA (6) inhibiting target gene transcription and
regulating the target gene. miRNA is closely associated with
multiple physiological processes in mammals, including cell
proliferation, migration and development (7). In other cases, miRNA serves a vital
regulatory role in certain functions, physiological activities
(including anti-inflammatory and anti-apoptotic effects) and cell
differentiation of stem cell (3,7).
DPSCs serve a crucial role in research on tooth
repair and regeneration. Multiple signal transduction pathways,
such as Notch and Wnt, can regulate DPSC proliferation and
differentiation (8). Typically, the
Wnt signaling pathway serves a key role in medical engineering
research, including signal regulation in tooth development and
tooth regeneration (9). Wnt1,
adenomatous polyposis coli (APC) and β-catenin are important
factors in the Wnt signaling pathway, which can affect the
interaction between dental epithelium and mesenchyme (10). Furthermore, the Wnt/β-catenin
signaling pathway impacts the development, proliferation and
differentiation of dentin and tooth root (10). The aim of the present study was to
identify an miRNA-based biomarker and therapeutic target for
skeletal myogenic differentiation of human adult DPSCs (ADSCs).
Materials and methods
Cell culture and transfection
A total of 12 Female C57/BL6 mice (age, 5–6 weeks;
weight, 16–18 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd., (Shanghai, China). Mice were housed at a
temperature of 22–23°C and a humidity of 55–60%, with a 12 h
light/dark cycle and free access to food and water. All mice were
used in the present study and anesthetized using 35 mg/kg
pentobarbital sodium (intraperitoneal inkection; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The present study was approved by
the Ethics Committee of Ninth People's Hospital, Shanghai JiaoTong
University School of Medicine (Shanghai, China). Mice were
sacrificed via decapitation, mandibular tissues were removed and
pulp tissues were separated and incubated with α-minimum essential
medium (a-MEM) supplemented with 5% fetal bovine serum (FBS),
0.004% DNase I, 1.5 mg/ml collagenase type I and 2 mg/ml dispase
(all Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 12 h at 37°C. The dissociated cells were separated, resultant
single-cell suspensions of dental pulp were counted and incubated
into six-well cell culture plates (BD Biosciences, Franklin Lakes,
NJ, USA) using a-MEM supplemented with 10% FBS (Invitrogen; Thermo
Fisher Scientific, Inc.) in a 5% CO2 atmosphere at 37°C
for 14 days.
Subsequently, cells were incubated with a-MEM with
2% FBS and 0.5 mM 5-Aza-20-deoxycytidine (Sigma-Aldrich; Merck
KGaA) for 1 day at 37°C and subsequently incubated with a-MEM with
10% FBS for 3 days at 37°C.
The sequences of each transfected miRNA were as
follows: miR-139-5p forward, 5′-UCUACAGUGCACGUGUCUCCAGU-3′ and
reverse, 5′-UGGAGACACGUGCACUGUAGAUU-3′; anti-miR-139-5p forward,
5′-CAGUACUUUUGUGUAGUACAA-3′ and reverse,
5′-CAGUACUUUUGUGUAGUACAA-3′; negative forward mimics,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. Each miRNA was purchased from Sangon
Biotech Co., Ltd (Shanghai, China). miR-139-5p (100 ng),
anti-miR-139-5p (100 ng) and negative mimics (100 ng) were
co-transfected into the cells with 1 ml Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following 6 h
transfection, the medium was replaced with a-MEM with 10% FBS and
treated with the Wnt inhibitor (1 µmol/l; Wnt-C59; MedChemExpress
USA, Monmouth Junction, NJ, USA) for 42 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cell samples using a
Total RNA kit (Tiangen Biotech Co., Ltd., Beijing, China). cDNA was
prepared using a SuperScript III First-Strand Synthesis Supermix
kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. miR-139-5p expression was measured using a
7500 Realtime PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with a Kapa SYBR Fast qPCR kit (Kapa Biosystems,
Inc., Wilmington, MA, USA). The Primer sequences utilized for
RT-qPCR were as follows: miR-139-5p forward,
5′-ACACTCCAGCTGGGTCTACAGTGCACGTG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCCGT-3′. The PCR conditions consisted
of an initial 3 min denaturation at 95°C, 40 cycles of 95°C for 30
sec, 58°C for 30 sec and 72°C for 30 sec. miR-139-5p expression was
calculated using the 2ΔΔCq method (11).
Cell viability assay and lactate
dehydrogenase (LDH) activity levels
At 24, 48 and 72 h following transfection, cells
were stained with MTT (20 µl; 5 mg/ml) for 4 h at 37°C. Dimethyl
sulfoxide was added to samples and incubated for 30 min at 37°C.
The absorbance was measured using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 492 nM.
At 48 h following transfection, cellular LDH
activity levels were measured using LDH activity kits (Beyotime
Institute of Biotechnology, Haimen, China) following the
manufacturer's protocol. The absorbance was measured using a
microplate reader at 450 nM.
Apoptosis assay
At 48 h following transfection, cells were washed
with PBS for 15 min and fixed with 4% paraformaldehyde for 15 min
at room temperature. Cells were then stained with 5 µl Annexin
V-APC and 5 µl propidium iodide (1 mg/ml; Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) for 15 min in darkness at room
temperature. Apoptosis rate was measured using a FACScan flow
cytometer (BD Biosciences) and analyzed using Flowjo 7.6.1 (FlowJo,
LLC, Ashland, OR, USA).
Western blot analysis
At 48 h following transfection, total protein was
extracted from the transfection cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) for 15 min at 4°C and protein was quantified using a
BCA assay (Beyotime Institute of Biotechnology). Protein samples
(30 µg) were loaded on 10–12% SDS-PAGE for electrophoresis and were
transferred onto polyvinylidene difluoride membranes. Membranes
were blocked with 5% non-fat milk in TBS-Tween 20 (TBST) for 1 h at
37°C and incubated with myocyte-specific enhancer factor 2C (MEF2C;
cat. no. sc-13268; 1:1,000), myogenic differentiation 1 (MyoD; cat.
no. sc-304, 1:1,000) and myosin heavy chain (MyHC; cat. no.
sc-20641; 1:1,000), anti-Wnt (cat. no. sc-50360; 1:1,000),
anti-β-catenin (cat. no. sc-376959; 1:1,000), and anti-GAPDH
antibodies (cat. no. sc-25778; 1:5,000; each Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Membranes
were washed with TBST for 15 min and subsequently incubated with
goat-anti-rabbit or anti-mouse horseradish peroxidase conjugated
immunoglobulin G antibodies (cat. nos. sc-2004 or sc-2005,
respectively; 1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for
1 h. Membranes were observed using BeyoECL Plus (Beyotime Institute
of Biotechnology) and analyzed using Image Lab 3.0 software
(Bio-Rad Laboratories, Inc.).
Luciferase reporter assays
100 ng of Wnt1 3′UTRs (forward,
5′-CCATGGGGCTCTGGGCGCTG-3′ and reverse, 5′-TTATAGATAAAAG-3′)
containing miRNA-139-5p (forward,
5′-ACACTCCAGCTGGGTCTACAGTGCACGTG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′) were amplified in psiCHECK™−2
Vectors (Promega Corporation, Madison, WI, USA) and co-transfected
into cells using 1 ml Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Following transfection for 48 h,
cells were lysed in Passive Lysis Buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) and luciferase activity was measured using a
DualGlo Luciferase Assay System (Promega Corporation).
Immunofluorescence microscopy
At 48 h following transfection, Cells were washed
with PBS and fixed with 4% paraformaldehyde for 15 min at room
temperature. Cells were incubated with 0.01% Tris-X100 in PBS for
15 min at room temperature and blocked with 5% bovine serum albumin
(Beyotime Institute of Biotechnology) in PBS for 1 h at room
temperature. Cells were then incubated with anti-Wnt (cat. no.
sc-5210; 1:1,000) and anti-β-catenin antibodies (cat. no. sc-65480;
Santa Cruz Biotechnology, Inc.) at 4°C overnight and washed with
PBS for 15 min. Cells were then incubated with 488 and 555
fluorescein isothiocyanate-conjugated goat anti-mouse secondary
antibodies for 1 h at 37°C and washed with PBS for 15 min. Cells
were stained with DAPI for 30 min at room temperature in darkness
and washed with PBS for 15 min. Images were captured using an
Olympus FluoView FV1000 confocal laser scanning microscope (Olympus
Corporation, Tokyo, Japan; magnification, ×200).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=3). Differences between groups were determined using Student's
t-test, or one-way analysis of variance with Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
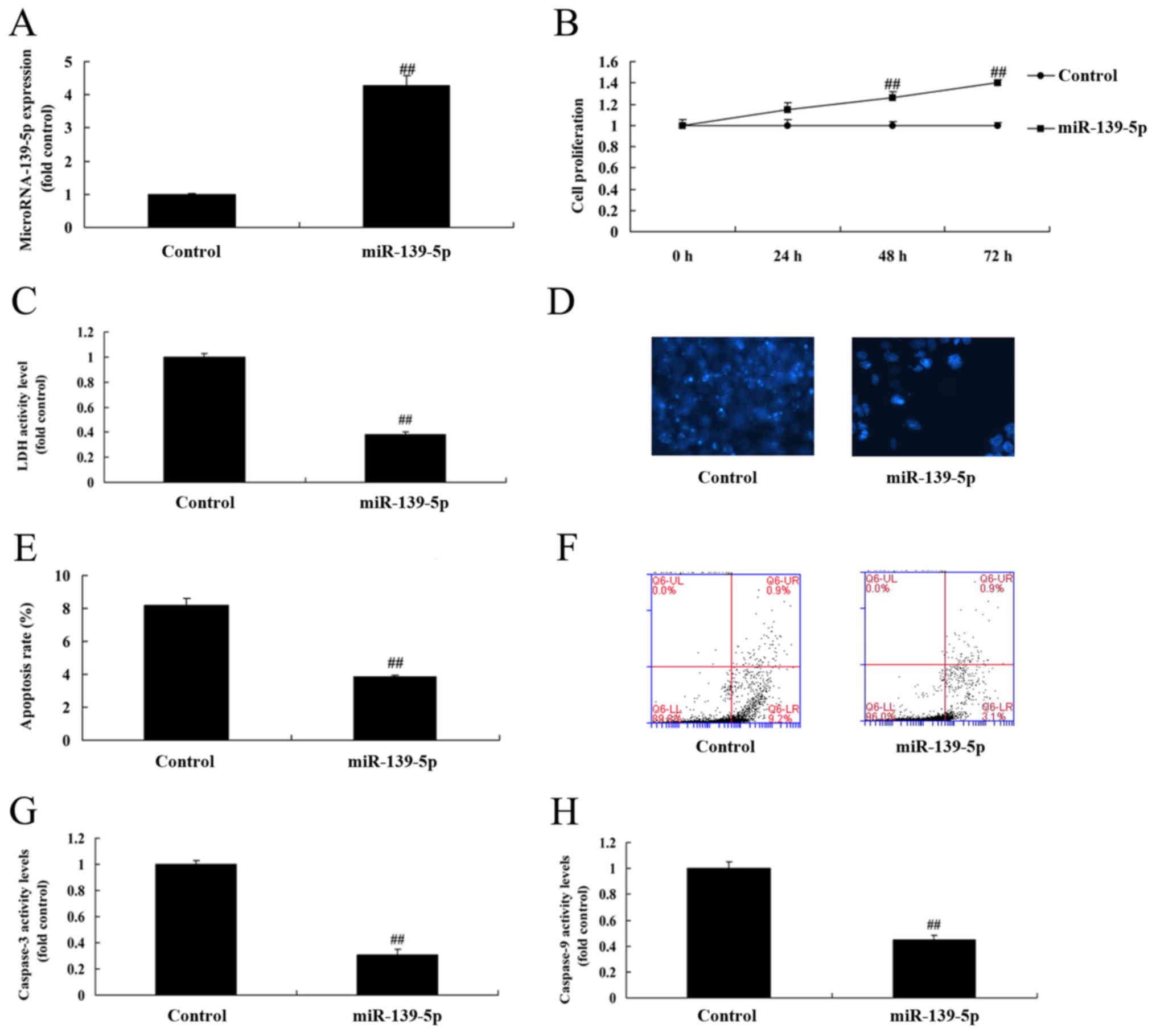
Overexpression of miR-139-5p promotes
cell growth of ADSC
The function of miR-139-5p on skeletal myogenic
differentiation of ADSCs was analyzed, and miR-139-5p mimics were
observed to increase the expression of miR-139-5p in ADSCs compared
with control negative group via RT-qPCR (Fig. 1A). Overexpression of miR-139-5p
promoted cell growth, inhibited LDH and caspase-3/9 activity
levels, and reduced apoptosis rate of ADSC, compared with the
control negative group (Fig.
1B-H).
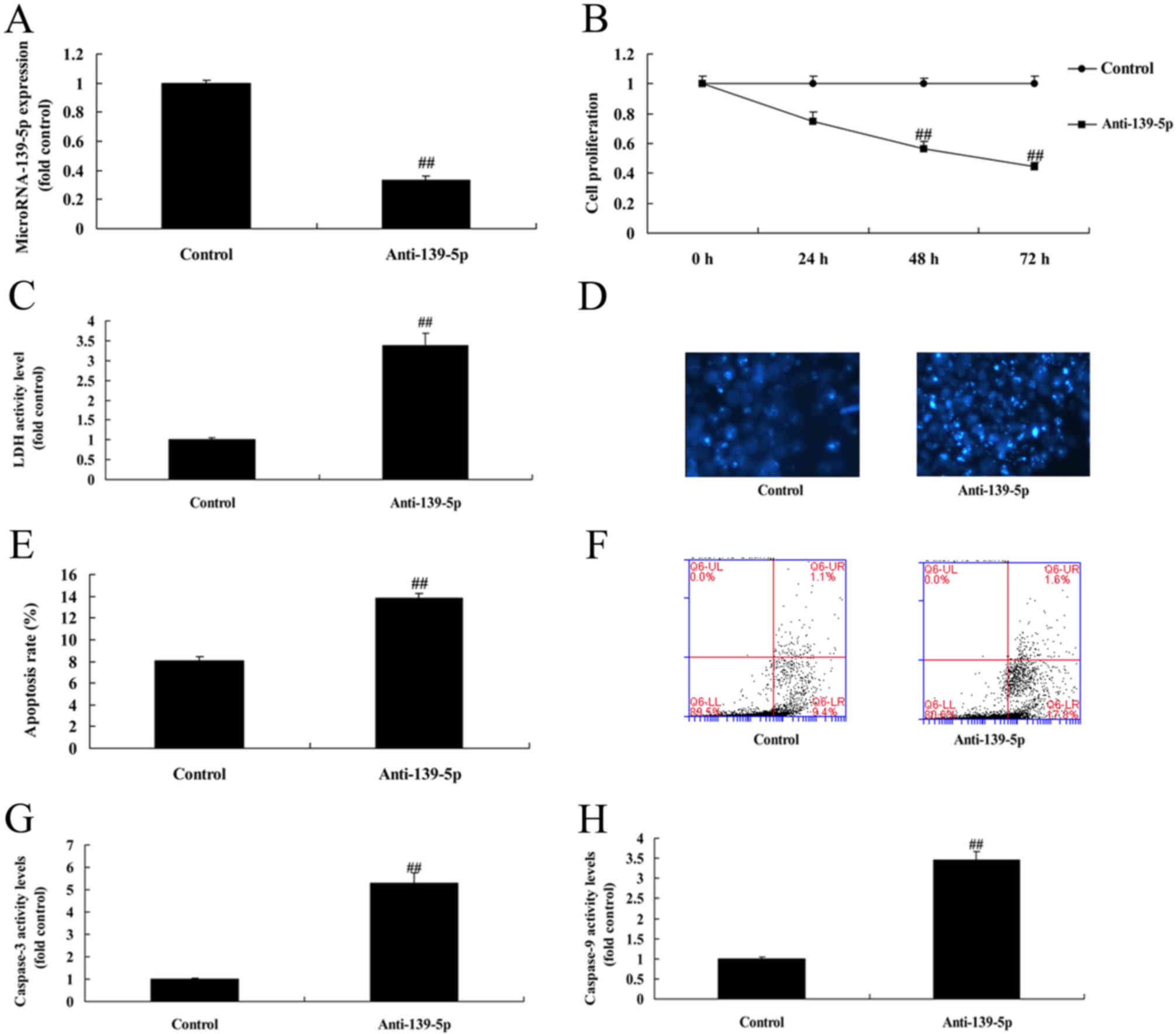
Downregulation of miR-139-5p inhibits
cell growth of ADSCs
Anti-miR-139-5p was used to decrease the protein
expression of miR-139-5p in ADSCs, compared with control negative
group (Fig. 2A). Downregulation of
miR-139-5p inhibited cell growth, increased LDH and caspase-3/9
activity levels, and promoted apoptosis rate of ADSCs, compared
with control negative group (Fig.
2B-H).
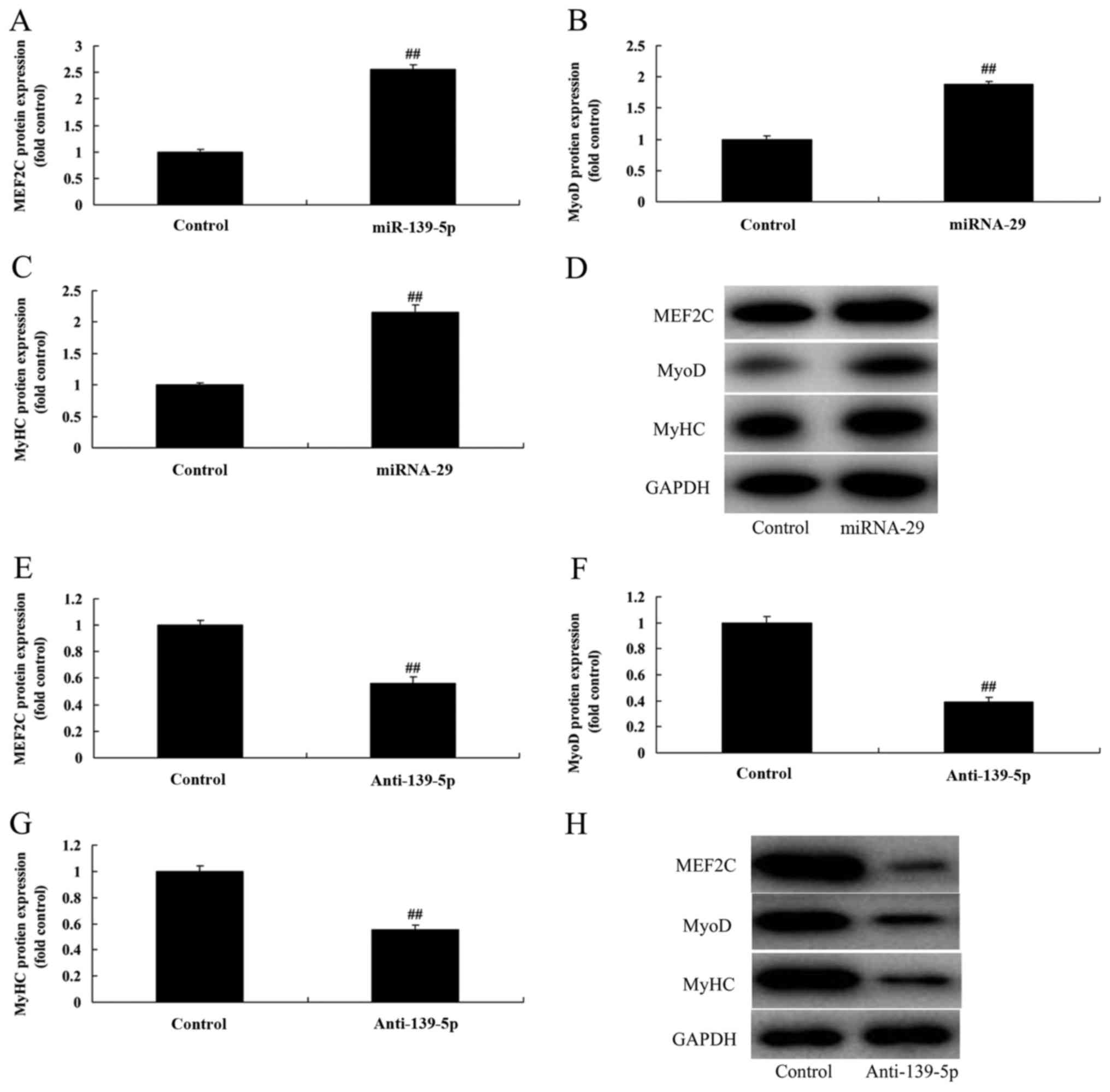
miR-139-5p regulates skeletal myogenic
differentiation of ADSCs
It was hypothesized that miR-139-5p would upregulate
skeletal myogenic differentiation of ADSC. As presented in Fig. 3A-D, overexpression of miR-139-5p
inducedMEF2C, MyoD and MyHC protein expression in ADSCs, compared
with control negative group. However, downregulation of miR-139-5p
suppressed MEF2C, MyoD and MyHC protein expression in ADSCs,
compared with control negative group (Fig. 3E-H). These results indicated that
overexpression of miR-139-5p induced skeletal myogenic
differentiation of ADSCs.
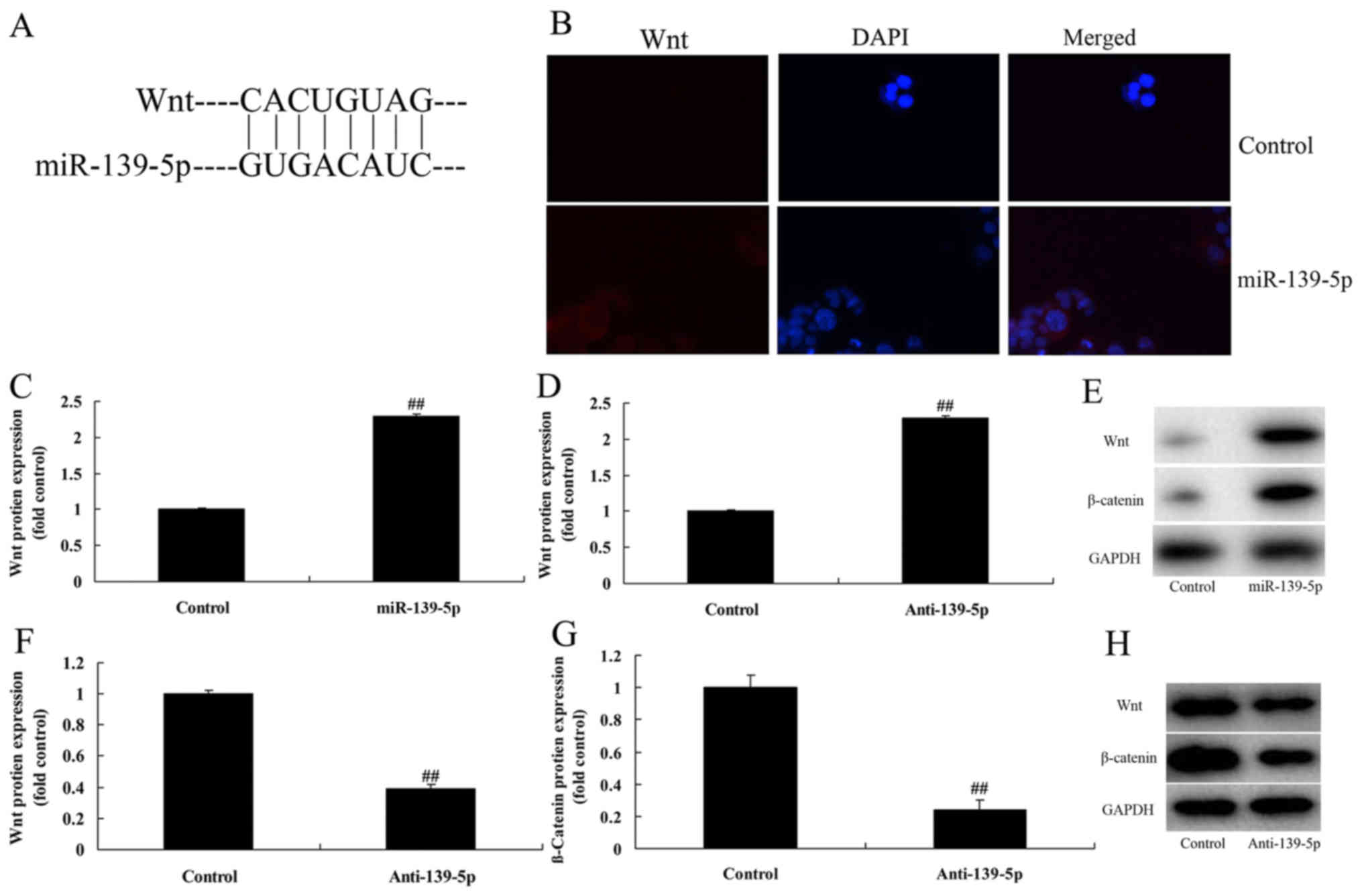
miR-139-5p regulates skeletal myogenic
differentiation of ADSC through Wnt/β-catenin signaling
pathway
The mechanism of miR-139-5p on skeletal myogenic
differentiation of ADSC was investigated, and miR-139-5p was
identified as a direct target of Wnt (Fig. 4A). Immunofluorescence microscopy
analysis demonstrated that overexpression of miR-139-5p induced Wnt
and β-catenin protein expression in ADSCs, compared with control
negative group (Fig. 4B).
Overexpression of miR-139-5p induced Wnt and β-catenin protein
expression in ADSCs, compared with control negative group (Fig. 4C-E). Downregulation of miR-139-5p
suppressed Wnt and β-catenin protein expression in ADSCs, compared
with control negative group (Fig.
4F-H). These data indicated that miR-139-5p regulates skeletal
myogenic differentiation of ADSCs through the Wnt/β-catenin
signaling pathway.
Wnt inhibitor reduces the effect of
miR-139-5p on skeletal myogenic differentiation of ADSCs
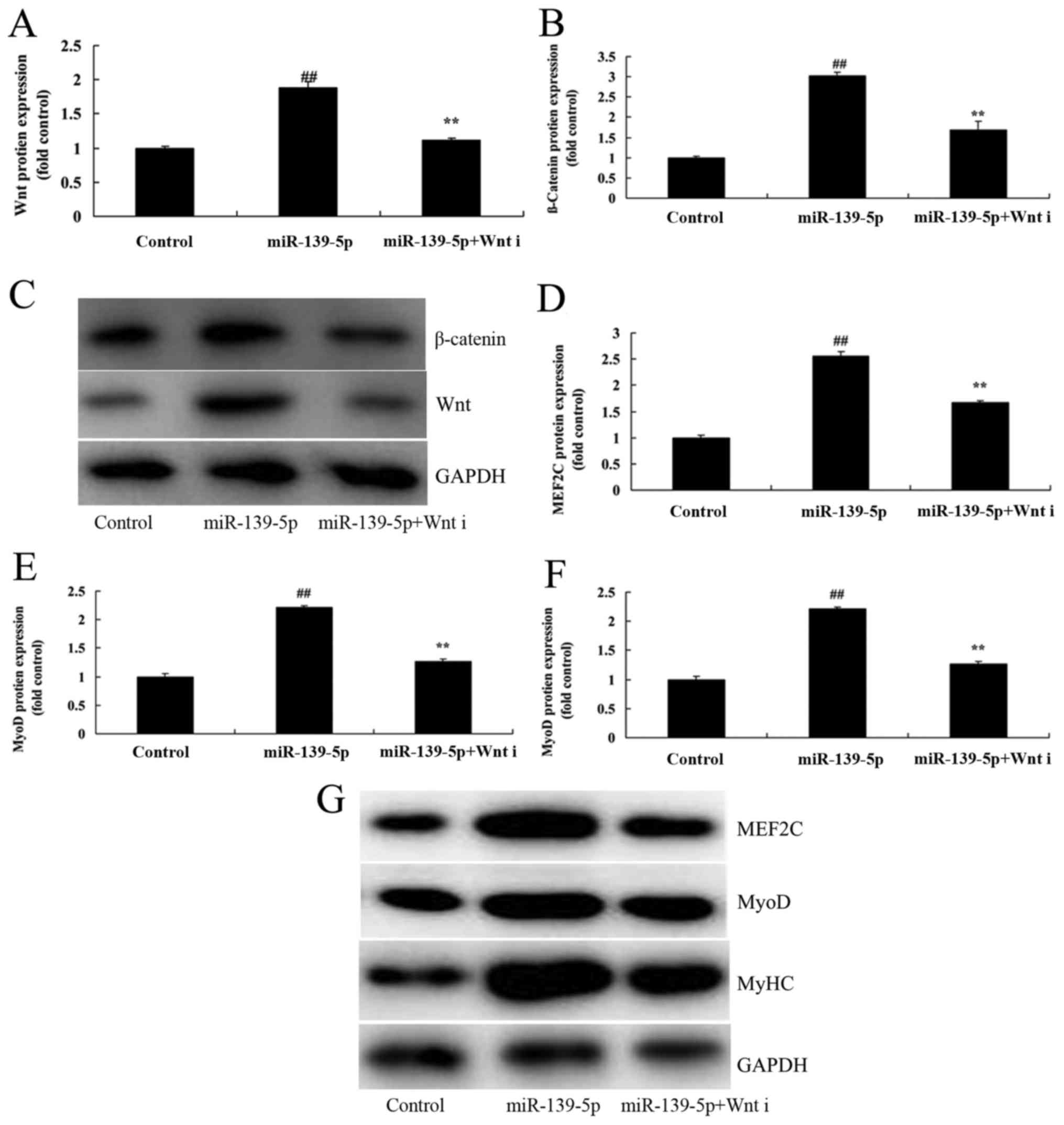
The role of Wnt/β-catenin signaling pathway on the
effect of miR-139-5p on skeletal myogenic differentiation of ADSCs
was investigated. As presented in Fig.
5A-C, Wnt inhibitor suppressed Wnt and β-catenin protein
expression in ADSCs induced by miR-139-5p, compared with the
miR-139-5p group. Wnt inhibitor also suppressed MEF2C, MyoD, and
MyHC protein expression in ADSCs induced by miR-139-5p, compared
with the miR-139-5p group (Fig.
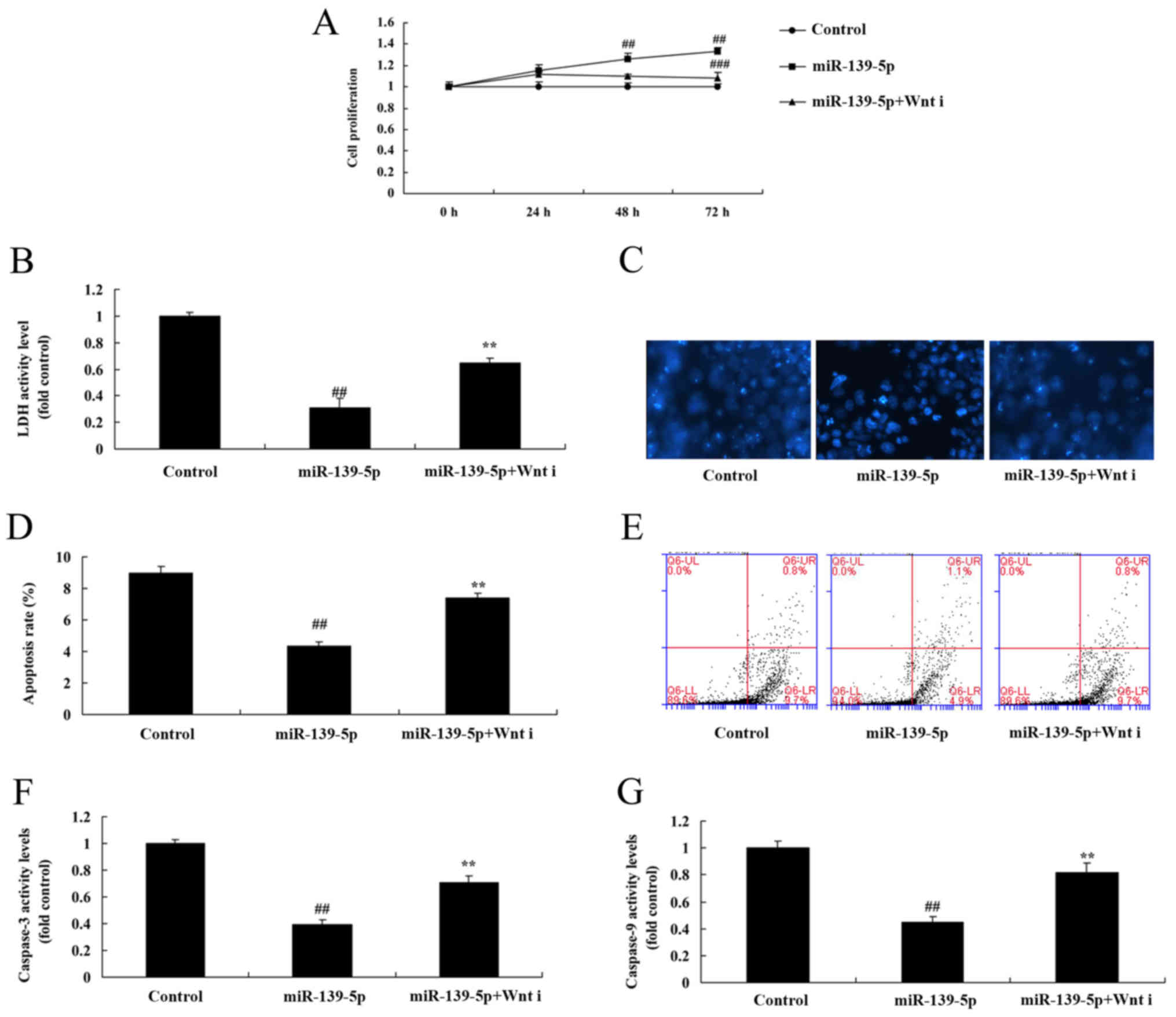
5D-G). Wnt inhibitor also reduced the effect of miR-139-5p on
cell growth, LDH and caspase-3/9 activity levels, and apoptosis
rate of ADSCs induced by miR-139-5p, compared with the miR-139-5p
group (Fig. 6).
Discussion
Endodontic disease is a common disease in the oral
cavity, with an incidence rate of 72% (4). It mainly results from irreversible
inflammatory response, liquidation or necrosis in dental pulp.
Eventually, the diseased dental pulp should be removed (4). Consequently, conventional root canal
therapy should be conducted to relieve the disease and repair tooth
function (12). Tissue engineering
technology has been rapidly developed in recent years (12). As a result, regenerative medicine has
gradually penetrated into all areas of medicine (12). Meanwhile, regenerative medical
research on oral endodontic disease has attracted attention from
scholars worldwide. The focus of research has typically been dental
pulp regeneration (6). miRNAs can
also regulate the expression of genes associated with dental germ
formation quantity (6). Researchers
have previously knocked out Dicer enzyme, which is essential for
the formation of mature miRNAs, and observed an increase in the
number of dental germs in mice, as well as partial edentia
(13). miRNAs also serve important
regulatory roles in dental pulp regeneration research (13). Results of the present study
demonstrated that overexpression of miR-139-5p promoted cell growth
and induced skeletal myogenic differentiation of ADSCs. Mi et
al (14) recently demonstrated
that miR-139-5p suppresses 3T3-L1 preadipocyte differentiation.
The Wnt signaling pathway is an extremely conserved
signal transduction pathway (9).
Furthermore, it serves a key role in the dynamic balance of
maintaining adult tissue (9). Recent
findings have suggested that Wnt signaling can also regulate stem
cell differentiation and self-renewal (9). In addition, recent research has also
indicated that inhibiting canonical Wnt signal transduction can
promote adipogenic differentiation of preadipocytes and
differentiation of adipocytes (9).
Upregulating peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
can activate the canonical Wnt pathway, and inhibit expression of
CCAAT/enhancer-binding protein-α and peroxisome
proliferator-activated receptor γ (9). Thus, it can suppress the adipogenic
differentiation of DPSCs (15).
These findings indicate that the Wnt pathway serves a key
regulatory role in the adipogenic differentiation of MSC (15). The Wnt pathway is an important target
of the adipogenic differentiation of pluripotent stem cells
(16). In the present study, it as
demonstrated that overexpression of miR-139-5p induced the
Wnt/β-catenin signaling pathway of ADSCs. Mi et al (17) recently reported that miR-139-5p
regulates myogenesis through blocking Wnt/β-catenin signaling
pathway.
Wnt protein ligand, Wnt receptor, β-catenin,
glycogen synthase kinase, axin and APC are the major members of the
Wnt signaling pathway (18).
Specifically, β-catenin is the important mediator of Wnt signal
transduction. APC also serves an important role in Wnt signal
transduction pathway (19).
Wnt/β-catenin is a canonical intracellular Wnt signaling pathway.
Previous studies have demonstrated that Wnt signal serves an
irreplaceable role in dental epithelium-mesenchyme interaction,
dentin and dental root development (18,20). The
present study demonstrated that Wnt inhibitor reduced the effect of
miR-139-5p on skeletal myogenic differentiation of ADSCs. Luo et
al (21) recently demonstrated
that miR-139-5p inhibits bladder cancer proliferation by targeting
the Wnt signaling pathway. However, this study is limited as only
in vitro experiments were performed. Future studies should
therefore perform in vivo experiments in order to confirm
these results.
In conclusion, the results of the present study
demonstrated that miR-139-5p elevates skeletal myogenic
differentiation of human ADSCs through Wnt/β-catenin signaling
pathway. miR-139-5p may therefore be a novel marker of skeletal
myogenic differentiation of human ADSCs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS designed the experiment; YX and GS performed the
experiments, analyzed the data and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ninth People's Hospital, Shanghai JiaoTong University
School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuo YR, Chien CM, Kuo MJ, Wang FS, Huang
EY and Wang CJ: Endothelin-1 expression associated with lipid
peroxidation and nuclear factor-κB activation in type 2 diabetes
mellitus patients with angiopathy and limb amputation. Plast
Reconstr Surg. 137:187e–195e. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsang KK, Kwong EW, Woo KY, To TS, Chung
JW and Wong TK: The anti-inflammatory and antibacterial action of
nanocrystalline silver and manuka honey on the molecular
alternation of diabetic foot ulcer: A comprehensive literature
review. Evid Based Complement Alternat Med. 2015:2182832015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang E, Gao B, Yang L, Wu X and Wang Z:
Notoginsenoside Ft1 promotes fibroblast proliferation via
PI3K/Akt/mTOR signaling pathway and benefits wound healing in
genetically diabetic mice. J Pharmacol Exp Ther. 356:324–332. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arya AK, Tripathi R, Kumar S and Tripathi
K: Recent advances on the association of apoptosis in chronic non
healing diabetic wound. World J Diabetes. 5:756–762. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folestad A, Ålund M, Asteberg S, Fowelin
J, Aurell Y, Göthlin J and Cassuto J: IL-17 cytokines in bone
healing of diabetic Charcot arthropathy patients: A prospective 2
year follow-up study. J Foot Ankle Res. 8:392015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang RH, Lin J, Hou XH, Cao R, Yu F, Liu
HQ, Ji AL, Xu XN, Zhang L and Wang F: Effect of docosahexaenoic
acid on hippocampal neurons in high-glucose condition: Involvement
of PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways.
Neuroscience. 274:218–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padiya R, Chowdhury D, Borkar R, Srinivas
R, Pal Bhadra M and Banerjee SK: Garlic attenuates cardiac
oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in
fructose-fed diabetic rat. PLoS One. 9:e942282014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang M, Liang L, Li L, Han K, Li Q, Peng
Y, Peng X and Zeng K: Increased miR-424-5p expression in peripheral
blood mononuclear cells from patients with pemphigus. Mol Med Rep.
15:3479–3484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu Q, Sun Y, Cui J and Xu T: Inducible
microRNA-214 contributes to the suppression of NF-κB-mediated
inflammatory response via targeting myd88 gene in fish. J Biol
Chem. 292:5282–5290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang G, Gao J, Jia Y, Yan L, Yu J and
Jiang Y: Oxysophoridine through intrathecal injection induces
antinociception and increases the expression of the GABAAα1
receptor in the spinal cord of mice. Planta Med. 78:874–880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kandhare AD, Ghosh P and Bodhankar SL:
Naringin, a flavanone glycoside, promotes angiogenesis and inhibits
endothelial apoptosis through modulation of inflammatory and growth
factor expression in diabetic foot ulcer in rats. Chem Biol
Interact. 219:101–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin X, Xu Z, Zhang Z, Li L, Pan Q, Zheng F
and Li H: Association of PI3K/AKT/mTOR pathway genetic variants
with type 2 diabetes mellitus in Chinese. Diabetes Res Clin Pract.
128:127–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mi L, Chen Y, Zheng X, Li Y, Zhang Q, Mo D
and Yang G: MicroRNA-139-5p suppresses 3T3-L1 preadipocyte
differentiation through notch and IRS1/PI3K/Akt insulin signaling
pathways. J Cell Biochem. 116:1195–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ZH, Zhang JL, Duan YL, Zhang QS, Li
GF and Zheng DL: MicroRNA-214 participates in the neuroprotective
effect of Resveratrol via inhibiting α-synuclein expression in
MPTP-induced Parkinson's disease mouse. Biomed Pharmacother.
74:252–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Liu J, Guo B, Liang C, Dang L, Lu C,
He X, Cheung HY, Xu L, Lu C, et al: Osteoclast-derived exosomal
miR-214-3p inhibits osteoblastic bone formation. Nat Commun.
7:108722016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mi L, Li Y, Zhang Q, Zhao C, Peng Y, Yang
G and Zheng X: MicroRNA-139-5p regulates C2C12 cell myogenesis
through blocking Wnt/β-catenin signaling pathway. Biochem Cell
Biol. 93:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu L, Yang XP, Janic B, Rhaleb NE,
Harding P, Nakagawa P, Peterson EL and Carretero OA: Ac-SDKP
suppresses TNF-α-induced ICAM-1 expression in endothelial cells via
inhibition of IκB kinase and NF-κB activation. Am J Physiol Heart
Circ Physiol. 310:H1176–H1183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Huang D, Yang F, Tian M, Wang Y,
Shen D, Wang Q, Chen Q and Zhang L: Long noncoding RNA highly
upregulated in liver cancer regulates the tumor necrosis
factor-α-induced apoptosis in human vascular endothelial cells. DNA
Cell Biol. 35:296–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Ha T, Hu Y, Lu C, Liu L, Zhang X,
Kao R, Kalbfleisch J, Williams D and Li C: MicroRNA-214 protects
against hypoxia/reoxygenation induced cell damage and myocardial
ischemia/reperfusion injury via suppression of PTEN and Bim1
expression. Oncotarget. 7:86926–86936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo H, Yang R, Li C, Tong Y, Fan L, Liu X
and Xu C: MicroRNA-139-5p inhibits bladder cancer proliferation and
self-renewal by targeting the Bmi1 oncogene. Tumour Biol.
39:10104283177184142017. View Article : Google Scholar : PubMed/NCBI
|