Introduction
The prevalence of cancer is increasing rapidly
(1) and research is focusing on the
exploration of novel anticancer treatments. Although the field is
growing rapidly, very few cancer drugs are able to pass clinical
trials (2). There are numerous types
of cancer cells, which are characterized based on either the source
of the cell or the development of cells due to gene mutations
(3). Various types of drugs may be
required for treatments based on the particular characteristics of
the cancer cells. As a result, novel anticancer treatments from
plants and marine invertebrates, including sea cucumbers were
explored.
In general, in vitro and in vivo
studies involving sea cucumbers have primarily focused on the
toxicity of active compounds on cancer cells by induction of
apoptosis or cell cycle arrest (4).
Compounds from sea cucumbers exhibiting anticancer properties have
been reported (5), including
colochiroside A from Colochirus anceps (6), ds-echinoside A from Pearsonothuria
graeffei (7), philinopside E
from Pentacta quadrangularis (8), sphingosine from Stichopus
variegatus (9) and stichoposide
C from Thelenota anax (10).
However, the mechanisms of action controlling cancer cell growth at
a molecular level remain unclear. The current study analyzed the
potential of these compounds as inhibitors of mouse double minute 2
homolog (MDM2) and C-X-C chemokine receptor type 4 (CXCR4). The
inhibition of these two targets simultaneously may induce a
synergistic effect, increasing treatment efficacy.
MDM2 serves a role in binding pro-apoptotic tumor
protein 53 (p53) and degrading it (11). Inhibiting the activity of MDM2 may
increase p53 levels in the cell, which are necessary for apoptosis
(11,12). CXCR4 belongs to the G-protein-coupled
receptor family that is involved in several pathways associated
with cancer and serves a role in controlling cell proliferation
(13). CXCR4 promotes survival of
various cell types (14) and serves
a critical role in tumorigenesis (15). Furthermore, it acts as receptor for
the C-X-C motif chemokine ligand 12 (CXCL12) which serves a role in
signal transduction for calcium uptake and enhances the activity of
mitogen-activated protein kinase (MAPK)1/MAPK3 (15,16).
CXCR4 was reported to be a potent inducer of apoptosis in acute
myeloid leukemia cell lines (14).
The protein has been a target in drug development (17) and cancer treatment (18,19), and
anti-CXCR4 antibodies were demonstrated to induce apoptosis in
hematologic malignancies (15). The
current study describes the potential of compounds from sea
cucumbers as MDM2 and CXCR4 inhibitors, aiming to reveal novel
insight into the mechanisms of inhibiting cancer cell growth.
Materials and methods
Preparation of molecule structures and
codes
The ligands used for docking analysis were
colochiroside A, ds-echinoside A, philinopside E, sphingosine,
stichoposide C,
1-(5-chloro-2-methylphenyl)-5-(3-chlorophenyl)-2-(3-methylphenyl)-1H-imidazole-4-carboxylic
acid, a tetra-substituted imidazole (an MDM2 inhibitor) and
chalcone-4 (a CXCR4 inhibitor). SMILES codes of the compounds were
converted to 3D structures in Protein Data Bank (PDB) format using
BIOVIA Discovery Studio 4.5 (20).
These structures were used for ligand docking. The 3D structure for
chalcone-4 was obtained from the binding database (https://www.bindingdb.org/bind/index.jsp) (21) and the 3D structure for the
substituted imidazole was obtained from the PDB (PDB ID, 4OQ3). The
receptor structures were retrieved from the PDB for MDM2 (PDB ID,
4OQ3) and CXCR4 (PDB ID, 3OE6). The proteins then were prepared by
BIOVIA Discovery Studio.
Ligand docking studies
Interactions between receptors and ligand were
analyzed by AutoDock Vina integrated in PyRx 0.8 (https://pyrx.sourceforge.io) (22,23). The
docking method was used to evaluate binding affinities and to
elucidate molecular mechanisms, and was performed according to
previous literature (24). Docking
was performed by setting receptors as rigid molecules and ligands
as flexible molecules within the active site. Results of docking
and bonding interactions were analyzed by BIOVIA Discovery Studio
(20).
Protein-protein interactions and
networks
Proteins that interact with CXCR4 were identified
using BioGRID database (https://thebiogrid.org/) (25). Protein-protein interaction networks
were examined using STRING (https://string-db.org/) (26).
Pathway analysis
Pathway analysis for CXCR4 was performed using Kyoto
Encyclopedia Gene and Genome (KEGG; http://www.genome.jp/kegg/) (27). The role of CXCR4 proteins in various
molecular pathways was identified using KEGG pathways databases
with STRINGdb 10.5 software. The database covers a range of
pathways that have been used as references for the determination of
gene or protein function within a cell (28).
Results
Ligand docking analysis
The results of the docking between CXCR4 or MDM2
with the five compounds identified in sea cucumbers revealed that
four of the compounds (Ds-echinoside A, Philinopside E,
Stichoposide C and Colochiroside A, with values of −9.0, −8.5, −9.2
and −8.5 kcal/mol, respectively) exhibited higher binding
affinities to CXCR4 compared with a known inhibitor (chalcone; −7.1
kcal/mol; Table I). Additionally,
two compounds (Ds-echinoside A and Philinopside E) were identified
to potentially bind to MDM2, with binding affinities of −7.1 and
−7.5 kcal/mol, respectively. The compounds (Ds-echinoside A and
Philinopside E) were predicted to inhibit MDM2 and to exhibit
binding energies higher than its inhibitor, imidazole. However, the
binding energies of these two molecules to MDM2 were similar to
those of chalcone bound to CXCR4 (−7.1 kcal/mol), but lower when
compared with protease bound to its inhibitor (−7.0 kcal/mol)
(29) and to the coline receptor
bound to its ligand (−6.0 kcal/mol) (30). Sphingosine exhibited lowest binding
affinities for CXCR4 and MDM2 (−5.8 kcal/mol and −5.2 kcal/mol,
respectively).
 | Table I.Binding affinity between compounds
from sea cucumbers and CXCR4 (PDB ID, 3OE6) or MDM2 (PDB ID,
4OQ3). |
Table I.
Binding affinity between compounds
from sea cucumbers and CXCR4 (PDB ID, 3OE6) or MDM2 (PDB ID,
4OQ3).
|
| Binding affinity
(kcal/mol) |
|---|
|
|
|
|---|
| Ligand Name | CXCR4 | MDM2 |
|---|
| Ds-echinoside A | −9.0 | −7.1 |
| Sphingosine | −5.8 | −5.2 |
| Philinopside E | −8.5 | −7.5 |
| Stichoposide C | −9.2 | −5.7 |
| Colochiroside A | −8.5 | −7.0 |
| CXCR4 Inhibitor
(Chalcone) | −7.1 | – |
| MDM2 Inhibitor
(Imidazoles) | – | −9.5 |
Further analysis focused on evaluating the
orientation of the compounds when interacting with the active site
of MDM2. This analysis describes a critical part in assessing the
potential of a compound for inhibiting MDM2. It was demonstrated
that two compounds (Ds-echinoside A and Philinopside E) from sea
cucumbers bound to the active site of MDM2 in a similar position to
the known inhibitor, a substituted imidazole (Fig. 1). The data suggested that
philinopside E and ds-echinoside A, extracted from Pentacta
quadrangularis and Pearsonothuria graeffei,
respectively, may have potential as MDM2 inhibitors.
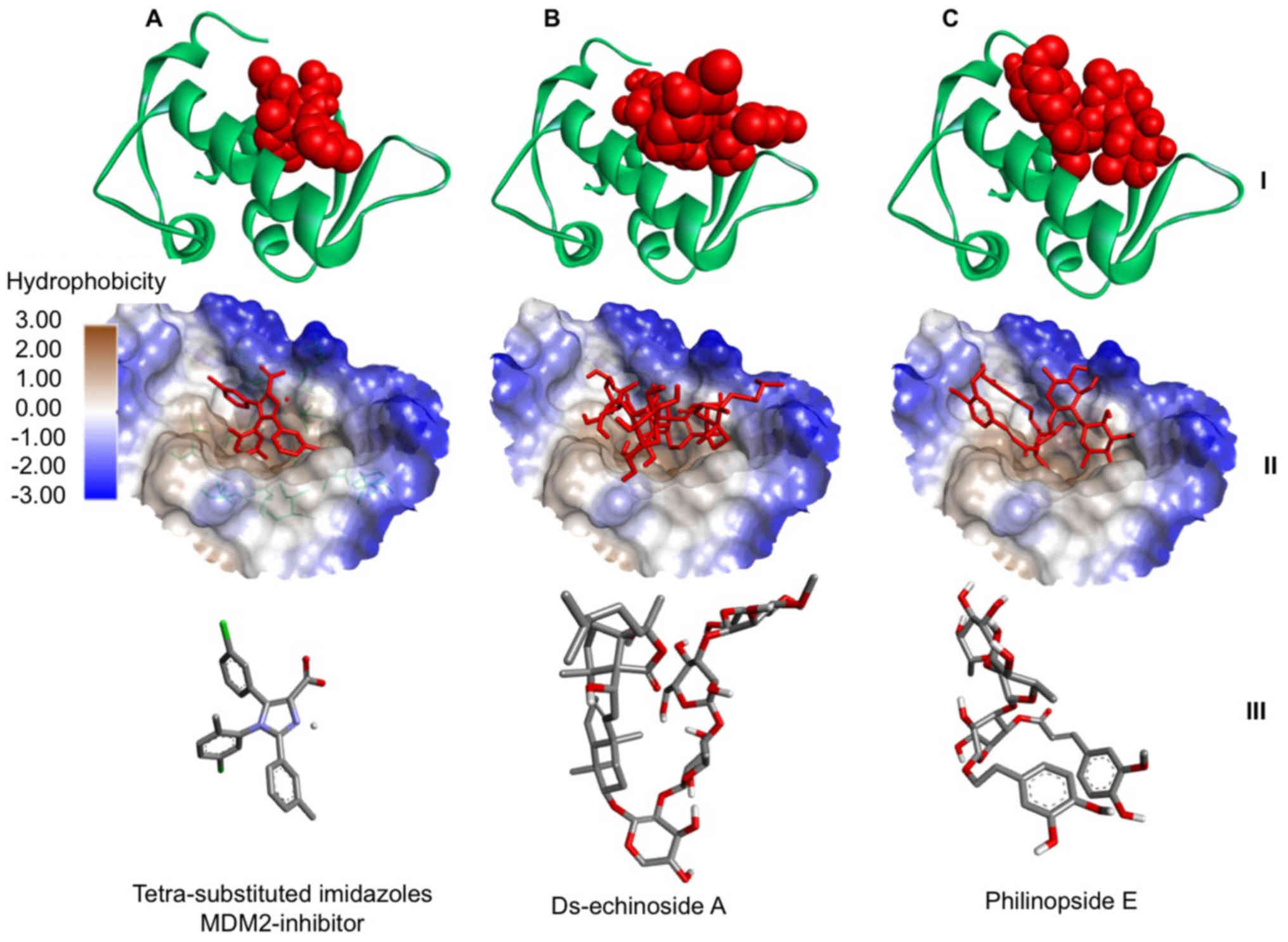 | Figure 1.Interaction between MDM2 and compounds
from sea cucumbers. Docking of (A) a tetra-substituted imidazole, a
known MDM2 inhibitor, (B) ds-echinoside A and (C) philinopside E to
MDM2. At the top, MDM2 presented in green ribbon structure with
ligands presented as red spheres. In the middle, hydrophobicity
surface map of the active site of MDM2 with the ligands presented
as red cylinders. At the bottom, cylinder representation of the
ligands with carbon atoms in grey, nitrogen in blue, chlorine in
green, oxygen in red and hydrogen in white, to emphasize ligand
orientation. MDM2, mouse double minute 2 homolog. |
The binding of ds-echinoside A, philinopside E,
stichoposide C and colochiroside A to CXCR4 were compared to the
binding of chalcone-4, a known CXCR4 inhibitor, to CXCR4 (Fig. 2). It was demonstrated that all
compounds bound to the active site of CXCR4 in a similar position
to chalcone-4. The data indicated that these compounds may have
potential as CXCR4 inhibitors.
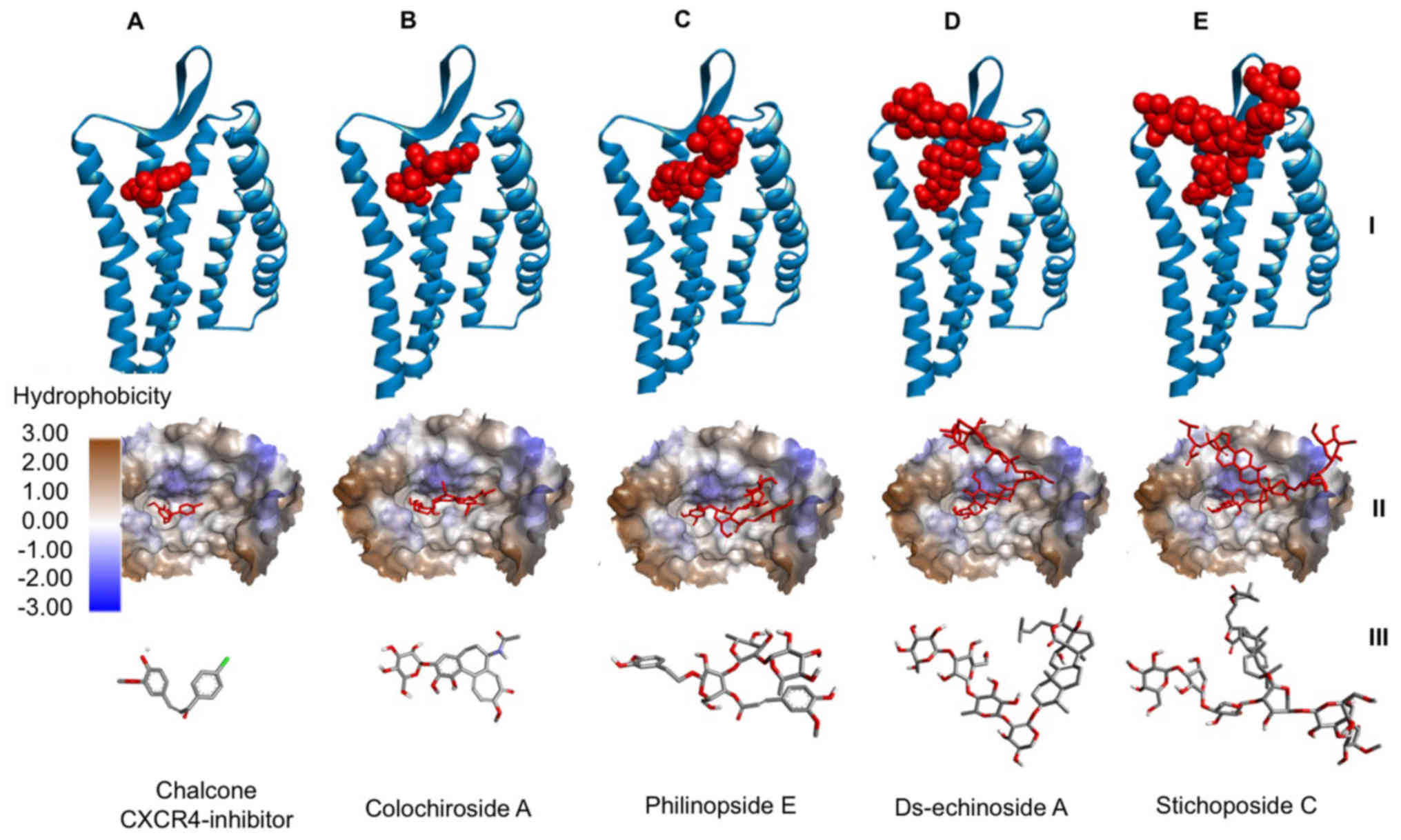 | Figure 2.Interaction between CXCR4 and
compounds from sea cucumbers. Docking of (A) chalcone-4, a known
CXCR4 inhibitor, (B) colochiroside A, (C) philinopside E, (D)
ds-echinoside A and (E) stichoposide C to CXCR4. At the top, CXCR4
presented in blue ribbon structure with ligands presented as red
spheres. In the middle, hydrophobicity surface map of the active
site of CXCR4 with the ligands presented as red cylinders. At the
bottom, cylinder representation of the ligands with carbon atoms in
grey, nitrogen in blue, chlorine in green, oxygen in red and
hydrogen in white, to emphasize ligand orientation. CXCR4, C-X-C
chemokine receptor type 4. |
Protein interaction and pathway
analysis
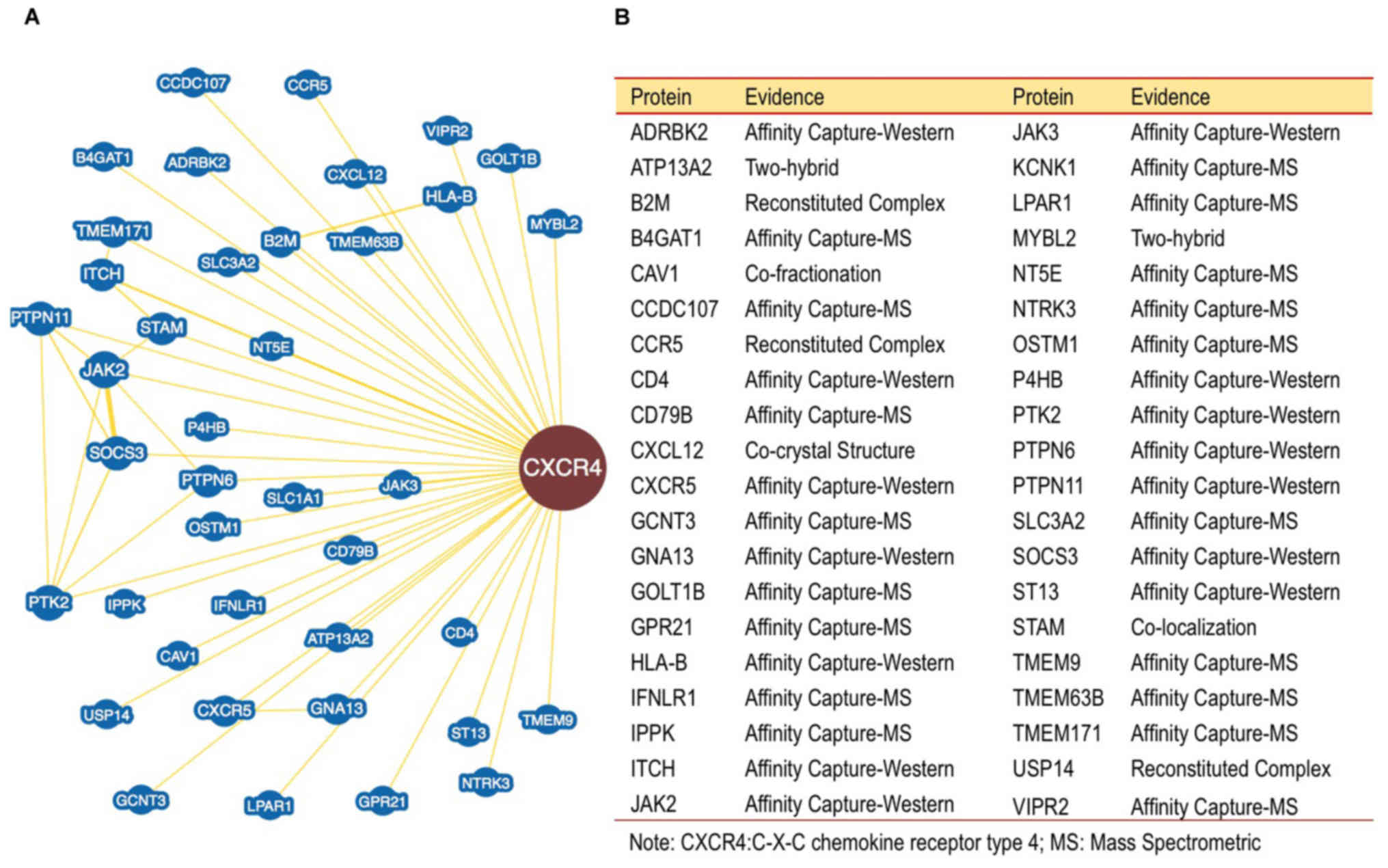
Furthermore, the binding of proteins to CXCR4 were
investigated. The data obtained using BioGRID revealed over 40
proteins interacting with CXCR4 (Fig.
3). This analysis is essential to map and resolve functions of
proteins that interact with CXCR4. The data may be used in further
pathway analysis to help understand the role of CXCR4 in the
mechanism of cancer cell growth regulation. The results of the
analysis are summarized as a map of proteins interacting with
CXCR4. Identified proteins may serve a role in the pathways
involved in the pathomechanism of cancer. The proteins are involved
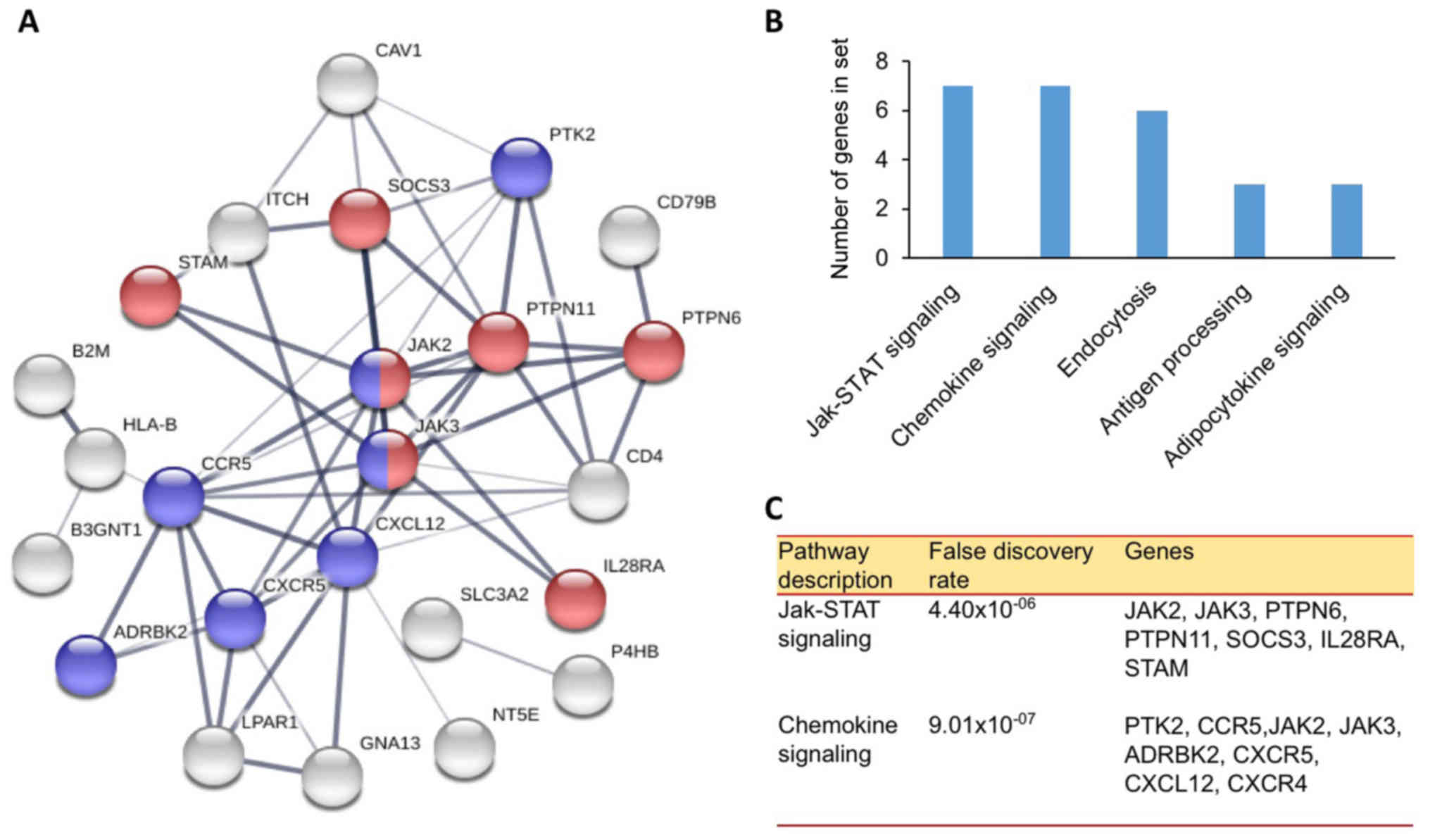
in two pathways associated with cancer cell signaling, including
the chemokine and Janus kinase (JAK) signal transducer and
activator of transcription (STAT) signaling pathway (Fig. 4). A minimum of 15 proteins
participates in these pathways, including protein tyrosine kinase 2
(PTK2), C-C chemokine receptor type 5, JAK2, JAK3, β-adrenergic
receptor kinase 2, CXCR5, CXCL12, CXCR4, protein tyrosine
phosphatase (PTP)6, PTPN11, suppressor of cytokine signaling 3,
interleukin-28 α receptor and signal transducing adapter molecule
1. These pathways serve a role in cell proliferation, angiogenesis,
cell growth, and metastasis (31).
Inhibition of these two pathways may inhibit cancer cell growth and
induce apoptosis.
The results of the current study indicated that two
out of the five chosen compounds, philinopside E and ds-echinoside
A, may inhibit MDM2 and CXCR4 (Fig.
5). Two other compounds, stichoposide C and colochiroside A,
were predicted to inhibit CXCR4. The data suggested that
philinopside E and ds-echinoside A may exhibit higher efficiencies
due to inhibiting two targets simultaneously. Inhibition of MDM2
may trigger apoptosis through p53 activation (32) and inhibition of CXCR4 may affect cell
proliferation and growth through JAK2/3-STAT and PTK2 signalling
pathways.
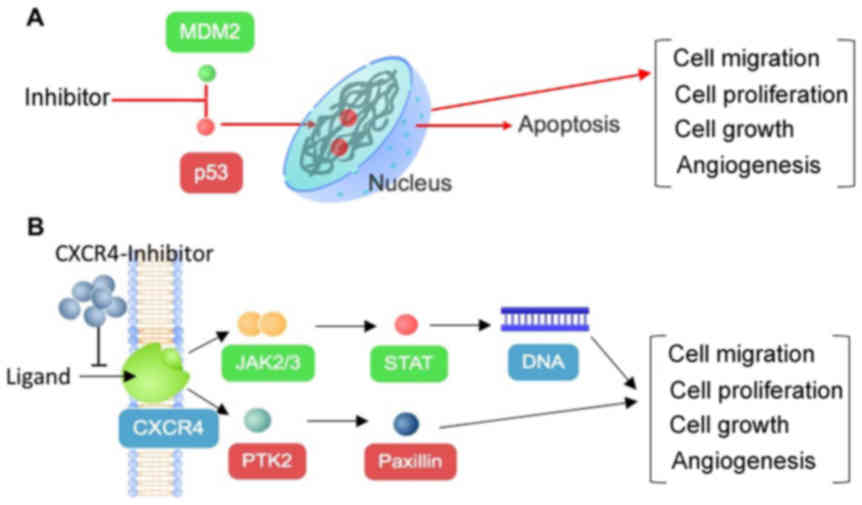 | Figure 5.Anticancer mechanism of compounds from
sea cucumbers through inhibition of (A) MDM2 or (B) CXCR4.
Inhibition of both proteins may lead to decreased cell migration,
cell proliferation, cell growth and angiogenesis, which may lead to
apoptosis. MDM2, mouse double minute 2 homolog; CXCR4, C-X-C
chemokine receptor type 4; p53, tumor protein 53; CXCL12, C-X-C
motif chemokine 12; JAK, Janus kinase; STAT, signal transducer and
activator of transcription; PTK2, protein tyrosine kinase 2. |
Discussion
Previous studies have demonstrated that sea
cucumbers contain compounds which exhibit anticancer properties and
are described as beneficial agents for human health (33). However, mechanisms of action
explaining the anticancer properties remain unclear. The current
study analyzed the anticancer mechanisms of compounds from five
species of sea cucumber (Table II)
using an in silico approach. These compounds included
colochiroside A, ds-echinoside A, philinopside E, sphingosine and
stichoposide C. Molecular docking was conducted to examine the
binding affinity between these compounds and MDM2 or CXCR4. MDM2
has been used as a target in cancer therapy (32). It serves a role in the degradation of
p53, a pro-apoptotic protein (32).
CXCR4 has also been described as a target in the development of
cancer treatments (13). The protein
is a receptor that regulates cell cycle, cell proliferation,
metastasis and angiogenesis (34).
 | Table II.Compounds from sea cucumbers
exhibiting anticancer activities. |
Table II.
Compounds from sea cucumbers
exhibiting anticancer activities.
| Author, year | Organism | Compound | Cell effect | (Refs.) |
|---|
| Zhang and Yi,
2011 | Colochirus
anceps | Colochiroside
A | Antitumor
activity | (6) |
| Zhao et al,
2011 | Pearsonothuria
graeffei | Ds-echinoside
A | Antimetastatic,
angiogenesis, apoptosis | (7) |
| Tian et al,
2007 | Pentacta
quadrangularis | Philinopside E | Antimetastatic,
angiogenesis, apoptosis | (8) |
| Sugawara et
al, 2006 | Stichopus
variegatus | Sphingosine | Apoptosis | (9) |
| Yun et al,
2012 | Thelenota
anax | Stichoposide C | Apoptosis, growth
inhibition | (10) |
Details on mechanisms of action for compounds from
sea cucumbers as anticancer treatments remain limited. An in-depth
docking analysis was conducted to evaluate the potential of
compounds from sea cucumbers as anticancer treatments through
inhibition of MDM2 and CXCR4. The findings indicated that four out
of the five chosen compounds from sea cucumbers are predicted to
inhibit CXCR4 and two of these further inhibit MDM2. However, based
on the in silico analysis performed in the current study,
sphingosine may not be a suitable inhibitor for CXCR4 or MDM2.
The data obtained from pathway analysis suggested
that the studied compounds may inhibit cancer cell growth through
the chemokine and JAK-STAT signaling pathway or p53 pathway
(4). These three pathways serve a
central role in the process of controlling cell cycle, migration
and apoptosis (13,31,32).
Therefore, the data indicated that the anticancer mechanism of the
active compound of sea cucumber occurs through inference with the
JAK-STAT and Chemokine signaling pathways.
CXCR4 is a receptor located on the cell surface,
functioning as a communicator between cells and their environment
(14). The receptor binds chemokines
and other growth factors, which then transmit signals into cells
through multiple pathways, including JAK2/3 and PTK2, which
regulate cell division (35). By
interrupting the signal transmission through CXCR4, inhibition of
cancer cell growth may occur. CXCR4 is known to bind CXCL12, which
influences calcium uptake and enhances MAPK1/MAPK3 (15,16) and
may be a suitable target in cancer treatment (17,18).
In conclusion, the current study suggested that
several compounds from sea cucumbers may have potential as MDM2 or
CXCR4 inhibitors. Philinopside E and ds-echinoside A were predicted
as MDM2 and CXCR4 inhibitors, while stichoposide C and
colochiroside A were predicted as CXCR4 inhibitors. The compounds
may be able to inhibit MDM2 and CXCR4 and induce apoptosis in
cancer cells. Further research should be conducted in vitro
to validate the activity of the studied compounds.
Acknowledgements
The authors would like to thank the Ministry of
Research, Technology and Higher Education for supporting the
present study.
Funding
The present study was funded by the Ministry of
Research, Technology and Higher Education, Republic of Indonesia
(grant no. 1598/K4/KM/2017).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SP and NW designed the study. TWL and NW conducted
the research and prepared the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khazaei S, Salehiniya H and
Mohammadian-Hafshejani A: Some facts about cancer in the world
using registered cancer in 2012. Iran J Public Health.
44:1559–1560. 2015.PubMed/NCBI
|
|
2
|
Chary KV and Pandian K: Accelerated
approval of drugs: Ethics versus efficacy. Indian J Med Ethics.
2:244–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pilié PG, Johnson AM, Hanson KL, Dayno ME,
Kapron AL, Stoffel EM and Cooney KA: Germline genetic variants in
men with prostate cancer and one or more additional cancers.
Cancer. 123:3925–3932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wargasetia TL, Permana S and Widodo: The
role of sea cucumber active compound and its derivative as an
anti-cancer agent. Curr Pharmacol Rep. 4:27–32. 2018. View Article : Google Scholar
|
|
5
|
Janakiram NB, Mohammed A and Rao CV: Sea
cucumbers metabolites as potent anti-cancer agents. Mar Drugs.
13:2909–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y and Yi Y: Studies on antitumor
activities of triterpene glycoside colochiroside A from sea
cucumber Colochirus anceps. Zhongguo Zhong Yao Za Zhi. 36:504–507.
2011.(In Chinese). PubMed/NCBI
|
|
7
|
Zhao Q, Liu Z, Xue Y, Wang JF, Li H, Tang
QJ, Wang YM, Dong P and Xue CH: Ds-echinoside A, a new triterpene
glycoside derived from sea cucumber, exhibits antimetastatic
activity via the inhibition of NF-κB-dependent MMP-9 and VEGF
expressions. J Zhejiang Univ Sci B. 12:534–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian F, Zhu CH, Zhang XW, Xie X, Xin XL,
Yi YH, Lin LP, Geng MY and Ding J: Philinopside E, a new sulfated
saponin from sea cucumber, blocks the interaction between kinase
insert domain-containing receptor (KDR) and alphavbeta3 integrin
via binding to the extracellular domain of KDR. Mol Pharmacol.
72:545–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugawara T, Zaima N, Yamamoto A, Sakai S,
Noguchi R and Hirata T: Isolation of sphingoid bases of sea
cucumber cerebrosides and their cytotoxicity against human colon
cancer cells. Biosci Biotechnol Biochem. 70:2906–2912. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yun SH, Park ES, Shin SW, Na YW, Han JY,
Jeong JS, Shastina VV, Stonik VA, Park JI and Kwak JY: Stichoposide
C induces apoptosis through the generation of ceramide in leukemia
and colorectal cancer cells and shows in vivo antitumor activity.
Clin Cancer Res. 18:5934–5948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nag S, Qin J, Srivenugopal KS, Wang M and
Zhang R: The MDM2-p53 pathway revisited. J Biomed Res. 27:254–271.
2013.PubMed/NCBI
|
|
12
|
Bally C, Adès L, Renneville A, Sebert M,
Eclache V, Preudhomme C, Mozziconacci MJ, de The H, Lehmann-Che J
and Fenaux P: Prognostic value of TP53 gene mutations in
myelodysplastic syndromes and acute myeloid leukemia treated with
azacitidine. Leuk Res. 38:751–755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Zhao H, Chen H and Yao Q: CXCR4 in
breast cancer: Oncogenic role and therapeutic targeting. Drug Des
Devel Ther. 9:4953–4964. 2015.PubMed/NCBI
|
|
14
|
Kremer KN, Peterson KL, Schneider PA, Meng
XW, Dai H, Hess AD, Smith BD, Rodriguez-Ramirez C, Karp JE,
Kaufmann SH and Hedin KE: CXCR4 chemokine receptor signaling
induces apoptosis in acute myeloid leukemia cells via regulation of
the Bcl-2 family members Bcl-XL, Noxa, and Bak. J Biol Chem.
288:22899–22914. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng SB, Zhang X, Paul D, Kays LM, Ye M,
Vaillancourt P, Dowless M, Stancato LF, Stewart J, Uhlik MT, et al:
Inhibition of CXCR4 by LY2624587, a fully humanized anti-CXCR4
antibody induces apoptosis of hematologic malignancies. PLoS One.
11:e1505852016.
|
|
16
|
Zheng T, Chou J, Zhang F, Liu Y, Ni H, Li
X, Zheng L, Tang T, Jin L and Xi T: CXCR4 3′UTR functions as a
ceRNA in promoting metastasis, proliferation and survival of MCF-7
cells by regulating miR-146a activity. Eur J Cell Biol. 94:458–469.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Narumi T, Tanaka T, Hashimoto C, Nomura W,
Aikawa H, Sohma A, Itotani K, Kawamata M, Murakami T, Yamamoto N
and Tamamura H: Pharmacophore-based small molecule CXCR4 ligands.
Bioorg Med Chem Lett. 22:4169–4172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fontanella R, Pelagalli A, Nardelli A,
D'Alterio C, Ieranò C, Cerchia L, Lucarelli E, Scala S and Zannetti
A: A novel antagonist of CXCR4 prevents bone marrow-derived
mesenchymal stem cell-mediated osteosarcoma and hepatocellular
carcinoma cell migration and invasion. Cancer Lett. 370:100–107.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang K, Li J, Yin J, Ma Q, Yan B, Zhang
X, Wang L, Wang L, Liu T, Zhang Y, et al: Targeted delivery of
CXCR4-siRNA by scFv for HER2(+) breast cancer therapy. Biomaterial.
59:77–87. 2015. View Article : Google Scholar
|
|
20
|
Dassault Systèmes BIOVIA, Discovery studio
modeling environment, Version 4.5. San Diego: Dassault Systèmes;
2015
|
|
21
|
Liu T, Lin Y, Wen X, Jorissen RN and
Gilson MK: BindingDB: A web-accessible database of experimentally
determined protein-ligand binding affinities. Nucleic Acids Res.
35:D198–D201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dallakyan S and Olson AJ: Small-molecule
library screening by docking with PyRx. Methods Mol Biol.
1263:243–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.PubMed/NCBI
|
|
24
|
Widodo, Wisnasari S, Saifur Rohman M, et
al: Alu insertion/deletion of ACE gene polymorphism might not
affect significantly the serum bradykinin level in hypertensive
patients taking ACE inhibitors. Egypt J Med Hum Genet. 18:187–191.
2017. View Article : Google Scholar
|
|
25
|
Chatr-Aryamontri A, Oughtred R, Boucher L,
Rust J, Chang C, Kolas NK, O'Donnell L, Oster S, Theesfeld C,
Sellam A, et al: The BioGRID interaction database: 2017 update.
Nucleic Acids Res. 45:D369–D379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein–protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:D109–D114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang MW, Lindstrom W, Olson AJ and Belew
RK: Analysis of HIV wild-type and mutant structures via in silico
docking against diverse ligand libraries. J Chem Inf Model.
47:1258–1262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shityakov S and Förster C: In silico
predictive model to determine vector-mediated transport properties
for the blood-brain barrier choline transporter. Adv Appl
Bioinforma Chem. 7:23–36. 2014.
|
|
31
|
Groner B and von Manstein V: Jak Stat
signaling and cancer: Opportunities, benefits and side effects of
targeted inhibition. Mol Cell Endocrinol. 451:1–14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Zeng SX and Lu H: Targeting
p53-MDM2-MDMX loop for cancer therapy. Subcell Biochem. 85:281–319.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bordbar S, Anwar F and Saari N: High-Value
components and bioactives from sea cucumbers for functional Foods-A
review. Mar Drugs. 9:1761–1805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi WT, Yang Y, Xu Y and An J: Targeting
chemokine receptor CXCR4 for treatment of HIV-1 infection, tumor
progression, and metastasis. Curr Top Med Chem. 14:1574–1589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Curnock AP, Logan MK and Ward SG:
Chemokine signalling: Pivoting around multiple phosphoinositide
3-kinases. Immunology. 105:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|