Introduction
As the second most common type of neurodegenerative
disorder, Parkinson's disease (PD) is characterized by evident
motor symptoms including rigidity, bradykinesia, resting tremor and
postural instability (1). These
symptoms are mainly due to the selective dopaminergic neuron loss
within basal ganglia structures (2).
Previous studies indicated that apoptosis, a
specific type of programmed cell death, contributed to the loss of
dopaminergic neurons during PD progression (3,4). In
mammalian cells, apoptosis is closely modulated by two different
signaling pathways including the receptor-dependent apoptotic
pathway and the mitochondria-mediated apoptotic pathway (5). Previously, the authors of the present
study demonstrated that apoptosis of dopaminergic neurons was
primarily achieved via the mitochondria-mediated apoptotic pathway
(6), however, the precise molecular
mechanisms remain to be elucidated.
A member of the family of GTPases, mitofusin-2
(MFN2), is widely distributed in numerous tissues including heart,
kidney, liver and brain (7). At the
subcellular level, MFN2 is located predominantly in the outer
membrane of the mitochondria, and is required for mitochondrial
fusion, integrity and metabolism (8,9).
Previous reports suggested that MFN2 was involved in the process of
mitochondria-mediated apoptosis and, therefore, contributed to the
pathogenesis of several neurological disorders including
Charcot-Marie-Tooth disease (CMT), ischemic stroke and Alzheimer's
disease (10–12). However, the role of MFN2 in the
apoptosis of dopaminergic neurons during the progression of PD
remains unclear.
The present study therefore aimed to assess whether
MFN2 participates in dopaminergic neuronal apoptosis using a
cellular model of PD induced by rotenone (13).
Materials and methods
Cell culture
A human neuroblastoma cell line SH-SY5Y was
purchased from American Type Culture Collection (Manassas, VA, USA;
stock no. CRL-2266). Cells were cultured in RPMI 1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented
with 10% fetal calf serum (Thermo Fisher Scientific, Inc.)
containing 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C, as previously described (13).
Lentiviral particles and cell
transduction
For MFN2 knockdown and overexpression, lentiviral
particles containing MFN2 short hairpin (sh) RNA (cat. no.
sc-43928-V), lentiviral particles encoding human wild-type MFN2
cDNA (sc-400536-LAC) and their control lentiviral particles (cat.
nos. sc-108080 and sc-437282, respectively) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The cell
transduction was performed as previously described (14). The efficiency of transduction was
determined by western blotting 72 h later as described below.
Rotenone treatment
SH-SY5Y cells were treated with vehicle (0.01% DMSO)
and rotenone (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 20,
100 or 500 nM dissolved in 0.01% DMSO) for 12 h, as previously
described (15). Subsequently, the
cells were collected and used for the experiments described
below.
Cell viability assay
The viability of SH-SY5Y cells was assessed by the
MTT method, as previously described (4). The SH-SY5Y cells were seeded at a
density of 1.5×105 cells/cm2 and received the
indicated treatment. Subsequently, MTT was added to the culture
medium to reach a final concentration of 0.5 mg/ml. After
incubation at 37° for 4 h, the culture medium containing MTT was
removed. DMSO was added into each well and the absorbance was
measured at a wavelength of 490 nm using a microplate reader.
Colorimetric assay for caspase-3
activity
A colorimetric assay was carried out as previously
described (3). The SH-SY5Y cells
were seeded at a density of 1.5×105
cells/cm2. After receiving the indicated treatment, the
cells were harvested and lysed in an extraction buffer (included in
the caspase-3 colorimetric assay kit mentioned below). The activity
of caspase-3 was detected by a colorimetric assay kit (cat. no.
ab39401; Abcam, Cambridge, UK) according to the manufacturer's
protocol.
Western blotting
Western blotting was carried out as previously
described (16). The SH-SY5Y cells
were seeded at a density of 1.5×105 cells/cm2
and received the indicated treatment. Subsequently, the cells were
harvested and lysed in an extraction buffer containing complete
protease inhibitor cocktail. Samples with equal amounts of protein
were separated via SDS-PAGE on a 10% gel, transferred to
nitrocellulose membranes and blocked with 5% bovine serum albumin
(Thermo Fisher Scientific, Inc.). Following washing, membranes were
incubated with a primary antibody against MFN2 (cat. no. ab101055;
1:500; Abcam) at 4°C overnight, washed again and incubated with a
horseradish peroxidase (HRP)-conjugated secondary antibody (cat.
no. A0208; 1:1,500; Beyotime Institute of Biotechnology, Haimen,
China) for 2 h. Following washing, protein bands were detected with
a chemiluminescent HRP substrate and exposed to an X-ray film. The
signal intensity of primary antibody binding was analyzed using
Quantity One software 4.6.2 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and normalized to a loading control GAPDH (cat. no.
sc-47724; 1:1,000; Santa Cruz Biotechnology, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The SH-SY5Y cells were seeded at a density of
1.5×105 cells/cm2 and received the indicated
treatment. Subsequently, total RNA was extracted from SH-SY5Y cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Equal amounts of total RNA were reverse transcribed in a final
volume of 10 µl with random primers under standard conditions
(described in the manufacturer's protocol) using the
PrimeScript® RT Master Mix (Takara Biotechnology Co.,
Ltd., Dalian, China). Reverse transcription reaction was carried
out as previously described (17).
Subsequently, qPCR reactions were performed with SYBR®
Premix Ex Taq™ (Takara Biotechnology Co., Ltd.) and a specific
primer (forward: 5′-TGGCTCAAGACTATAAGCTGCG-3′, reverse:
5′-GAGGACTACTGGAGAAGGGTGG-3′) to detect MFN2 mRNA expression
levels. The thermo cycling conditions were the same as previously
described (17). GAPDH (forward:
5′-AAGGTGAAGGTCGGAGTCAAC-3′, reverse: 5′-GGGGTCATTGATGGCAACAATA-3′)
was used as an internal control. Relative levels were determined
using the 2−ΔΔCq method (18).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least four independent experiments. Statistical significance
was detected by one-way analysis of variance followed by Turkey's
post hoc test using SPSS software 18.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Apoptosis contributes to the decrease
in viability of SH-SY5Y cells induced by rotenone
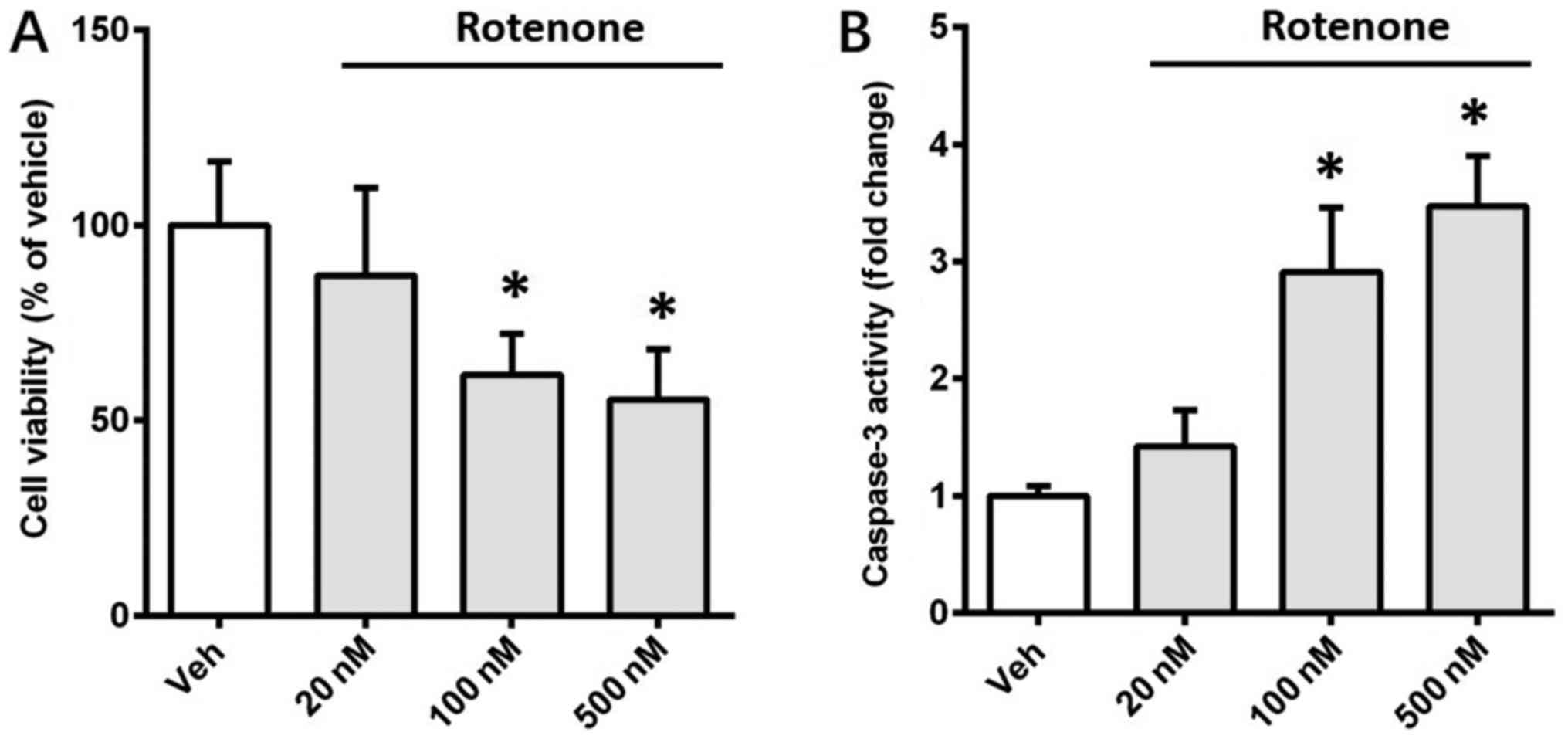
The effects of rotenone on the viability of SH-SY5Y
cells were assessed in the present study. Cells were incubated with
different doses of rotenone (20, 100 and 500 nM), and MTT assay was
used to evaluate cell viability after 12 h. Rotenone (100 and 500
nM) significantly reduced the viability of SH-SY5Y cells by 38.3%
(P<0.05) and 45.6% (P<0.05), respectively (Fig. 1A). This result implied that rotenone
induced cell death in a dose-dependent manner. To investigate
whether apoptosis contributed to the rotenone-induced cell death,
activity of caspase-3, a crucial executioner of apoptosis, was
detected using a colorimetric assay kit. Rotenone (100 and 500 nM)
significantly increased caspase-3 activity 2.91-fold (P<0.05)
and 3.47-fold (P<0.05), respectively (Fig. 1B). These observations indicated that
apoptosis contributed to the death of SH-SY5Y cells induced by
rotenone.
Expression of MFN2 in SH-SY5Y cells is
stable following treatment with rotenone
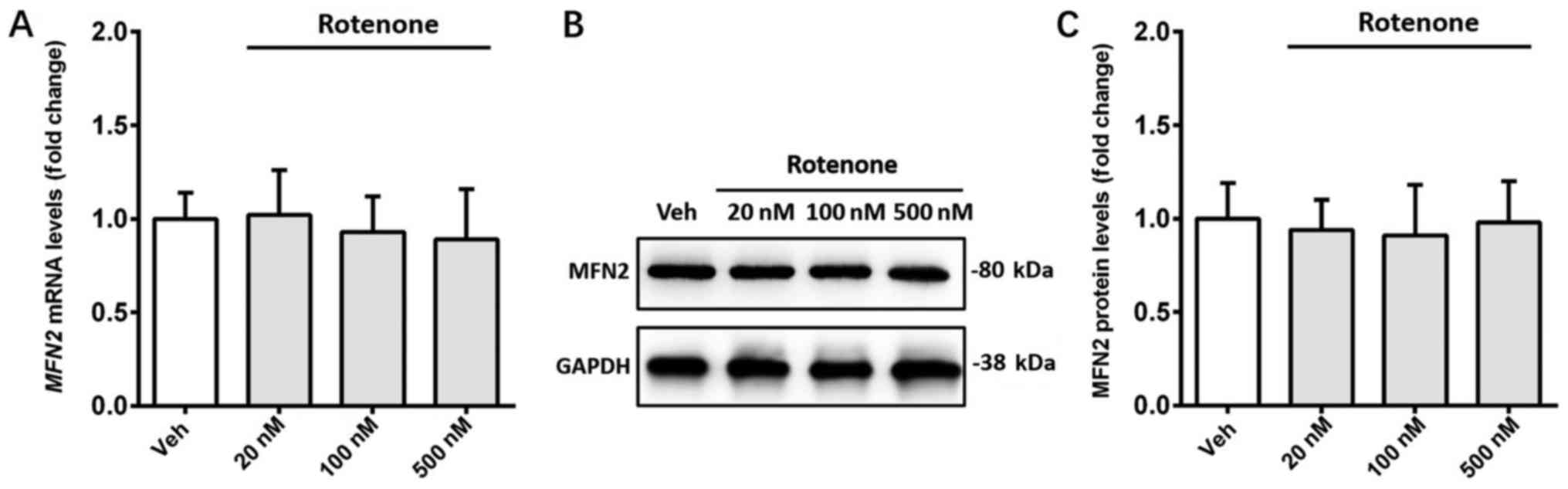
The effect of rotenone on the expression of MFN2 was
detected in SH-SY5Y cells. Treatment with rotenone (20, 100 and 500
nM) did not significantly affect the mRNA and protein expression
levels of MFN2 compared with the vehicle control (Fig. 2A-C).
Knockdown of MFN2 exacerbates
apoptosis induced by rotenone in SH-SY5Y cells
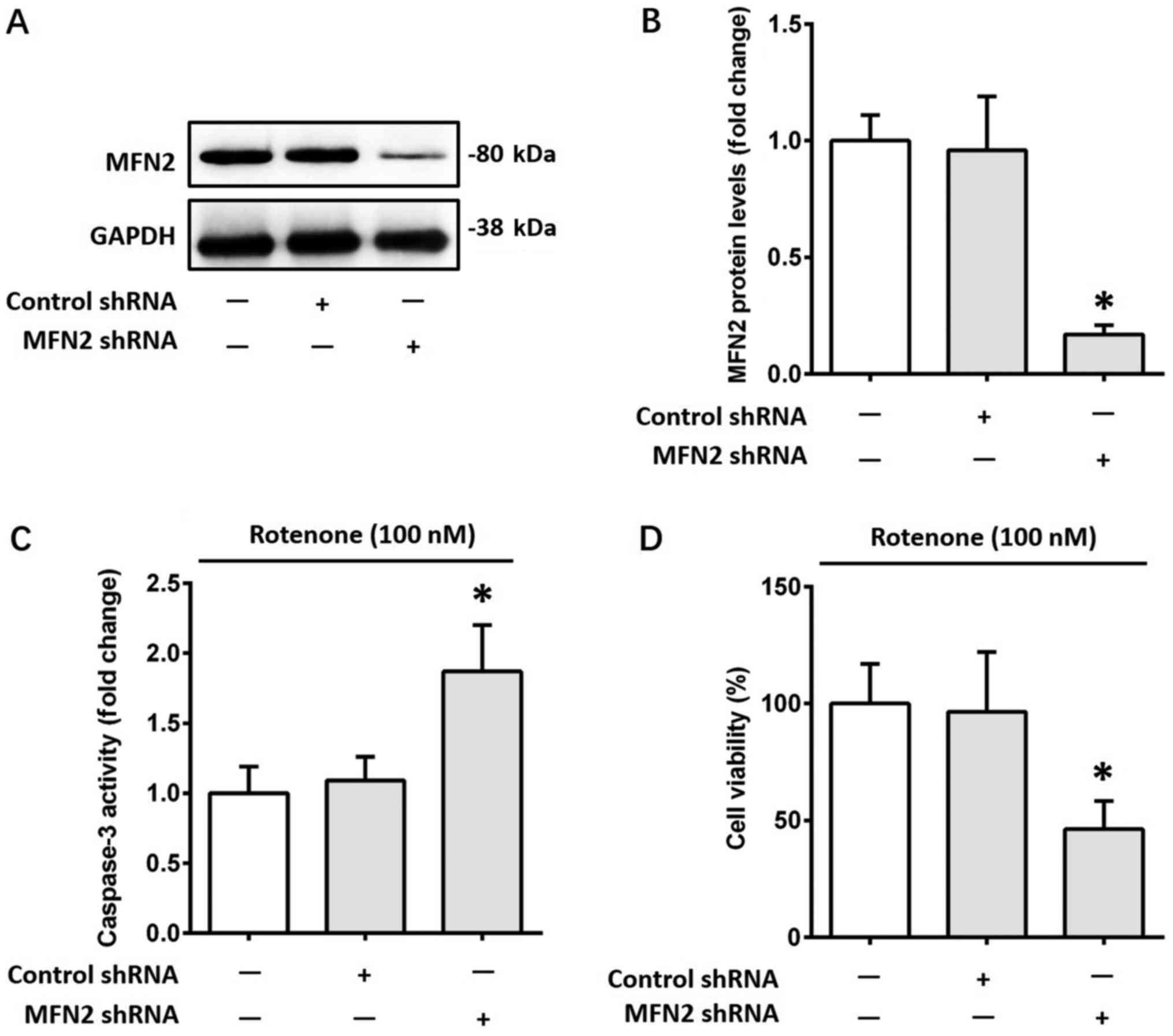
To further investigate the role of MFN2 in
rotenone-induced apoptosis, a lentiviral transduction strategy was
used to knock down MFN2 expression in SH-SY5Y cells. The
alterations in MFN2 protein expression levels were confirmed by
western blotting (Fig. 3A and B).
Subsequently, cells were incubated with 100 nM rotenone for 12 h.
Knockdown of MFN2 further increased the activity of caspase-3
1.72-fold (P<0.05) in SH-SY5Y cells following treatment with
rotenone, compared with the control shRNA group (Fig. 3C). Furthermore, MFN2 knockdown
further reduced the viability of SH-SY5Y cells by 52% (P<0.05)
following treatment with rotenone, compared with the control shRNA
group (Fig. 3D). Knockdown of MFN2
did not significantly affect the viability of SH-SY5Y cells
untreated with rotenone (data not shown). Taken together, the above
results indicated that MFN2 knockdown exacerbated cell apoptosis
caused by rotenone, suggesting that MFN2 may serve a protective
role against rotenone-induced cell apoptosis.
Overexpression of MFN2 ameliorates
apoptosis induced by rotenone in SH-SY5Y cells
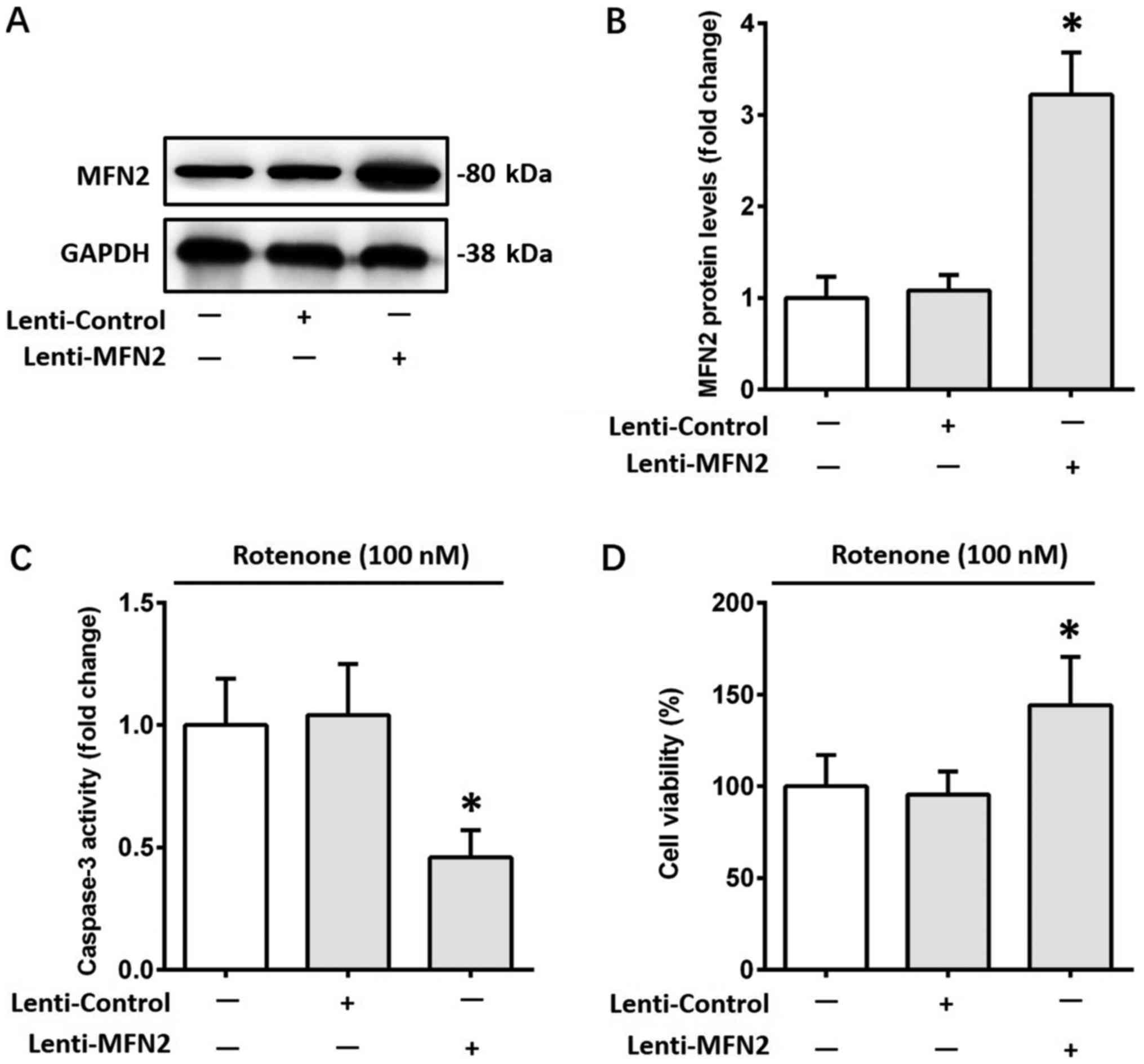
To validate the protective role of MFN2 against
rotenone-induced cell apoptosis, MFN2 was overexpressed in SH-SY5Y
cells using a lentiviral strategy. The alterations in MFN2 protein
levels were confirmed by western blotting (Fig. 4A and B). Subsequently, cells were
incubated with 100 nM rotenone for 12 h. The increase in caspase-3
activity induced by rotenone was attenuated by MFN2 overexpression
(P<0.05; Fig. 4C). Furthermore,
overexpression of MFN2 ameliorated the rotenone-induced reduction
in cell viability (P<0.05; Fig.
4D). MFN2 overexpression did not significantly affect the
viability of SH-SY5Y cells without rotenone treatment (data not
shown).
Discussion
Rotenone, a mitochondrial complex I inhibitor, was
reported to reproduce a number of neuropathological features of PD,
including loss of dopaminergic neurons (19). In the present study, rotenone
resulted in decreased viability of human neuroblastoma SH-SY5Y
cells, in a dose-dependent manner. Furthermore, expression levels
of caspase-3, a crucial executioner of apoptosis, were markedly
increased following treatment with rotenone, suggesting that
apoptosis contributed to the loss of SH-SY5Y cells. These results
are in agreement with previous reports by the authors of the
present study, where rotenone induced apoptosis in human and mouse
dopaminergic cell lines (13,15). It
has been previously demonstrated that apoptosis is closely
modulated by two pathways: The receptor-dependent apoptotic pathway
and the mitochondria-mediated apoptotic pathway (5). Recently, the authors of the present
study suggested that apoptosis of dopaminergic neurons was
primarily achieved via the mitochondria-mediated apoptotic pathway,
since cytochrome c, a mitochondrial pro-apoptotic factor, was
observed in the cytoplasm of dopaminergic neurons (6). However, the underlying molecular
mechanisms remain unclear.
MFN2, a mitochondrial protein that belongs to the
family of GTPases, is widely distributed in numerous tissues and
organs including heart, kidney, liver, and brain (7). At the subcellular level, MFN2 is
located predominantly in the outer membrane of the mitochondria
(8). A previous study has indicated
that MFN2 was required for several physiological processes of
mitochondria including fusion and metabolism (9). Furthermore, several lines of evidence
indicated that MFN2 may be involved in the process of
mitochondria-mediated apoptosis and participated in the
pathogenesis of several neurological disorders (10–12).
Loss-of-function mutations of MFN2 gene led to the onset of CMT
type 2A, a neurological disease characterized by the degeneration
of axons in the peripheral nervous system (20). In an in vitro model of
ischemic stroke, decreased MFN2 expression was closely associated
with increased neuronal apoptosis (21). In addition, reduced MFN2 level was
associated with increased oxidative stress and apoptosis of neurons
during neurodegeneration (22,23). In
the present study, knockdown of MFN2 in a cellular model of PD
induced by rotenone aggravated cell apoptosis. The above results
implied a protective role of MFN2 against apoptosis. To further
validate the antiapoptotic effect of MFN2 in the in vitro
model of PD, MFN2 was overexpressed in SH-SY5Y cells prior to
treatment with rotenone. For the first time to the best of the
authors' knowledge, it was demonstrated that MFN2 ameliorated
apoptosis induced by rotenone. This observation was supported by
previous reports that MFN2 overexpression attenuated neuronal
apoptosis under the conditions of ischemic stroke or
neurodegeneration (21–23), indicating the antiapoptotic effect of
MFN2.
The present study has certain limitations. The
antiapoptotic effect of MFN2 was evaluated in a cellular model of
PD. Therefore, the results require further confirmation in
vivo using animal models of PD. Additionally, the precise
signaling underlying the antiapoptotic effect of MFN2 was not
identified in the present study and this should be investigated in
the future.
In conclusion, the present study indicated that the
expression of MFN2 was stable following treatment with rotenone in
a cellular model of PD. Using a lentiviral knockdown and
overexpression strategy, it was demonstrated that MFN2 prevented
rotenone-induced cell death by amelioration of apoptosis. These
results revealed a protective role of MFN2 against apoptosis in an
in vitro model of PD and may be used to establish MFN2 as a
potential therapeutic target for the treatment of this disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81771140), Natural Science
Foundation of Jiangsu Province (grant no. BK20151084), Key Research
and Development Project of Jiangsu Province (grant no. BL2014014),
‘Six Talent Summit’ Foundation of Jiangsu Province (grant no.
2016-WSN-180), Youth Medical Talent Program of Jiangsu Province
(grant no. QNRC2016068), Medical Innovation Team of Jiangsu
Province (grant no. CXTDA2017030), and Nanjing Medical Science and
Technology Development Foundation for Distinguished Young Scholars
(grant no. JQX17008).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and TJ designed the present study. YY and LX
performed the experiments. ZO and XX analyzed the data and prepared
all figures. TJ wrote the manuscript. All authors have read and
approved this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tysnes OB and Storstein A: Epidemiology of
Parkinson's disease. J Neural Transm (Vienna). 124:901–905. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schapira AH: Neurobiology and treatment of
Parkinson's disease. Trends Pharmacol Sci. 30:41–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao Q, Jiang T, Zhao HR, Wu L, Tian YY, Ou
Z, Zhang L, Pan Y, Lu J and Zhang YD: Activation of autophagy
contributes to the Angiotensin II-triggered apoptosis in a
dopaminergic neuronal cell line. Mol Neurobiol. 53:2911–2919. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao HR, Jiang T, Tian YY, Gao Q, Li Z,
Pan Y, Wu L, Lu J and Zhang YD: Angiotensin II triggers apoptosis
via enhancement of NADPH Oxidase-dependent oxidative stress in a
dopaminergic neuronal cell line. Neurochem Res. 40:854–863. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:pii: a0060802015.
View Article : Google Scholar
|
|
6
|
Ou Z, Jiang T, Gao Q, Tian YY, Zhou JS, Wu
L, Shi JQ and Zhang YD: Mitochondrial-dependent mechanisms are
involved in angiotensin II-induced apoptosis in dopaminergic
neurons. J Renin Angiotensin Aldosterone Syst. 17:pii2016.
View Article : Google Scholar
|
|
7
|
Schrepfer E and Scorrano L: Mitofusins,
from mitochondria to metabolism. Mol Cell. 61:683–694. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Brito OM and Scorrano L: Mitofusin 2: A
mitochondria-shaping protein with signaling roles beyond fusion.
Antioxid Redox Signal. 10:621–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zorzano A, Hernández-Alvarez MI, Sebastián
D and Muñoz JP: Mitofusin 2 as a driver that controls energy
metabolism and insulin signaling. Antioxid Redox Signal.
22:1020–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stuppia G, Rizzo F, Riboldi G, Del Bo R,
Nizzardo M, Simone C, Comi GP, Bresolin N and Corti S: MFN2-related
neuropathies: Clinical features, molecular pathogenesis and
therapeutic perspectives. J Neurol Sci. 356:7–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Yi C, Li X, Wang J, Zhou F and Chen
X: Overexpression of Mitofusin2 decreased the reactive astrocytes
proliferation in vitro induced by oxygen-glucose
deprivation/reoxygenation. Neurosc Lett. 639:68–73. 2017.
View Article : Google Scholar
|
|
12
|
Wang X, Su B, Lee HG, Li X, Perry G, Smith
MA and Zhu X: Impaired balance of mitochondrial fission and fusion
in Alzheimer's disease. J Neurosci. 29:9090–9103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu L, Luo N, Zhao HR, Gao Q, Lu J, Pan Y,
Shi JP, Tian YY and Zhang YD: Salubrinal protects against
rotenone-induced SH-SY5Y cell death via ATF4-parkin pathway. Brain
Res. 1549:52–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L,
Tan MS, Gu LZ, Wang HF, Ding ZZ, Zhang YD and Yu JT: Upregulation
of TREM2 ameliorates neuropathology and rescues spatial cognitive
impairment in a transgenic mouse model of Alzheimer's disease.
Neuropsychopharmacology. 39:2949–2962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Wu L, Jiang T, Wang Y, Zhao H, Gao
Q, Pan Y, Tian Y and Zhang Y: Angiotensin AT2 receptor stimulation
inhibits activation of NADPH oxidase and ameliorates oxidative
stress in rotenone model of Parkinson's disease in CATH.a cells.
Neurotoxicol Teratol. 47:16–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang T, Zhang YD, Chen Q, Gao Q, Zhu XC,
Zhou JS, Shi JQ, Lu H, Tan L and Yu JT: TREM2 modifies microglial
phenotype and provides neuroprotection in P301S tau transgenic
mice. Neuropharmacology. 105:196–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang T, Zhang YD, Gao Q, Zhou JS, Zhu XC,
Lu H, Shi JQ, Tan L, Chen Q and Yu JT: TREM1 facilitates microglial
phagocytosis of amyloid beta. Acta Neuropathol. 132:667–683. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson ME and Bobrovskaya L: An update on
the rotenone models of Parkinson's disease: Their ability to
reproduce the features of clinical disease and model
gene-environment interactions. Neurotoxicology. 46:101–116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bombelli F, Stojkovic T, Dubourg O,
Echaniz-Laguna A, Tardieu S, Larcher K, Amati-Bonneau P, Latour P,
Vignal O, Cazeneuve C, et al: Charcot-Marie-Tooth disease type 2A:
From typical to rare phenotypic and genotypic features. JAMA
Neurol. 71:1036–1042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng C, Rao W, Zhang L, Wang K, Hui H,
Wang L, Su N, Luo P, Hao YL, Tu Y, et al: Mitofusin 2 ameliorates
hypoxia-induced apoptosis via mitochondrial function and signaling
pathways. Int J Biochem Cell Biol. 69:29–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park J, Choi H, Min JS, Kim B, Lee SR, Yun
JW, Choi MS, Chang KT and Lee DS: Loss of mitofusin 2 links
beta-amyloid-mediated mitochondrial fragmentation and Cdk5-induced
oxidative stress in neuron cells. J Neurochem. 132:687–702. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao F, Wang W, Wang C, Siedlak SL,
Fujioka H, Tang B and Zhu X: Mfn2 protects dopaminergic neurons
exposed to paraquat both in vitro and in vivo: Implications for
idiopathic Parkinson's disease. Biochim Biophy Acta.
1863:1359–1370. 2017. View Article : Google Scholar
|