Introduction
Gestational hypertension is a disease that occurs
during pregnancy and leads to symptoms including hypertension,
preeclampsia, eclampsia and chronic hypertension (1,2). This
disease adversely affects maternal and fetal health (3). Gestational hypertension is one of the
leading causes of maternal, fetal and neonatal morbidity and
mortality (4,5). A previous report has suggested that the
incidence rate of gestational hypertension is 7–12% in China, which
is higher than the worldwide average (3.2–5.0%) (6). It has been demonstrated that clinically
assisted reproductive technology treatment may increase the
incidence of gestational hypertension and preeclampsia (7). In addition, there are a number of
factors that induce gestational hypertension and have been
identified to serve a role in the etiology of these hypertensive
disorders, including work stress, depression and anxiety (8,9).
Furthermore, reports have indicated that vasodilation-converting
enzyme and α-adrenergic receptor serve a crucial role in the
process of gestational hypertension and may be potential targets
for the treatment of patients with gestational hypertension in the
clinic (10–12).
Antihypertensive drugs are typically used in
clinical treatment for remission of patients with hypertension
during the gestation period (13).
Notably, the mechanisms of formation of gestational arterial
hypertension for women during pregnancy have previously been
investigated and results have indicated that
vasodilation-converting enzyme contributes to the progression of
gestational hypertension (14).
Therefore, the angiotensin-converting enzyme inhibitor was
investigated and the efficacy for hypertension in the clinic has
been studied. Tovar-Rodriguez et al (12) have previously investigated a
combination therapy with an angiotensin-converting enzyme inhibitor
and a diuretic that was highly effective in hypertension.
Furthermore, the influence of the treatment of
angiotensin-converting enzyme inhibitor on baroreflex sensitivity
and flow-mediated vasodilation of the brachial artery has also been
demonstrated previously in essential hypertension (15). Captopril is an antihypertensive agent
that targets angiotensin converting enzyme for the treatment of
gestational hypertension (16). In
the present study, the efficacy of captopril for the treatment of
gestational hypertension, compared with prazosin, was
investigated.
Prazosin is an α-adrenergic receptor inhibitor,
which is used for the treatment of hypertension and heart failure
(17). It has previously been
revealed that a higher expression of α-adrenergic receptor is
associated with the aggravation of gestational hypertension and
α-adrenergic receptor blockers are beneficial for patients with
hypertension (18). The present
study further analyzed the therapeutic effects of prazosin on
patients with gestational hypertension compared with captopril.
Although the effects of prazosin on patients with diabetic
nephropathy may induce positive α-adrenergic receptor
autoantibodies, the efficacy of prazosin is evident on the
improvement of gestational hypertension (19). Furthermore, it was observed that
prazosin as an α-adrenergic receptor blocker, significantly reduced
hypertension, heart failure and symptoms induced by gestational
hypertension.
The aim of the present clinical study was to
evaluate the combined treatment of captopril and prazosin for
patients with gestational hypertension in a relatively large sample
of pregnant outpatients (n=324) who had undergone gestational
hypertension. The present study also investigated the potential
molecular mechanism of captopril and prazosin in the processes of
gestational hypertension. The results indicate that combination
treatment with captopril and prazosin is more efficient for
gestational hypertension than treatment with either captopril or
prazosin. These clinical outcomes indicate that a therapeutic
regimen of captopril and prazosin may be a potential therapeutic
option for patients with gestational hypertension.
Materials and methods
Ethics statement
The current phase-II study (approval no.
HMCH20090236-A4) was performed between February 2009 and August
2011 in accordance with the recommendations in the Guide for
Haidian Maternal and Child Healthcare. The present study was
approved by the Ethics Committee of Haidian Maternal and Child
Health Care Center (Beijing, China). All patients were required to
review trial protocols and amendments and to provide written
informed consent.
Patients
A total of 324 patients with gestational
hypertension (aged 22–42 years old) were randomly divided into four
groups (Captopril, n=92; Prazosin, n=88; Combination, n=94;
Placebo, n=50) and double-blind trails were conducted once daily in
the Haidian Maternal and Child Healthcare unit. Patients were
recruited between February 2009 and August 2011. A detailed
description of the inclusion/exclusion criteria, allocation method
and other details were provided in previously published studies
(20,21). All patients were divided into four
groups following the principle of random double-blind and control
experiments. Patients with gestational hypertension received
treatment with captopril (n=92) and prazosin (n=88), combination
therapy of captopril and prazosin (n=94) or placebo (n=50).
Patients with gestational hypertension received captopril (0.5
mg/kg, n=5; 1.0 mg/kg, n=5; 1.5 mg/kg, n=6; 2.0 mg/kg, n=6; 2.5
mg/kg, n=10; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
prazosin (0.02 mg/kg, n=5; 0.06 mg/kg, n=5; 0.10 mg/kg, n=7; 0.14
mg/kg, n=8; 0.18 mg/kg, n=7; Sigma-Aldrich), combined treatment
(Captopril: 0.06 mg/kg and prazosin, 1.0 mg/kg) or a placebo
(starch; Sigma-Aldrich; 1.0 mg/kg) that were administered orally.
Patient trials were performed in a comfortable room.
Study design
The present double-blind study was performed in
three phases: i) The baseline phase (consisting of 1-week of
dose-titration treatment); ii) the double-blind treatment phase
(consisting of 2-week dose-titration treatment); and iii) the
2-week post-treatment phase for patients with gestational
hypertension who volunteered to continue to complete the study. A
total of 39 patients ceased participation between phases II and III
due to adverse side effects, including headache, angioedema, rash,
pruritus, amblygeustia, palpitation, fever and tachycardia.
Patients with gestational hypertension were randomized to undergo
the double-blind treatment with captopril, prazosin, combined
treatment or placebo, which were administered once daily. Patients
with gestational hypertension continued to receive treatment with
0.06 mg/kg captopril, 1.0 mg/kg prazosin, combined therapy
(Captopril: 0.06 mg/kg and Prazosin, 1.0 mg/kg) or placebo to
achieve the final investigation throughout the post-treatment
phase.
ELISA
Blood samples were collected from patients following
4-weeks treatment. Serum was separated using centrifugation at
6,000 × g at 4°C for 15 min. Commercial ELISA kits were used to
measure plasma concentration levels of vasodilation-converting
enzyme using a BACE1 ELISA kit (cat. no. MA1-744; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and an α-adrenergic receptor
ELISA kit (cat. no. PA5-32660; Thermo Fisher Scientific, Inc.) in
patients with gestational hypertension, following the
manufacturer's protocol. Final results were recorded at 450 nm on
an ELISA plate reader.
Activity of angiotensin
vasodilation-converting enzyme
Activity of vasodilation-converting enzyme was
analyzed in patients following treatment. The activity of
vasodilation-converting enzyme was conducted according to a
previous report (22). The serum
activity of angiotensin converting enzyme was determined using the
spectrophotometric method using the synthetic substrate Hip-Gly-Gly
(Thermo Fisher Scientific, Inc.) (23).
Outcome measures
An automated sphygmomanometer was used to assess the
function of patients with gestational hypertension. The
artery-to-vein ratio was used to analyze the efficacy of captopril,
prazosin and combination treatment for patients with gestational
hypertension. Clinical gestational hypertension parameters were
evaluated as described in a previous study (24). The data of gestational hypertension
was recorded and the degree of hypertension was calculated.
Efficacy and safety assessments
Efficacy assessments, including the median
percentage reduction scores and response rate were analyzed in
patients with gestational hypertension from the baseline and
double-blind phases in the presence of each group's respective
treatment. In addition, overall safety and pharmacokinetic analysis
were conducted according to previous clinical studies (25,26).
Furthermore, at least one safety assessment of the most frequent
treatment-emergent adverse events was conducted in all randomized
patients following the administration of treatment. A dose-response
analysis was conducted when the last dose of drugs was
administered.
Evaluation of toxicity
The toxicities of captopril and prazosin were
assessed using the National Gestational Hypertension Institute
Common Toxicity Criteria (27). A
biochemical profile measurement of blood pressure and urinalysis
were performed every 2 days during gestational hypertension
treatment periods. Furthermore, electrocardiograms and biochemical
detection were performed every 3 days. Toxicity was defined as any
of the drug-related toxicities defined in a previous study
(28).
Statistical analysis
Data are presented as the mean ± standard error of
the mean, unless otherwise stated. Statistical significance of
differences between mean values was assessed by Student's t-test
for unpaired data. Comparisons of data between multiple groups were
performed using one-way analysis of variance followed by a post-hoc
Turkey honest significant difference test. Continuous variables are
presented as the mean and 95% confidence interval (CI). Treatment
effect is presented as median reduction in knee osteoarthritis over
the treatment period. Robust nonparametric Hodges-Lehmann estimates
of median drugs treatment effects and 95% CI are provided.
Responder rates and treatment-emergent adverse events were analyzed
by χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 324 patients participated in the present
clinical investigation to analyze the efficacy of captopril and
prazosin in the treatment of gestational hypertension. The mean age
of patients with gestational hypertension was 33.6±9.8 years old.
The characteristics of patients with gestational hypertension are
summarized in Table I. In addition,
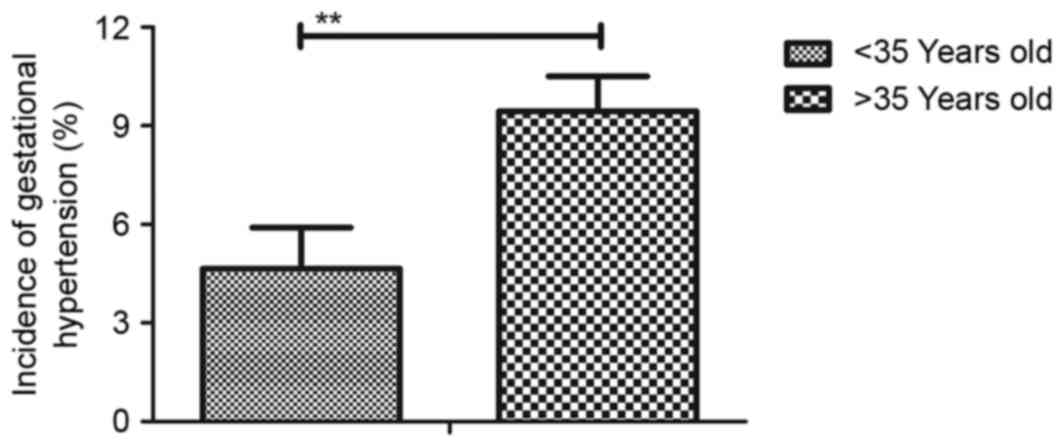
it was revealed that the percentage of patients with gestational
hypertension who are ≥35 years old was significantly higher than
those <35 years old (Fig. 1).
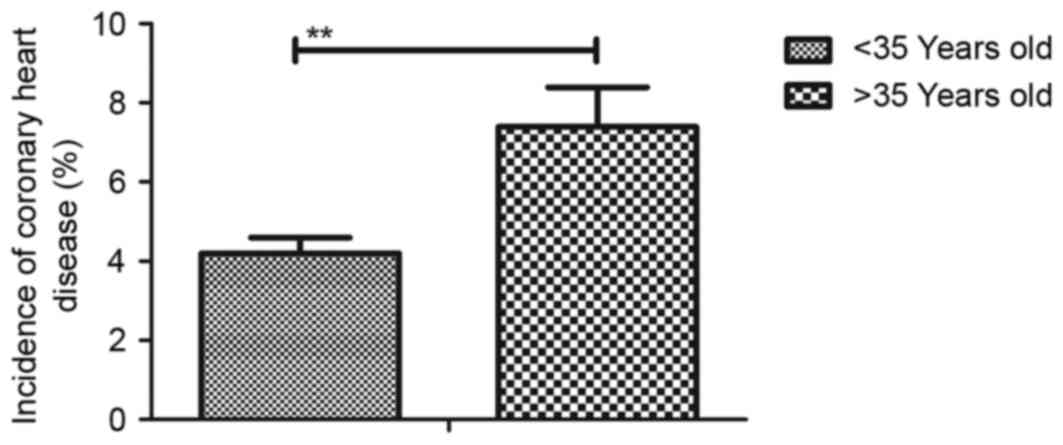
Furthermore, incidence rate of coronary heart disease induced by
gestational hypertension was significantly higher in patients aged
>35 years old (Fig. 2). A total
of 285 patients continued to complete the double-blind treatment
period and the post-treatment phase.
 | Table I.Characteristics of patients with
gestational hypertension. |
Table I.
Characteristics of patients with
gestational hypertension.
|
| Groups |
|---|
|
|
|
|---|
| Variable | Captopril | Captopril | Combination | Placebo |
|---|
| Patients, n
(%) | 92 (28.4) | 88 (27.2) | 94 (29.0) | 50 (15.4) |
| Age, mean ± SD | 29.2±7.2 | 29.5±6.4 | 32.5±8.1 | 35.2±9.2 |
| Blood pressure (mm
Hg) | 144±12 | 139±10 | 154±8 | 148±10 |
| Coronary heart
disease, n (%) | 6 (6.5) | 5 (5.7) | 7 (7.4) | 3 (6.0) |
| Edema
(mm3) |
26.13±14.37 |
17.26±10.68 |
36.24±14.66 | 10.20±4.63 |
| 24 h protein,
mg |
35.45±20.19 |
24.03±14.79 |
52.45±19.50 | 13.49±6.48 |
| Creatinine
clearance, ml/min |
85.45±12.45 |
74.24±14.76 |
82.78±12.68 | 92.34±8.06 |
| Serum urea,
mmol/l |
3.67±0.48 |
3.86±0.73 |
3.65±0.43 |
3.49±0.56 |
Duration of treatment, dose-limiting
toxicities (DLT) and maximum tolerated dose (MTD) of captopril and
prazosin
To analyze the optimal dose of captopril and
prazosin in patients with gestational hypertension, the duration of
treatment, DLT and MTD of captopril and prazosin were identified.
The duration of treatment with captopril and prazosin was 4 weeks
in patients with gestational hypertension. The dosing cohorts were
0.5, 1.0, 1.5, 2.0 and 2.5 mg/kg for captopril and 0.02, 0.06,
0.10, 0.14 and 0.18 mg/kg for prazosin. The doses of 1.5 and 2.0
mg/kg were identified as the MTD and DLT of captopril,
respectively. In addition, 0.10 and 0.14 mg/kg of prazosin were
identified as the MTD and DLT of prazosin, respectively. MTD and
DLT were assessed according to the Common Toxicity Criteria
(version 2.0) of the National Cancer Institute, National Institutes
of Health (29). It was observed
that the lowest-dose cohorts of antihypertensive agents presented
the lowest number of captopril or prazosin dose reductions. Common
treatment-emergent adverse events of captopril were headache,
angioedema, rash, pruritus, amblygeustia, palpitation, fever and
tachycardia (Table II). The common
treatment-emergent adverse events of prazosin were palpitation,
orthostatic hypotension, nausea, tinnitus, vomiting, sleepiness and
diarrhea (Table III). Notably, the
majority of patients with gestational hypertension required a
reduced drug dosage to combat cumulative toxicity following
treatment with the MTD dose (data not shown). Therefore, patients
were administered 1.0 mg/kg captopril and 0.06 mg/kg prazosin to
complete the present study.
 | Table II.Treatment-emergence adverse events of
captopril with an overall incidence ≥10%. |
Table II.
Treatment-emergence adverse events of
captopril with an overall incidence ≥10%.
| Adverse event | Total (n=32) | 0.5–1.0 mg/kg
(n=10) | 1.0–1.5 mg/kg
(n=12) | 2.0 mg/kg
(n=10) |
|---|
| Headache | 4 | 0 | 2 | 2 |
| Angioedema | 4 | 1 | 1 | 2 |
| Rash | 5 | 1 | 2 | 2 |
| Pruritus | 4 | 1 | 1 | 2 |
| Amblygeustia | 4 | 1 | 1 | 2 |
| Palpitation | 4 | 1 | 1 | 2 |
| Fever | 4 | 1 | 1 | 2 |
| Tachycardia | 5 | 1 | 2 | 2 |
 | Table III.Treatment-emergence adverse events of
prazosin with an overall incidence ≥10%. |
Table III.
Treatment-emergence adverse events of
prazosin with an overall incidence ≥10%.
| Adverse event | Total (n=32) | 0.02–0.06 mg/kg
(n=10) | 0.10–0.14 mg/kg
(n=15) | 0.18 mg/kg
(n=7) |
|---|
| Palpitation | 5 | 1 | 2 | 2 |
| Orthostatic
hypotension | 4 | 1 | 1 | 2 |
| Nausea | 6 | 1 | 2 | 3 |
| Tinnitus | 5 | 1 | 2 | 2 |
| Vomiting | 5 | 1 | 1 | 3 |
| Sleepiness | 4 | 1 | 1 | 2 |
| Diarrhea | 4 | 1 | 1 | 2 |
Treatment-emergent adverse events of
comprehensive treatment of captopril and prazosin
The treatment-emergent adverse events of
comprehensive treatment of captopril and prazosin were analyzed in
patients with gestational hypertension. Patients with gestational
hypertension in each group received at least one dose of their
respective group's treatment and a post-baseline safety evaluation,
which included analysis of toxic events. As depicted in Table IV, following the last dose of
captopril and prazosin, the most common treatment-emergent adverse
events in single agent treatment and combination treatment were
rash and palpitation (≥10% each). These side effects were catabolic
and were resolved following termination of the treatment.
 | Table IV.Treatment-emergent adverse events for
hypertension and proteinuria by common toxicity criteria grade. |
Table IV.
Treatment-emergent adverse events for
hypertension and proteinuria by common toxicity criteria grade.
| Adverse event | Total (n=32) | Captopril
(n=10) | Prazosin
(n=10) | Combination
(n=12) |
|---|
| Rash | 9 | 2 | 3 | 4 |
| Grade
1 | 3 | 1 | 1 | 1 |
| Grade
2 | 2 | 0 | 1 | 1 |
| Grade
3 | 4 | 1 | 1 | 2 |
| Palpitation | 10 | 5 | 4 | 2 |
| Grade
1 | 4 | 1 | 1 | 2 |
| Grade
2 | 3 | 1 | 1 | 1 |
| Grade
3 | 3 | 0 | 1 | 2 |
Efficacy of comprehensive treatment of
captopril and prazosin for gestational hypertension
Following analysis of the treatment-emergent adverse
events of comprehensive treatment of captopril and prazosin, the
therapeutic effects of captopril and/or prazosin were examined on
blood pressure, proteinuria, artery-to-vein ratio and edema for
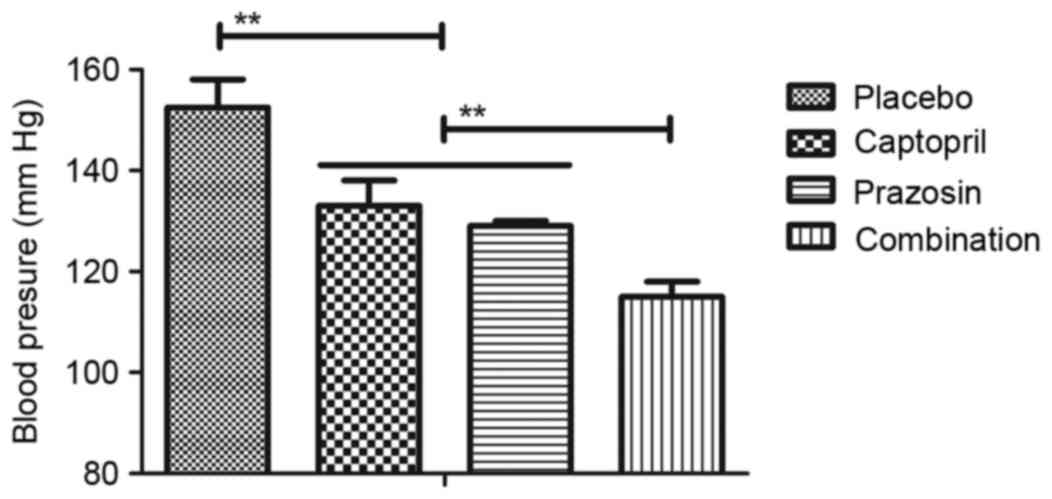
patients with gestational hypertension. As depicted in Fig. 3, it was observed that blood pressure
was significantly improved in patients treated with captopril or
prazosin compared with placebo. However, combination treatment of
captopril and prazosin was significantly more effective compared
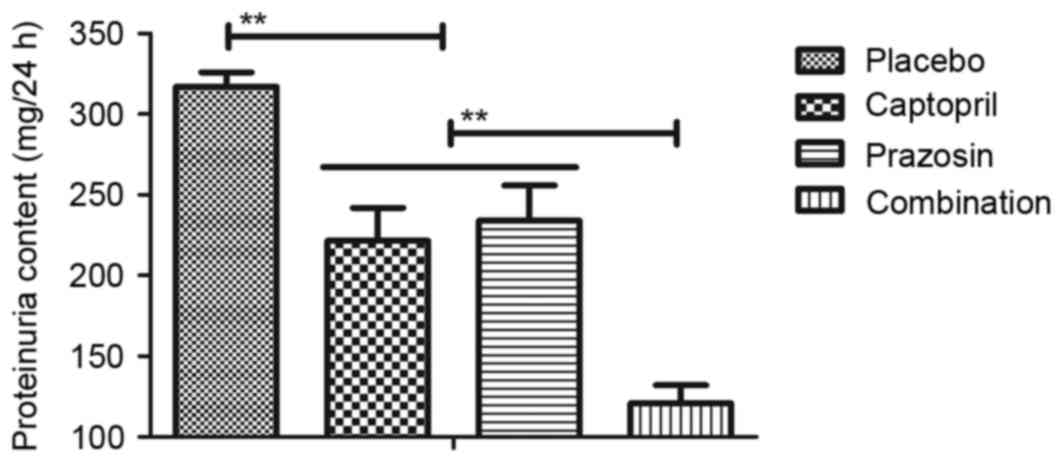
with either captopril or prazosin alone. Similar results were also
observed in proteinuria levels in patients with gestational
hypertension, which recovered to healthy levels (80–120 mm Hg) in
the captopril and/or prazosin groups, compared with placebo
treatment (Fig. 4). In addition, it
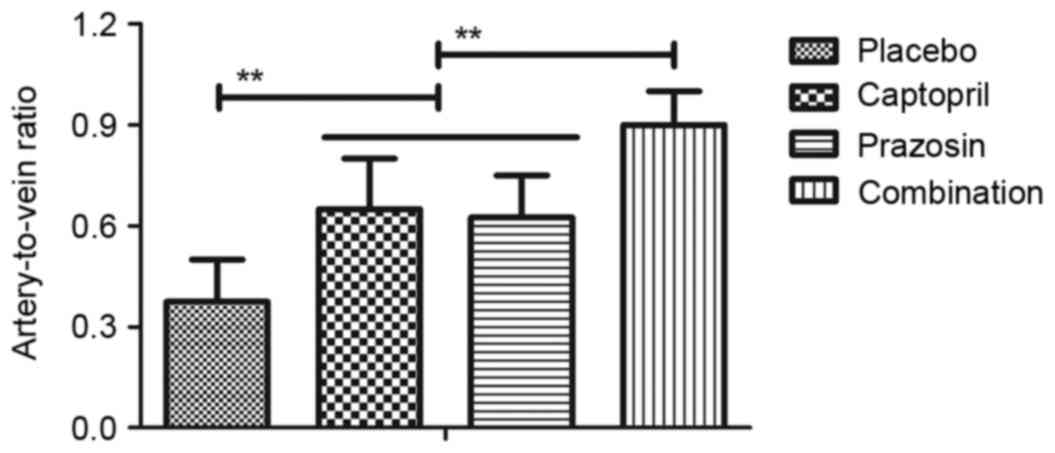
was revealed that the artery-to-vein ratio was significantly
increased by the treatment of captopril or prazosin compared with
placebo and combination treatment induced a significant increase
compared with either single agent (Fig.
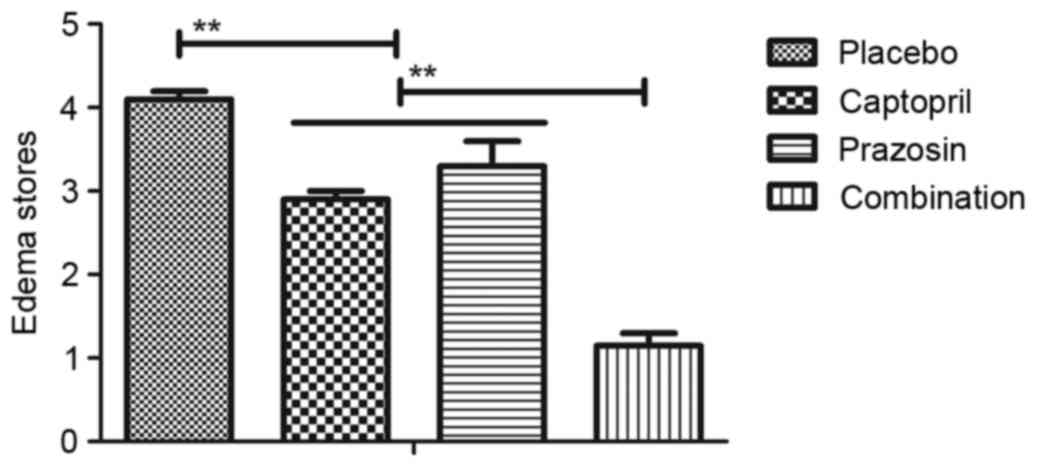
5). Furthermore, the edema stores of patients with gestational
hypertension were significantly reduced following treatment with
captopril or prazosin compared with placebo and combination
treatment induced a significant decrease compared with either
single agent (Fig. 6). Together,
these clinical outcomes revealed that combination therapy with
captopril and prazosin significantly ameliorated the clinical
features of gestational hypertension.
Analysis of plasma concentration of
vasodilation-converting enzyme and α-adrenergic receptor in
patients with gestational hypertension
The expression and activity of
vasodilation-converting enzyme and α-adrenergic receptor was
subsequently detected in patients with gestational hypertension
following treatment with captopril and/or prazosin. As demonstrated
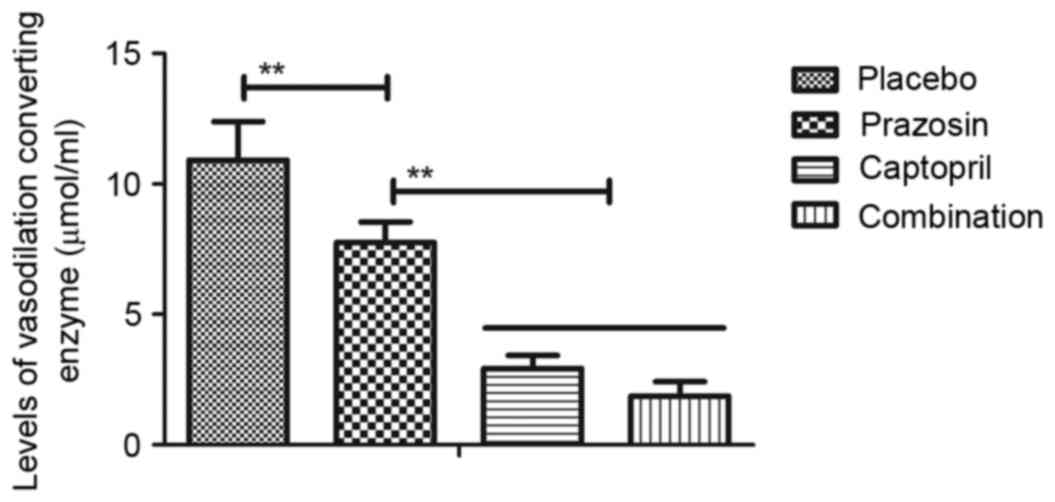
in Fig. 7, compared with the placebo
group, the plasma concentration of vasodilation-converting enzyme
was significantly downregulated following prazosin treatment.
Treatment with captopril alone or combination treatment led to a
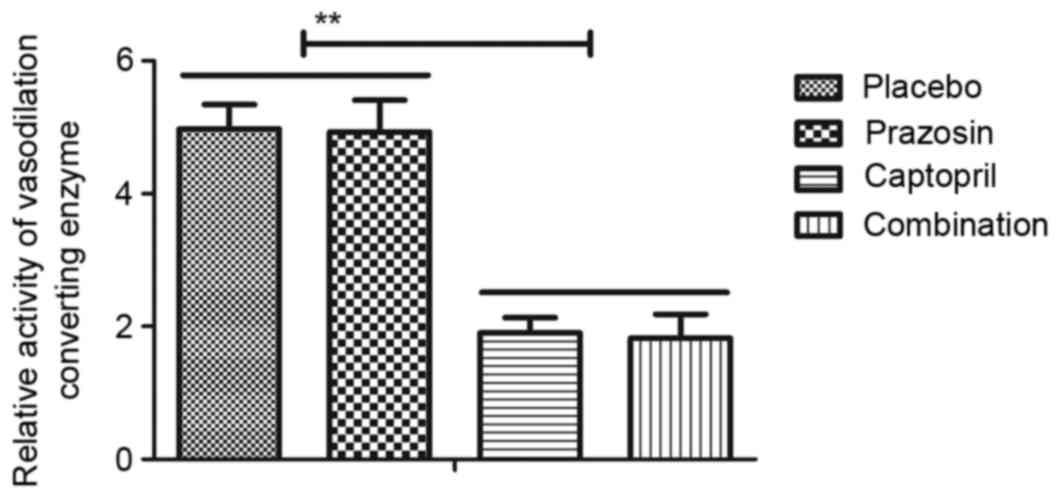
significant decrease compared with prazosin treatment alone. It was
observed that the activity of vasodilation-converting enzyme was
also significantly inhibited in patients with gestational
hypertension following treatment with captopril or combination
treatment in comparison with both the placebo and prazosin groups
(Fig. 8). In addition, it was
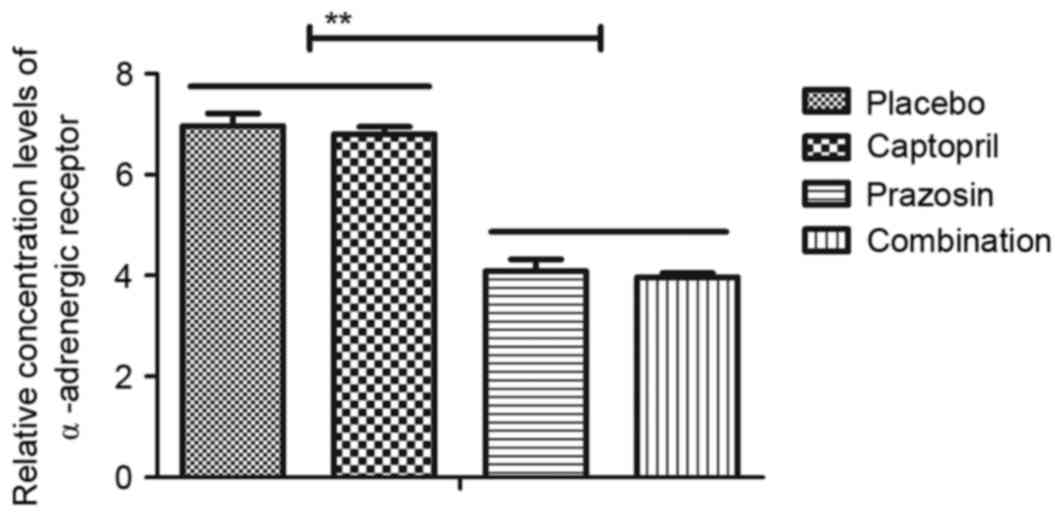
revealed that the plasma concentration levels of α-adrenergic
receptor in patients with gestational hypertension were
downregulated following treatment with prazosin or combination
treatment in comparison with both the placebo and captopril groups
(Fig. 9). Together, these clinical
outcomes indicate that expression levels of vasodilation-converting
enzyme and α-adrenergic receptor may be downregulated by captopril
and prazosin, respectively, which contribute to the recovery of
gestational hypertension.
Pharmacodynamics analysis
After examining the efficacy of comprehensive
treatment of captopril and prazosin, the pharmacodynamics of
captopril and prazosin were analyzed in patients with gestational
hypertension during the clinical therapeutic period. The plasma
concentration levels of captopril and prazosin were analyzed in
patients with gestational hypertension following treatment with
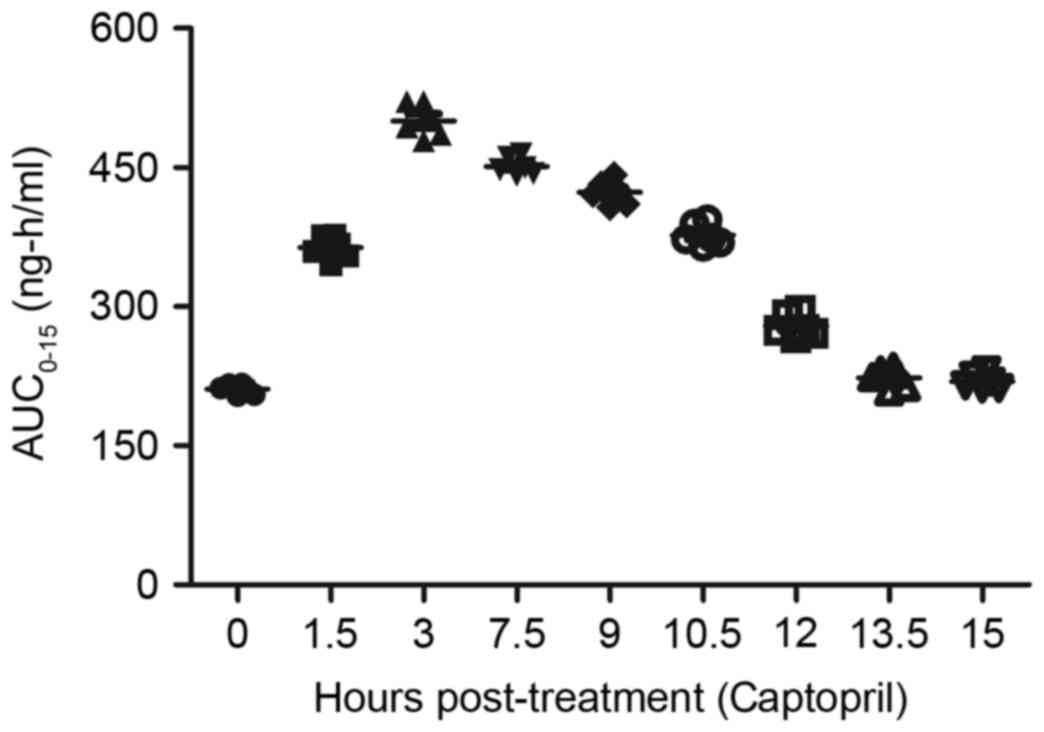
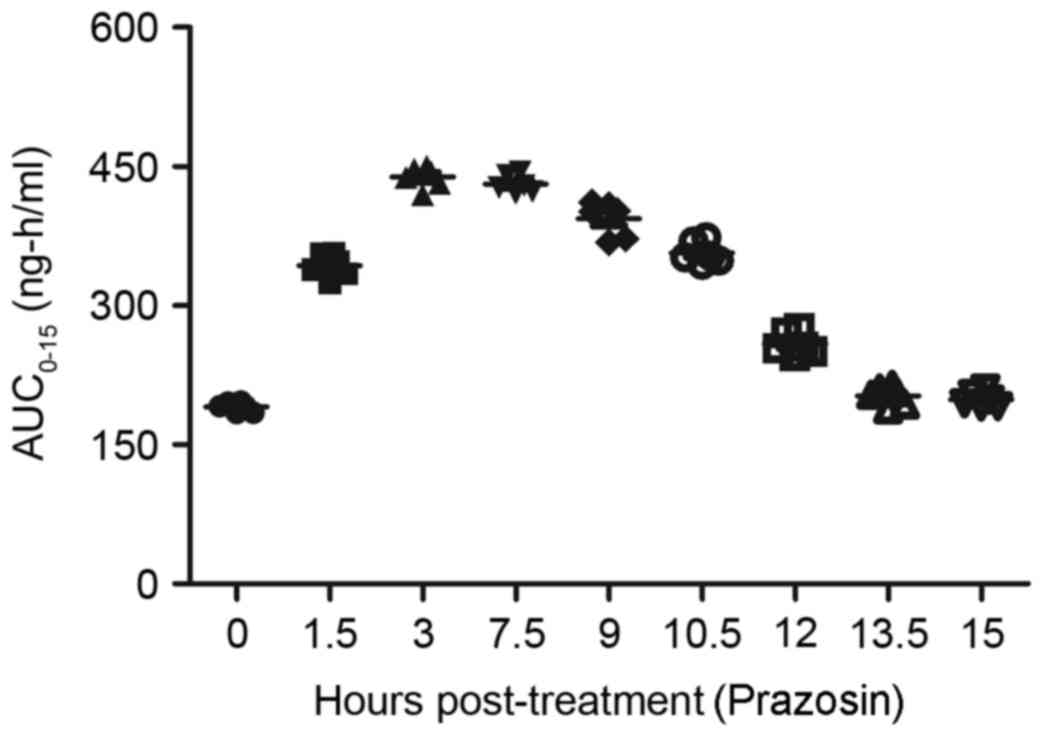
captopril and/or prazosin. As depicted in Fig. 10, the plasma concentration levels of
captopril peaked at 3 h post-treatment. In addition, the plasma
concentration levels of prazosin reached a maximum 3–7.5 h
following administration (Fig. 11).
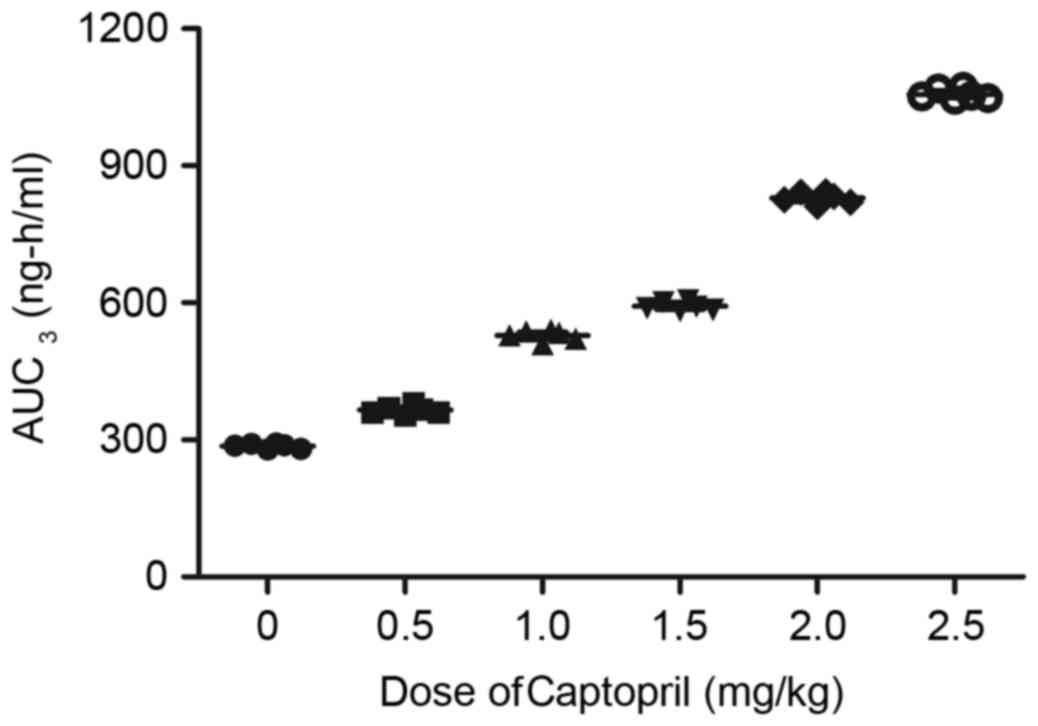
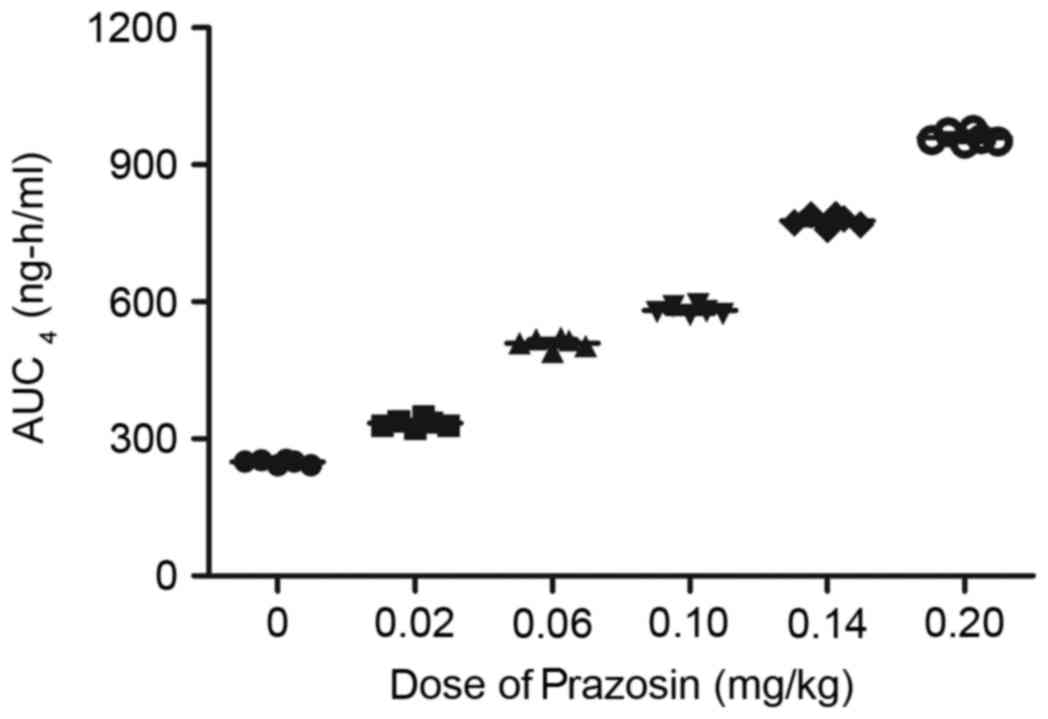
Furthermore, the maximum concentrations (Cmax) of
captopril or prazosin were observed to increase linearly with
increasing dosages (Figs. 12 and
13). The present study presented
that the median terminal elimination half-time (t½) of captopril or
prazosin ranged between 1 and 12 h at the indicated dose.
Furthermore, there was no drug accumulation observed after patients
received captopril or prazosin by observing the Cmax
values. Together, these data indicate that captopril and prazosin
exhibited efficient concentration and functional times.
Discussion
Previous studies have indicated that
vasodilation-converting enzyme and α-adrenergic receptor serve a
crucial role in the initiation and development of patients with
gestational hypertension, which respectively target angiotensin and
α-adrenergic receptor and subsequently lead to gestational
hypertension (18,30). In the present study, the association
among the plasma concentration levels of vasodilation-converting
enzyme and α-adrenergic receptor and hypertension was investigated
in patients with gestational hypertension. In addition, the
efficacies of the antihypertensive drugs captopril and prazosin,
which target vasodilation-converting enzyme and α-adrenergic
receptor, respectively, have been examined in patients with
gestational hypertension.
Following a 4-week baseline, patients with
gestational hypertension were randomized to a double-blind
treatment with captopril, prazosin, combined therapy or placebo
once daily. Although previous results indicated that treatment with
captopril or prazosin was able to regulate the plasma
concentrations of vasodilation-converting enzyme and α-adrenergic
receptor, respectively (19,31), to the best of our knowledge, the
clinical outcomes of combined captopril and prazosin have not been
investigated in previous studies. Therefore, the present study
aimed to evaluate the clinical application of combined treatment
with captopril and prazosin. The safety, treatment-emergent adverse
events, positive observations and pharmacodynamics of the
comprehensive treatment of captopril and prazosin were also
evaluated to make a comprehensive assessment for the therapeutic
effects for patients with gestational hypertension. Treatment
responses have assessed median percent reduction in hypertension
that was improved with comprehensive treatment of captopril and
prazosin compared to a single agent and placebo. Furthermore, the
overall incidence of treatment-emergent adverse events in the
presence of captopril and prazosin were rash and palpitation during
the treatment of gestational hypertension, which is consistent with
previous reports (32,33). The clinical data of the present study
have demonstrated that captopril and prazosin are able to alleviate
gestational hypertension and hypertension-related indictors.
Captopril is an inhibitor of vasodilation-converting
enzyme that is used as a clinical oral drug for the treatment of
hypertension in a number of countries. A number of previous studies
have investigated the therapeutic effects of captopril on patients
with gestational hypertension in clinical. Woodworth et al
(29) have demonstrated the effect
of long-term captopril therapy on patients with hypertension and
diabetes determined by the biochemical parameters of intrarenal
blood flow and renal function. In addition, Van Guilder et
al (30) have indicated that
comprehensive effects of low-dose oral spironolactone and captopril
therapy present more benefits for spontaneous hypertension and
heart failure. Furthermore, Reusz et al (31) have recently suggested the influence
of captopril treatment on occupational activity of engine operators
with hypertension. These outcomes indicate that captopril is an
efficient drug for the treatment of hypertension in preclinical and
clinical experiments. The outcomes also confirm the efficacy of
captopril on gestational hypertension. Furthermore, the
treatment-emergent adverse events and pharmacodynamics of Captopril
were evaluated and studied to systematically analyze the drug
metabolism during the treatment for patients with gestational
hypertension. The outcomes indicate that captopril is a relatively
efficient antihypertensive drug for the treatment of gestational
hypertension.
Prazosin is an α-adrenergic receptor inhibitor that
can significantly inhibit vasoconstriction by targeting the
α-adrenergic receptor in the vascular endothelial cells. Previous
studies have indicated that prazosin inhibits the activity of
α-adrenergic receptor leading to the improvement of activity of
vascular endothelial cells in patients with hypertension (34,35). In
addition, Franklin et al (27) have demonstrated the efficacy and
tolerance of prazosin in patients with hypertension and non-insulin
dependent diabetes. Furthermore, Joglekar and Nanivadekar (34) also compared the efficacies of oral
therapy with combined enalapril, prazosin and hydrochlorothiazide
in the acute treatment of severe hypertension in Nigerian patients.
The present study investigated the efficacies of combined treatment
of captopril and prazosin for the treatment of gestational
hypertension and it was demonstrated that prazosin treatment also
inhibits the serum concentration levels of α-adrenergic receptor in
patients with gestational hypertension. These results of the
present study revealed that captopril and prazosin used in
combination had a higher efficacy than when the drugs were
administered alone in patients being treated for gestational
hypertension. Furthermore, pharmacodynamic analysis indicated that
captopril and prazosin may be maintained at efficient
concentrations in patients with gestational hypertension.
In conclusion, the present study investigated the
clinical efficacy of combination treatment with captopril and
prazosin in patients with gestational hypertension in a phase-II
clinical study. Although previous studies have identified a number
of drugs that exhibit direct effects on gestational hypertension,
it is important to investigate the overall role of captopril and
prazosin in affecting hypertension and hypertension-related
indicators (36,37). The clinical outcomes presented that
captopril and prazosin revealed novel options in gestational
hypertension management and an increasing number of clinical
reports demonstrate promising results. Of note, this clinical
analysis indicates that pharmacokinetic interactions of captopril
and prazosin are important determinants in optimizing therapy for
gestational hypertension. Therefore, clinicians require monitoring
the clinical responses and tolerability when patients undergo
treatment with captopril and prazosin. Overall, the observations of
the present study indicate that patients with gestational
hypertension comprehensively treated with captopril and prazosin
demonstrated beneficial effects on gestational hypertension.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
BH designed and performed experiments. XD, HI and JZ
analyzed the data.
Ethics approval and consent to
participate
The current phase-II study (approval no.
HMCH20090236-A4) was performed between February 2009 and August
2011 in accordance with the recommendations in the Guide for
Haidian Maternal and Child Healthcare. The present study was
approved by the Ethics Committee of Haidian Maternal and Child
Health Care Center (Beijing, China). All patients were required to
review trial protocols and amendments and to provide written
informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kennedy DA, Woodland C and Koren G: Lead
exposure, gestational hypertension and pre-eclampsia: A systematic
review of cause and effect. J Obstet Gynaecol. 32:512–517. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang YH, Chen YP, Liang CC, Chang YL and
Hsieh CC: Impetigo herpetiformis with gestational hypertension: A
case report and literature review. Dermatology. 222:221–224. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu YC, Sun Y and Yang HX: Chronic
hypertension superimposed on preeclampsia at 13 gestational weeks:
A case report with review. Chin Med J (Engl). 125:2067–2069.
2012.PubMed/NCBI
|
|
4
|
Schoenaker DA, Soedamah-Muthu SS and
Mishra GD: The association between dietary factors and gestational
hypertension and pre-eclampsia: A systematic review and
meta-analysis of observational studies. BMC Med. 12:1572014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pengo MF, Rossi GP and Steier J:
Obstructive sleep apnea, gestational hypertension and preeclampsia:
A review of the literature. Curr Opin Pulm Med. 20:588–594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zainul Rashid MR, Lim JF, Nawawi NH,
Luqman M, Zolkeplai MF, Rangkuty HS, Mohamad Nor NA, Tamil A, Shah
SA, Tham SW and Schindler AE: A pilot study to determine whether
progestogen supplementation using dydrogesterone during the first
trimester will reduce the incidence of gestational hypertension in
primigravidae. Gynecol Endocrinol. 30:217–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YA, Chughtai AA, Farquhar CM, Pollock
W, Lui K and Sullivan EA: Increased incidence of gestational
hypertension and preeclampsia after assisted reproductive
technology treatment. Fertil Steril. 105:920–926.e922. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua X, Zhang J, Guo Y, Shen M, Gaudet L,
Janoudi G, Walker M and Wen SW: Effect of folic acid
supplementation during pregnancy on gestational
hypertension/preeclampsia: A systematic review and meta-analysis.
Hypertens pregnancy. 35:447–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basaran A, Basaran M, Topatan B and Martin
JN Jr: Effect of chorionic villus sampling on the occurrence of
preeclampsia and gestational hypertension: An updated systematic
review and meta-analysis. J Turk Ger Gynecol Assoc. 17:65–72. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Li J and Wang K: Clinical
analysis of the therapeutic effect of patients with gestational
hypertension combined with obstructive sleep apnea hypopnea
syndrom. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
28:1521–1522. 2014.(In Chinese). PubMed/NCBI
|
|
11
|
Sakar MN, Atay AE, Demir S, Bakir VL,
Demir B, Balsak D, Akay E, Ulusoy AI and Verit FF: Association of
endothelial nitric oxide synthase gene G894T polymorphism and serum
nitric oxide levels in patients with preeclampsia and gestational
hypertension. J Mater Fetal Neonatal Med. 28:1907–1911. 2015.
View Article : Google Scholar
|
|
12
|
Tovar-Rodriguez JM, Medel-Lagunes Idel C,
Acosta-Altamirano G and Vargas-Hernandez VM: Clinic characteristics
of patients with trophoblastic gestational disease complicate with
hypertension. Ginecol Obstet Mex. 81:578–586. 2013.(In Spanish).
PubMed/NCBI
|
|
13
|
Vasapollo B, Novelli GP, Gagliardi G,
Tiralongo GM, Pisani I, Manfellotto D, Giannini L and Valensise H:
Medical treatment of early-onset mild gestational hypertension
reduces total peripheral vascular resistance and influences
maternal and fetal complications. Ultrasound Obstet Gynecol.
40:325–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidovich IM, Bloshchinskaia IA and
Petrichko TA: Gestational arterial hypertension. Mechanisms of
formation. Treatment with normodipin. Ter Arkh. 75:50–54. 2003.(In
Russian).
|
|
15
|
Munakata M, Aihara A, Nunokawa T, Ito N,
Imai Y, Ito S and Yoshinaga K: The influence of one-year treatment
by angiotensin converting enzyme inhibitor on baroreflex
sensitivity and flow-mediated vasodilation of the brachial artery
in essential hypertension-comparison with calcium channel blockers.
Clin Exp Hypertens. 25:169–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y and Zhu J: Quality of life of
patients with mild hypertension treated with captopril: A
randomized double-blind placebo-controlled clinical trial. Chin Med
J (Engl). 112:302–307. 1999.PubMed/NCBI
|
|
17
|
Rudner S and Browne P: Case report:
Management of secondary hypertension in a feline with the use of
transdermal prazosin. Int J Pharm Compd. 14:488–491.
2010.PubMed/NCBI
|
|
18
|
Iwatsubo K and Umemura S: Alpha adrenergic
receptor blockers for patients with hypertension. Nihon Rinsho.
6:294–299. 2006.(In Japanese).
|
|
19
|
Zhao LS and Xu CY: Effect of prazosin on
diabetic nephropathy patients with positive α1-adrenergic receptor
autoantibodies and refractory hypertension. Exp Ther Med.
9:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raeissadat SA, Rayegani SM, Hassanabadi H,
Fathi M, Ghorbani E, Babaee M and Azma K: Knee osteoarthritis
injection choices: Platelet-rich plasma (PRP) versus hyaluronic
acid (A one-year randomized clinical trial). Clin Med Insights
Arthritis Musculoskelet Disord. 8:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez-Merchan EC: Intraarticular
injections of platelet-rich plasma (PRP) in the management of knee
osteoarthritis. Arch Bone Jt Surg. 1:5–8. 2013.PubMed/NCBI
|
|
22
|
Goetz RM and Holtz J: Enhanced
angiotensin-converting enzyme activity and impaired
endothelium-dependent vasodilation in aortae from hypertensive
rats: Evidence for a causal link. Clin Sci (Lond). 97:165–174.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paiva L, Lima E, Neto AI and Baptista J:
Angiotensin I-converting enzyme (ACE) inhibitory activity of fucus
spiralis macroalgae and influence of the extracts storage
temperature-A short report. J Pharm Biomed Anal. 131:503–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mattar MM, Morad MA, El Husseiny NM, Ali
NH and El Demerdash DM: Correlation between JAK2 allele burden and
pulmonary arterial hypertension and hematological parameters in
philadelphia negative JAK2 positive myeloproliferative neoplasms.
An egyptian experience. Ann Hematol. 95:1611–1616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gobbi A, Lad D and Karnatzikos G: The
effects of repeated intra-articular PRP injections on clinical
outcomes of early osteoarthritis of the knee. Knee Surg Sports
Traumatol Arthrosc. 23:2170–2177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filardo G, Kon E, DI Matteo B, DI Marino
A, Sessa A, Merli ML and Marcacci M: Leukocyte-poor PRP application
for the treatment of knee osteoarthritis. Joints. 1:112–120.
2013.PubMed/NCBI
|
|
27
|
Franklin HR, Simonetti GP, Dubbelman AC,
ten Bokkel Huinink WW, Taal BG, Wigbout G, Mandjes IA, Dalesio OB
and Aaronson NK: Toxicity grading systems. A comparison between the
WHO scoring system and the common toxicity criteria when used for
nausea and vomiting. Ann Oncol. 5:113–117. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boss DS, Glen H, Beijnen JH, Keesen M,
Morrison R, Tait B, Copalu W, Mazur A, Wanders J, O'Brien JP, et
al: A phase I study of E7080, a multitargeted tyrosine kinase
inhibitor, in patients with advanced solid tumours. Br J Cancer.
106:1598–1604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woodworth T, Furst DE, Alten R, Bingham CO
III, Yocum D, Sloan V, Tsuji W, Stevens R, Fries J, Witter J, et
al: Standardizing assessment and reporting of adverse effects in
rheumatology clinical trials II: The rheumatology common toxicity
criteria v.2.0. J Rheumatol. 34:1401–1414. 2007.PubMed/NCBI
|
|
30
|
Van Guilder GP, Pretorius M, Luther JM,
Byrd JB, Hill K, Gainer JV and Brown NJ: Bradykinin type 2 receptor
BE1 genotype influences bradykinin-dependent vasodilation during
angiotensin-converting enzyme inhibition. Hypertension. 51:454–459.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reusz GS and Kis E, Cseprekál O, Szabó AJ
and Kis E: Captopril-enhanced renal scintigraphy in the diagnosis
of pediatric hypertension. Pediatr Nephrol. 25:185–189. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanon O, Giacomino A, Troy S, Bernaud C,
Girerd X and Weber S: Efficacy of and tolerance to prolonged
release prazosin in patients with hypertension and non-insulin
dependent diabetes. Ann Cardiol Angeiol (Paris). 49:390–396.
2000.(In French). PubMed/NCBI
|
|
33
|
Hall DR, Odendaal HJ, Steyn DW and Smith
M: Nifedipine or prazosin as a second agent to control early severe
hypertension in pregnancy: A randomised controlled trial. BJOG.
107:759–765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Joglekar SJ and Nanivadekar AS: A
randomized, controlled, multicenter study to compare prazosin GITS
with enalapril in hypertensive patients with diabetes mellitus.
Bombay Hypertension Study Group. J Assoc Physicians India. 1:52–62.
1998.PubMed/NCBI
|
|
35
|
Joglekar SJ, Jaguste V and Nanivadekar AS:
Prazosin GITS vs atenolol in patients with hypertension and normal
lipid profile: A randomized, controlled multicenter study.
Hyderabad Hypertension Study Group. J Assoc Physicians India.
1:41–51. 1998.PubMed/NCBI
|
|
36
|
Morishita S, Hagihara M, Itabashi M, Ishii
Y, Yamamoto W, Numata A, Motohashi K, Matsumoto K, Fujisawa S and
Nakajima H: Development of pulmonary arterial hypertension during
oral dasatinib therapy for chronic myelogenous leukemia. Rinsho
Ketsueki. 57:999–1003. 2016.PubMed/NCBI
|
|
37
|
Galappatthy P, Waniganayake YC, Sabeer MI,
Wijethunga TJ, Galappatthy GK and Ekanayaka RA: Leg edema with
(S)-amlodipine vs conventional amlodipine given in triple therapy
for hypertension: A randomized double blind controlled clinical
trial. BMC Cardiovasc Disord. 16:1682016. View Article : Google Scholar : PubMed/NCBI
|