Introduction
The ovaries are known to age more rapidly in
comparison with other organs. Numerous women present premature
ovarian failure (POF), with ~1 in 1,000 women considered to suffer
from POF by 30 years of age, while this occurrence is reported to
increase to ~1 in 100 women by 40 years of age (1). The primary cause of POF remains
unknown; however, it is associated with a number of congenital
conditions or a gene abnormality (19%), autoimmunity (19%),
iatrogenic causes (37%) or unidentified/unknown causes (25%) in
patients worldwide (2).
Reactive oxygen intermediates, including oxygen
ions, superoxides, singlet oxygen and peroxides, are considered to
affect ovarian aging and even cause premature aging (3). Ovarian aging is induced by an
escalation in the levels of oxygen radicals, leading to failure of
the antioxidant defense system, which in turn initiates and
regulates the release of apoptotic factors that cause ovarian
granulosa cell death (4). B-cell
lymphoma 2 (Bcl-2) is an anti-apoptosis protein that protects cells
by preventing a proteolytic cascade dependent on Caspase3, a type
of cysteine-aspartic acid protease, which in turn prevents cell
death (4). A surge in reactive
oxygen species (ROS) not only has detrimental effects, such as
protein oxidation, nucleic acid damage and eventual premature cell
death, but also negatively impacts the reproductive abilities
(5). ROS are formed throughout
ovulatory processes and have biological roles during ovulation,
such as supporting the ovum release. However, a significantly
increased ROS levels can cause oxidative stress (OS) and induce the
death of oocyte granulosa cells, as well as oocytes. A number of
previous studies investigating OS have reported that enhanced ROS
levels caused oocyte destruction, maturation of the ovum and
degradation of oocyte superiority (6,7).
Under normal conditions, human cellular systems
limit the release of excessive amounts of oxygen intermediates,
inhibiting their levels and supporting cell repair (8–11). A
woman's ability to reproduce is highly influenced by OS and other
events, such as the menopause. It has previously been hypothesized
that OS may be responsible for decreasing fertility in women
(12). Furthermore, OS serves a
major role throughout the 9-month gestation period (13), in normal vaginal delivery (14,15), as
well as in the commencement of normal and preterm labor (16,17).
Lipid peroxidation, and reduced ATP and protein formation are some
of the effects of excess oxygen radical levels (18). In addition, ROS are involved in the
rupture of Graafian follicles, and are known to serve major effects
during the maturation of oocytes, steroid hormone production,
gamete embedding and also during the formation of an embryo.
Furthermore, ROS are known to help maintain the corpus luteum in
order to prevent abortion and later support luteolysis (19–23).
The human immune system is able to alter the
expression levels of cell protective enzymes in response to
specific stimuli as a defense mechanism (24,25). One
of the roles of nuclear factor erythroid 2-related factor 2 (Nrf2)
is to induce the production of cellular defense proteins. Nrf2
stimulates a wide range of cellular defense processes, which lead
to the destruction of harmful factors that are detrimental to human
health. It is a member of the cap-n-collar subdivision of basic
region-leucine zipper-type family of enzymes responsible for
regulating the transcription frequency (26). Nrf2 binds to one of the smallest Maf
macromolecules (sMaf), and the Nrf2-sMaf macromolecular complex
then binds to antioxidant response elements (AREs) or electrophile
response elements, which are located in the surveillance sections
of DNAs coding for several cell defense-associated proteins
(26–29).
A previous study suggested that during OS induced by
cell tension or stress, the Nrf2 protein may be involved in
chemical sensing, as well as transcription, supporting redox
balancing and functioning with Kelch-like ECH-associated protein 1
(30). The stability of cells,
primarily when challenged by external stimuli, including toxins or
reactive oxygen radicals, is constantly adjusted by the secretion
of numerous macromolecules that assist in the response to stimuli
by acting as transporters to eliminate harmful stimuli from the
host's system (31), and Nrf2 serves
a key role in this process. The Nrf2-ARE signaling pathway
stimulates a defensive mechanism during cell stress induced by OS
(32), and is therefore considered
to serve an important role in the process of ovarian aging. During
the continuous process of aging, apoptosis is the main cause of
follicle loss from the ovaries (33).
The present study evaluated Nrf2 protein
localization and release in mice of diverse age groups. The aim of
the present study was to determine the association between the
release of Nrf2 and apoptosis-associated proteins, including
Caspase3 and Bcl-2, in the ovaries of different age groups of
mice.
Materials and methods
Laboratory animals
Adult ICR mice (n=32; age, 12 weeks old; weight,
25–30 g) were acquired from the Animal Center of Wenzhou Medical
University (Wenzhou, China) and reproduced in the Animal Center of
the Second Affiliated Hospital of Wenzhou Medical University. The
adult mice (male to female ratio, 1:2) were housed in a plastic
cage for 1 week, maintained at a controlled temperature of 21–24°C
and a 12-h light/dark cycle. Food and fresh water were available to
the mice ad libitum. Pregnant mice were observed daily to
determine whether they had delivered. All procedures were performed
in accordance with the guidelines for laboratory animal use and
welfare of Wenzhou Medical University, and the experimental
protocol was approved by the Ethics Committee Board of the Second
Affiliated Hospital of Wenzhou Medical University (approval no.
wydw2013-0002).
Mouse ovaries of the offspring were obtained at
different stages of growth for immunohistochemical procedures. In
accordance with the reproductive physiology of female mice, the
ovaries were obtained at 4 days (young group; n=12), 3 weeks
(prepubertal group; n=10), 8 weeks (sexual maturity group; n=10),
12 weeks (childbearing age group; n=12) and 40 weeks (older age
group; n=9) of age for use in subsequent experiments. Briefly, mice
were sacrificed and the freshly excised ovaries were fixed with 4%
paraformaldehyde for 1 h. The ovaries were then cleaned with water
and dehydrated by passing through a series of 70, 80, 95 and 100%
alcohol for 5 min each time. Ovarian tissues were then washed in
xylene and finally embedded in paraffin.
Immunohistochemical assay
The paraffin-embedded ovaries were sectioned at 4 µm
and placed on glass slides. Next, the slides were dipped in xylene
in order to remove the paraffin and transferred through a
descending ethanol series (100, 95, 70 and 55%) for 3–4 min. Slides
were then heated in a solution of citrate buffer in a microwave at
a 100°C for 10 min, in order to perform antigen retrieval.
Following cooling to room temperature, slides were washed three
times with PBS (5 min each) and then treated with 3%
H2O2 in methanol for ~10 min to quench endogenous H2O2
activity. Subsequently, tissues were blocked using 10% fetal bovine
serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 20
min, placed in a dark environment, washed with PBS and then
incubated with Nrf2 primary antibody (cat. no. ab62352; Abcam,
Cambridge, MA, USA) in Primary Antibody Dilution Buffer (1:500
dilution) at 4°C overnight. For the negative control, PBS was used
instead of the antibody. Following overnight incubation, the slides
were kept in a warm water bath at 37°C for at least 30 min, which
was followed by washing three time with PBS for 5 min each and then
incubation with the secondary antibody (1:20,000; cat. no. 14709;
Cell Signaling Technology, Inc., Danvers, MA, USA) for at least 20
min at room temperature. The samples were washed thrice with PBS,
stained with 3,3′-diaminobenzidine (Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) and then stained with hematoxylin for 2 min,
followed by dehydration in alcohol for 5 min each. Finally, the
slides were mounted using a mounting solution. Photomicrographs
were captured with a Nikon digital camera (Nikon Corporation,
Tokyo, Japan) and SPOT Advanced Plus software (SPOT Imaging;
Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Western blot analysis
Mouse ovaries from the different age groups were
collected at the aforementioned time points. Protein extraction
buffers (radioimmunoprecipitation assay and phenylmethane sulfonyl
fluoride; Sigma-Aldrich, Merck KGaA) were added to the tissues, and
the protein concentrations were quantified using a bicinchoninic
acid kit. The samples were then homogenized, centrifuged at 12,000
× g at 4°C for 30 min, then subjected to ultra-sonication and the
supernatant was aspirated again via centrifugation at 4,000 × g at
4°C for 20 min. The sample was used to perform quantification of
the protein expression. A total of 40 µg protein samples from the
different age groups of mice were subjected to 12% SDS-PAGE and
then transferred to polyvinylidene difluoride membranes, which were
activated with methanol for 1–2 min. Next, the membranes were
blocked with 5% skimmed milk at room temperature for 2 h and then
incubated at 4°C overnight with the Nrf2 (cat. no. ab62352; Abcam),
Bcl-2 (cat. no. ab692; Abcam) and Caspase3 (cat. no. ab13847;
Abcam) primary antibodies at 1:500 dilution, with human β-actin
(cat no. 85845; Cell Signaling Technology, Inc.) serving as the
internal reference. Membranes were subsequently washed three times
with Tris-buffered saline/Tween 20 for 5 min each and then
incubated with goat anti-rabbit immunoglobulin G secondary antibody
(Alexa Fluor 488; cat. no. ab150077; 1:1,000 dilution; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 2 h in room
temperature. The membranes were washed with TBST and the grey bands
of proteins were evaluated using enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.). The intensity of the
bands was determined semi-quantitatively through colorimetry
detection with AlphaEaseFC software (Genetic Technologies, Inc.,
Miami, FL, USA), and the changes in protein band intensity were
normalized to β-actin.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA isolation was performed using TRIzol reagent
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RT was then performed with the ReverTra Ace qPCR RT Kit
(Toyobo Life Science, Osaka, Japan) according to the manufacturer's
protocol, using oligo-dT-primers and 1 µg RNA per reaction as a
template. The isolated RNA was measured at 260/280 nm using a
NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.)
to assess RNA quality and quantity. qPCR analyses were run and
analyzed using the ABI 7500 quantitative PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the Thunderbird
SYBR qPCR Mix (Toyobo Life Science). The qPCR cycling conditions
were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec, 60°C for 15 sec and 60°C for 1 min. The primer sequences
used were as follows: Nrf2 forward, 5′-CAGCATGATGGACTTGGA-3′, and
reverse, 5′-TGAGACACTGGTCACACT-3′; GAPDH forward,
5′-GGTCGGAGTCAACGGATTTG-3′, and reverse,
5′-ATGAGCCCCAGCCTTCTCCAT-3′. The relative expression of Nrf2 was
normalized to GADPH expression and calculated according to the
2−ΔΔCq method described by Livak and Schmittgen
(34).
Statistical analysis
Statistical analysis was performed using IBM SPSS
software (version 22.0; IBM Corp., Armonk, NY, USA). The
differences between groups were analyzed using Student's t-test or
with one-way analysis of variance, followed by the
Student-Newman-Keuls post hoc test. The results are expressed as
the mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
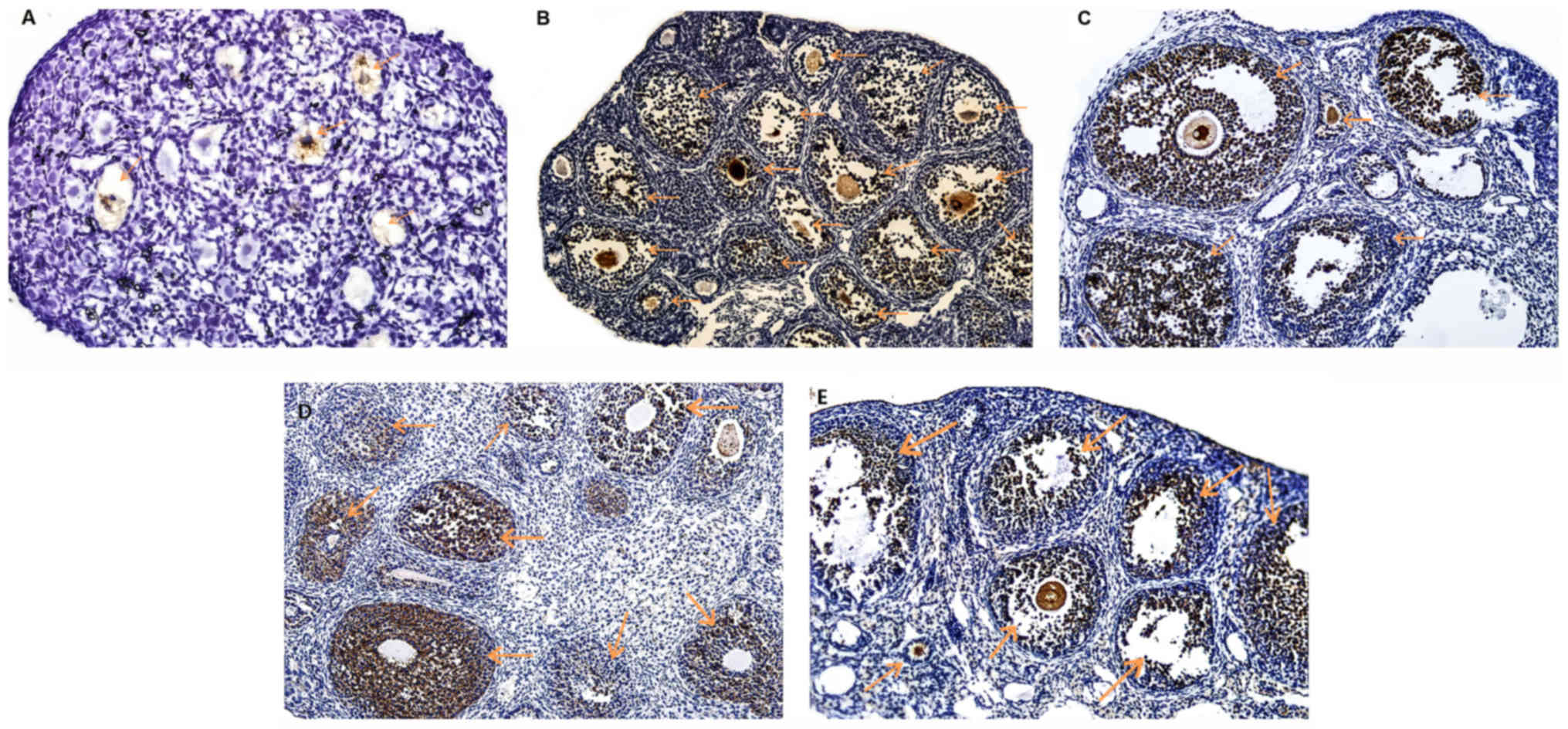
Nrf2 protein localization in mouse
ovaries
The results indicated that Nrf2 protein was
expressed in the ovarian tissue of all age groups.
Immunohistochemical staining revealed that Nrf2 protein was
predominantly expressed in follicular cells (granulosa cells),
secondary follicles and antral follicles in the oocytes, while
lower levels of expression were also observed in primary and
primordial follicles (Fig. 1). The
expression of Nrf2 protein in the ovarian tissues of the 4-day-old
group was significantly lower when compared with that in the
ovarian tissues of 3, 8, 12 and 40-week-old mice.
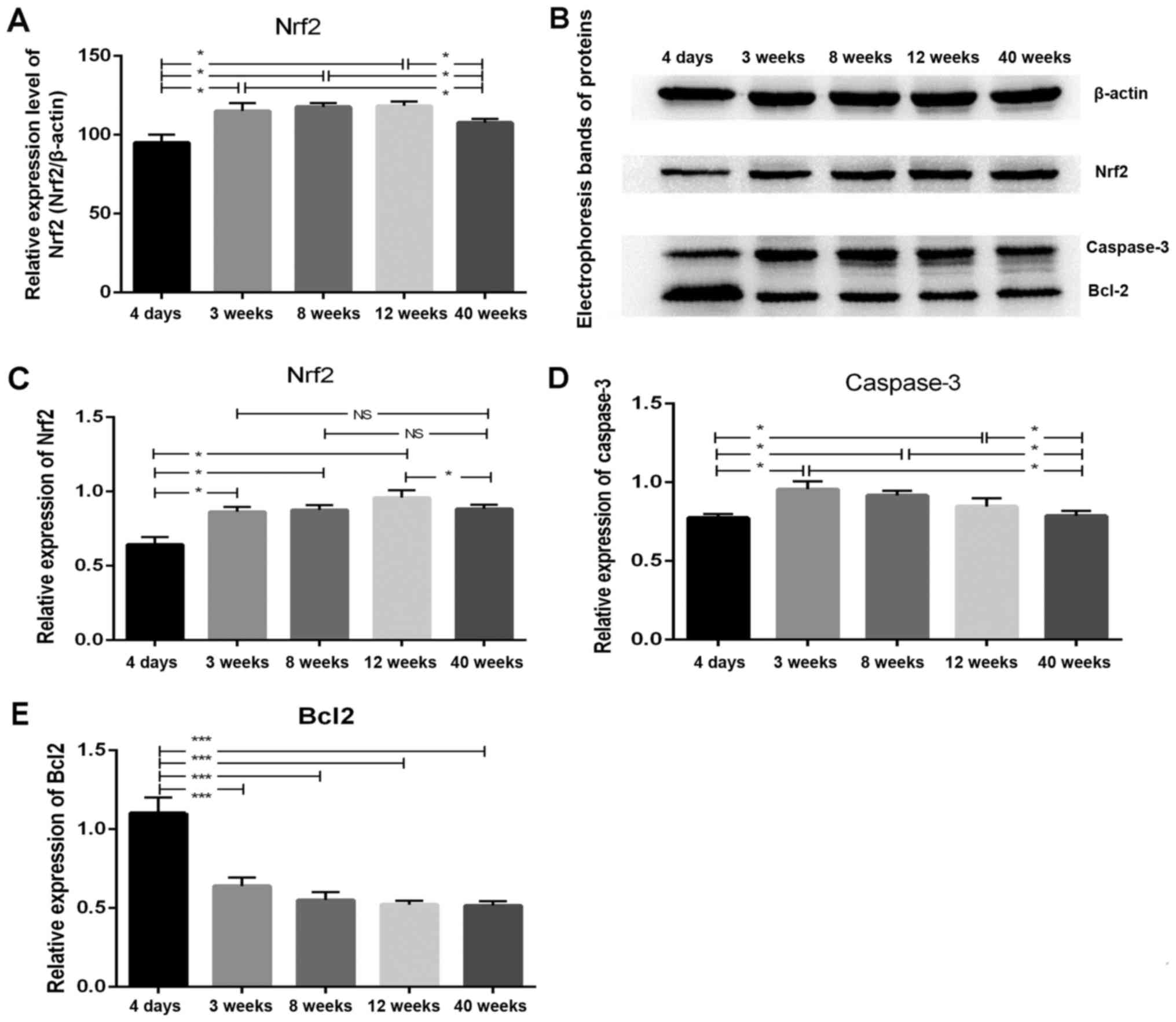
Expression of Nrf2 in the mouse
ovaries
Significant differences in the expression of Nrf2
were observed when comparing the results of the different age
groups. As shown in Fig. 2A, the
relative expression of Nrf2 mRNA in mouse ovarian tissues was
initially increased until ~12 weeks of age and then decreased with
the increase in the age of mice. Furthermore, multiple comparisons
among the different age groups revealed that the expression of Nrf2
protein in the ovaries of 4-day-old mice was significantly lower
compared with that observed in the ovaries of the mice aged 3, 8
and 12 weeks (P<0.05; Fig. 2B and
C). In addition, Nrf2 protein expression in the ovarian tissues
of the 40-week-old group was markedly reduced when compared with
that observed in the 12-week-old mice (P<0.05).
Expression of apoptosis-associated
proteins Caspase3 and Bcl-2 in the mouse ovaries
Western blot analysis revealed significant
differences in the expression levels of Caspase3, a pro-apoptotic
protein, and Bcl-2, an anti-apoptotic protein, among different age
groups. As shown in Fig. 2D, with
increasing age, the expression of the pro-apoptotic protein
Caspase3 was initially increased, reached a peak level at 3 weeks
and then decreased. The expression of Caspase3 in the ovaries of
4-day-old mice was markedly lower when compared with that in the 3,
8 and 12-week groups (P<0.05). In addition, the expression of
Caspase3 in the ovarian tissue of 40-week-old mice was
significantly reduced when compared with that in 3, 8 and 12-week
groups (P<0.05). However, no significant differences in Caspase3
expression were identified among the 3, 8 and 12-week-old mouse
groups (P=0.162). As presented in Fig.
2E, the expression of the anti-apoptotic protein Bcl-2 in the
ovarian tissues of 4-day-old mice was significantly higher in
comparison with that observed in the 3, 8, 12 and 40-week-old mice
(P<0.001). However, no significant differences in Bcl-2 protein
expression were identified among the 3, 8, 12 and 40-week-old
groups (P=0.55).
Discussion
The ovaries are important for the production of sex
steroidal hormones, such as progesterone and estrogen, and in
reproduction. In women, the functional ability of the ovaries is
gradually reduced with increasing age. Currently, failure of
ovarian function due to the continuous aging process is
unpreventable. Advancing age is a major factor in the release of
excess ROS; coupled with aging, the defense mechanism is impaired
at the cellular level, which initiates oxidative destruction
(35). An excess output of free
oxygen ions and intermediates causes cellular damage, which cannot
be attenuated by the impaired defense system of the host. Such
disequilibrium has a severe effect, stimulating the production of
elements associated with cell death and the release of other
enzymes, such as cytochrome c (36), which are accountable for the decline
in ovarian function observed during ovarian aging. Nrf2 is an
important protein that adjusts the production of proteins
associated with cytoprotection in response to harmful stressors
from external sources (30). Nrf2
protein functions as a detector whose main action is to induce the
inhibition of oxidation in various organs in the body, including
the ovaries (37,38).
Low levels of Nrf2 may impede the inhibition of
oxidation and may even cause reduced antioxidant support (39,40).
Previous studies have revealed that ovarian aging is associated
with decreased ovarian antioxidant defense capacity and increased
oxidative damage (41–45). When activated, the Nrf2-ARE signaling
pathway may have an important effect on the progression of aging
(46). Glutamyl cysteine synthetase
and heme oxygenase-1 are the macromolecules that assist in the
restriction of oxidation and are induced by Nrf2 activation
(47,48). In atrazine (ATR)-induced ovarian
aging in rats, the number of ovarian atretic follicles increased
with an increase in ATR concentration, while Nrf2 protein content
and its expression in the nucleus were also significantly increased
(49). In addition, in a 4-vinyl
cyclohexene diepoxide (VCD)-induced ovarian failure in a rat model,
it was demonstrated that the decrease in Nrf2 markedly increased OS
and increased ovarian toxicity (50). It was also revealed that an increase
in Nrf2 protein expression protected mouse ovarian cells from VCD
ovarian toxicity (49). Therefore,
the protein expression level of Nrf2 in ovarian tissues and during
ovarian aging has certain relevance. The upregulation of Nrf2
protein in ovarian cells improves the antioxidant capacity, reduces
OS and protects ovarian function. Zhao et al (49) reported that a severe oxidative
reaction was influenced by ATR in the ovaries of rats. Nrf2, as a
transcription factor, regulates the enzymes necessary to hinder
oxidation and has a major role in protecting cells. When rat
ovaries were continually exposed to ATR, Nrf2 action increased,
thus, it may be an important antioxidant factor for future curative
strategies (48).
In the present study, mice of different ages were
employed to simulate the process of natural aging. Nrf2 protein was
expressed in the ovarian tissues of mice at all ages, specifically
in the follicular cells and secondary follicles, as well as in the
antral follicles. In the mice aged 3, 8 and 12 weeks old, the
expression of Nrf2 protein was higher when compared with that
observed in mice aged 4 days and 40 weeks old; however, no
significant difference in the protein expression of Nrf2 was
observed between the 3, 8 and 12-week age groups. Thus, it is
speculated that there may be a correlation between Nrf2 protein
expression and ovarian reproductive function, and that Nrf2 protein
may be highly associated with the protection of ovarian
reproductive function. During the young (4 days old), growth (3–12
weeks old) and older (40 weeks old) periods of aging, as the
reproductive function of the ovaries increased, the Nrf2 protein
content in the ovarian tissue also exhibited an increasing and then
a decreasing trend.
Detection of the apoptosis-associated proteins
Caspase3 and Bcl-2 in the ovarian tissues of mice in the different
age groups was also performed in the current study. The results
demonstrated that the expression of the apoptosis inhibitor Bcl-2
decreased with increasing age, while the expression of the
pro-apoptosis protein Caspase3 was affected by the age of mice. The
Bcl-2 family mediates programmed cell death and is comprised of
proteins that are pro- or anti-apoptotic in nature, thereby either
preventing or inducing cell death (51). Depending upon the external or
internal stimuli, the process of cell death is triggered by a
series of actions, including the activation of important proteins
known as Caspases and activation of numerous mediators from
mitochondria, which are responsible for the destiny of a cell
(51).
In conclusion, the results of the present study
supported and confirmed the existence of a correlation between Nrf2
protein and ovarian function. Upregulated expression of Nrf2
protein in ovarian tissue can protect ovarian function and may
delay ovarian aging. The results raised the possibility that Nrf2
protein signaling may be required for anti-OS processes during
ovarian aging. Furthermore, the results of the present study, which
used mice of different ages to represent the natural aging process,
provided support for the hypothesis that increased ovarian OS may
be responsible for premature ovarian senescence. However, the
current study did have certain limitations, including a small group
size, the use of mouse tissues and cells, which may not be
representative of the condition in humans; the specific mechanism
remains unclear. Thus, further research is required in order to
resolve these issues and elucidate the underlying mechanism.
Acknowledgements
The authors would like to thank all the doctors of
the Reproductive Health Center Department of The Second Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) for
providing all the necessary information required for this
study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no.
3250008A0976-1850).
Availability of data and materials
The data sets supporting the conclusions of the
present study are included in this published article. The raw data
stored on the main electronic data storage system of the Second
Affiliated Hospital of Wenzhou Medical University are available
from the corresponding author on reasonable request.
Authors' contributions
NS analyzed the raw data and wrote the manuscript.
NS and AB performed the main experiment. YZ and XL collected the
raw data. JL conceptualized and designed the study, and provided
supervision. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee Board of The Second Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hernández-Angeles C and Castelo-Branco C:
Early menopause: A hazard to a woman's health. Indian J Med Res.
143:420–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenton AJ: Premature ovarian
insufficiency: Pathogenesis and management. J Midlife Health.
6:147–153. 2015.PubMed/NCBI
|
|
3
|
Pan JS, Hong MZ and Ren JL: Reactive
oxygen species: A double-edged sword in oncogenesis. World J
Gastroenterol. 15:1702–1707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sukur YE, Kivançli IB and Ozmen B: Ovarian
aging and premature ovarian failure. J Turk Ger Gynecol Assoc.
15:190–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3:282005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goud AP, Goud PT, Diamond MP, Gonik B and
Abu-Soud HM: Reactive oxygen species and oocyte aging: Role of
superoxide, hydrogen peroxide and hypochlorous acid. Free Radic
Biol Med. 44:1295–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi
KW and Oh KS: Detection of reactive oxygen species (ROS) and
apoptosis in human fragmented embryos. Hum Reprod. 13:998–1002.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pierce JD, Cackler AB and Arnett MG: Why
should you care about free radicals? RN. 67:38–43. 2004.PubMed/NCBI
|
|
9
|
Szczepanska M, Koźlik J, Skrzypczak J and
Mikolajczyk M: Oxidative stress may be a piece in the endometriosis
puzzle. Fertil Steril. 79:1288–1293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Langendonckt A, Casanas-Roux F and
Donnez J: Oxidative stress and peritoneal endometriosis. Fertil
Steril. 77:861–870. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal A and Allamaneni SS: Role of free
radicals in female reproductive diseases and assisted reproduction.
Reprod Biomed Online. 9:338–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Bruin JP, Dorland M, Spek ER, Posthuma
G, van Haaften M, Looman CW and te Velde ER: Ultrastructure of the
resting ovarian follicle pool in healthy young women. Biol Reprod.
66:1151–1160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myatt L and Cui X: Oxidative stress in the
placenta. Histochem Cell Biol. 122:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fainaru O, Almog B, Pinchuk I, Kupferminc
MJ, Lichtenberg D and Many A: Active labour is associated with
increased oxidisibility of serum lipids ex vivo. BJOG. 109:938–941.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mocatta TJ, Winterbourn CC, Inder TE and
Darlow BA: The effect of gestational age and labour on markers of
lipid and protein oxidation in cord plasma. Free Radic Res.
38:185–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wall PD, Pressman EK and Woods JR Jr:
Preterm premature rupture of the membranes and antioxidants: The
free radical connection. J Perinat Med. 30:447–457. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pressman EK, Cavanaugh JL, Mingione M,
Norkus EP and Woods JR: Effects of maternal antioxidant
supplementation on maternal and fetal antioxidant levels: A
randomized, double-blind study. Am J Obstet Gynecol. 189:1720–1725.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ray SD, Lam TS, Rotollo JA, Phadke S,
Patel C, Dontabhaktuni A, Mohammad S, Lee H, Strika S, Dobrogowska
A, et al: Oxidative stress is the master operator of drug and
chemically-induced programmed and unprogrammed cell death:
Implications of natural antioxidants in vivo. Biofactors.
21:223–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki T, Sugino N, Fukaya T, Sugiyama S,
Uda T, Takaya R, Yajima A and Sasano H: Superoxide dismutase in
normal cycling human ovaries: Immunohistochemical localization and
characterization. Fertil Steril. 72:720–726. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jozwik M, Wolczynski S, Jozwik M and
Szamatowicz M: Oxidative stress markers in preovulatory follicular
fluid in humans. Mol Hum Reprod. 5:409–413. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishikawa M: Oxygen radicals-superoxide
dismutase system and reproduction medicine. Nihon Sanka Fujinka
Gakkai Zasshi. 45:842–848. 1993.(In Japanese). PubMed/NCBI
|
|
22
|
Vega M, Johnson MC, Díaz HA, Urrutia LR,
Troncoso JL and Devoto L: Regulation of human luteal
steroidogenesis in vitro by nitric oxide. Endocrine. 8:185–191.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugino N, Takiguchi S, Kashida S, Karube
A, Nakamura Y and Kato H: Superoxide dismutase expression in the
human corpus luteum during the menstrual cycle and in early
pregnancy. Mol Hum Reprod. 6:19–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itoh K, Igarashi K, Hayashi N, Nishizawa M
and Yamamoto M: Cloning and characterization of a novel erythroid
cell-derived CNC family transcription factor heterodimerizing with
the small Maf family proteins. Mol Cell Biol. 15:4184–4193. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friling RS, Bensimon A, Tichauer Y and
Daniel V: Xenobiotic-inducible expression of murine glutathione
S-transferase Ya subunit gene is controlled by an
electrophile-responsive element. Proc Natl Acad Sci USA.
87:6258–6262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsuoka F, Motohashi H, Ishii T,
Aburatani H, Engel JD and Yamamoto M: Genetic evidence that small
maf proteins are essential for the activation of antioxidant
response element-dependent genes. Mol Cell Biol. 25:8044–8051.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rushmore TH, Morton MR and Pickett CB: The
antioxidant responsive element. Activation by oxidative stress and
identification of the DNA consensus sequence required for
functional activity. J Biol Chem. 266:11632–11639. 1991.PubMed/NCBI
|
|
30
|
Kobayashi A, Ohta T and Yamamoto M: Unique
function of the Nrf2-Keap1 pathway in the inducible expression of
antioxidant and detoxifying enzymes. Methods Enzymol. 378:273–286.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bryan HK, Olayanju A, Goldring CE and Park
BK: The Nrf2 cell defence pathway: Keap1-dependent and -independent
mechanisms of regulation. Biochem Pharmacol. 85:705–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tilly JL: Emerging technologies to control
oocyte apoptosis are finally treading on fertile ground.
ScientificWorldJournal. 1:181–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Davies KJA and Forman HJ:
Oxidative stress response and Nrf2 signaling in aging. Free Radic
Biol Med. 88:314–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tatone C, Amicarelli F, Carbone MC,
Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P and
Focarelli R: Cellular and molecular aspects of ovarian follicle
ageing. Hum Reprod Update. 14:131–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu X, Roberts JR, Apopa PL, Kan YW and Ma
Q: Accelerated ovarian failure induced by 4-vinyl cyclohexene
diepoxide in Nrf2 null mice. Mol Cell Biol. 26:940–954. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: Detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chan JY and Kwong M: Impaired expression
of glutathione synthetic enzyme genes in mice with targeted
deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys
Acta. 1517:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leung L, Kwong M, Hou S, Lee C and Chan
JY: Deficiency of the Nrf1 and Nrf2 transcription factors results
in early embryonic lethality and severe oxidative stress. J Biol
Chem. 278:48021–48029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim J and Luderer U: Oxidative damage
increases and antioxidant gene expression decreases with aging in
the mouse ovary. Biol Reprod. 84:775–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tarín JJ: Potential effects of
age-associated oxidative stress on mammalian oocytes/embryos. Mol
Hum Reprod. 2:717–724. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamatani T, Falco G, Carter MG, Akutsu H,
Stagg CA, Sharov AA, Dudekula DB, VanBuren V and Ko MS:
Age-associated alteration of gene expression patterns in mouse
oocytes. Hum Mol Genet. 13:2263–2278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wiener-Megnazi Z, Vardi L, Lissak A,
Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M
and Dirnfeld M: Oxidative stress indices in follicular fluid as
measured by the thermochemiluminescence assay correlate with
outcome parameters in in vitro fertilization. Fertil Steril. 82
Suppl 3:S1171–S1176. 2004. View Article : Google Scholar
|
|
45
|
Das S, Chattopadhyay R, Ghosh S, Ghosh S,
Goswami SK, Chakravarty BN and Chaudhury K: Reactive oxygen species
level in follicular fluid-embryo quality marker in IVF? Hum Reprod.
21:2403–2407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lim J, Ortiz L, Nakamura BN, Hoang YD,
Banuelos J, Flores VN, Chan JY and Luderer U: Effects of deletion
of the transcription factor Nrf2 and benzo [a]pyrene treatment on
ovarian follicles and ovarian surface epithelial cells in mice.
Reprod Toxicol. 58:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moinova HR and Mulcahy RT: Up-regulation
of the human gamma-glutamylcysteine synthetase regulatory subunit
gene involves binding of Nrf-2 to an electrophile responsive
element. Biochem Biophys Res Commun. 261:661–668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alam J, Wicks C, Stewart D, Gong P,
Touchard C, Otterbein S, Choi AM, Burow ME and Tou J: Mechanism of
heme oxygenase-1 gene activation by cadmium in MCF-7 mammary
epithelial cells. Role of p38 kinase and Nrf2 transcription factor.
J Biol Chem. 275:27694–27702. 2000.PubMed/NCBI
|
|
49
|
Zhao F, Li K, Zhao L, Liu J, Suo Q, Zhao
J, Wang H and Zhao S: Effect of Nrf2 on rat ovarian tissues against
atrazine-induced anti-oxidative response. Int J Clin Exp Pathol.
7:2780–2789. 2014.PubMed/NCBI
|
|
50
|
Wright LE, Frye JB, Lukefahr AL, Marion
SL, Hoyer PB, Besselsen DG and Funk JL: 4-Vinylcyclohexene
diepoxide (VCD) inhibits mammary epithelial differentiation and
induces fibroadenoma formation in female Sprague Dawley rats.
Reprod Toxicol. 32:26–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|