Introduction
Knee osteoarthritis (KOA) is a common disease in the
elderly population (1). Due to the
nonrenewable nature of the articular cartilage, once KOA exists,
the quality of life in elderly patients is largely affected
(1–3). According to a statistical analysis, the
incidence of KOA in China has reached 3–15.6%, and the annual cost
of treatment for KOA is a heavy burden for the family and society
(4). Currently, early treatment of
KOA mainly depends on conservative drug treatments. At the advanced
stage, patients usually undergo artificial total knee arthroplasty
(5,6). However, there are a many controversies
regarding surgery for patients younger than 60 years of age since
surgical trauma will bring many disadvantages. Moreover,
postoperative rehabilitation treatment requires a long time to
ensure the recovery of knee joint function in patients. Hence,
early detection and prevention of KOA are of great importance for
high-risk patients.
Interleukin (IL-17) is a novel cytokine family
secreted mainly by CD4+ T helper 17 cells (Th17),
including IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F
(7,8). By binding the corresponding receptors
on the cell surface, IL-17 is widely involved in bone metabolism
(9,10). Multiple studies have confirmed that
IL-17 is the main factor leading to osteoarthritis bone injury
(9–11). Clinical data also indicate that the
levels of IL-17 and IL-17R are higher in the synovial fluid of
patients with arthritis (11).
Furthermore, high levels of IL-17 and TNFα are correlated with poor
prognosis for osteoarthritis patients (12,13).
However, the underlying mechanism by which IL17 is highly
upregulated in osteoarthritis has been poorly understood.
MicroRNAs (miRNAs/miRs) are small noncoding RNAs
with approximately 23 nucleotides that extensively participate in
posttranscriptional regulation by binding the 3′ untranslated
region (3′UTR) of mRNA (14,15). Due to the ability to be resistant to
RNase, increasing evidence has suggested the use of circulating
miRNAs as potential biomarkers for various diseases (16). For instance, increased levels of
miR-29c, miR-93, and miR-126 are shown to be related to the
progression of osteoarthritis (16).
Abnormal expression of miR-136 has been widely
reported in the development of tumors and metabolic diseases
(17,18). However, we still lack knowledge
regarding whether miR-136 is involved in the progression of KOA.
The current study explored the plasma level of miR-136 in KOA
patients and attempted to determine if it could be used as a
potential biomarker to screen KOA patients from healthy
controls.
Materials and methods
Patient samples
This study was approved by the Ethics Committee of
Rizhao People's Hospital (Rizhao, China) and performed in
accordance with the Declaration of Helsinki. All participants gave
their informed consent.
In total, 50 patients with knee OA were recruited
from February 2016 to December 2016 at the Department of Joint
Surgery, Rizhao People's Hospital, according to the 1986
classification of osteoarthritis of the knee in the diagnostic
criteria of the American Rheumatism Association (19). Diagnosis of osteoarthritis was
performed according to criteria of the American College of
Rheumatology (19). Exclusion
criteria were: i) Symptoms suggestive of any other chronic
inflammatory disease; ii) diabetes; iii) history of corticosteroid
treatment; iv) any other form of arthritis; v) cancer; and vi)
family history of osteoarthritis. X-ray examinations were carried
out to divide patients into early, middle and late KOA stages. All
participants were examined with bilateral knee plain radiographs
(bilateral anteroposterior and lateral) using an SD 3000 Synchro
Stand. Two independent radiologists evaluated radiographic changes
related to OA using the K/L grading system (20). KOA was defined as a K/L grade ≥2 in
both knees. In addition, health volunteers who were 35 years of age
and gender matched at the Department of Physical Examination were
enrolled in the study as health controls (HCs). The details of KOA
patients and healthy controls are listed in Table I.
 | Table I.Comparison of patients baseline data
between groups. |
Table I.
Comparison of patients baseline data
between groups.
| Characteristic | KOAs (n=74) | HCs (n=79) | P-value |
|---|
| Age (mean ±SE,
years) | 65.8±7.3 | 66.2±6.9 | 0.451 |
| Sex (female/male,
n) | 40/34 | 42/37 | 0.298 |
| BMI (mean ±SE,
kg/m2) | 26.7±1.5 | 26.8±1.2 | 0.353 |
| K/L grade
(2/3/4) | 22/29/23 | – |
|
Sample acquisition and RNA
extraction
A 5 ml aliquot of blood was collected from all
participants and directly into sodium citrate tubes. Total RNA was
isolated with RNAVzol LS (Vigorous, Beijing, China) according to
the manufacturer's instructions for isolating small RNAs. The
quality, quantity and integrity of RNA were monitored using a
NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA).
qPCR validation
RNA was reverse transcribed into cDNA using a
PrimeScript OneStep RT-qPCR kit (C28025-032; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The detailed RT-qPCR
procedure is described as follows: 95°C for 10 min; followed by 50
cycles of 95°C for 10 sec, 55°C for 10 sec, 72°C for 5 sec, 99°C
for 1 sec, 59°C for 15 sec, and 95°C for 1 sec; and then cooled to
40°C. The relative expression levels were calculated with the
2−∆∆Cq method (21), and
the experiments were repeated in triplicate. The specific primers
used in the current study are listed as follows: miR-136-RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACAC; U6-RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAATATG; miR-136-F:
GCGCTGGAGTGTGACAATGGTG; U6-F: GCGCGTCGTGAAGCGTTC; Universal reverse
primer: GTGCAGGGTCCGAGGT.
Enzyme-linked immunosorbent assay
(ELISA)
The serum samples were used to quantify the level of
IL-17 using an enzyme-linked immunosorbent assay according to the
manufacturer's instructions (R&D Systems, Inc., Minneapolis,
MN, USA). Samples were read at a 450 nm wavelength using a
microplate reader (Model 3550; Thermo Fisher Scientific, Inc.).
Cell culture
293 cells were seeded at a density of
1.5×104 cells/cm2 and cultured in Dulbecco's
modified Eagle's medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% heat-inactivated fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.), streptomycin (100 mg/ml,
Gibco; Thermo Fisher Scientific, Inc.), and penicillin (100 U/ml;
Gibco; Thermo Fisher Scientific, Inc.) in an incubator at 37°C with
5% CO2.
miRNA target prediction and
dual-luciferase reporter assay
miRNA targets were predicted using TargetScan
(https://www.targetscan.org). The 3′UTR
of IL-17, which contains the predicted target site for miR-136, was
cloned into the pmirGLO luciferase reporter vector (Promega
Corporation, Madison, WI, USA), which was cleaved at SacI
and XhoI sites. The details of the PCR procedures are
described as follows: A hot start step at 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec, 55°C for 45 sec and 72°C for 30
sec.
Prior to conducting the dual reporter assay,
5×104 293 cells/well were seeded in 24-well plates with
500 µl DMEM and cultured for 18 h. The cells were transfected with
the modified firefly luciferase reporter vector (500 ng/µl) and
mixed with Vigofect transfection reagent (Vigorous, Beijing, China)
according to the manufacturer's protocol. After continuous exposure
to miR-136/pmirGLO-IL-17-3′UTR or NC/pmirGLO blank vector for 48 h,
the firefly and Renilla luciferase activities were measured
with the Dual-Luciferase® Reporter Assay system (Promega
Corporation) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Cell transfection
miR-136 mimic, inhibitor and negative control (NC)
were purchased from Genepharma Co., Ltd. (Shanghai, China). In
brief, 6×105 cells were equally seeded in 6-well plates
with 2 ml DMEM containing serum and antibiotics. At the same time,
miR-136 mimic, inhibitor or NC was mixed with HiPerFect
transfection reagent (Qiagen, Inc., Valencia, CA, USA) and
incubated at room temperature for 10 min. The complex was then
transfected into the cells for 48 h.
Western blotting
Cell protein was extracted using
radioimmunoprecipitation lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and was collected
following centrifugation at 12,000 × g for 30 min at 4°C. A
bicinchoninic protein assay kit (Pierce; Thermo Fisher Scientific,
Inc.) was used to determine the protein concentration. A total of
15 µg protein was loaded per lane, separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with 8% non-fat dry milk at 4°C overnight. Following
three washes with PBS with Tween 20 (5 min/wash), the membranes
were incubated with the following primary antibodies at 4°C
overnight: IL-17 (cat no. 13838; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) and GAPDH (cat. no. 5174;
1:1,000; Cell Signaling Technology, Inc.). Following several washes
with TBST, the membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and anti-mouse Immunoglobulin G
(IgG) or HRP-conjugated mouse anti-goat IgG (all 1:5,000; Zhongshan
Gold Bridge Biological Technology Co., Beijing, China) for 2 h at
room temperature and then washed. The blots were then incubated
with horseradish peroxidase (HRP)-conjugated anti-IgG secondary
antibody (1:5,000; OriGene Technologies, Inc., Beijing, China) for
2 h at room temperature and then washed, followed by detection with
enhanced chemiluminescent substrate (EMD Millipore, Billerica, MA,
USA). GAPDH was used as an internal control. ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used for
density analysis.
Receiver operating characteristic
(ROC) curves
An ROC curve was established to interpret the
ability of miR-136 in discriminating KOA patients from healthy
controls. In brief, the fluorescence signals of each reaction well
were collected quantitatively, and the Cq values were recorded. The
expression of miR-136 in KOA patients relative to healthy control
was expressed as 2−∆∆Cq. ΔCq is the difference of Cq
values of the target gene to the internal reference U6 in each
sample, namely ∆Cq=Cq (miR-136)-Cq (U6), ∆∆Cq=(Cq miR-136-Cq U6)
KOA-(Cq miR-136-Cq U6) normal. To perform ROC analysis,
2−∆∆Cq was recorded parallel to the diagnostic results
of gold standard, that is healthy control=0, KOA patients=1. Then,
the area under the curve (AUC), sensitivity and specificity were
computed in order to validate the diagnostic application of miR-136
as KOA biomarkers in contrast to the diagnostic results of gold
standard (version 20.0, SPSS, Inc., Chicago, Illinois).
Statistics
The data are represented as the mean ± standard
error. The two-tailed unpaired Student's t-tests were used for
comparisons of the two groups. For multiple groups comparisons,
one-way analysis of variance followed by Tukey's post hoc test was
used. Statistical tests were performed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Reduced plasma miR-136 and increased
serum IL-17 levels in KOA patients
First, we examined the plasma levels of miR-136 in
KOA patients and healthy controls. Compared with healthy controls
(1±1.07), the plasma levels of miR-136 were significantly increased
to 0.55±0.37, 0.26±0.27, and 0.08±0.17 for patients at the early,
middle and late KOA stages, respectively (Fig. 1A). Next, serum IL-17 levels were
determined using an ELISA kit. In contrast to healthy controls
(3.23±2.86 ng/ml), the levels of IL-17 for patients at the early,
middle and late KOA stages were 34.37±8.93, 69.65±15.41, and
96.42±21.45 ng/ml, respectively (Fig.
1B).
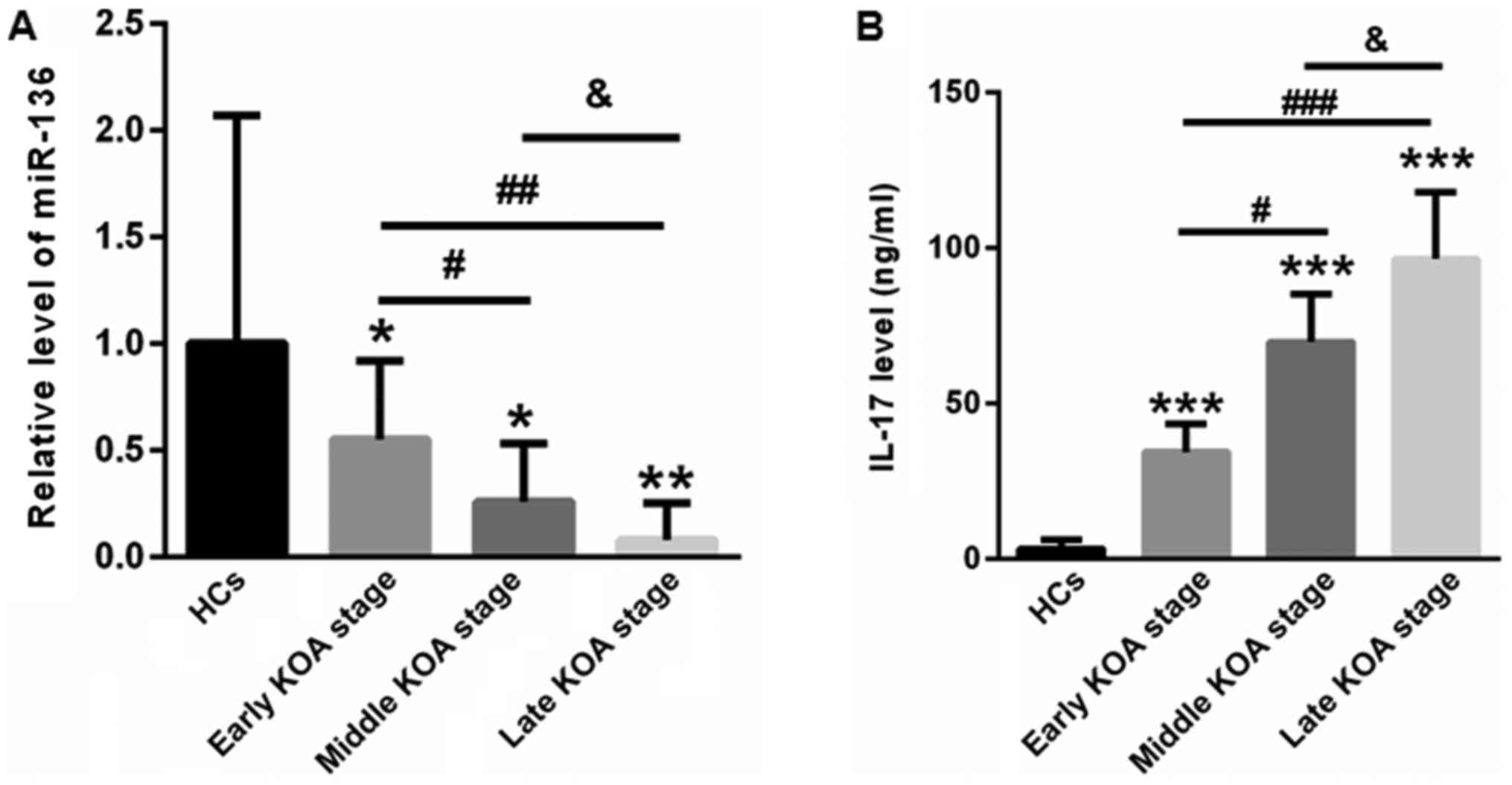 | Figure 1.Plasma miR-136 levels were reduced and
serum IL-17 levels were increased in KOA patients compared with
those in healthy controls. (A) qPCR analysis showed that plasma
levels of miR-136 were significantly decreased in patients at the
early, middle and late KOA stages, respectively. (B) In contrast to
healthy controls, the levels of IL-17 were increased in patients at
the early, middle and late KOA stages. *P<0.05, **P<0.01,
***P<0.001 vs. healthy control; #P<0.05,
##P<0.01, ###P<0.001;
&P<0.05 vs. as indicated. miR, microRNA; IL,
interleukin; KOA, knee osteoarthritis. |
miR-136 was reduced along with
increased K/L grades
Moreover, we examined miR-136 and Il-17 levels based
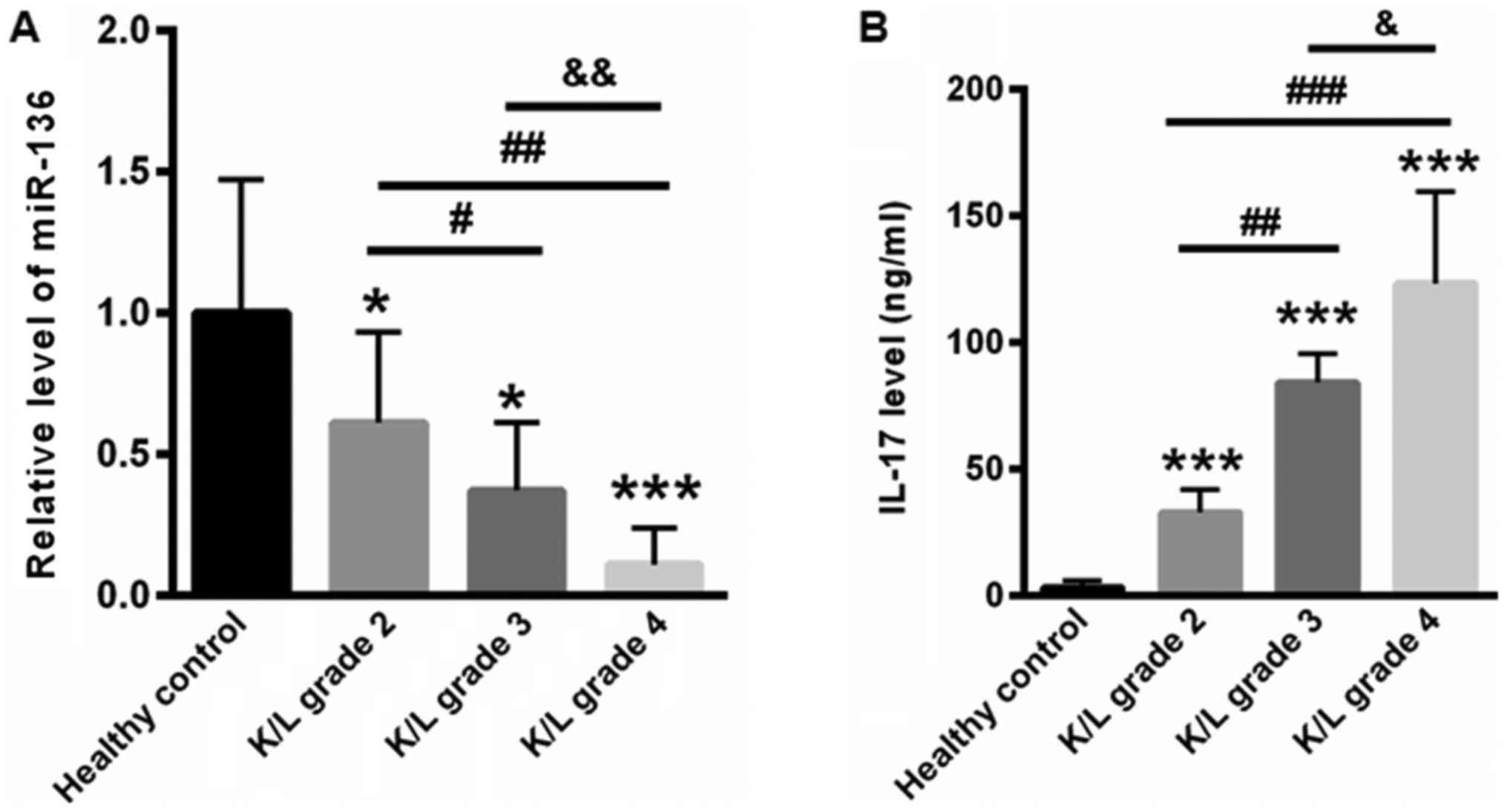
on the K/L grade. Our data showed that the level of plasma miR-136
was 1±0.47 but was much lower in the K/L grade 2 (0.61±0.32), grade
3 (0.37±0.24), and grade 4 (0.11±0.13) groups (Fig. 2A). In addition, we determined the
serum IL-17 levels according to the K/L grade. Compared with
healthy controls (3.23±2.86 ng/ml), the levels of IL-17 for K/L
grade 2, 3, and 4 KOA patients were 32.87±8.97, 84.32±11.45, and
123.34±36.45 ng/ml, respectively (Fig.
2B).
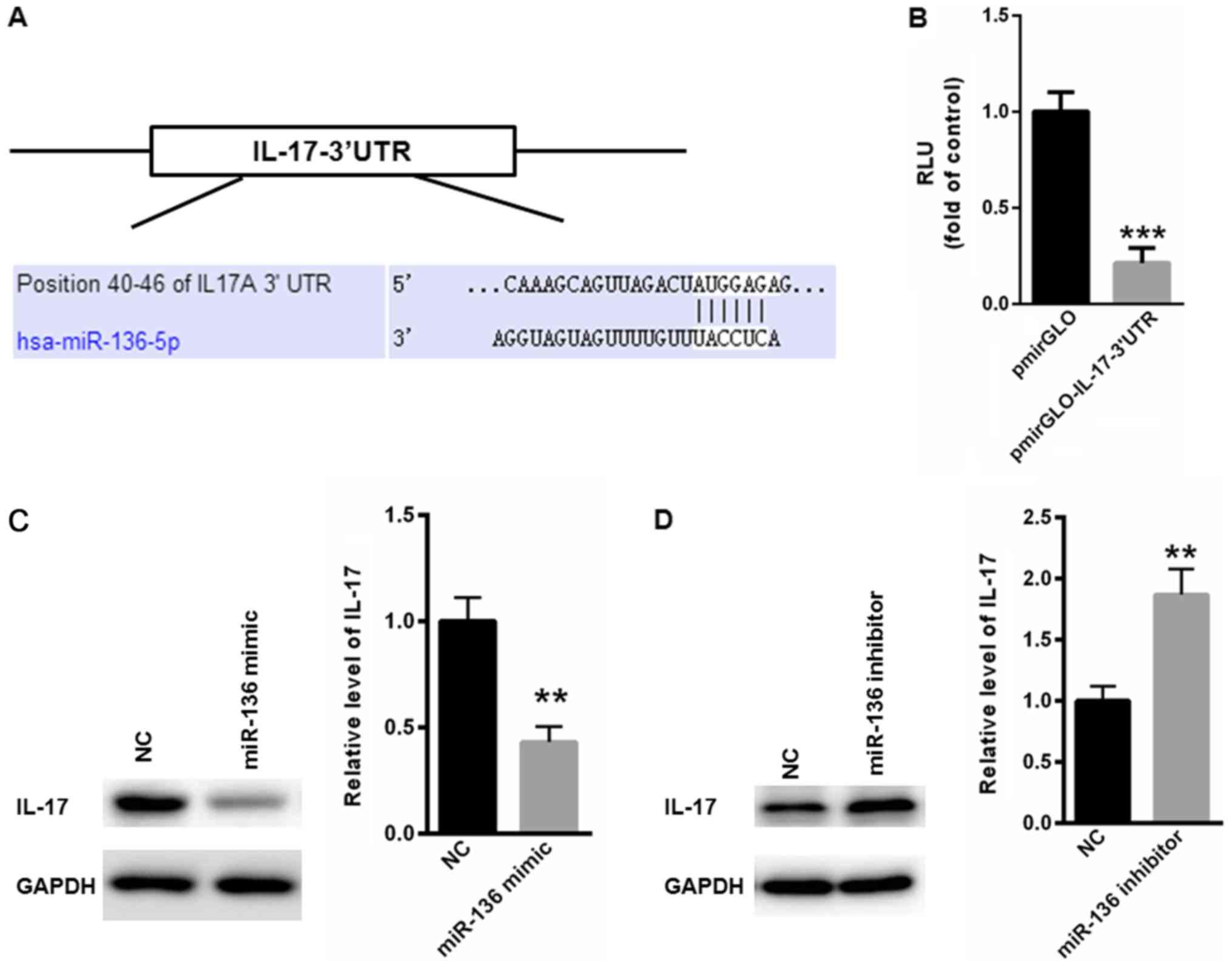
IL-17 is a target gene of miR-136
The above observations motivated us to explore the
underlying mechanism by which miR-136 levels were negatively
correlated with the serum levels of IL-17. TargetScan analysis
showed a conserved binding site of miR-136 in the 3′UTR of IL-17
(Fig. 3A). Dual-luciferase reporter
assay showed that miR-136 significantly suppressed the relative
luciferase activity of pmirGLO-IL-17-3′UTR, compared with the blank
pmirGLO plasmid (Fig. 3B). The
western blot assay also indicated that overexpression of miR-136
significantly suppressed the protein level of IL-17 (Fig. 3C), while inhibition of miR-136
significantly increased the expression of IL-17 (Fig. 3D). These data validated that IL-17
was a target gene of miR-136.
miR-136 could be used to screen KOA
patients from healthy controls
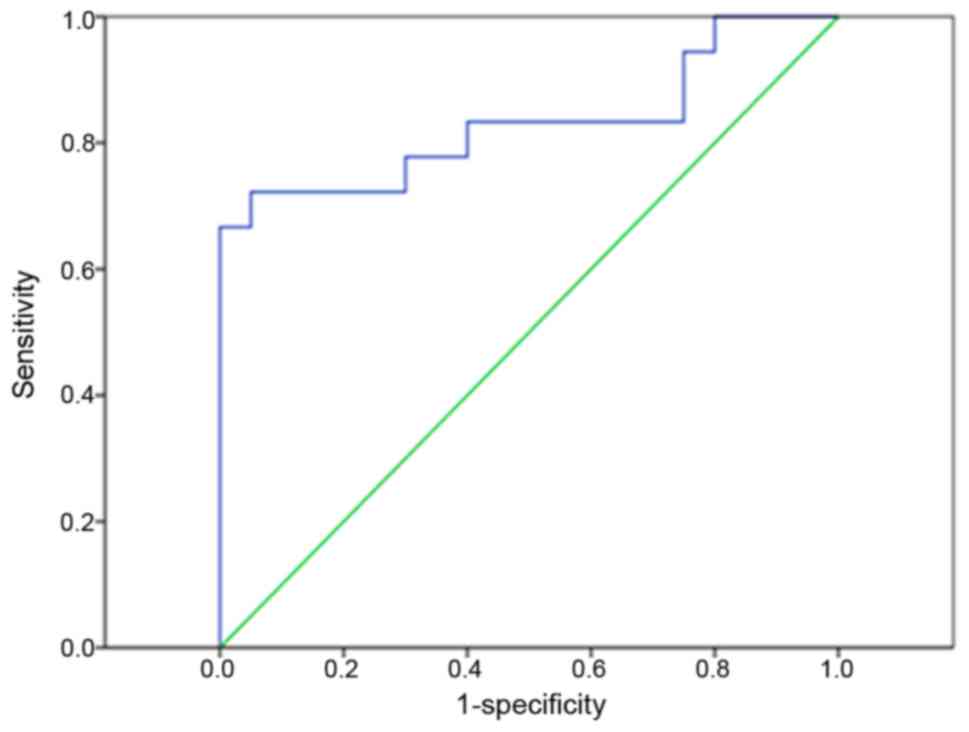
We then evaluated whether miR-136 could be used as a
potential biomarker for patients with KOA. ROC analysis showed that
the plasma miR-136 level could be used to screen KOA patients from
healthy controls, with an ROC curve area of 0.935 (95% confidence
interval: 0.817–1.000; P<0.0001) (Fig. 4).
Discussion
KOA, a chronic progressive disease, is a common
disease in the elderly. With the advent of an aging society, the
incidence of this disease is increasing (22,23).
Undoubtedly, it is of great significance to improve the quality of
life of patients with KOA (5).
Increasing evidence has associated key roles of miRs
with the progression of KOA (24,25). For
example, miR-9 is shown to modulate the development of KOA via the
NF-kappaB1 pathway in chondrocytes (24). miR-29a is also reported to suppress
synovitis in the pathogenesis of KOA by targeting VEGF (25). Abnormal expression of miR-136-5p
after spinal cord injury has been identified. He et al
reported that overexpression of miR-136-5p effectively enhanced
inflammatory factors and chemokines via activating NF-κB/A20
signaling in the IL-17-mediated inflammatory response both in
vitro and in vivo (26).
In IL-17-stimulated astrocytes, miR-136-5p is also found to
increase inflammatory responses via suppressing the expression of
A20 (27). However, whether miR-136
is involved in IL-17 induced inflammatory responses in KOA patients
has never been explored. In the current study, we mainly focused on
miR-136, which has been extensively studied in various diseases.
However, there have been limited studies on the role of miR-136 in
the pathology of KOA. Here, we showed novel data that the plasma
miR-136 level was significantly decreased in the plasma of KOA
patients. Moreover, the reduction in plasma miR-136 levels was
negatively correlated with the severity of KOA.
As a potent inflammatory factor, IL-17 plays an
important role in the development of a variety of inflammatory
diseases, and it is also an important regulator in the development
of bone metabolism and bone diseases (28). It is suggested that IL-17 can
regulate osteoblast differentiation and bone salt deposition.
Meanwhile, through the stimulation of osteoblasts, bone cells, and
stromal cells, IL-17 can enhance osteoclast activity and promote
bone degradation (12,29). Targeting IL-17 or IL-17 receptors
(IL-17R) is becoming a hot spot for the treatment of inflammatory
diseases and inflammatory bone disease (29,30).
Chen et al conducted a retrospective study and reported that
Serum IL-17 concentrations were much higher in Han Chinese patients
with primary KOA (n=98) than those in healthy control (n=50)
(29). Deligne et al explored
the expression of IL-17 in the inflamed and the non-inflamed area
of each synovium sample (n=20) from human osteoarthritic knee
tissues (30). They demonstrated the
expression of IL-17 is much higher in the the inflamed than that of
the non-inflamed area (30). Both of
them pointed out that IL-17 may be effective in the early
prevention and therapy for KOA patients (29,30).
Therefore, in the future, it will be important to study the signal
transduction and pathogenic mechanisms of IL-17/IL-17R in order to
find a new therapeutic target for KOA therapy.
In the current study, we also evaluated the level of
IL-17 in the serum of KOA patients and healthy controls. Our data
showed that the increase in serum IL-17 levels positively
correlated with the severity of KOA. The negative correlation
between IL-17 and miR-136 promoted us to explore its underlying
mechanism. Interestingly, we found that IL-17 is a target gene of
miR-136. Further analysis showed that plasma miR-136 levels could
be used as a biomarker to screen KOA patients from healthy
controls.
In summary, for the first time, we found that plasma
miR-136 levels were significantly decreased in KOA patients. More
importantly, by targeting IL-17, miR-136 could be used as a
potential biomarker for KOA patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Scientific Research Starting Foundation of Rizhao People's Hospital
(grant no. 20160823).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL performed the experiments and analyzed the data.
ZQ, NG, XT, DC performed part of the RT-qPCR experiments. WS
designed the experiments, analyzed the data and gave final approval
of the version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Rizhao People's Hospital (Rizhao, China) and
all the patients have provided written informed consent for this
study.
Patient consent for publication
Informed consent for participation in the study or
use of their tissue was obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evaniew AL and Evaniew N: Knee
osteoarthritis: Therapeutic alternatives in primary care. World J
Orthop. 8:187–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monfort J, Pujol J, Contreras-Rodriguez O,
Llorente-Onaindia J, López-Solà M, Blanco-Hinojo L, Vergés J,
Herrero M, Sánchez L, Ortiz H, et al: Effects of chondroitin
sulfate on brain response to painful stimulation in knee
osteoarthritis patients. A randomized, double-blind,
placebo-controlled functional magnetic resonance imaging study. Med
Clin (Barc). 148:539–547. 2017.(In English, Spanish).
|
|
3
|
Niu J, Clancy M, Aliabadi P, Vasan R and
Felson DT: Metabolic syndrome, its components, and knee
osteoarthritis: The Framingham Osteoarthritis Study. Arthritis
Rheumatol. 69:1194–1203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Leeuwen DM, van de Bunt F, de Ruiter
CJ, van Schoor NM, Deeg DJH and Emanuel KS: Functioning without
cartilage: Older people with radiographic knee osteoarthritis who
self-report no functional limitations do score lower on a
performance battery. J Aging Phys Act. 25:570–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rongen JJ, Rovers MM, van Tienen TG, Buma
P and Hannink G: Increased risk for knee replacement surgery after
arthroscopic surgery for degenerative meniscal tears: A
multi-center longitudinal observational study using data from the
osteoarthritis initiative. Osteoarthritis Cartilage. 25:23–29.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oo WM, Linklater JM and Hunter DJ: Imaging
in knee osteoarthritis. Curr Opin Rheumatol. 29:86–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang K, Xu J, Cai J, Zheng S, Han W,
Antony B and Ding C: Serum levels of interleukin-17 and adiponectin
are associated with infrapatellar fat pad volume and signal
intensity alteration in patients with knee osteoarthritis.
Arthritis Res Ther. 18:1932016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Xu J, Cai J, Zheng S, Yang X and
Ding C: Serum levels of resistin and interleukin-17 are associated
with increased cartilage defects and bone marrow lesions in
patients with knee osteoarthritis. Mod Rheumatol. 27:339–344. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suurmond J, Dorjée AL, Boon MR, Knol EF,
Huizinga TW, Toes RE and Schuerwegh AJ: Mast cells are the main
interleukin 17-positive cells in anticitrullinated protein
antibody-positive and -negative rheumatoid arthritis and
osteoarthritis synovium. Arthritis Res Ther. 13:R1502011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Baarsen LG, Lebre MC, van der Coelen
D, Aarrass S, Tang MW, Ramwadhdoebe TH, Gerlag DM and Tak PP:
Heterogeneous expression pattern of interleukin 17A (IL-17A),
IL-17F and their receptors in synovium of rheumatoid arthritis,
psoriatic arthritis and osteoarthritis: Possible explanation for
nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 16:4262014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Southam L, Heath O, Chapman K and Loughlin
J: Association analysis of the interleukin 17 genes IL17A and IL17F
as potential osteoarthritis susceptibility loci. Ann Rheum Dis.
65:556–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attur MG, Patel RN, Abramson SB and Amin
AR: Interleukin-17 up-regulation of nitric oxide production in
human osteoarthritis cartilage. Arthritis Rheum. 40:1050–1053.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Honorati MC, Bovara M, Cattini L,
Piacentini A and Facchini A: Contribution of interleukin 17 to
human cartilage degradation and synovial inflammation in
osteoarthritis. Osteoarthritis Cartilage. 10:799–807. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu W, Zhang W, Li F, Guo F and Chen A:
miR-139 is up-regulated in osteoarthritis and inhibits chondrocyte
proliferation and migration possibly via suppressing EIF4G2 and
IGF1R. Biochem Biophys Res Commun. 474:296–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu X, Lin J, Jin J, Qian W and Weng X:
Hsa-miR-15a exerts protective effects against osteoarthritis by
targeting aggrecanase-2 (ADAMTS5) in human chondrocytes. Int J Mol
Med. 37:509–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borgonio Cuadra VM, González-Huerta NC,
Romero-Córdoba S, Hidalgo-Miranda A and Miranda-Duarte A: Altered
expression of circulating microRNA in plasma of patients with
primary osteoarthritis and in silico analysis of their pathways.
PLoS One. 9:e976902014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin R, Xu S, Lin X and Shen M: MiR-136
controls neurocytes apoptosis by regulating Tissue Inhibitor of
Metalloproteinases-3 in spinal cord ischemic injury. Biomed
Pharmacother. 94:47–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan M, Li X, Tong D, Han C, Zhao R, He Y
and Jin X: miR-136 suppresses tumor invasion and metastasis by
targeting RASAL2 in triple-negative breast cancer. Oncol Rep.
36:65–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and Therapeutic Criteria Committee of the
American Rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pas HI, Winters M, Haisma HJ, Koenis MJ,
Tol JL and Moen MH: Stem cell injections in knee osteoarthritis: A
systematic review of the literature. Br J Sports Med. 51:1125–1133.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pihl K, Englund M, Lohmander LS, Jørgensen
U, Nissen N, Schjerning J and Thorlund JB: Signs of knee
osteoarthritis common in 620 patients undergoing arthroscopic
surgery for meniscal tear. Acta Orthop. 88:90–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu R, Liu N, Luo S, Huang W, Zha Z and
Yang J: MicroRNA-9 regulates the development of knee osteoarthritis
through the NF-kappaB1 pathway in chondrocytes. Medicine
(Baltimore). 95:e43152016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko JY, Lee MS, Lian WS, Weng WT, Sun YC,
Chen YS and Wang FS: MicroRNA-29a counteracts synovitis in knee
osteoarthritis pathogenesis by targeting VEGF. Sci Rep. 7:35842017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He J, Zhao J, Peng X, Shi X, Zong S and
Zeng G: Molecular mechanism of MiR-136-5p targeting NF-κB/A20 in
the IL-17-mediated inflammatory response after spinal cord injury.
Cell Physiol Biochem. 44:1224–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng X, Shi X, Zhao J, He J, Li K, Cen Z,
Wu Y, Zong S and Zeng G: The effects of miR-136-5p-mediated
regulation of A20 in astrocytes from cultured spinal cord cultured
cells in vitro. Cell Physiol Biochem. 41:1596–1604. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Retraction note: Mast cells are the main
interleukin 17-positive cells in anticitrullinated protein
antibody-positive and -negative rheumatoid arthritis and
osteoarthritis synovium. Arthritis Res Ther. 17:3542015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen B, Deng Y, Tan Y, Qin J and Chen LB:
Association between severity of knee osteoarthritis and serum and
synovial fluid interleukin 17 concentrations. J Int Med Res.
42:138–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deligne C, Casulli S, Pigenet A, Bougault
C, Campillo-Gimenez L, Nourissat G, Berenbaum F, Elbim C and Houard
X: Differential expression of interleukin-17 and interleukin-22 in
inflamed and non-inflamed synovium from osteoarthritis patients.
Osteoarthritis Cartilage. 23:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|