Introduction
The transradial approach (TRA) is widely preferred
in coronary procedures and is the recommended choice of access
particularly in the invasive treatment of acute coronary syndromes
(1). Lower rates of major vascular
complications, improved patient comfort and shorter hospital stay
are the major advantages of TRA compared to transfemoral access
(2); vascular complications,
including hemorrhage or pseudoaneurysm are rarely encountered
(3). The most important
disadvantages are radial artery spasm (RAS) (4) and RA occlusion (RAO) (5).
A small RA diameter (RAD), difficult access,
inexperienced operators and the presence of peripheral arterial
disease have been identified as predictors of RA access failure
(6). In a recently published study,
female sex, older age and short stature were reported as enhancing
factors of technical failure risk in TR angiography (7). The RA is a small-sized artery whose
diameter ranges from 1.15 to 3.95 mm (8). Smaller RADs have been associated with
vascular complications (9,10). Multiple puncture attempts increase
the risk of vasospasm and may result in hemorrhage, dissection,
pseudoaneurysm, thrombosis and pain. Radial sheath insertion may
lead to endothelial damage and multiple puncture attempts, and
increase the risk of endothelial dysfunction (11). A RAD/sheath diameter (<1 mm)
indicated to be a risk factor for RAS (12) and RAO (13). To prevent this damage and safely
complete the procedure, vasodilator medications are mainly used.
Various maneuvers and medications have been used to increase RAD
and enhance RA cannulation (14–16). The
strategy of increasing radial artery flow by ulnar artery
compression (UAC) has been previously reported (17). In a recently published study, ulnar
occlusion for 30 min increased the RAD and facilitated access
(18). However, application of UAC
for 30 min is not feasible in each patient. The aim of the
preliminary study was to assess the effect of 1 min of ipsilateral
transient UAC on RAD.
Patients and methods
Patients
A total of 151 consecutive patients who were
referred to the Department of Cardiology (Trakya University
Hospital, Edirne, Turkey) for coronary angiography between December
2016 and July 2017 were included in the present study. The
procedures of the current study were not a part of the routine
coronary angiography and USG was performed the day before the
coronary angiography procedure. Patients with hemodynamic
instability, non-palpable UAs or RAs, those who were on
hemodialysis or with a history of RA access were excluded. The
present study was approved by the Scientific Research Ethics
Committee of Trakya University (approval no. 2016/286). All
patients provided written informed consent. The study was performed
in accordance with the Declaration of Helsinki.
The patients' demographic data and the presence of
coronary artery disease risk factors were all recorded.
Evaluation of the effect of UAC on
RAD
RA ultrasonography was performed on the day before
the coronary angiography procedure, by an experienced radiologist
after 10 min of supine rest (baseline) using a 6–18 MHz linear
array transducer (Mylab 70 XVG; Esaote Medical Systems SpA, Genova,
Italy). RAD was measured from two-dimensional gray scale transverse
images at the wrist level, 2 cm proximal to the styloid process.
Following the resting measurements, UAC was applied by manual
compression for 1 min at the level of the wrist joint, at Guyon's
canal, with complete obliteration of the UA as confirmed through
ultrasonographic assessment. During UAC, the RA was continuously
observed. The RA peak systolic flow velocity was also measured at
baseline and during UAC. The RAD was measured at the end of the UAC
(1st minute) and at 1 min after stopping UAC (2nd minute). All
images and patient numbers were digitally recorded and the
measurements were performed offline. An experienced sonographer,
who was blinded to the study design, measured the diameters and
blood flow. The average of two values was accepted as the RAD. To
evaluate inter-observer variability, the recorded images of 30
randomly selected patients were analyzed by another sonographer.
Intra-observer variability was also evaluated by having 10 randomly
selected patients re-measured by the same operator.
The sample size was determined according to the
estimated increase in the RAD. In a recently published study by
Zhou et al (18), 30 min of
UAC caused a 0.11-mm increase in the RAD. A 0.15-mm increase in the
RAD was assumed in the present study. For an estimated statistical
power of 80%, 114 patients were sufficient for a 0.15-mm increase
to reach statistical significance.
Statistical analysis
Statistical analysis was performed using SPSS
version 20 for Windows (IBM Corp., Armonk, NY, USA). Categorical
variables are expressed as numbers and percentages. A
Kolmogor-Smirnov test was used to assess the distribution of
variables. Normally distributed continuous variables are expressed
as the mean ± standard deviation and were compared using the
independent-samples t-test. Non-normally distributed continuous
variables were compared using the Mann-Whitney U test. Categorical
values were compared with χ2 test. The change in the RAD
and the peak systolic velocity at baseline, during and after UAC
were calculated using one-way repeated-measures analysis of
variance followed by Bonferroni's post-hoc test for multiple
comparisons. Wilcoxon's signed rank test was used for comparison of
the peak RA velocity at baseline with that during UAC. For
assessing the correlation of non-normally distributed variables,
Spearman's rank test was used to calculate the correlation
coefficient (r) and its significance. Inter- and intra-observer
reliability were evaluated using Kappa (κ) statistics. P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
A total of 151 patients were enrolled in the present
study. The characteristics of the patients at baseline are
presented in Table I. The mean
resting RAD was 2.45±0.41 mm. The baseline characteristics of
female and male, and diabetic and non-diabetic patients were also
compared and are presented in Tables
II and III. The RAD was
significantly smaller in diabetic vs. non-diabetic patients
(2.35±0.43 vs. 2.50±0.39 mm, P=0.024) and in women vs. men
(2.25±0.38 vs. 2.56±0.38 mm, P<0.001). A weak but significant
correlation of baseline RAD with the body height and weight was
identified (r=0.313, P<0.001; and r=0.328, P<0.001,
respectively; data not shown). However, the correlation between the
body mass index and the baseline RAD was not significant (r=0.137,
P=0.093; data not shown).
 | Table I.Demographic and clinical data of the
patients (n=151). |
Table I.
Demographic and clinical data of the
patients (n=151).
| Characteristic | Value |
|---|
| Age (years) | 63 (33–84) |
| Male/female | 97/54 |
| BMI
(kg/m2) | 28.5±5.2 |
| Height (cm) | 165.2±8.9 |
| Weight (kg) | 77.6±14.6 |
| Diabetes
mellitus | 55 (36.4) |
| Hypertension | 128 (84.8) |
| Hyperlipidemia | 113 (74.8) |
| Smoker | 55 (36.4) |
| CAD | 137 (90.7) |
| β-blockers | 104 (68.9) |
| Ca-channel
blockers | 74 (49) |
| ACE-I/ARB | 99 (65.6) |
| Long-acting
nitrates | 41 (27.2) |
 | Table II.Demographic, clinical data and radial
artery diameter by sex. |
Table II.
Demographic, clinical data and radial
artery diameter by sex.
| Characteristic | Male | Female | P-value |
|---|
| N (%) | 97 (36.4) | 54 (63.6) |
|
| Age (years) | 63 (33–81) | 62 (36–84) |
0.910 |
| Height (cm) | 169.2±7.4 | 158.1±7.1 | <0.001 |
| Weight (kg) | 80.1±14.1 | 72.9±14.9 |
0.006 |
| BMI
(kg/m2) | 28.0±4.6 | 29.3±6.1 |
0.159 |
| Diabetes
mellitus | 30 (30.9) | 25 (46.3) |
0.060 |
| Hypertension | 82 (84.5) | 46 (85.2) |
0.915 |
| Hyperlipidemia | 76 (78.4) | 37 (68.5) |
0.182 |
| Smoker | 43 (44.3) | 12 (22.2) |
0.007 |
| CAD | 94 (96.9) | 43 (79.6) | <0.001 |
| β-blockers | 69 (71.1) | 35 (64.8) |
0.421 |
| Ca-channel
blockers | 46 (47.4) | 28 (51.9) |
0.602 |
| ACE-I/ARB | 64 (66.0) | 35 (64.8) |
0.885 |
| Long-acting
nitrates | 23 (23.7) | 18 (33.3) |
0.203 |
| RAD (mm) | 2.56±0.38 | 2.25±0.38 | <0.001 |
 | Table III.Demographic, clinical data and radial
artery diameter based on diabetes diagnosis. |
Table III.
Demographic, clinical data and radial
artery diameter based on diabetes diagnosis.
| Characteristic | Diabetic | Not diabetic | P-value |
|---|
| N (%) | 55 (36.4) | 96 (63.6) |
|
| Age (years) | 63(33–81) | 62 (38–84) |
0.227 |
| Male/Female | 30/25 | 67/29 |
0.060 |
| Height (cm) | 163.2±8.9 | 166.4±8.8 |
0.035 |
| Weight (kg) | 81.5±13.9 | 75.4±14.6 |
0.011 |
| BMI
(kg/m2) | 30.7±5.6 | 27.2±4.5 | <0.001 |
| Hypertension | 53 (96.4) | 75 (78.1) | <0.001 |
| Hyperlipidemia | 46 (83.6) | 67 (69.8) |
0.059 |
| Smoker | 15 (21.3) | 40 (41.7) |
0.077 |
| CAD | 55 (100) | 82 (85.4) |
0.003 |
| β-blockers | 43 (78.2) | 61 (63.5) |
0.062 |
| Ca-channel
blockers | 26 (47.3) | 48 (50) |
0.747 |
| ACE-I/ARB | 43 (78.2) | 56 (58.3) |
0.014 |
| Long-acting
nitrates | 20 (36.4) | 21 (21.9) |
0.054 |
| RAD (mm) | 2.35±0.43 | 2.50±0.39 |
0.024 |
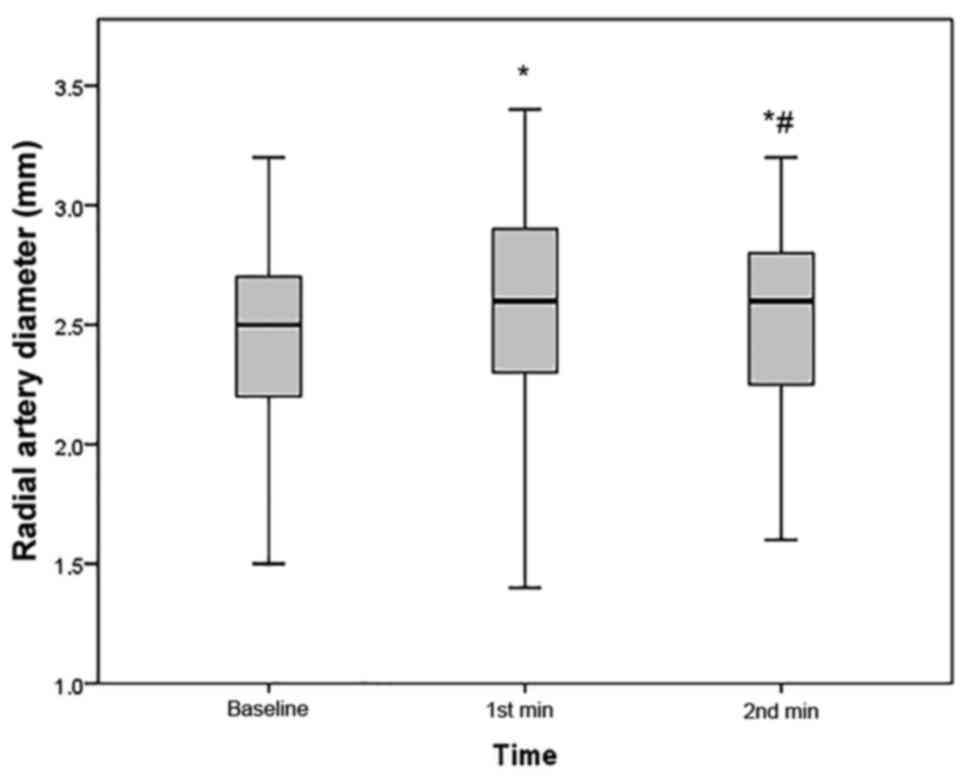
Efficacy of 1 min of UAC to increase
the RAD
Compared with the baseline, the RAD significantly
increased after UAC (2.45±0.41 vs. 2.62±0.41 mm, P<0.001;
Fig. 1). The increase in RAD was
0.17±0.09 mm. In comparison with that immediately after 1 min of
UAC, the mean RAD at 1 min after stopping UAC was significantly
decreased (2.62±0.41 vs. 2.55±0.40 mm, P<0.001); however, it was
still larger than the baseline RAD (P<0.001; Fig. 1). The RA peak systolic flow velocity
also significantly increased during UAC (35.3±8.9 vs. 60.3±19.2
cm/sec, P<0.001).
Regarding intra-observer reliability, as assessed
using κ statistics, an almost perfect agreement was determined
(κ=0.819), while assessment of inter-observer reliability indicated
a substantial agreement (κ=0.710).
Discussion
The present study demonstrated that transient
ipsilateral UAC for 1 min increases the RAD and the RA peak
systolic flow velocity. TR access is the recommended choice for
coronary procedures, but there are certain limitations, e.g., RAS
and RAO. The thicker muscular layer and the predominance of α
receptors make the RA naturally prone to spasm. To overcome
vasospasm, several techniques and co-medications have been used
(4,19,20).
Sedation, smaller-size hydrophilic sheaths and catheters, and
vasodilatory cocktails are widely used to prevent spasm.
Hypotension and small-sized, deeply located RAs
increase the difficulty of successful puncture, particularly for
less experienced operators. Pre-procedural anxiety, pain during
local anesthetic injection or puncture attempts may also cause
vasospasm. Sedatives given to overcome anxiety may cause
hypotension and loss of RA pulse, which complicates successful
puncture. As the number of attempts increases, the RA also becomes
more susceptible to spasm (21).
A small-diameter RA is the most important factor
that affects successful access. Similar to the results of previous
studies, the RAD determined in the present study ranged from
1.3–3.2 mm (mean value, 2.45±0.41 mm) (8). The RAD is significantly smaller in
women and patients with diabetes, and may be as small as 1.3 mm,
smaller than 5-F sheaths or catheters that are used in the majority
of coronary procedures performed via the radial route. It was
indicated that a smaller RAD, particularly an RA internal diameter
to sheath outer diameter ratio of <1, is a major predictor of
RAS (12). Of note, for patients of
small stature, diabetics and female patients, RA ultrasonography
prior to coronary angiography may be helpful to prevent access site
complications.
Various drugs and maneuvers have been used to dilate
the RA and facilitate radial RA (14–16).
Transient ipsilateral UAC, by directing blood flow to non-UAs,
mainly increases radial flow (17).
UAC was reported to be effective in reducing RAO after TRA
(22–25). In a recent study by Kaplanoglu and
Beton (26), the effects of 1 min of
UAC on radial artery diameter and consecutive radial artery
compression on ulnar artery diameter were assessed. Their results
were in line with those of the present study, indicating an
increase in the RAD (2.2+0.4 vs. 2.4+04 mm, P<0.001) and blood
flow with UAC. In addition to this previous study, the present
study demonstrated that the RAD at 1 min after the end of UAC was
still significantly larger than the baseline value. In another
recently published study, UAC for 30 min significantly increased
the RAD (2.28±0.44 vs. 2.39±0.50 mm, P=0.042) (18). The authors stated that UAC
facilitated radial access with fewer puncture attempts and reduced
vascular access time. In contrast to the abovementioned study, UAC
was applied in the present study for 1 min only, and this shorter
time was also sufficient to increase the RAD. Based on the study by
Zhou et al (18), it may be
assumed that the enlargement in the RAD achieved in the present
study may also facilitate RA access and decrease RAS; however, this
requires further investigation.
Of note, the present study had certain limitations.
First, it was a single-center study. It was demonstrated that UAC
for 1 min was effective in dilating the RA, but further studies
with clinical endpoints are required to assess the real-life
consequences of UAC. Finally, although RAD was still larger than
the baseline measurement at 1 min after UAC, data regarding the
duration of RA enlargement are currently lacking.
In conclusion, the application of UAC for 1 min
increases the RAD, which may be performed as a strategy to
facilitate the TRA in coronary procedures. Further prospective,
randomized studies with clinical endpoints are required to assess
the feasibility of this method.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAY and EY performed the enrolment of the patients,
data collection and ultrasound imaging. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Scientific
Research Ethics Committee of Trakya University. All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ibanez B, James S, Agewall S, Antunes MJ,
Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA,
Halvorsen S, et al: 2017 ESC Guidelines for the management of acute
myocardial infarction in patients presenting with ST-segment
elevation: The Task Force for the management of acute myocardial
infarction in patients presenting with ST-segment elevation of the
European Society of Cardiology (ESC). Eur Heart J. 39:119–177.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhat FA, Changal KH, Raina H, Tramboo NA
and Rather HA: Transradial versus transfemoral approach for
coronary angiography and angioplasty-A prospective, randomized
comparison. BMC Cardiovasc Disord. 17:232017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tatli E, Buturak A, Cakar A, Vatan BM,
Degirmencioglu A, Agac TM, Kilic H, Gunduz H and Akdemir R: Unusual
vascular complications associated with transradial coronary
procedures among 10,324 patients: Case based experience and
treatment options. J Interv Cardiol. 28:305–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwok CS, Rashid M, Fraser D, Nolan J and
Mamas M: Intra-arterial vasodilators to prevent radial artery
spasm: A systematic review and pooled analysis of clinical studies.
Cardiovasc Revasc Med. 16:484–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dharma S, Kedev S, Patel T, Rao SV,
Bertrand OF and Gilchrist IC: Radial artery diameter does not
correlate with body mass index: A duplex ultrasound analysis of
1706 patients undergoing trans-radial catheterization at three
experienced radial centers. Int J Cardiol. 228:169–172. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guedes A, Dangoisse V, Gabriel L, Jamart
J, Chenu P, Marchandise B and Schroeder E: Low rate of conversion
to transfemoral approach when attempting both radial arteries for
coronary angiography and percutaneous coronary intervention: A
study of 1,826 consecutive procedures. J Invasive Cardiol.
22:391–397. 2010.PubMed/NCBI
|
|
7
|
Trobs M, Achenbach S, Plank PM, Marwan M,
Röther J, Klinghammer L, Blachutzik F and Schlundt C: Predictors of
technical failure in transradial coronary angiography and
intervention. Am J Cardiol. 120:1508–1513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo BS, Yoon J, Ko JY, Kim JY, Lee SH,
Hwang SO and Choe KH: Anatomical consideration of the radial artery
for transradial coronary procedures: Arterial diameter, branching
anomaly and vessel tortuosity. Int J Cardiol. 101:421–427. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abazid RM, Smettei OA, Mohamed MZ, Kattea
MO, Suresh A, Beshir Y and Sakr H: Radial artery ultrasound
predicts the success of transradial coronary angiography. Cardiol
J. 24:9–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattea V, Salomon C, Menck N, Lauten P,
Malur FM, Schade A, Steinborn F, Costello-Boerrigter L, Neumeister
A and Lapp H: Low rate of access site complications after
transradial coronary catheterization: A prospective ultrasound
study. Int J Cardiol Heart Vasc. 14:46–52. 2016.PubMed/NCBI
|
|
11
|
Tak BT, Balci KG, Erken H, Gerede DM, Tak
S, Göksülük H, Turhan S and Erol C: Evaluation of endothelial
dysfunction with flow-mediated dilatation after transradial
coronary angiography. Acta Cardiol. 72:305–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Heijden D, van Leeuwen MA,
Janssens GN, Hermie J, Lenzen MJ, Ritt MJ, van de Ven PM, Kiemeneij
F and van Royen N: Endothelial dysfunction and the occurrence of
radial artery spasm during transradial coronary procedures: The
ACRA-Spasm study. EuroIntervention. 12:1263–1270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha SK, Jha MJ, Mishra V, Thakur R, Goel
A, Kumar A, Singh AK, Sachan M, Varma CM and Krishna V: Radial
artery occlusion-incidence, predictors and Long-term outcome after
TRAnsradial catheterization: Clinico-Doppler ultrasound-based study
(RAIL-TRAC study). Acta Cardiol. 72:318–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beyer AT, Ng R, Singh A, Zimmet J, Shunk
K, Yeghiazarians Y, Ports TA and Boyle AJ: Topical nitroglycerin
and lidocaine to dilate the radial artery prior to transradial
cardiac catheterization: A randomized, placebo-controlled,
double-blind clinical trial: The PRE-DILATE study. Int J Cardiol.
168:2575–2578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majure DT, Hallaux M, Yeghiazarians Y and
Boyle AJ: Topical nitroglycerin and lidocaine locally vasodilate
the radial artery without affecting systemic blood pressure: A
dose-finding phase I study. J Crit Care. 27:532.e9–e13. 2012.
View Article : Google Scholar
|
|
16
|
Ünal S, Açar B, Yayla Ç, Balci MM, Ertem
AG, Kara M, Maden O, Temizhan A, Tola M and Balbay Y: Manual
heating of the radial artery (Balbay maneuver) to facilitate radial
puncture prior to transradial coronary catheterization. Rev Port
Cardiol. 36:409–414. 2017.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pancholy SB, Heck LA and Patel T: Forearm
arterial anatomy and flow characteristics: A prospective
observational study. J Invasive Cardiol. 27:218–221.
2015.PubMed/NCBI
|
|
18
|
Zhou ZM, Yan ZX, Nie B, Guo YH and Zhou
YJ: Transient ulnar artery compression facilitates transradial
access. Medicine (Baltimore). 95:e54912016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koga S, Ikeda S, Futagawa K, Sonoda K,
Yoshitake T, Miyahara Y and Kohno S: The use of a
hydrophilic-coated catheter during transradial cardiac
catheterization is associated with a low incidence of radial artery
spasm. Int J Cardiol. 96:255–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tatli E, Yilmaztepe MA, Vural MG, Tokatli
A, Aksoy M, Ağaç MT, Çakar MA, Gündüz H and Akdemir R: Cutaneous
analgesia before transradial access for coronary intervention to
prevent radial artery spasm. Perfusion. 33:110–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia DA, Zhou YJ, Shi DM, Liu YY, Wang JL,
Liu XL, Wang ZJ, Yang SW, Ge HL, Hu B, et al: Incidence and
predictors of radial artery spasm during transradial coronary
angiography and intervention. Chin Med J (Engl). 123:843–847.
2010.PubMed/NCBI
|
|
22
|
Koutouzis MJ, Maniotis CD, Avdikos G,
Tsoumeleas A, Andreou C and Kyriakides ZS: Ulnar artery transient
compression facilitating radial artery patent hemostasis (ULTRA): A
novel technique to reduce radial artery occlusion after transradial
coronary catheterization. J Invasive Cardiol. 28:451–454.
2016.PubMed/NCBI
|
|
23
|
Tian J, Chu YS, Sun J and Jiang TM: Ulnar
artery compression: A feasible and effective approach to prevent
the radial artery occlusion after coronary intervention. Chin Med J
(Engl). 128:795–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bernat I, Bertrand OF, Rokyta R, Kacer M,
Pesek J, Koza J, Smid M, Bruhova H, Sterbakova G, Stepankova L and
Costerousse O: Efficacy and safety of transient ulnar artery
compression to recanalize acute radial artery occlusion after
transradial catheterization. Am J Cardiol. 107:1698–1701. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pancholy SB, Bernat I, Bertrand OF and
Patel TM: Prevention of radial artery occlusion after transradial
catheterization: The PROPHET-II randomized trial. JACC Cardiovasc
Interv. 9:1992–1999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaplanoglu H and Beton O: Flow and
diameter changes of forearm arteries during temporary unilateral
reciprocal occlusion: A prospective observational study. J Clin
Ultrasound. 45:197–203. 2017. View Article : Google Scholar : PubMed/NCBI
|