Introduction
Pelvic organ prolapse (POP), presenting symptoms
including uterine prolapse, bladder prolapseand rectal prolapse, is
a common and distressing health problem in adult women, which can
affect ~50% of women over the age of 50 (1). In the USA, the total number of women
who undergo surgery for POP is projected to increase from 166,000
(in 2010) to 245,970 in 2050 (2) and
the incidence would peak in women aged between 60 to 79 years
(3,4). The annual cost for POP surgery was $1.4
billion between 1996 and 2005 (5).
The precise pathogenesis of POP is still unknown, but it is
believed to be multifactorial. Environmental factors, including
age, hormonal status, constipation, smoking, obesity, prior
surgery, increased infant birth weight, episiotomy, vaginal parity
and extended second stage of labor, have been identified as key
risk factors in the development of POP (6). Other factors, including chronic
illnesses that increase intra-abdominal pressure, underlying
neurological disease and a decrease of estrogen receptor in the
pelvic supportive tissue, are also considered to be risk factors
(7,8). However, environmental factors alone
cannot explain the development and progression of POP. For example,
severe female pelvic floor dysfunction has been reported in
nulliparous women with minimal risk factors, while a large number
of multiparous women do not develop POP (9–11).
Oxidative stress is caused by an imbalance of
reactive oxygen species (ROS) and antioxidant defense systems in a
cell, tissue or organ (12). ROS,
including the superoxide radical anion (O2−),
hydroxyl radical and hydrogen peroxide
(H2O2), are produced as byproducts of normal
cellular metabolism (13). They are
reactive molecules due to their unpaired electrons and can react
spontaneously with biomolecules such as DNA, RNA, protein and
lipids, leading to cell death and disease (14,15).
Oxidative stress damage occurs when levels of ROS exceed the cell's
antioxidant defense capacity (16).
As previously reported, isoprostanes, reliable biomarkers of
oxidative stress, are higher in women with uterine prolapse
compared with non-prolapse women, in the cardinal ligament and
urine samples (17). Selenium is a
key component of glutathione peroxidase (GPx), which can indirectly
reflect antioxidant capacity (18).
It was previously demonstrated that serum selenium concentration is
significantly lower in pelvic organ prolapse-affected buffaloes
compared with control group buffaloes (19). Recently, a genetic predisposition
investigation revealed that oxidative-related genes are associated
with POP (20). In addition, Visco
and Yuan (21) demonstrated that the
gene expression of DSCR-1, an antioxidative related gene, and its
gene product calcipressin 1, which can protect cells from oxidative
stress, was underexpressed in pubococcygeus muscle from women with
prolapse. This indicated that the pelvic floor may suffer from
oxidative stress, leading to the damage of pelvic floor tissues. In
addition, oxidative stress has been demonstrated to regulate matrix
metalloproteinase (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs), thus leading to decreased collagen and
elastin synthesis in fibroblasts and smooth muscle cells (22–25). It
has also been documented that abnormal collagen metabolism is
involved in the molecular pathology of POP (26–30).
Therefore, in the present study, it was hypothesized that oxidative
stress may be involved in the pathogenesis of POP.
Several specific oxidative stress markers are
required to assess redox status. 8-hydroxydeoxyguanosine (8-OHdG)
is the most studied product of oxidative DNA damage, produced via
hydroxylation at the C-8 position of the guanine base on DNA by
extremely active hydroxyl radicals (31). 4-hydroxynonenal (4-HNE), a product of
cell membrane lipid peroxidation, can be generated by oxidative
stimuli and has been detected in numerous diseases, including
diabetes and Parkinson's disease (32–33). The
formation of 8-OHdG, 4-HNE and 4-HNE-protein conjugation have
become common oxidative biomarkers used to estimate ROS-induced DNA
and lipid damage in vivo with high precision (34–37).
In the endogenous defense system against ROS damage
in humans, GPx and superoxide dismutase (SOD) are the two critical
antioxidant enzymes (38). There are
three well-known forms of SOD, namely cytosolic copper/zinc SOD
(CuZnSOD, SOD1), mitochondrial SOD (MnSOD, SOD2) as well as
extracellular CuZnSOD (SOD3). MnSOD is located in the mitochondria,
a major site of ROS production, which indicates that MnSOD may
serve a key function in the antioxidant defense system (38). MnSOD is the only mitochondrial matrix
enzyme that transforms O2−, generated from
complexes I and III of the electron transport chain, into
H2O2, which then diffuses out of the
mitochondrial matrix and is subsequently neutralized to
H2O and O2 by GPx1 (39). Of the four GPx isoenzymes, GPx1 is
the most abundant. It is a selenium-dependent enzyme that protects
cells against oxidative damage through scavenging
H2O2 and other organic peroxides with reduced
glutathione (40). Thus, MnSOD and
GPx1 serve the most important role in maintaining equilibrium
between oxidative and antioxidative activity under normal
physiological conditions (38–40).
The uterosacral and cardinal ligaments are important
parts of the pelvic floor support system to the cervix and the
upper vagina (41). Thus, in the
present study, the oxidative stress biomarkers, 8-OHdG and 4-HNE,
and the major antioxidative enzymes, MnSOD and GPx1, were evaluated
in the cardinal ligaments. The aim of the present study was to
investigate the oxidative status of pelvic supportive tissue in POP
and further demonstrate that oxidative stress is involved in the
pathogenesis of POP.
Patients and methods
Patients
The present study was conducted in the Department of
Obstetrics and Gynecology, Renmin Hospital of Wuhan University
(Wuhan, China), and approved by the Institutional Ethic Committee
of the hospital. Informed consent was obtained from all patients.
From January 2012 to December 2013, cardinal ligament tissue was
obtained from 60 female patients (age range, 42–69 years old)
undergoing hysterectomy at Renmin Hospital of Wuhan University. The
patients were divided into three groups: 20 patients range from 47
to 69 year old who were undergoing hysterectomy for cervical
intraepithelial neoplasia (CIN) II or CIN III (42) were included as the control group; 16
women (age range, 42–63 years old) who were diagnosed with POP II
according to the POP-Q system (43)
were included in the POP II group; and 24 women range from 48–66
years old who were diagnosed with POP III or POP IV were included
in the POP III–IV group.
Patients with malignancy, hormone-related diseases
(including leiomyoma, endometriosis and adenomyosis), diabetes,
asthma or cardiovascular disease were excluded since these
disorders are known to be associated with oxidative stress
(44–47).
Immunohistochemistry
All tissue samples were fixed in 4% paraformaldehyde
at 37°C for 8 h, then embedded in paraffin. Samples were cut into
4-µm thick sections and heated at 62°C for 2 h. Each sample was
passed through xylene and ethanol series to remove paraffin. Then,
samples were incubated with 1% H2O2 to
prevent endogenous peroxidase activities. Sections were boiled in
sodium citrate antigen retrieval buffer (10 mmol/l; pH<6.0;
95°C) for 20 min and then cooled down to room temperature. Then,
specimens were washed with PBS and placed in the
immunohistochemistry container. Immunohistochemistry was performed
using antibodies against 8-OHdG (mouse monoclonal antibody; 1:100;
Abcam, Cambridge, UK; cat. no. ab62623) and 4-HNE (rabbit
polyclonal antibody; 1:100; Abcam; cat. no. ab46545), following the
manufacturer's protocols. Secondary antibodies were goat anti-mouse
polyclonal horseradish peroxidase (HRP)-conjugated Immunoglobulin G
(IgG; 1:1,000; cat. no. ab97023) and goat anti-rabbit polyclonal
HRP-conjugated IgG (1:1,000; cat. no. ab97051; both Abcam). The
staining procedure was performed using DAB (DAB-0031; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) according to the DAB detection
kit protocol. All immunohistochemical images were obtained using a
BH-2 light microscope (Olympus Corporation, Tokyo, Japan) and
analyzed using Image J2× 2.1.4.7 software (National Institutes of
Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from freshly collected
cardinal ligament tissue with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), following the
manufacturer's protocol. The obtained RNA concentration and purity
were detected by OD260/280 nm absorption ratio. The extracted RNA
was stored at −80°C until cDNA synthesis. cDNA synthesis was
performed using a RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The synthesized cDNA was stored at −20°C prior to qPCR. Specific
primer pairs were designed as follows: MnSOD forward,
5′-GACATATGAAGCACAGCCTCCCCGACC-3′ and reverse,
5′-GCAAGCTTGCATAACGATCGTGGTTTAC-3′; GPx1 forward,
5′-CGCTTCCAGAGCATTGACATC-3′ and reverse,
5′-CGAGGTGGTATTTTCTGTAAGATCA-3′. β-actin (forward,
5′-GTTGCTATCCAGGCTGTG-3′ and reverse, 5′-TGATCTTGATCTTCATTGTG-3′)
served as the internal control. qPCR was performed in triplicate
using the standard PCR kit of SYBR-Green Premix Ex Taq (Clontech
Laboratories, Inc., Mountainview, CA, USA) and the ABI Prism 7500
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 30 sec at 95°C, followed
by 40 cycles for 5 sec at 95°C and 34 sec at 60°C, 15 sec at 95°C,
1 min at 60°C, 15 sec at 95°C and 15 sec at 60°C. Relative gene
expression was analyzed using the 2−ΔΔCq method
(48) with a correction for
different amplification efficiencies.
Western blot analysis
Western blot analysis was performed according to
standard procedures. Total protein was extracted from cardinal
ligament tissue and quantified using an enhanced BCA protein assay
kit (cat. no. P0010; Beyotime Institute of Biotechnology, Haimen,
China). Equal amounts of protein samples (20 µg) from each sample
were separated in a 10% SDS-polyacrylamide gel with 5% stacking gel
in SDS-Tris-glycine running buffer. Then the proteins were
transferred to a polyvinylidene difluoride membrane by standard
procedures. The membranes were blocked with 5% nonfat dry milk in
TBS for 1 h at room temperature and incubated overnight with
primary antibodies at 4°C: MnSOD (rabbit polyclonal antibody, cat.
no. 06-984, 1:1,000; EMD Millipore, Billerica, MA, USA) and GPx1
(rabbit polyclonal antibody; cat. no. ab22604; 1:1,000; Abcam).
Antibodies against GAPDH were also utilized (rabbit polyclonal
antibody; cat. no. ab9485; 1:1,000; Abcam). After washing with
TBS-Tween-20 (TBST) three times, membranes were incubated with
secondary fluorescence antibodies (IRDye 800CWgoat anti-rabbit
secondary antibodies; cat. no. 926-32211; 1:10,000; LI-COR
Biosciences, Lincoln, NE, USA) for 1 h at room temperature. After
rewashing with TBST three times at room temperature, the
immunoreactive bands were detected and analyzed using the odyssey
3.0 image system software (LI-COR Biosciences).
Measurement of total antioxidant
capacity (T-AOC) and enzyme activity of SOD and GPx
The T-AOC assay kit (Nanjing jiancheng
Bioengineering Institute, Nanjing, China; cat. no. A015) and SOD
enzyme activity assay kit (Nanjing Jiancheng Bioengineering
Institute; cat. no. A001-3) and GPx enzyme activity assay kit
(Nanjing jiancheng Bioengineering Institute; cat. no. A005) were
purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing,
China. The assays were performed according to the manufacturer's
protocol.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 13.0 statistical analysis software (SPSS, Inc., Chicago, IL,
USA) was used to analyze the results. One-way analysis of variance
was performed to compare the means among groups and the post-hoc
LSD test was used to make multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
8-OHdG and 4-HNE are overexpressed in
patients with POP III–IV
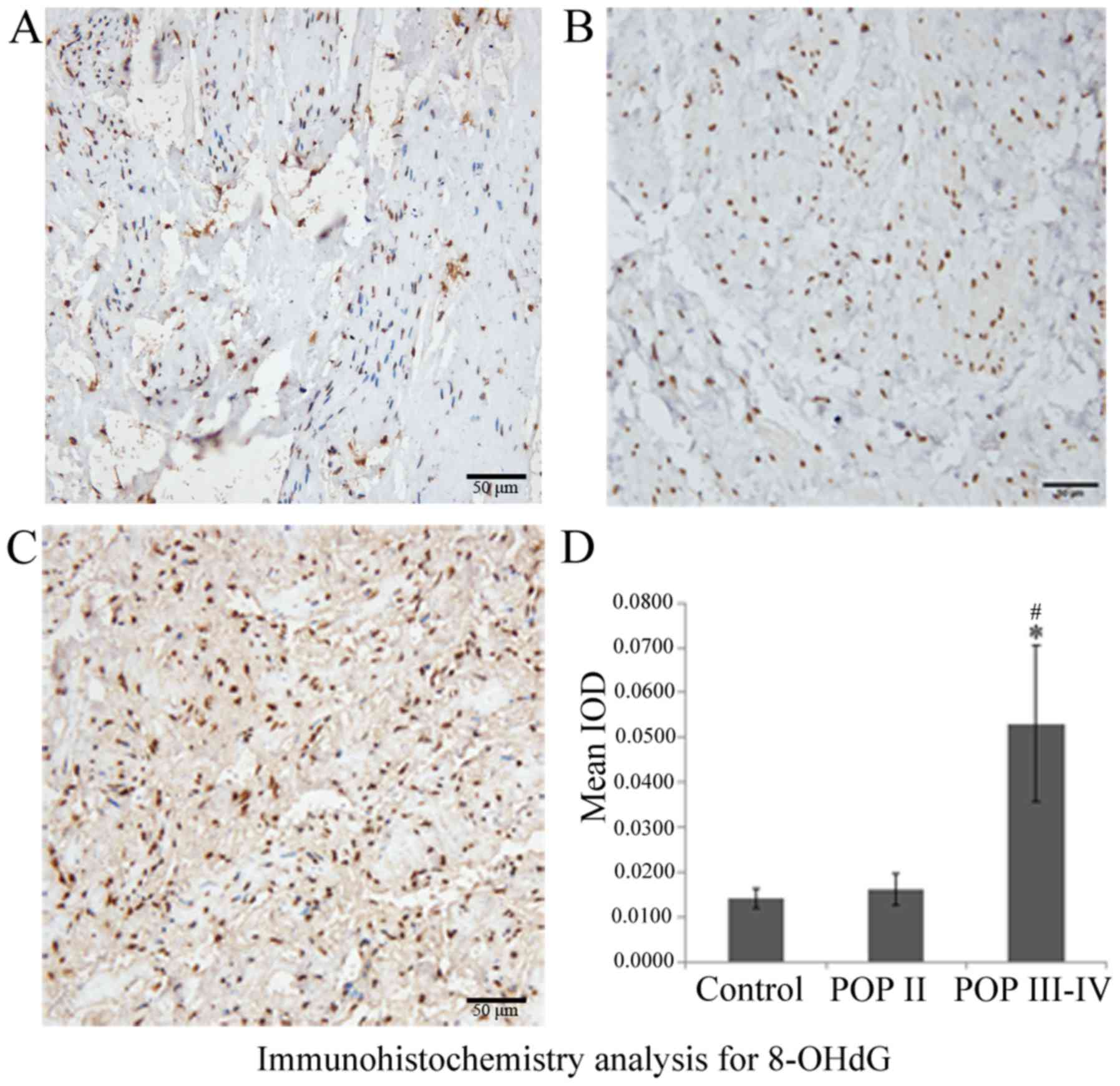
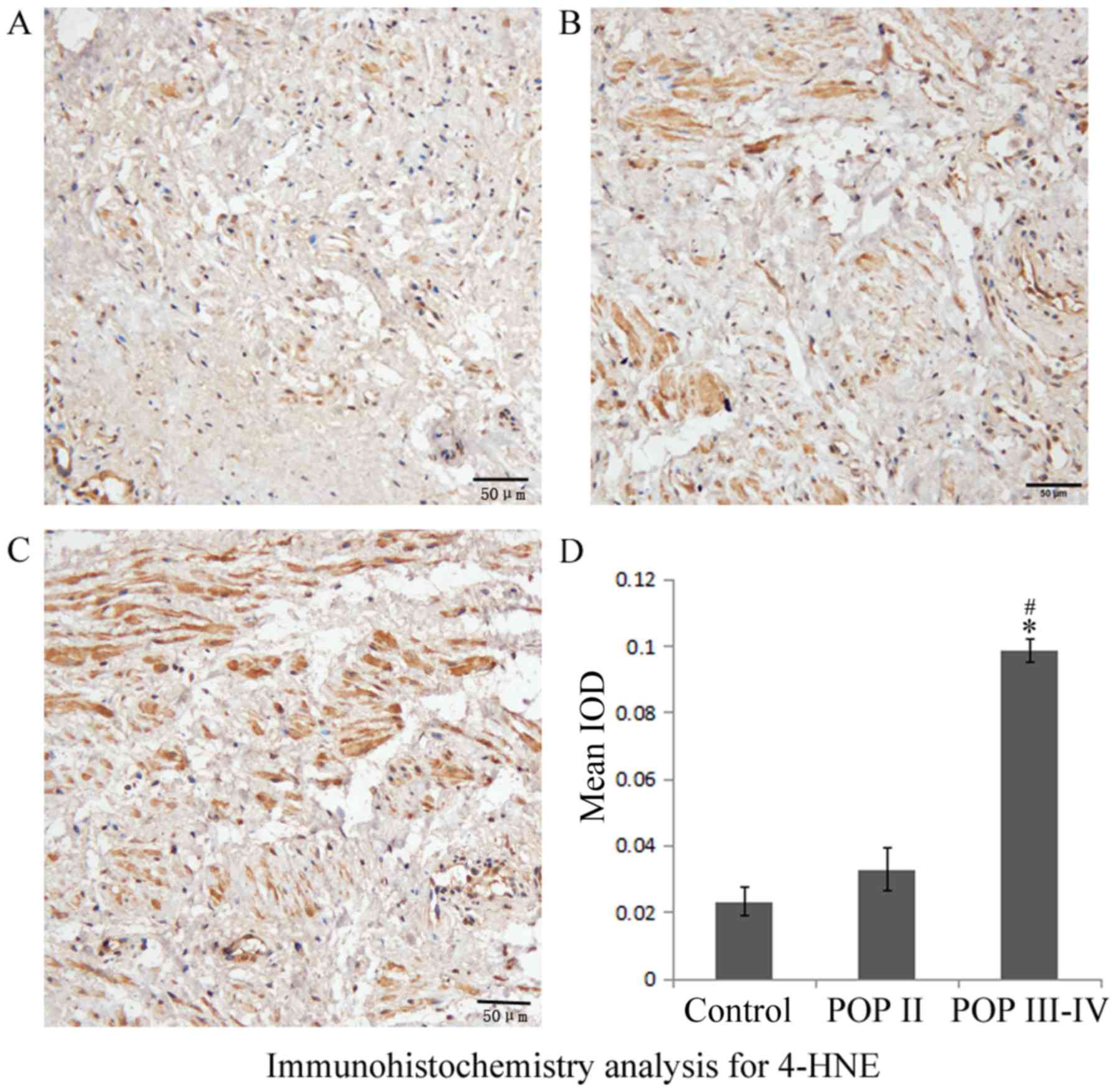
Immunohistochemistry results indicated that 8-OHdG
was expressed in the nuclei and 4-HNE was expressed in the
cytoplasm of cardinal ligaments, respectively (Figs. 1 and 2). The expression of 8-OHdG and 4-HNE was
significantly higher in the POP III–IV group compared with the POP
II group (P<0.05; Figs. 1D and
2D) and the control group
(P<0.05; Figs. 1D and 2D). No significant differences were
observed between the POP II group and the control group. These
results indicated that the oxidative markers 8-OHdG and 4-HNE are
expressed at higher levels in patients with severe POP compared
with patients with mild POP or healthy controls.
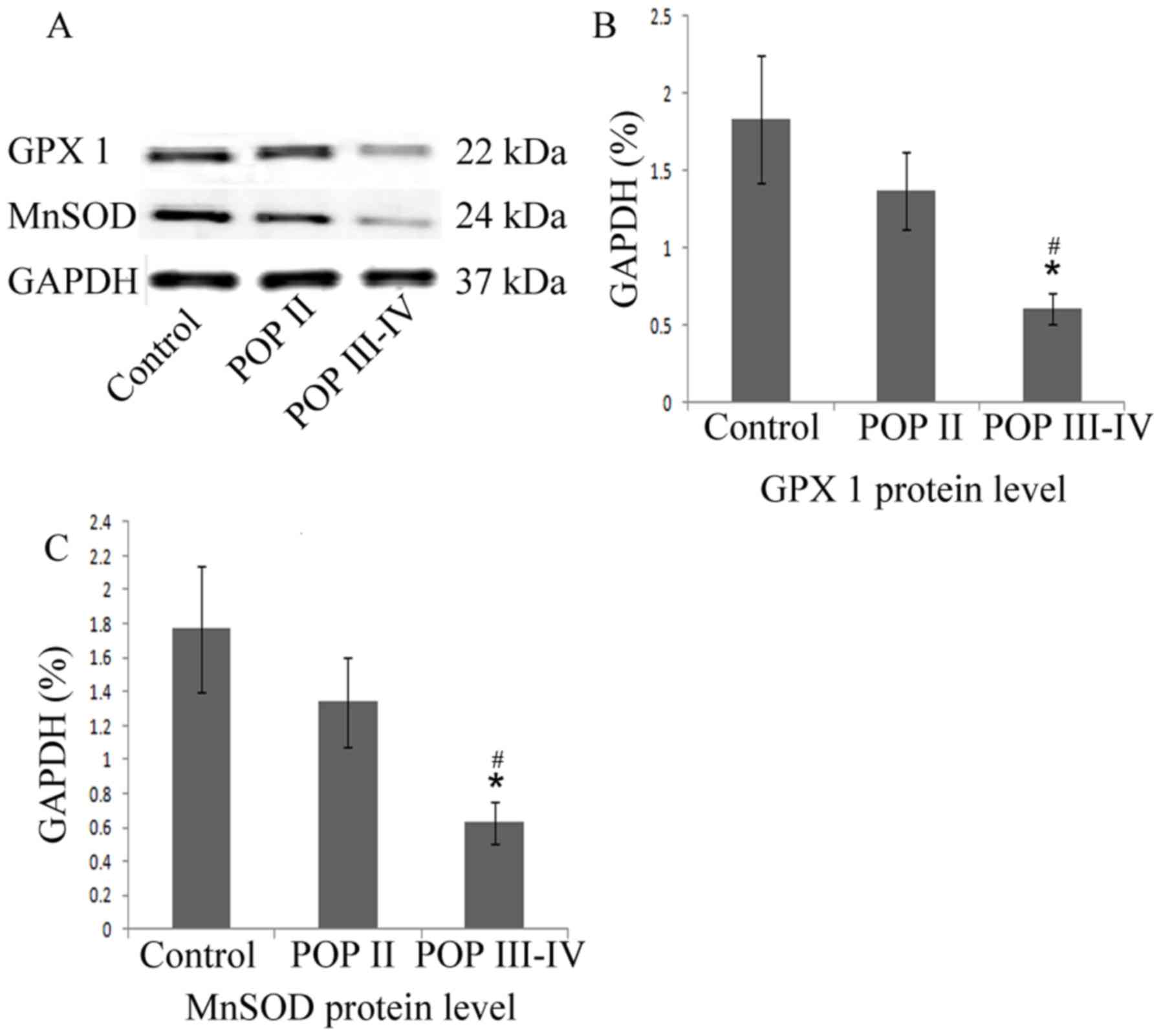
MnSOD and GPx1 protein level is
decreased in patients with POP III–IV
As indicated in the western blot analysis (Fig. 3), the MnSOD and GPx1 protein levels
were significantly lower in the POP III–IV group compared with the
POP II group and the control group (P<0.05). No significant
differences were observed between the control group and the POP II
group. These results indicated that the antioxidative proteins
MnSOD and GPx1 are expressed at lower levels in patients with
severe POP compared with patients with mild POP or healthy
controls.
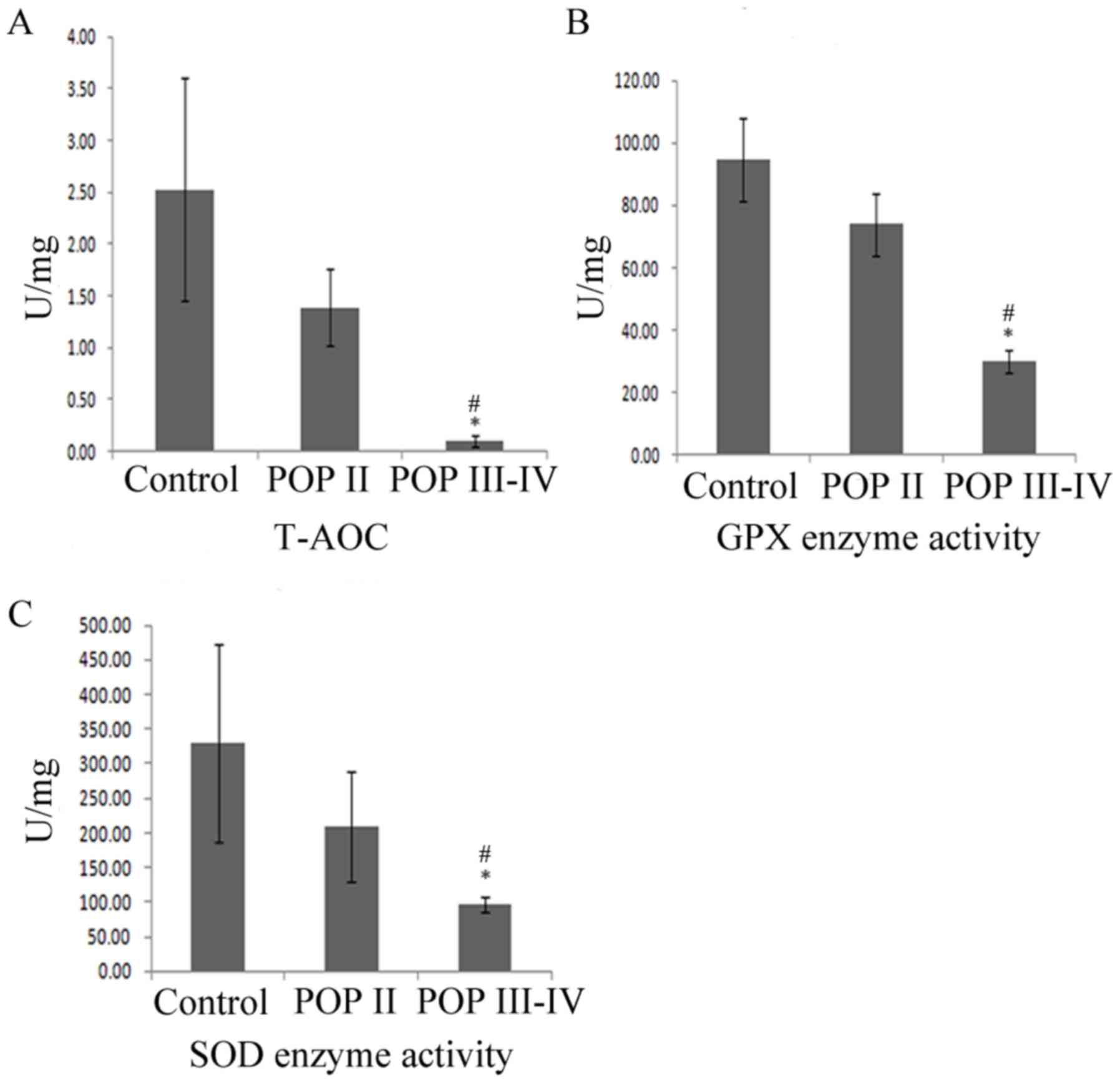
T-AOC, SOD and GPx enzyme activity is
decreased in patients with POP III–IV
In order to detect the primary defense capacity to
oxidative stress, T-AOC, SOD and GPx enzyme activity was detected
among the three groups. As shown in Fig.
4, the T-AOC, SOD and GPx enzyme activity was significantly
lower in the POP III–IV group compared with the POP II group and
the control group (P<0.05). No significant differences were
observed between the control group and the POP II group
(P>0.05). These results indicated that T-AOC, SOD and GPx enzyme
activity was lower in patients with severe POP compared with
patients with mild POP or healthy controls.
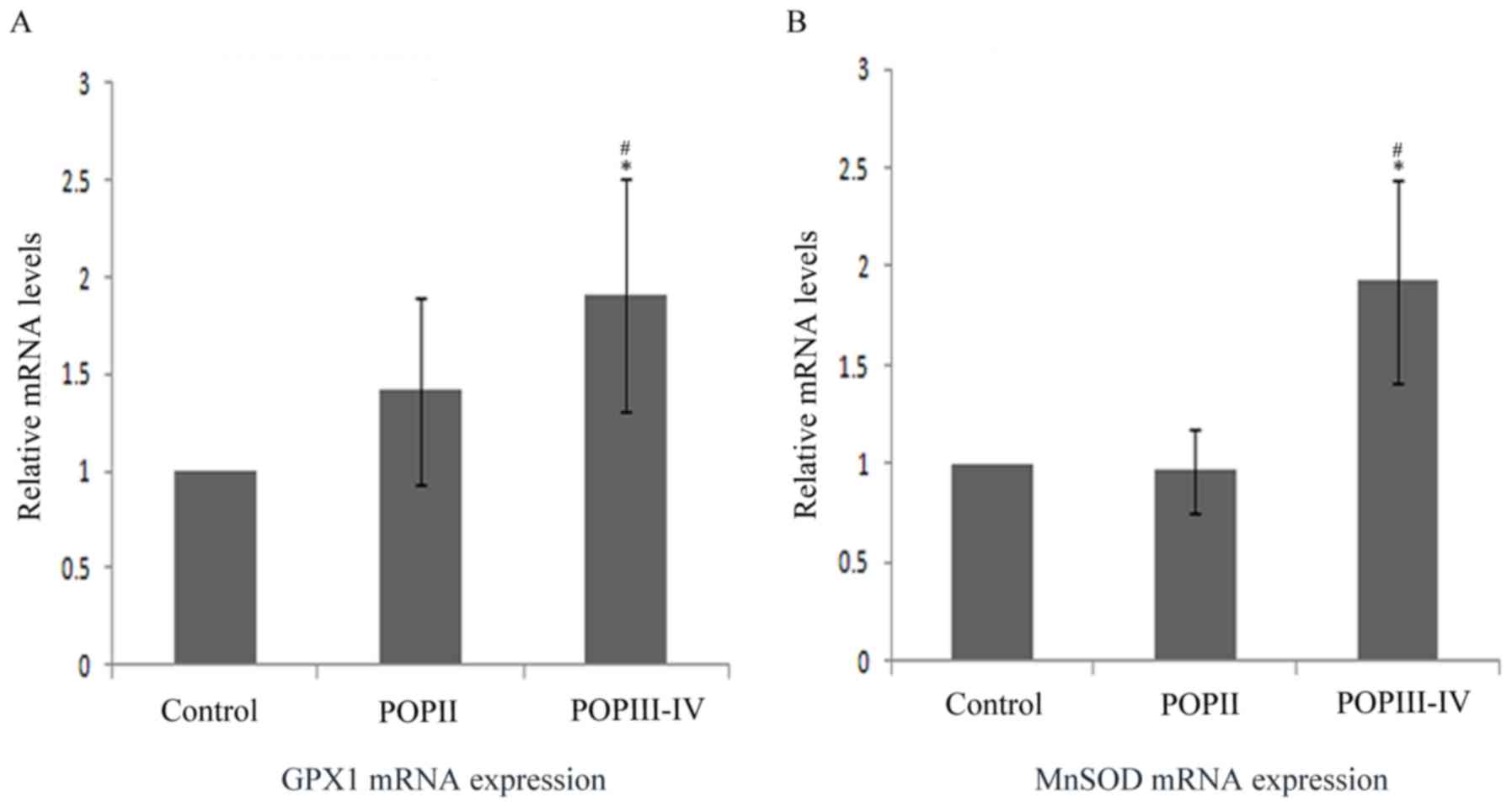
MnSOD and GPx1 mRNA level is increased
in POP group
The aforementioned results suggested that MnSOD and
GPx1 protein level and enzyme activity were lower in the POP III–IV
group compared with the control group. Thus, the mRNA level was
also compared among groups by RT-qPCR (Fig. 5). The results indicated that MnSOD
and GPx1 mRNA expression was significantly higher in the POP III–IV
group compared with the POP II group and the control group
(P<0.05). No significant differences were observed between the
control group and the POP II group.
Discussion
POP is primarily characterized by biomechanical
defects of pelvic supportive tissues and increased apoptotic cell
death and altered extracellular matrix (ECM) metabolism may be
involved (49,50). It has been documented that oxidative
stress is a common mediator of apoptosis in numerous cell types and
it has been observed in the pelvic supportive tissues of women with
POP (51).
Oxidative stress results from an imbalance of ROS
and antioxidant defense systems in a cell, tissue or organ and has
been identified to be involved in multiple diseases (52–56).
Pregnancy, childbirth, age, obesity, constipation and smoking are
well-established risk factors for POP, and these risk factors have
been demonstrated to be associated with oxidative stress (6–8).
Therefore, it was speculated that oxidative stress may be involved
in the pathogenesis of POP.
Previous studies have reported that oxidative stress
may be involved in the pathogenesis of POP and certain
antioxidative-related genes, including Adapt 78 and DSCR-1 have
been identified to be associated with POP (17,20–21).
However, none of these studies detected the oxidative/antioxidative
status in the pelvic supportive ligament of patients with POP. The
purpose of the current study was to investigate the oxidative
damage and antioxidative status of the pelvic supportive ligament
in patients with POP, and further demonstrate that oxidative stress
may be involved in the pathogenesis of POP.
In the present study, it was demonstrated that
oxidative damage markers, 8-OHdG and 4-HNE, were increased in the
pelvic supportive ligament of patients with severe POP compared
with controls, while the protein levels of the major antioxidative
enzymes, MnSOD and GPx1, were decreased. T-AOC, SOD and GPx enzyme
activity were also decreased in patients with severe POP. From
these observations, it may be concluded that in patients with
severe POP, oxidative damage is increased, while the antioxidative
defense system is weakened. These results supported previous
findings that oxidative stress is associated with the pathogenesis
of POP.
Oxidative stress reflects an excessive
bioavailability of ROS, which is the result of an imbalance between
production and destruction of ROS. ROS such as
O2− and H2O2 have been
demonstrated to serve important roles as signal transduction
intermediates (57–66). For instance, ROS have been
demonstrated to activate downstream signaling molecules, induce the
expression of redox-sensitive genes and regulate cell proliferation
and migration, which has been demonstrated to be associated with
POP (57–62). ROS can also induce mitochondrial
damage and dysfunction, resulting in impairment of the Krebs cycle
and activation of apoptotic pathways (58,63–66),
while oxidative stress-induced apoptotic cell death is reported to
be involved in the pathological generation of oxidative
stress-related diseases (67,68).
Previous studies have focused on ROS, the synthesis and
decomposition process of ECM. ROS have been demonstrated to
regulate MMPs through changes in expression and by direct
interactions with Zn-thiol groups (69), activating MMP secretion (25) or through nitration of cysteine
residues in the autoinhibitory domain (70) and suppressed collagen production in
fibroblasts (71). An association
between the expression of MMP-2 and TIMP-2 in the uterosacral
ligament and the occurrence of uterine prolapse has also been
demonstrated (72). Conversely,
reactive nitrogen species, such as ONOO−, can induce
nitration of TIMP-4 and then attenuate its inhibitory activity
against MMP-2 activity in cells (73). Therefore, oxidative stress may be
involved in the pathogenesis of POP through multiple mechanisms,
including cell apoptosis and the synthesis and decomposition
process of ECM, which are associated with POP.
8-OHdG and 4-HNE are oxidative damage markers of DNA
and lipids, respectively (34–37).
However, 8-OHdG and 4-HNE may also serve other functions. For
example, 8-OHdG has gained much attention due to its mutagenic
potential to pair with adenine, instead of cytosine, resulting in
G:C to T:A transversions if the damage is not repaired before DNA
replication (34,74). This may constitute 20–30% of the
deoxyguanosine damage in DNA, equivalent to 5–11% of the total DNA
nucleoside damage and has been demonstrated to be associated with
many oxidative-related diseases or processes, including cancer,
aging and neurodegeneration (75,76).
4-HNE, which was initially considered as merely a toxic end-product
of lipid peroxidation (LPO) derived from oxidized ω-6
polyunsaturated fatty acids such as arachidonic acid, has been
demonstrated to be an important second messenger signaling
molecule; alterations in the intracellular levels of 4-HNE are
associated with signaling for proliferation, transformation,
apoptosis and differentiation in numerous cell types (77–81).
Chaudhary et al (82)
demonstrated that 4-HNE can cause toxicity to cells through
apoptosis and necrosis in a dose-dependent manner. Furthermore,
through interactions with cell membrane receptors as well as
transcription repressors, 4-HNE could have extensive effects on the
expression of genes regulating multiple cellular processes,
including oxidative stress (82).
Therefore, 4-HNE may be a key mediator of oxidative stress-induced
apoptosis. As a result, aside from being markers of oxidative
damage to DNA and lipids, 8-OHdG and 4-HNE can also cause cell and
tissue injury multiple mechanisms, which may lead to disease,
including POP. This may partly explain the increased levels of
8-OHdG and 4-HNE in the pelvic supportive ligament of patients with
POP III–IV in the present study.
Antioxidant defense system enzymes, including GPx
and SOD, reduce oxidative stress through inactivation of ROS. GPx1
detoxifies H2O2 and lipid peroxides using
reduced glutathione to protect cells against oxidative and
nitrosative stress (83). GPx1
knockdown mice (GPX1−/−) exhibit increased mitochondrial
ROS production and oxidative mitochondrial DNA damage (84). MnSOD is an important first defense
against mitochondrial oxidative stress and damage to mitochondrial
integrity (85). Homozygous MnSOD
knockout (Sod2−/−) mice were reported to be neonatal
lethal, produce low levels of cellular ATP, exhibit low
O2 consumption and generate increased levels of
superoxide (86). Meanwhile,
enhancement of MnSOD protein levels in Sod2 transgenic mice notably
reduced markers of oxidative stress and protected against
age-related increases of proapoptotic signaling, including Bax and
cleaved caspase-3. It also reduced the number of apoptotic nuclei
and the amount of DNA fragmentation in mouse hearts (87). Additionally, MnSOD could serve a key
function in protecting RNA and DNA, thus maintaining normal protein
expression, through interacting with RNA or DNA, or interacting
with proteins involved in DNA repair, apoptosis, translation and
metabolic function (88,89). The overexpression of MnSOD appears to
increase mitochondrial antioxidative capacity and reduce apoptosis,
which has been demonstrated in the liver, brain and skeletal muscle
(90–93).
Previous research into the pathogenesis of POP has
demonstrated that alterations in ECM metabolism and cell apoptosis
are involved (27–30,49). It
has been proposed that alterations in the smooth muscle content and
function may contribute to the development of POP (94–97).
Takacs et al (50)
demonstrated that the smooth muscle component of the cervical
uterosacral ligaments (USL) is decreased significantly and the
apoptosis rate is increased in women with uterine prolapse.
Decreased smooth muscle content of the USL due to increased
apoptosis may also serve a key function in the pathogenesis of POP.
On the basis of previous research, it is obvious that oxidative
stress, oxidative damage molecules such as 4-HNE and antioxidative
enzymes such as MnSOD can regulate or influence cell apoptosis or
MMPs and TIMPs, which are associated with ECM metabolism. Decreased
protein level and activity of antioxidants GPx1 and MnSOD, as in
the present study, is likely to lead to DNA, lipid, protein and
mitochondrial function damage and disrupted electron transport,
which in turn would lead to increased ROS production. This could
form a feedback loop and finally lead to the development of
POP.
The observations in the current study support the
proposal that oxidative stress is involved in the pathogenesis of
POP. This may be through regulating or influencing cell apoptosis
and ECM protein metabolism, thus influencing the content and
function of the pelvic supportive tissue in POP women. Furthermore,
with the decrease of antioxidative capacity, oxidative stress
increases, which further leads to increased oxidative damage in the
antioxidative defense system, forming a negative feedback loop.
Therefore, different degrees of antioxidative capacity could lead
to different extents of oxidative stress and thus contribute to
different severities of POP. This would mean that the most damaged
antioxidative defense capacity in the severe POP group would lead
to the greatest oxidative injures in cell and tissue, probably
through damage to mitochondria, ECM metabolism and induced cell
apoptosis. Therefore, this would result in a greater extent of
damage to the pelvic supportive tissue compared with mild POP or
healthy controls.
Notably, in the present study, protein expression
and enzyme activity of MnSOD and GPx1 were decreased, while the
mRNA expression level of the two enzymes increased in patients with
POP compared with the control group. As discussed above, ROS can
activate downstream signaling targets and induce the expression of
redox-sensitive genes. Thus, ROS accumulates in cells and tissue,
and activates the expression of protective proteins when oxidative
stress occurs, including MnSOD and GPx1. The mRNA expression of
these proteins also increases. The specific mechanism for the
decrease of MnSOD and GPx1 protein expression and enzyme activity
in women with POP remains unknown. Further studies are therefore
required to elucidate this.
As previously reported, selenium-enriched food and
exercise training is effective in upregulating GPx and MnSOD
activity, as well as reducing apoptosis in a range of tissues
(98–101). The antioxidant enzyme is a
potential candidate for the treatment of ROS-related disease,
suggesting that selenium-enriched food and exercise training may
reduce or delay the occurrence of POP.
In conclusion, the present study demonstrated that
oxidative damage is increased in women with severe POP, while the
antioxidative defense capacity is decreased. Oxidative stress may
be involved in the pathogenesis of POP via regulation of cell
apoptosis and ECM metabolism, but the underlying mechanisms require
further investigation. The methods that eliminateoxidative damage
or enhance antioxidant capacity may therefore be beneficial to
delaying the progress of or for the treatment of POP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270684) and the
Foundation of Collaborative and Innovation Projects of Wuhan
University School of Medicine (grant no. 523-266078).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF conceived and designed the experiments and wrote
the manuscript. LH participated in designing the experiments,
revised it critically for important intellectual content and gave
their final approval of the version to be published. CL, QY, QZ, YL
helped with the western blot and PCR experiments. BL, DW, WW and HS
helped with tissue collection. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted in the Department of
Obstetrics and Gynecology, Renmin Hospital of Wuhan University and
approved by the Institutional Ethic Committee of the hospital.
Informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Samuelsson EC, Victor FT, Tibblin G and
Svärdsudd KF: Signs of genital prolapse in a Swedish population of
women 20 to 59 years of age and possible related factors. Am J
Obstet Gynecol. 180:299–305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JM, Kawasaki A, Hundley AF, Dieter AA,
Myers ER and Sung VW: Predicting the number of women who will
undergo incontinence and prolapse surgery, 2010 to 2050. Am J
Obstet Gynecol. 205:230.e1–e5. 2011. View Article : Google Scholar
|
|
3
|
Boyles SH, Weber AM and Meyn L: Procedures
for pelvic organ prolapse in the United States, 1979–1997. Am J
Obstet Gynecol. 188:108–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah AD, Kohli N, Rajan SS and Hoyte L:
The age distribution, rates, and types of surgery for pelvic organ
prolapse in the USA. Int Urogynecol J Pelvic Floor Dysfunct.
19:421–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown JS, Waetjen LE, Subak LL, Thom DH,
Van den Eeden S and Vittinghoff E: Pelvic organ prolapse surgery in
the United States, 1997. Am J Obstet Gynecol. 186:712–716. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikolova G, Lee H, Berkovitz S, Nelson S,
Sinsheimer J, Vilain E and Rodríguez LV: Sequence variant in the
laminin gamma1 (LAMC1) gene associated with familial pelvic organ
prolapse. Hum Genet. 120:847–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olsen AL, Smith VJ, Bergstrom JO, Colling
JC and Clark AL: Epidemiology of surgically managed pelvic organ
prolapse and urinary incontinence. Obstet Gynecol. 89:501–506.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu L, Lang J, Feng R, Chen J and Wong F:
Estrogen receptor in pelvic floor tissues in patients with stress
urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct.
15:340–343. 2004.PubMed/NCBI
|
|
9
|
Buchsbaum GM, Chin M, Glantz C and Guzick
D: Prevalence of urinary incontinence and associated risk factors
in a cohort of nuns. Obstet Gynecol. 100:226–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchsbaum GM, Duecy EE, Kerr LA, Huang LS
and Guzick DS: Urinary incontinence in nulliparous women and their
parous sisters. Obstet Gynecol. 106:1253–1258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bump RC and Norton PA: Epidemiology and
natural history of pelvic floor dysfunction. Obstet Gynecol Clin
North Am. 25:723–746. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
George G, Joseph J and Ganjifrockwalaa F:
Decreased total antioxidant levels and increased oxidative stress
in South African type 2 diabetes mellitus patients. J Endocrinol
Metab Diabetes South Africa. 22:21–25. 2017. View Article : Google Scholar
|
|
13
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
14
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diplock AT: Antioxidants and disease
prevention. Mol Aspects Med. 15:293–376. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai-Turton M and Luderer U: Opposing
effects of glutathione depletion and follicle-stimulating hormone
on reactive oxygen species and apoptosis in cultured preovulatory
rat follicles. Endocrinology. 147:1224–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choy KW, Liu YM, Chu CY, Wang CC, Lui WT,
Lee LL, Pang MW, Rogers MS and Yip SK: High isoprostane level in
cardinal ligament-derived fibroblasts and urine sample of women
with uterine prolapse. BJOG. 115:1179–1183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brigelius-Flohé R and Maiorino M:
Glutathione peroxidases. Biochim Biophys Acta. 1830:3289–3303.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akhtar MS, Lodhi LA, Ahmad I, Qureshi ZI
and Muhammad G: Serum trace mineral variations in Nili-Ravi
buffaloes suffering with prepartum vaginal prolapse in two
different agro-ecological zones of Punjab, Pakistan.
Theriogenology. 77:1328–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Kim EJ, Jeon MJ, Kim H, Moon YJ
and Bai SW: Association between the poly(ADP-ribose) polymerase-1
gene polymorphism and advanced pelvic organ prolapse. Menopause.
21:177–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Visco AG and Yuan L: Differential gene
expression in pubococcygeus muscle from patients with pelvic organ
prolapse. Am J Obstet Gynecol. 189:102–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akhtar K, Broekelmann TJ, Miao M, Keeley
FW, Starcher BC, Pierce RA, Mecham RP and Adair-Kirk TL: Oxidative
and nitrosative modifications of tropoelastin prevent elastic fiber
assembly in vitro. J Biol Chem. 285:37396–37404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1429. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fisher GJ, Datta S, Wang Z, Li XY, Quan T,
Chung JH, Kang S and Voorhees JJ: c-Jun-dependent inhibition of
cutaneous procollagen transcription following ultraviolet
irradiation is reversed by all-trans retinoic acid. J Clin Invest.
106:663–670. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siwik DA, Pagano PJ and Colucci WS:
Oxidative stress regulates collagen synthesis and matrix
metalloproteinase activity in cardiac fibroblasts. Am J Physiol
Cell Physiol. 280:C53–C60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang J, Zhu L, Sun Z and Chen J: Clinical
study on collagen and stress urinary incontinence. Clin Exp Obstet
Gynecol. 29:180–182. 2002.PubMed/NCBI
|
|
27
|
Liapis A, Bakas P, Pafiti A,
Frangos-Plemenos M, Arnoyannaki N and Creatsas G: Changes of
collagen type III in female patients with genuine stress
incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod
Biol. 97:76–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ewies AA, Al-Azzawi F and Thompson J:
Changes in extracellular matrix proteins in the cardinal ligaments
of post-menopausal women with or without prolapse: A computerized
immunohistomorphometric analysis. Hum Reprod. 18:2189–2195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goepel C, Johanna Kantelhardt E, Karbe I,
Stoerer S and Dittmer J: Changes of glycoprotein and collagen
immunolocalization in the uterine artery wall of postmenopausal
women with and without pelvic organ prolapse. Acta Histochem.
113:375–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Söderberg MW, Falconer C, Byström B,
Malmström A and Ekman G: Young women with genital prolapse have a
low collagen concentration. Acta Obstet Gynecol Scand.
83:1193–1198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cooke MS, Evans MD, Dizdaroglu M and Lunec
J: Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB
J. 17:1195–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lupachyk S, Shevalye H, Maksimchyk Y, Drel
VR and Obrosova IG: PARP inhibition alleviates diabetes-induced
systemic oxidative stress and neural tissue 4-hydroxynonenal adduct
accumulation: Correlation with peripheral nerve function. Free
Radic Biol Med. 50:1400–1409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoritaka A, Hattori N, Uchida K, Tanaka M,
Stadtman ER and Mizuno Y: Immunohistochemical detection of
4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl
Acad Sci USA. 93:pp. 2696–2701. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Halliwell B: Why and how should we measure
oxidative DNA damage in nutritional studies? How far have we come?
Am J Clin Nutr. 72:1082–1087. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kasai H: Analysis of a form of oxidative
DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular
oxidative stress during carcinogenesis. Mutat Res. 387:147–163.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alary J, Guéraud F and Cravedi JP: Fate of
4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol
Aspects Med. 24:177–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dwivedi S, Sharma A, Patrick B, Sharma R
and Awasthi YC: Role of 4-hydroxynonenal and its metabolites in
signaling. Redox Rep. 12:4–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oberley TD and Oberley LW: Antioxidant
enzyme levels in cancer. Histol Histopathol. 12:525–535.
1997.PubMed/NCBI
|
|
39
|
Wallace DC: Mitochondrial diseases in man
and mouse. Science. 283:1482–1488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arthur JR: The glutathione peroxidases.
Cell Mol Life Sci. 57:1825–1835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeLancey JO: Anatomie aspects of vaginal
eversion after hysterectomy. Am J Obstet Gynecol. 166:1717–1728.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martin CM and O'Leary JJ: Histology of
cervical intraepithelial neoplasia and the role of biomarkers. Best
Pract Res Clin Obstet Gynaecol. 25:605–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bump RC, Mattiasson A, Bø K, Brubaker LP,
DeLancey JO, Klarskov P, Shull BL and Smith AR: The standardization
of terminology of female pelvic organ prolapse and pelvic floor
dysfunction. Am J Obstet Gynecol. 175:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Santulli P, Borghese B, Lemaréchal H,
Leconte M, Millischer AE, Batteux F, Chapron C and Borderie D:
Increased serum oxidative stress markers in women with uterine
leiomyoma. PLoS One. 8:e720692013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scutiero G, Iannone P, Bernardi G,
Bonaccorsi G, Spadaro S, Volta CA, Greco P and Nappi L: Oxidative
stress and endometriosis: A systematic review of the literature.
Oxid Med Cell Longev. 2017:72652382017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lugogo NL, Bappanad D and Kraft M:
Obesity, metabolic dysregulation and oxidative stress in asthma.
Biochim Biophys Acta. 1810:1120–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dikalov SI and Ungvari Z: Role of
mitochondrial oxidative stress in hypertension. Am J Physiol Heart
Circ Physiol. 305:H1417–H1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takacs P, Gualtieri M, Nassiri M,
Candiotti K and Medina CA: Vaginal smooth muscle cell apoptosis is
increased in women with pelvic organ prolapse. Int Urogynecol J
Pelvic Floor Dysfunct. 19:1559–1564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takacs P, Nassiri M, Gualtieri M,
Candiotti K and Medina CA: Uterosacral ligament smooth muscle cell
apoptosis is increased in women with uterine prolapse. Reprod Sci.
16:447–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim EJ, Chung N, Park SH, Lee KH, Kim SW,
Kim JY, Bai SW and Jeon MJ: Involvement of oxidative stress and
mitochondrial apoptosis in the pathogenesis of pelvic organ
prolapse. J Urol. 189:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Perl A: Oxidative stress in the pathology
and treatment of systemic lupus erythematosus. Nat Rev Rheumatol.
9:674–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lindblom R, Higgins G, Coughlan M and de
Haan JB: Targeting mitochondria and reactive oxygen Species-driven
pathogenesis in diabetic nephropathy. Rev Diabet Stud. 12:134–156.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nita M and Grzybowski A: The role of the
reactive oxygen species and oxidative stress in the pathomechanism
of the Age-related ocular diseases and other pathologies of the
anterior and posterior eye segments in adults. Oxid Med Cell
Longev. 2016:31647342016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Styskal J, Van Remmen H, Richardson A and
Salmon AB: Oxidative stress and diabetes: What can we learn about
insulin resistance from antioxidant mutant mouse models? Free Radic
Biol Med. 52:46–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Baas AS and Berk BC: Differential
activation of mitogen-activated protein kinases by H2O2 and O2- in
vascular smooth muscle cells. Circ Res. 77:29–36. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ushio-Fukai M, Alexander RW, Akers M and
Griendling KK: p38 Mitogen-activated protein kinase is a critical
component of the redox-sensitive signaling pathways activated by
angiotensin II. Role in vascular smooth muscle cell hypertrophy. J
Biol Chem. 273:15022–15029. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ushio-Fukai M, Alexander RW, Akers M, Yin
Q, Fujio Y, Walsh K and Griendling KK: Reactive oxygen species
mediate the activation of Akt/protein kinase B by angiotensin II in
vascular smooth muscle cells. J Biol Chem. 274:22699–22704. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sundaresan M, Yu ZX, Ferrans VJ, Irani K
and Finkel T: Requirement for generation of H2O2 for
platelet-derived growth factor signal transduction. Science.
270:296–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zafari AM, Ushio-Fukai M, Akers M, Yin Q,
Shah A, Harrison DG, Taylor WR and Griendling KK: Role of
NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular
hypertrophy. Hypertension. 32:488–495. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ushio-Fukai M, Zafari AM, Fukui T,
Ishizaka N and Griendling KK: p22phox is a critical component of
the superoxide-generating NADH/NADPH oxidase system and regulates
angiotensin II-induced hypertrophy in vascular smooth muscle cells.
J Biol Chem. 271:23317–23321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ballinger SW, Patterson C, Yan CN, Doan R,
Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman
BA and Runge MS: Hydrogen peroxide- and peroxynitrite-induced
mitochondrial DNA damage and dysfunction in vascular endothelial
and smooth muscle cells. Circ Res. 86:960–966. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kiningham KK, Oberley TD, Lin S, Mattingly
CA and St Clair DK: Overexpression of manganese superoxide
dismutase protects against mitochondrial-initiated poly(ADP-ribose)
polymerase-mediated cell death. FASEB J. 13:1601–1610. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Čáp M, Váchová L and Palková Z: Reactive
oxygen species in the signaling and adaptation of multicellular
microbial communities. Oxid Med Cell Longev. 2012:9767532012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Scherz-Shouval R and Elazar Z: Regulation
of autophagy by ROS: Physiology and pathology. Trends Biochem Sci.
36:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vernon PJ and Tang D: Eat-me: Autophagy,
phagocytosis, and reactive oxygen species signaling. Antioxid Redox
Signal. 18:677–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Owens MW, Milligan SA, Jourd'heuil D and
Grisham MB: Effects of reactive metabolites of oxygen and nitrogen
on gelatinase A activity. Am J Physiol. 273:L445–L450.
1997.PubMed/NCBI
|
|
70
|
Viappiani S, Nicolescu AC, Holt A, Sawicki
G, Crawford BD, León H, van Mulligen T and Schulz R: Activation and
modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and
glutathione. Biochem Pharmacol. 77:826–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim NN, Villegas S, Summerour SR and
Villarreal FJ: Regulation of cardiac fibroblast extracellular
matrix production by bradykinin and nitric oxide. J Mol Cell
Cardiol. 31:457–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liang CC, Huang HY and Chang SD: Gene
expression and immunoreactivity of elastolytic enzymes in the
uterosacral ligaments from women with uterine prolapse. Reprod Sci.
19:354–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Donnini S, Monti M, Roncone R, Morbidelli
L, Rocchigiani M, Oliviero S, Casella L, Giachetti A, Schulz R and
Ziche M: Peroxynitrite inactivates human-tissue inhibitor of
metalloproteinase-4. FEBS Lett. 582:1135–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mayne ST: Antioxidant nutrients and
chronic disease: Use of biomarkers of exposure and oxidative stress
status in epidemiologic research. J Nutr. 133 Suppl 3:933S–940S.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kulms D, Zeise E, Poeppelmann B and
Schwarz T: DNA damage, death receptor activation and reactive
oxygen species contribute to ultraviolet radiation-induced
apoptosis in an essential and independent way. Oncogene.
21:5844–5851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kaneko T, Tahara S and Matsuo M:
Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of
oxidized DNA damage, during aging. Mutat Res. 316:277–285. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Awasthi YC, Sharma R, Sharma A, Yadav S,
Singhal SS, Chaudhary P and Awasthi S: Self-regulatory role of
4-hydroxynonenal in signaling for stress-induced programmed cell
death. Free Radic Biol Med. 45:111–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cheng JZ, Singhal SS, Saini M, Singhal J,
Piper JT, Van Kuijk F, Zimniak P, Awasthi YC and Awasthi S: Effects
of mGST A4 transfection on 4-hydroxynonenal-mediated apoptosis and
differentiation of K562 human erythroleukemia cells. Arch Biochem
Biophys. 372:29–36. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li J, Sharma R, Patrick B, Sharma A,
Jeyabal PV, Reddy PM, Saini MK, Dwivedi S, Dhanani S, Ansari NH, et
al: Regulation of CD95 (Fas) expression and Fas-mediated apoptotic
signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry.
45:12253–12264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma R, Brown D, Awasthi S, Yang Y,
Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV and
Awasthi YC: Transfection with 4-hydroxynonenal-metabolizing
glutathione S-transferase isozymes leads to phenotypic
transformation and immortalization of adherent cells. Eur J
Biochem. 271:1690–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sharma R, Sharma A, Dwivedi S, Zimniak P,
Awasthi S and Awasthi YC: 4-Hydroxynonenal self-limits fas-mediated
DISC-independent apoptosis by promoting export of Daxx from the
nucleus to the cytosol and its binding to Fas. Biochemistry.
47:143–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chaudhary P, Sharma R, Sharma A, Vatsyayan
R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S and Awasthi
YC: Mechanisms of 4-hydroxy-2-nonenal induced pro-and
anti-apoptotic signaling. Biochemistry. 49:6263–6275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Forgione MA, Weiss N, Heydrick S, Cap A,
Klings ES, Bierl C, Eberhardt RT, Farber HW and Loscalzo J:
Cellular glutathione peroxidase deficiency and endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 282:H1255–H1261.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Thu VT, Kim HK, Ha SH, Yoo JY, Park WS,
Kim N, Oh GT and Han J: Glutathione peroxidase 1 protects
mitochondria against hypoxia/reoxygenation damage in mouse hearts.
Pflugers Arch. 460:55–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jang YC, Pérez VI, Song W, Lustgarten MS,
Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, et al:
Overexpression of Mn superoxide dismutase does not increase life
span in mice. J Gerontol A Biol Sci Med Sci. 64:1114–1125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li
Y, Qi W, Zhang BX and Van Remmen H: Loss of manganese superoxide
dismutase leads to abnormal growth and signal transduction in mouse
embryonic fibroblasts. Free Radic Biol Med. 49:1255–1262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kwak HB, Lee Y, Kim JH, Van Remmen H,
Richardson AG and Lawler JM: MnSOD overexpression reduces fibrosis
and Pro-apoptotic signaling in the aging mouse heart. J Gerontol A
Biol Sci Med Sci. 70:533–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Eldridge A, Fan M, Woloschak G, Grdina DJ,
Chromy BA and Li J: Manganese superoxide dismutase interacts with a
large scale of cellular and mitochondrial proteins in low-dose
radiation-induced adaptive radioprotection. Free Radic Biol Med.
53:1838–1847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Smolik AC, Bengez-Pudja L, Cheng I and
Mascotti DP: Characterization of E. coli manganese superoxide
dismutase binding to RNA and DNA. Biochim Biophys Acta.
1844:2251–2256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Holley AK, Dhar SK, Xu Y and St Clair DK:
Manganese superoxide dismutase: Beyond life and death. Amino Acids.
42:139–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Silva JP, Shabalina IG, Dufour E, Petrovic
N, Backlund EC, Hultenby K, Wibom R, Nedergaard J, Cannon B and
Larsson NG: SOD2 overexpression: Enhanced mitochondrial tolerance
but absence of effect on UCP activity. EMBO J. 24:4061–4070. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Motoori S, Majima HJ, Ebara M, Kato H,
Hirai F, Kakinuma S, Yamaguchi C, Ozawa T, Nagano T, Tsujii H and
Saisho H: Overexpression of mitochondrial manganese superoxide
dismutase protects against radiation-induced cell death in the
human hepatocellular carcinoma cell line HLE. Cancer Res.
61:5382–5388. 2001.PubMed/NCBI
|
|
93
|
Suzuki K, Murtuza B, Sammut IA, Latif N,
Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H and Yacoub
MH: Heat shock protein 72 enhances manganese superoxide dismutase
activity during myocardial ischemia-reperfusion injury, associated
with mitochondrial protection and apoptosis reduction. Circulation.
106 12 Suppl 1:I270–I276. 2002.PubMed/NCBI
|
|
94
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word RA: Morphometric properties of the posterior vaginal wall
in women with pelvic organ prolapse. Am J Obstet Gynecol.
187:1501–1509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word R: Morphometric analysis of smooth muscle in the anterior
vaginal wall of women with pelvic organ prolapse. Am J Obstet
Gynecol. 187:56–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gabriel B, Denschlag D, Göbel H, Fittkow
C, Werner M, Gitsch G and Watermann D: Uterosacral ligament in
postmenopausal women with or without pelvic organ prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 16:475–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ozdegirmenci O, Karslioglu Y, Dede S,
Karadeniz S, Haberal A, Gunhan O and Celasun B: Smooth muscle
fraction of the round ligament in women with pelvic organ prolapse:
A computer-based morphometric analysis. Int Urogynecol J Pelvic
Floor Dysfunct. 16:39–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bermingham EN, Hesketh JE, Sinclair BR,
Koolaard JP and Roy NC: Selenium-enriched foods are more effective
at increasing glutathione peroxidase (GPx) activity compared with
selenomethionine: A meta-analysis. Nutrients. 6:4002–4031. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kwak HB, Song W and Lawler JM: Exercise
training attenuates age-induced elevation in Bax/Bcl-2 ratio,
apoptosis, and remodeling in the rat heart. FASEB J. 20:791–793.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kwak HB, Kim JH, Joshi K, Yeh A, Martinez
DA and Lawler JM: Exercise training reduces fibrosis and matrix
metalloproteinase dysregulation in the aging rat heart. FASEB J.
25:1106–1117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lawler JM, Kwak HB, Kim JH and Suk MH:
Exercise training inducibility of MnSOD protein expression and
activity is retained while reducing prooxidant signaling in the
heart of senescent rats. Am J Physiol Regul Integr Comp Physiol.
296:R1496–R1502. 2009. View Article : Google Scholar : PubMed/NCBI
|