Introduction
Hypoxic ischemic brain damage (HIBD) occurs
primarily in newborns, and is characterized as a brain lesion
caused by a number of factors during the perinatal period (1,2). It is a
brain injury syndrome that causes neonatal death, mental
retardation in children and epilepsy (1,2). HIBD is
a common central nervous system disease in neonatal infants
(2). Currently, 25% of children who
survive moderate-to-severe HIBD will have permanent neurological
deficits. At present, there is no accepted treatment method for
HIBD, and the mechanism of the disease remains to be elucidated,
thus the disease often has a poor prognosis (3). Therefore, it is critical that an
effective neuroprotective therapy is developed.
It has previously been demonstrated that retinoic
acid (RA), which is a key metabolite of vitamin A (VA), and its
derivatives regulate the apoptosis and proliferation of tumor cells
(4). The PC12 cell line consists of
rat pheochromocytoma cells, the main secretion products of which
are catecholamines, including dopamine and norepinephrine (5). PC12 cell membranes contain nerve growth
factor receptor and the cells exhibit typical nerve cell
characteristics; as such, they are used as an in vitro model
for the study of numerous nervous system diseases, including
Parkinson's disease and Alzheimer's disease (5,6). It has
been established that the main form of neuron death following HIBD
is apoptosis (7). Imbalances in the
expression of genes in the B-cell lymphoma 2 (Bcl-2) family located
in the mitochondrion, and in the expression of their encoded
proteins, are key events in the mitochondrial apoptotic pathway
(8) that lead to damage of cellular
structure and function. A previous study on HIBD in rats indicated
that there is an association between the level of VA nutrition and
the repair of the nervous system following injury (9). The mechanism primarily functions
through the regulation of RA receptor α (RAR-α) nuclear receptors,
thereby affecting Ca2+ levels in nerve cells and
affecting learning and memory functions (9,10).
The protective effects of all-trans RA (ATRA) on
oxygen-glucose deprivation (OGD) damage may be partly mediated by
regulation of the mitochondrial apoptotic pathway, and promotion of
the repair of nerve cells (10). RNA
interference (RNAi) technology may be used to efficiently degrade
the endogenous target gene mRNA, which is homologous with small
interfering RNA (si)RNA, and silence the expression of target
genes, in order to study the function of specific target genes
(11). In the present study,
recombinant adenovirus RAR-α-siRNA (Ad-siRAR-α) was used to inhibit
RAR-α expression, in order to investigate whether ATRA exerts
anti-apoptotic effects through the RAR-α pathway. Furthermore, the
present study aimed to explore the regulatory effect of RAR-α on
the apoptosis rate of OGD-induced PC12 cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), high
glucose and DMEM, no glucose were purchased from Beijing
Qingdatianyi Biological Technology Co., Ltd. (Beijing, China).
Hank's balanced salt solution was purchased from Hyclone (GE
Healthcare, Logan, UT, USA). Fetal bovine serum (FBS), human serum
(HS) and TrypLE were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RNeasy Mini kit was purchased
from Qiagen GmbH (Hilden, Germany). PrimeScript™ RT
Reagent kit (Perfect Real Time) and the DNA ladder were purchased
from Takara Biotechnology Co., Ltd. (Dalian, China). Primers for
polymerase chain reaction (PCR) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). SuperReal PreMix Plus (SYBR
Green) was purchased from Tiangen Biotech Co., Ltd. (Beijing,
China). ATRA standard was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). ReadyPrep™ Protein Extraction kit
(Total Protein) was purchased from Bio-Rad Laboratories, Inc.
(Hercules, California, USA). Primary antibodies for Bcl-2 (cat. no.
sc-509), Bcl-2-associated X protein (Bax; cat. no. sc-20067) and
β-actin (cat. no. sc-47778), and goat anti-rat IgG-HRP secondary
antibodies (cat. no. sc-2065) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Goat anti-rabbit
FITC-conjugated secondary antibodies (cat. no. TA130021) were
purchased from OriGene Technologies, Inc. (Beijing, China). Bovine
serum albumin was purchased from Solarbio Science and Technology
Co., Ltd (Beijing, China). Annexin V-Propidium Iodide (PI)
Apoptosis Detection kit was purchased from BestBio Biotechnology
Co., Ltd. (Shanghai, China). JC-1 Mitochondrial Membrane Potential
kit was purchased from Beyotime Institute of Biotechnology (Haimen,
China). Earle's balanced salt solution (EBSS) sugar-free culture
medium was purchased from Hyclone (GE Healthcare).
Instruments
StepOne 2.1 Real-Time PCR system was purchased from
Applied Biosystems (Thermo Fisher Scientific, Inc.).
Mastercycler® nexus flat eco was purchased from
Eppendorf (Hamburg, Germany). A Odyssey® Fc Imaging
system (LI-COR Biosciences, Lincoln, NE, USA), BD
Accuri™ C6 Plus flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA), and Qubit™ 3 Fluorometer (Thermo Fisher
Scientific, Inc.) were also used in the present study.
Culture of PC12 cells
PC12 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
PC12 cells were transferred into high glucose DMEM containing 5%
FBS, 10% HS, 1×105 U/l penicillin and 100 mg/l
streptomycin, then incubated in a constant temperature incubator at
37°C, saturated humidity, 5% CO2 + 95% O2;
the medium was changed every 2 days. When cells reached 80–90%
confluence, they were digested with 1 ml TrypLE solution at 37°C
for 16–20 h, and centrifuged at room temperature and 252 × g for 5
min (12). The cell suspension was
prepared in the high glucose DMEM medium, and the cell
concentration was adjusted to 5×104 cells/ml and
transferred to the culture dish. Passages were proceeded when the
adherent cells approached 80% confluence. Cells were divided into
four groups: ATRA, OGD, ATRA + OGD and control. A 100 mM stock
solution of ATRA was prepared with dimethylsulfoxide (DMSO) and
diluted to the indicated final concentration (4 µmol/l) with high
glucose DMEM prior to adding to the culture medium. To induce OGD
injury, the PC12 cell culture medium (high glucose DMEM) was
replaced by glucose-free DMEM. The cells were then incubated (95%
N2 and 5% CO2) for 4 h at 37°C. Cells in the
OGD and ATRA groups were subjected to OGD injury and 4 µmol/l ATRA,
respectively, for 4 h at 37°C. Cells in the OGD + ATRA group were
subjected to OGD injury in glucose-free DMEM containing 4 µmol/l
ATRA. The control cells were washed with high glucose DMEM and
incubated in a normoxic incubator in 100 µl DMSO for 4 h.
Ad-siRAR-α and RFP transduction of
PC12 cells
Ad-siRAR-α was designed and constructed by the
present authors. Three different pairs of sequences of siRNA were
designed according to the gene sequence of rat RAR-α (NM_031528.2)
provided by GenBank (ncbi.nlm.nih.gov/genbank), as shown in Table I. The adenovirus shuttle plasmid
pSES-HUS-s RAR-α gifted by Professor Tong-Chuan He (Molecular
Oncology Lab, Department of Orthopaedic Surgery and Rehabilitation
Medicine, University of Chicago Medical Center, Chicago, IL, USA)
was constructed and recombined with a skeleton plasmid, pAd-Easy-1
(Stratagene; Agilent Technologies GmbH, Waldbronn, Germany), to
obtain Ad-siRAR-α recombinant adenovirus in 293 cells (American
Type Culture Collection, Manassas, VA, USA) using
ViraDuctin™ Adenovirus Transduction Reagent (Cell
Biolabs, Inc., San Diego, CA, USA) according to the manufacturer's
protocol. The inhibitory effect of the siRNA was verified by
semi-quantitative RT-PCR. pAd-Easy-1 alone was transfected as
aforementioned and used as the negative control for the
semi-quantitative RT-PCR.
 | Table I.siRAR-α sequences of RAR-α genes. |
Table I.
siRAR-α sequences of RAR-α genes.
| Name | Sequence
(5′-3′) |
|---|
| siRAR-α | Fwd:
ACCAAGGAGTCGGTGCGAAATTTT |
|
| Rev:
ATTTCGCACCGACTCCTTGGTTTT |
| siRAR-α2 | Fwd:
AGCAAGTACACTACGAACAATTTT |
|
| Rev:
ATTGTTCGTAGTGTACTTGCTTTT |
| siRAR-α3 | Fwd:
ACCTCATCTGTGGAGACCGATTTT |
|
| Rev:
ATCGGTCTCCACAGATGAGGTTTT |
Three pairs of different Ad-siRAR-α recombinant
adenoviruses were mixed in equal proportions (2 µl) and transduced
into PC12 cells at 70% confluence, with pSES-HUS adenovirus as the
negative control. RFP was incubated with cells treated with ATRA
and OGD for 4 h at 37°C. Viral transduction was subsequently
verified using fluorescence microscopy by red fluorescence
observation, and the fluorescence was quantified by ImageJ software
(version 1.51d; National Institutes of Health, Bethesda, MD, USA)
under three different fields of view with magnification of ×200.
PC12 cells were washed with Hank's solution following 36 h of viral
transduction at 37°C. Following the transduction, the cells in the
siRAR-α + ATRA + OGD group and RFP + ATRA + OGD group were
subsequently subjected to OGD injury in glucose-free DMEM
containing 4 µM ATRA, then incubated (95% N2 and 5%
CO2) for 4 h at 37°C. The control cells were washed with
high glucose DMEM and incubated in a normoxic incubator in 100 µl
DMSO for 4 h.
Detection of inhibition rate of
siRAR-α in PC12 cells by semi-quantitative reverse transcription
PCR (RT-PCR)
RNA was extracted from all cells using an RNeasy
Mini kit and reverse-transcribed into cDNA using
PrimeScript™ RT Reagent kit (Perfect Real Time)
according to the manufacturers' protocols. PCR amplification was
performed using a SuperReal PreMix Plus (SYBR-Green) and specific
primers for RAR-α in rats (Table
II) under the following conditions: 94°C For 2 min; 10 cycles
at 93°C for 20 sec, 65–55°C for 20 sec (each cycle reduced by 1°C)
and 72°C for 20 sec; 28 cycles at 93°C for 20 sec, 56°C for 20 sec
and 72°C for 20 sec, and 72°C for 2 min. β-actin was used as the
internal control. Finally, RAR-α mRNA expression level in
transduced PC12 cells was analysed by separating total RNA in a
1.5% agarose gel with ethidium bromide staining and quantified
software (Image Studio™ Lite, version 4.0) embedded in
Odyssey® Fc Imager (version 1.0.17; both LI-COR
Biosciences).
 | Table II.Primer sequences for polymerase chain
reaction analysis of RAR-α expression. |
Table II.
Primer sequences for polymerase chain
reaction analysis of RAR-α expression.
| Gene name | Primer sequence
(5′-3′) |
|---|
| RAR-α | Fwd:
GACTCCGCTTTGGAATGG |
|
| Rev:
ACTGCTGCTCTGGGTCTCG |
| β-actin | Fwd:
GCATAGCCACGCTTGTTCTTGAAG |
|
| Rev:
GAACCGCTCATTGCCGATAGTG |
Annexin V-PI flow cytometry detection
of apoptosis
PC12 cells wrere gently rinsed with Hank's solution
(2 ml) and the flushing fluid was collected in each test tube. The
cells with different treatments were digested with 0.5 ml TrypLE
solution at 37°C until 70% of the cells were detached and a glass
straw attached to a rubber head was used to blow cells into
corresponding test tubes. Tubes were centrifuged at 4°C and 252 × g
for 4 min, then the supernatant of each tube was discarded. Annexin
V-fluorescein isothiocyanate and PI staining were performed on cell
sediments according to the Annexin V-PI kit protocol. The apoptosis
rate of each group was detected using a flow cytometer and analyzed
using FCS Express Flow Cytometry Lite RUO software (version 6; De
Novo Software, Glendale, CA, USA). The incidence of apoptosis for
all groups was measured three times.
Detection of mitochondrial
transmembrane potential (MMP) by JC-1
Cells were collected using the aforementioned
method, and the supernatant was discarded following centrifugation.
The freshly prepared JC-1 working solution and high gluocose DMEM
were added to the PC12 cell pellet at a ratio of 1:1. Following
mixing, the mixture was incubated in a humidified atmosphere at
37°C, 5% CO2 + 95% O2 incubator for 20 min.
The mixture was centrifuged for 4 min at 252 × g, 4°C and the
supernatant was discarded, followed by washing with 1X PBS twice
(8–10 min/wash). The PC12 cells were resuspended in 300 µl 1X PBS
buffer, and the MMP was measured a flow cytometer and FCS Express
Flow Cytometry Lite RUO software (version 6). The above experiments
were repeated three times.
RT-quantitative (q)PCR detection of
Bax and Bcl-2 expression in PC12 cells with OGD-induced injury
following Ad-siRAR-α transduction
Following OGD-induced injury, PC12 cells were washed
with 2 ml Hank's solution and subjected to mRNA extraction
according to the protocol of the RNeasy Mini kit. The extracted RNA
was added to RQ1 RNase-free DNase (Promega Corporation, Madison,
WI, USA) to remove DNA contamination. Following the detection of
RNA concentration using the Qubit RNA HS Assay kit and Qubit 3
Fluorometer according to the manufacturer's protocol, the RNA was
reverse transcribed into cDNA using the PrimeScript Reverse
Transcription kit (reaction conditions: 37°C for 15 min, then 85°C
for 5 sec), and the obtained cDNA was diluted 10 times with water
and preserved at −20°C. qPCR was performed to detect the mRNA
expression level of Bax and Bcl-2 in a PCR system using SuperReal
PreMix Plus (SYBR Green). The thermocycling conditions were as
follows: 95°C for 3 min, 45 cycles of 95°C for 20 sec, 55°C for 20
sec and 72°C for 20 sec, then 65°C for 5 sec with β-actin as the
internal reference gene. PCR primers were designed using Primer
Premier 5.0 software (Premier Biosoft International, Palo Alto, CA,
USA). The specific primer sequences are shown in Table III. Band intensity values for Bcl-2
and Bax were normalized to those of β-actin and the relative
expression was calculated using the 2−ΔΔCq method
(13).
 | Table III.Primer sequences for polymerase chain
reaction analysis of apoptosis-related gene expression. |
Table III.
Primer sequences for polymerase chain
reaction analysis of apoptosis-related gene expression.
| Gene name | Primer sequence
(5′-3′) |
|---|
| Bax | Fwd:
ATTTCCAGACACCGAGGG |
|
| Rev:
TAAGCCAAATGTAGCAAGG |
| Bcl-2 | Fwd:
CGGGAGAACAGGGTATGA |
|
| Rev: CAGGC
TGGAAGGAGAAGAT |
| β-actin | Fwd:
GCATAGCCACGCTTGTTCTTGAAG |
|
| Rev: GAACCGC
TCATTGCCGATAGTG |
Western blot analysis of protein
expression in PC12 cells with OGD-induced injury following
Ad-siRAR-α transduction
PC12 cells were washed twice with PBS and protein
was extracted according to the protocol of ReadyPrep Protein
Extraction kit (Total Protein) kit. The concentration was
determined by bicinchoninic acid assay, and equal amounts of
protein (25 µg/lane) were separated by 12% SDS-PAGE. The proteins
were transferred to polyvinylidene fluoride membranes and blocked
for 1 h with 5% bovine serum albumin TBS-Tween-20 (TBST) solution
at room temperature. The TBST solution was used to wash the
membrane three times (8–10 min/wash), and membranes were incubated
in TBST solution with primary antibodies directed against Bcl-2,
Bax and β-actin (all 1:1,000) overnight at 4°C. Following three
washes of the membrane in TBST solution (8–10 min/wash),
horseradish peroxidase-labeled secondary antibodies (goat
anti-rabbit, 1:500; goat anti-rat, 1:800) were added and incubated
for 1 h at room temperature. Pierce™ ECL Plus Western
Blotting Substrate (Thermo Fisher Scientific, Inc.) was used to
visualize the bands, which were quantified using Image Studio Lite
software (version 4.0).
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for one-way analysis of variance to compare multiple
groups, with Student-Newman-Keuls analysis as a post-hoc test for
multiple comparisons. A Student's t-test was used to compare
differences between two groups. Experimental data are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Evaluation of Ad-siRAR-α
transduction
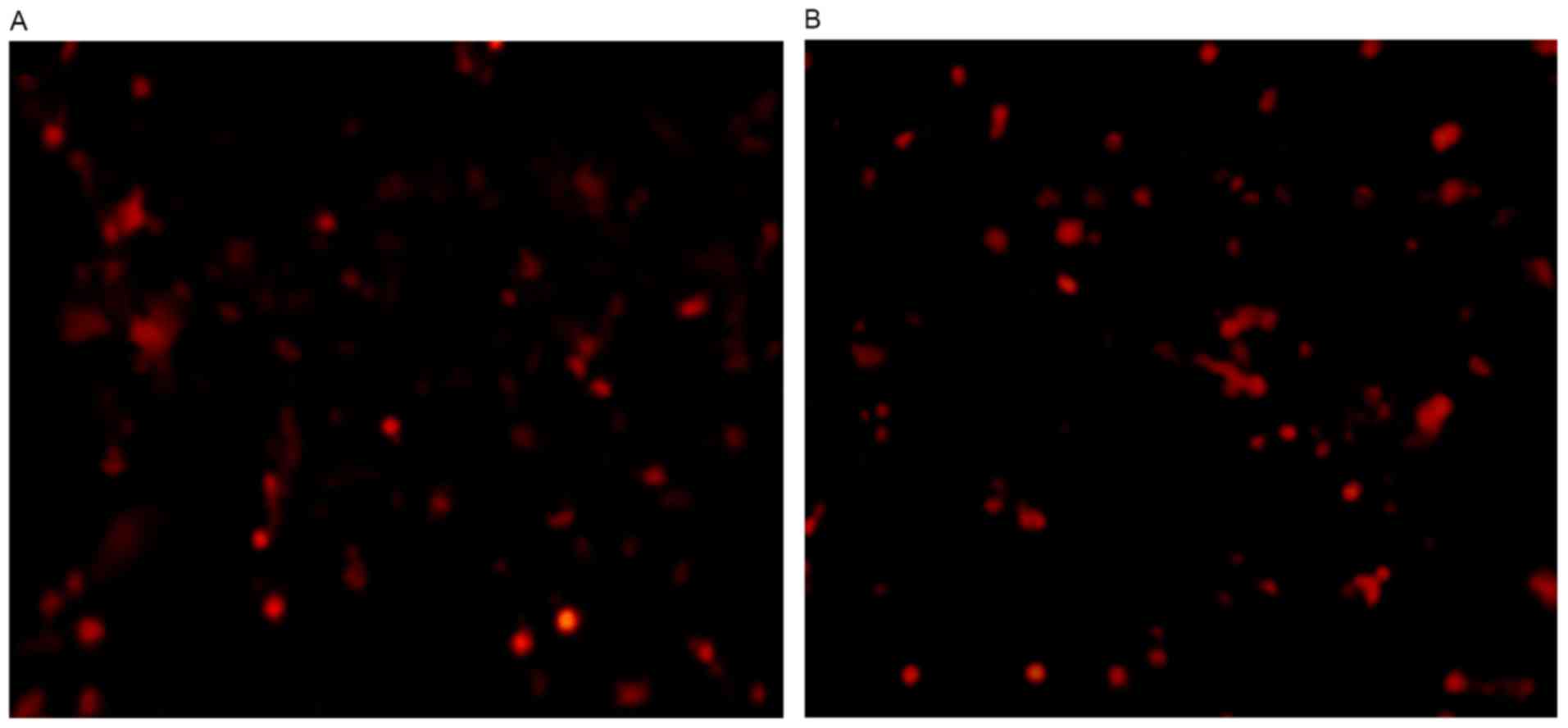
When the Ad-siRAR-α recombinant adenovirus was
transduced by Ad-siRAR-α for 36 h, it was identified that >70%
of the cells exhibited red fluorescence under fluorescent
microscope observation (Fig. 1).
This indicated that PC12 cells had been transduced with
Ad-siRAR-α.
Expression of RAR-α mRNA in PC12 cells
following transduction with Ad-siRAR-α
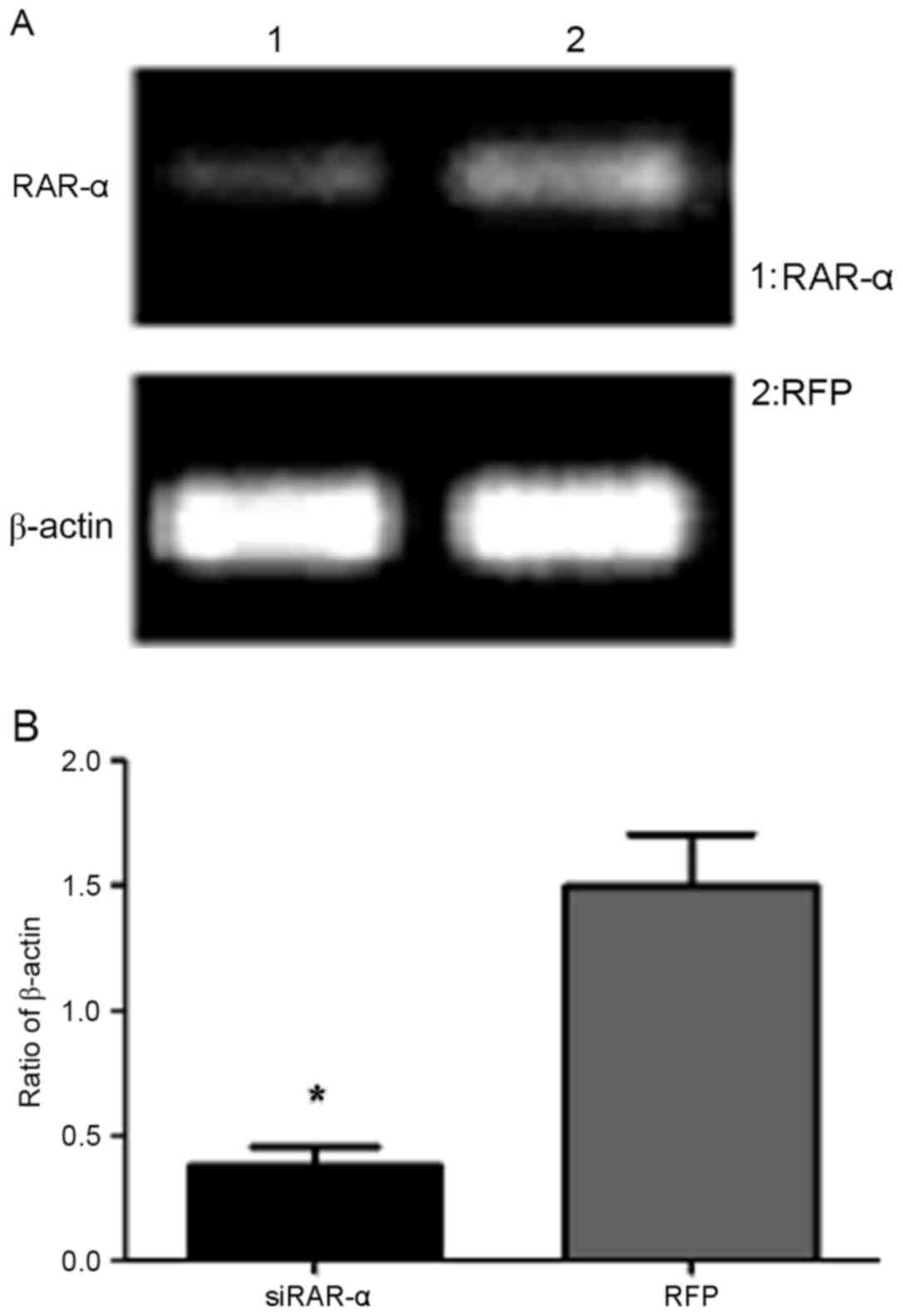
Semi-quantitative RT-PCR results indicated that the
expression of RAR-α mRNA was significantly decreased in PC12 cells
following transduction with Ad-siRAR-α compared with the control
group, which was transfected with adenovirus blank vector
(P<0.05; Fig. 2). This suggested
that Ad-siRAR-α was able to effectively suppress RAR-α gene
expression.
Expression of RAR-α in PC12 cells
following transduction with Ad-siRAR-α and OGD-induced injury
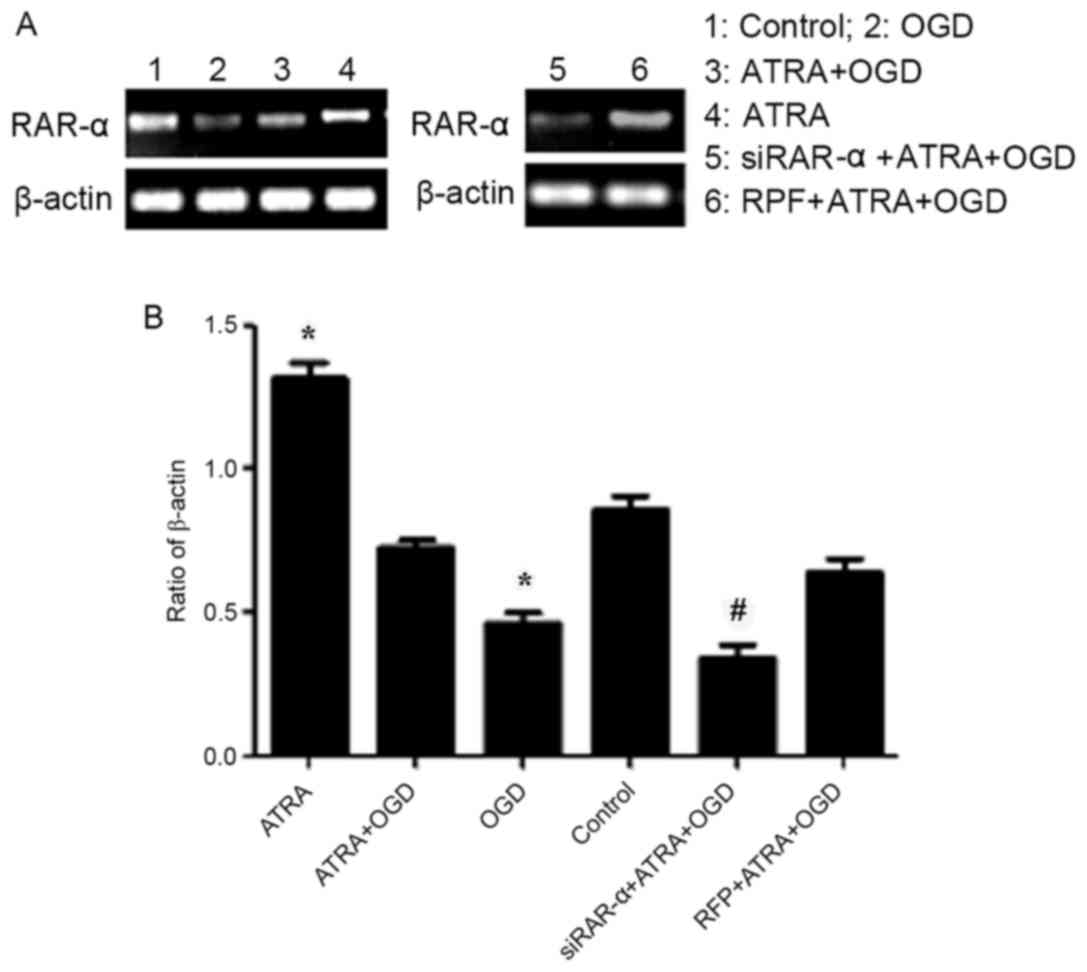
As presented in Fig.
3, the results of semi-quantitative RT-PCR indicated that RAR-α
mRNA expression was significantly increased in PC12 cells following
exposure to 4 µmol/l ATRA (P<0.05), whereas the expression of
RAR-α in OGD-injured cells was significantly decreased compared
with the control group cells (P<0.05). However, compared with
the control group, no significant difference was observed in the
expression of RAR-α mRNA in OGD-induced PC12 cells treated with 4
µmol/l ATRA. Similarly, RAR-α mRNA expression level in the PC12
cells transduced with Ad-siRAR-α was significantly lower compared
with the group that was transduced with adenovirus vector
(P<0.05). These results indicate that OGD-induced injury
down-regulated the RAR-α mRNA expression level and the Ad-siRAR-α
vector was successfully transduced.
Apoptosis rate of PC12 cells following
transduction with Ad-siRAR-α and OGD-induced injury
As shown in Fig. 4,
the apoptosis rate of PC12 cells following transduction with
Ad-siRAR-α and OGD-induced injury was significantly higher compared
with the adenovirus-transfected OGD-induced injury group
(P<0.05). This indicates that down-regulation of RAR-α can
induce the apoptosis of PC12 cells.
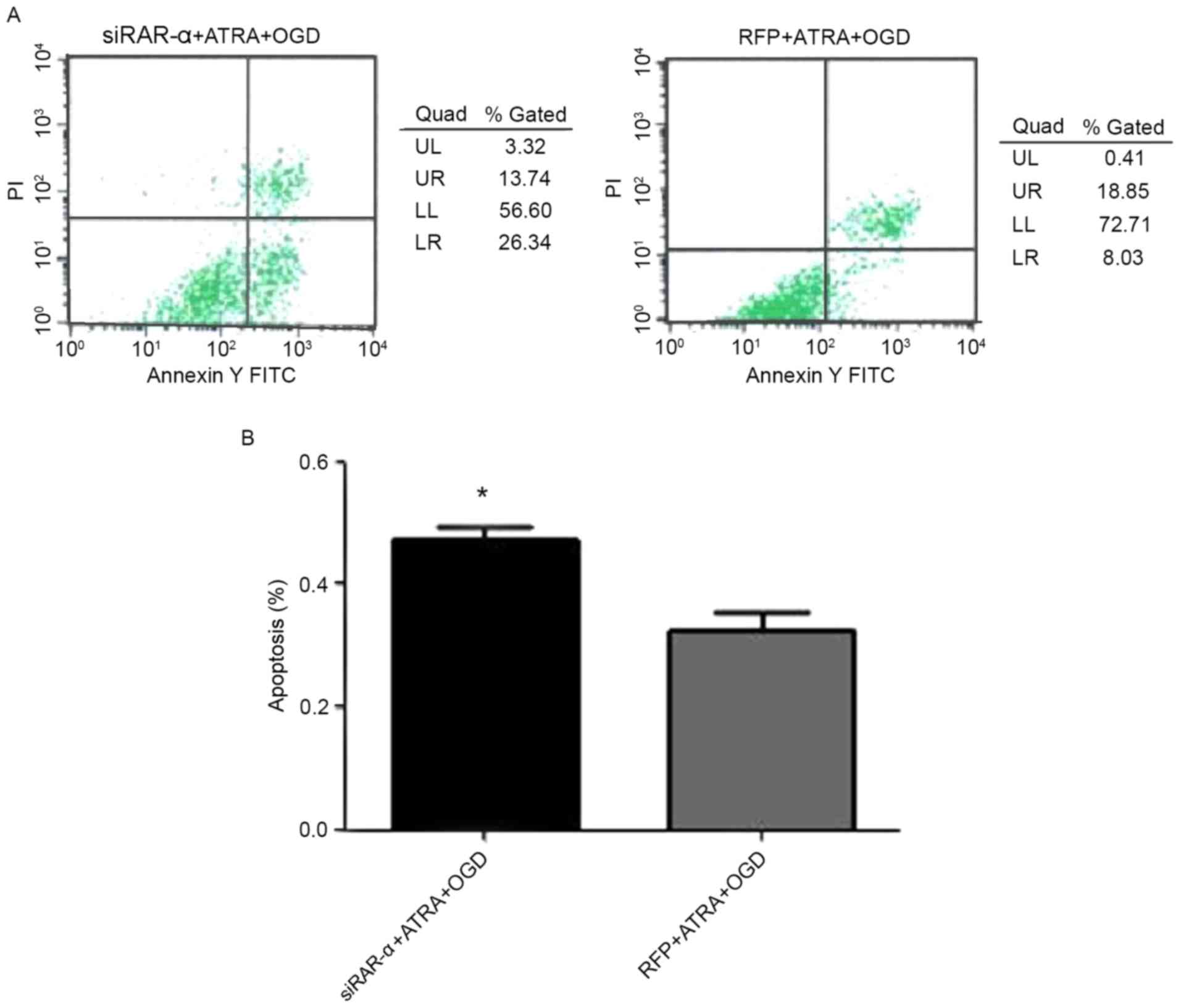 | Figure 4.Effect of recombinant adenovirus
siRAR-α transduction on the apoptosis rate of PC12 cells following
OGD treatment. (A) Apoptosis rate was detected by flow cytometry.
(B) Quantification of apoptosis rate. *P<0.05 vs. RFP + ATRA +
OGD group. RAR-α, retinoic acid receptor α; siRAR-α, RAR-α small
interfering RNA; ATRA, all-trans retinoic acid; OGD, oxygen-glucose
deprivation; RFP, red fluorescent protein; FITC, fluorescein
isothiocyanate; PI, propidium iodide; UL, upper left; UR, upper
right; LL, lower left; LR, lower right. |
Effect of Ad-siRAR-α on MMP in PC12
cells following OGD-induced injury
JC-1 is an ideal fluorescent probe for the detection
of MMP, and the decrease of MMP is a landmark event in the early
stage of mitochondrial apoptosis (14). When MMP is normal, JC-1 forms a
polymer in the mitochondrial matrix, producing red fluorescence.
When MMP decreases, JC-1 becomes a monomer, resulting in green
fluorescence (15). In the present
study, it was investigated whether different expression levels of
RAR-α were able to influence MMP in PC12 cells with OGD-induced
injury. It was identified that in PC12 cells transduced with
Ad-siRAR-α, the detection rate of green fluorescence was
significantly increased (P<0.05; Fig.
5), indicating a decrease in MMP compared with the control. In
Fig. 5A, the images indicate the MMP
at a certain time, and cannot reveal the trend changes in membrane
potential. The two axes in the figure represent the green
fluorescence detected by the FL1-H channel, and the green
fluorescence detected by the FL2-H channel. The detection rate of
green fluorescence indicates the degree of decrease in MMP. MMP was
decreased in the two groups and no red fluorescence was
observed.
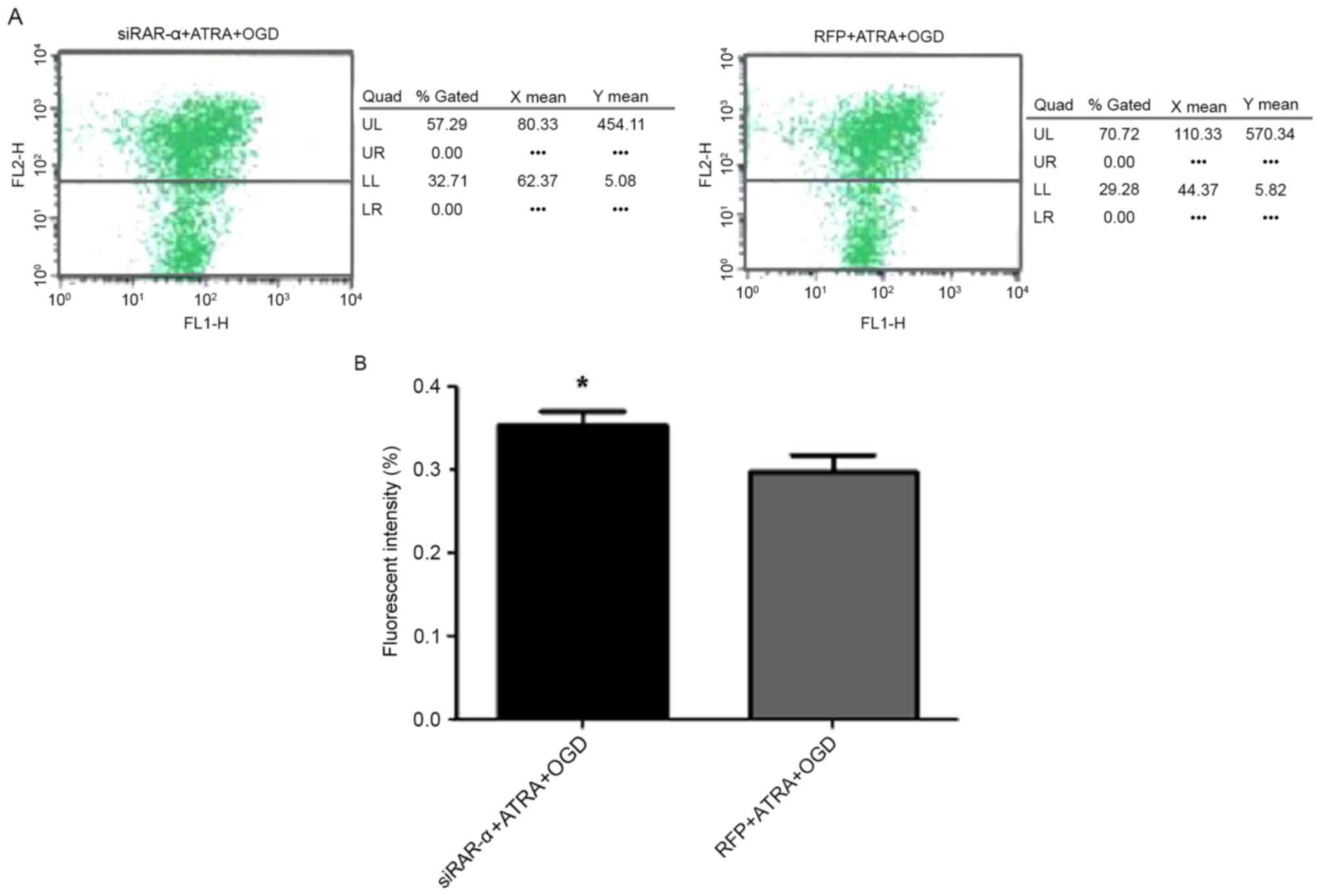 | Figure 5.Effect of recombinant adenovirus
siRAR-α transduction on MMP in PC12 cells following OGD treatment.
(A) Detection of MMP. (B) Quantification of fluorescence in PC12
cells. *P<0.05 vs. RFP + ATRA + OGD group. MMP, mitochondrial
membrane potential; RAR-α, retinoic acid receptor α; siRAR-α, RAR-α
small interfering RNA; ATRA, all-trans retinoic acid; OGD,
oxygen-glucose deprivation; RFP, red fluorescent protein; UL, upper
left; UR, upper right; LL, lower left; LR, lower right. |
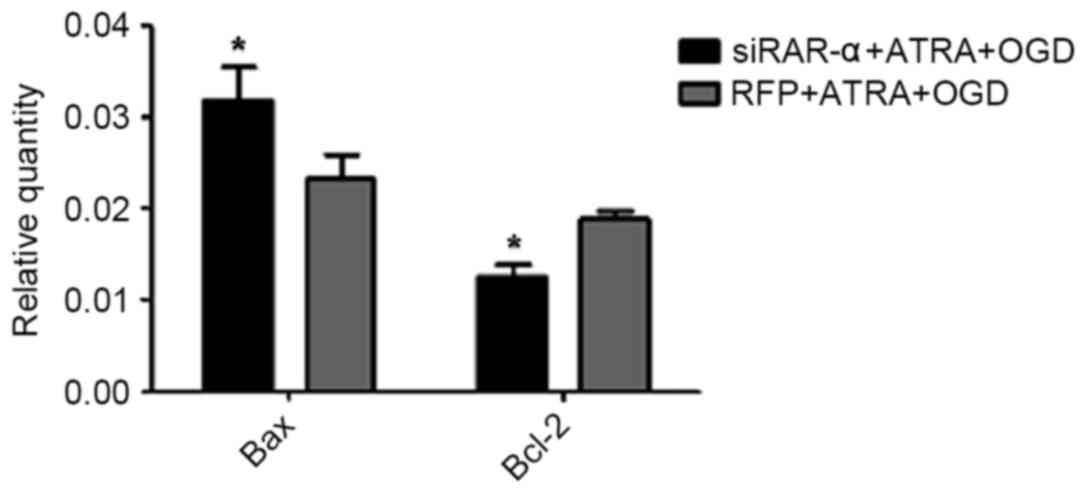
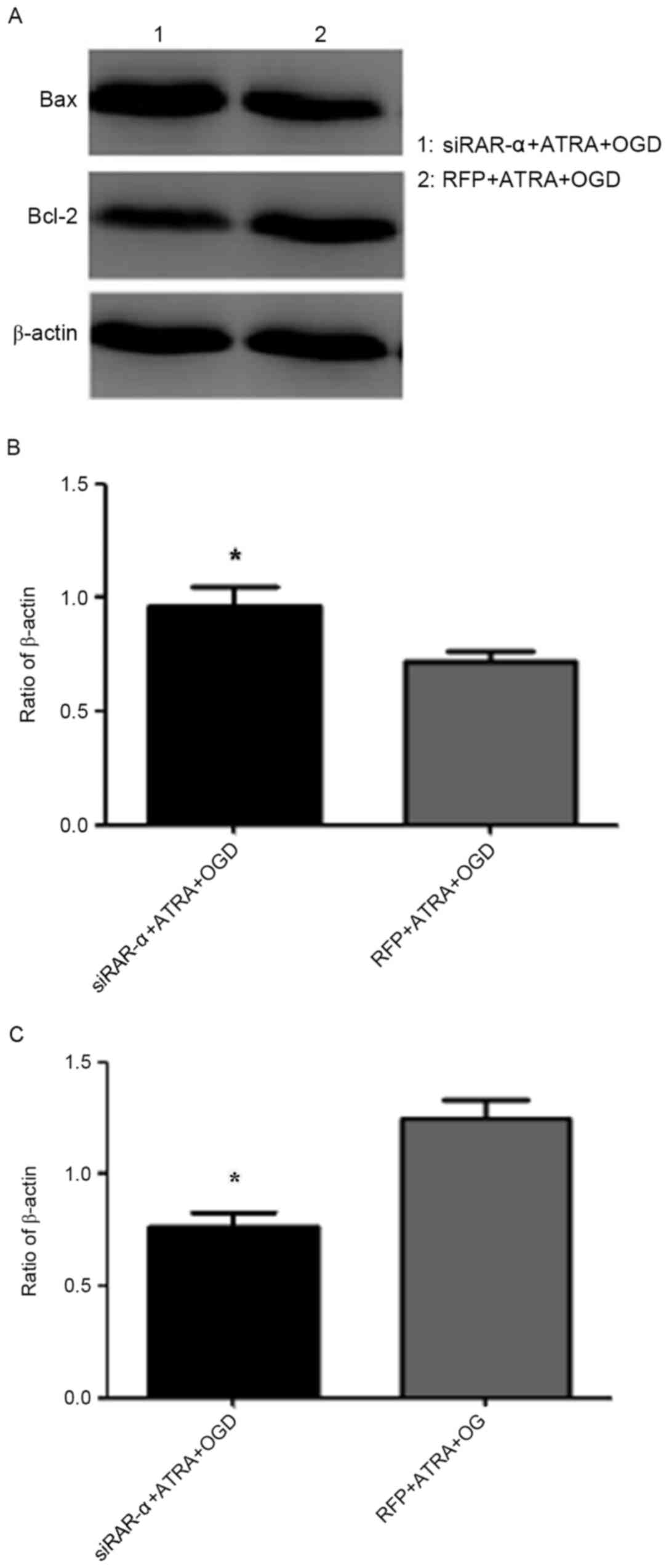
Effect of Ad-siRAR-α on Bax and Bcl-2
mRNA expression in PC12 cells following OGD-induced injury
The flow cytometry results described above indicated
that Ad-siRAR-α was able to significantly increase the apoptosis
rate in OGD-induced PC12 cells. Therefore, the current study aimed
to explore whether Ad-siRAR-α also affected the expression of Bax
and Bcl-2, which are key molecules in apoptosis. As presented in
Fig. 6, Ad-siRAR-α significantly
increased the Bax mRNA expression level (P<0.05) and
significantly decreased the Bcl-2 mRNA level (P<0.05) compared
with the control group of RFP + ATRA + OGD.
Effect of Ad-siRAR-α on Bax and Bcl-2
protein expression in PC12 cells following OGD-induced injury
As presented in Fig.
7, the protein expression of apoptosis factor Bax in the
siRAR-α + ATRA + OGD group was significantly higher compared with
the RFP + ATRA + OGD group (P<0.05), whereas the expression of
anti-apoptotic Bcl-2 protein was significantly lower compared with
the control group (P<0.05). These results were consistent with
the mRNA levels in Fig. 6. These
results suggest that siRAR-α promotes apoptosis in OGD-induced PC12
cells, and that OGD-induced apoptosis is, at least in part,
mediated by RAR-α expression.
Discussion
RA serves a key function in embryonic development,
particularly in neural system development (16,17). It
is the primary active substance of VA in the body, which acts via
regulating downstream gene transcription through signal
transduction of RARs (16,17). RARs may be divided into three
subtypes: α, β and γ. RARs and retinoid X receptors (RXRs)
typically form heterodimers, which may combine with RA response
elements of target genes to directly regulate the transcription of
target genes (including HOX, surfactant protein B and forkhead box
P3) (18). RXRs and RARs may also
form homodimers between themselves, or form homodimers combined
with peroxisome proliferator-activated receptor, thyroid hormone
receptor, vitamin D receptor, actin-related protein 1 or nerve
growth factor IB (16,18). As the central nervous system
develops, the RA signaling pathway is active in numerous brain
tissues (19), and different RA
receptors express specific functions in specific regions (20). Previous studies have demonstrated
that during the embryonic development of the nervous system, RA is
able to associate with corresponding receptors to take part in the
division and maintenance of nerve cells (19,21,22). RA
is able to activate different receptors, induce neuronal precursor
cells to differentiate into specific nerve cells (21), and also serves a key function in the
regional distribution of neural plate craniocaudal axis (22). RAR-α is a major RAR expressed during
hippocampal development in rats, and serves a key function in
learning and memory ability (23).
Previous studies by the present authors have
indicated that VA supplementation in rats with marginal VA
deficiency (MVAD) are not able to restore the learning and memory
ability of rats during postnatal development, indicating that the
MVAD from gestation may have irreversible effects on the brain
development of newborn rats (24,25).
Shinozaki et al (26) have
previously reported that ATRA may significantly reduce the
apoptosis rate of nerve cells in the hippocampus region in rats and
that this effect may be weakened by RAR-specific antagonists.
Following spinal cord injury in adult rats, exogenous RA is able to
activate the RAR-β2 receptor, and stimulate the growth of nerve
axons and repair of the peripheral nervous system (27). Furthermore, a study by the present
authors on HIBD neonatal rats demonstrated that the VA nutrition
level is associated with the repair function of the injured
neurons, the primary mechanism of which is to inhibit the
activation of Ca2+ in nerve cells through the regulation
of RAR-α nuclear receptors (9). In
the present study, it was also observed that OGD-induced injury
induced a decrease in RAR-α expression level, whereas ATRA
incorporation induced an increase of RAR-α, which indicated that
RAR-α may serve a function in the anti-apoptotic effect of ATRA
against OGD-induced injury.
RNAi is a phenomenon that inhibits specific gene
expression. When double-stranded RNA that is homologous to the
endogenous mRNA coding region is introduced into the cell, the mRNA
is degraded to cause silencing of gene expression, which is a key
method for studying gene function (11). The establishment of highly efficient
siRNA has allowed RNAi technology to be widely used in the study of
numerous diseases, including Alzheimer's disease, Parkinson's
disease and amyotrophic lateral sclerosis (28). In the current study, following
transduction with Ad-siRAR-α, RAR-α expression in PC12 cells was
significantly inhibited. By using Ad-siRAR-α transduction, flow
cytometry analysis indicated that the apoptotic rate of OGD-injured
PC12 cells was increased. The MMP also decreased following
Ad-siRAR-α transduction. These results indicated that low
expression of RAR-α is able to enhance the rate of apoptosis in
injured cells, and inhibit the repair function of cells. Previous
results have indicated that in the process of neurological damage,
RAR-α primarily promotes the proliferation of astrocytes and
oligodendrocytes, and serves a key function in the final maturation
process (21). Katsuki et al
(29) previously proposed that RA
may enhance the activity of brain-derived neurotrophic factor by
upregulating RAR-α expression, thereby protecting against
dopaminergic neuronal injury in the mesencephalon, which is
consistent with the results of the current study.
HIBD injury is typically divided into three phases:
Primary cell injury stage, energy recovery stage and late-onset
cell injury stage (30). The
previous study demonstrated that during the late-onset cell injury
stage, mitochondrial dysfunction serves a key function and induces
secondary failure of cellular energy metabolism. Bcl-2, which is
located on the mitochondrial membrane, is a proto-oncogene that has
been identified in lymphoma leukemia cells. Bcl-2 is a highly
conserved eukaryotic gene, and is expressed at low levels in normal
cells (31). Bax and Bcl-2 are two
key apoptosis-regulating genes of the Bcl-2 gene family with
opposite functions. Downregulation of Bax and upregulation of Bcl-2
increase the permeability of mitochondrial membrane, which releases
cytochrome C from the mitochondria, activates apoptosis-inducible
factors and ultimately triggers apoptosis (32). In the current study, it was indicated
that ATRA served an anti-apoptosis function by regulating an
endogenous apoptosis signaling pathway, and the expression of RAR-α
was inhibited by transduction with Ad-siRAR-α. It was also
identified that Bcl-2 gene expression was downregulated and Bax
gene expression upregulated in the mitochondrial apoptosis
signaling pathway, and the results were consistent at the mRNA and
protein level. Previous studies have demonstrated that Bcl-2 and
Bax are targets of RA signals (33–35). In
addition, in thymic tumor cells and pancreatic cancer cells, RA has
been demonstrated to induce the upregulation of suppressor gene p53
to increase Bcl-2 expression and inhibit apoptosis; however, the
specific mechanism remains unclear (36,37).
In conclusion, ATRA has anti-apoptosis and other
protective effects on OGD-induced injury. In the present study,
through the method of silencing RAR-α expression in PC12 cells by
Ad-siRAR-α transduction, it was demonstrated that the
anti-apoptosis effect of ATRA on OGD-induced injury is achieved via
increasing the expression of anti-apoptotic factor Bcl-2 and
decreasing the expression of pro-apoptotic factor Bax. This is
achieved, at least in part, by inhibiting the mitochondrial
apoptosis signaling pathway via upregulation of RAR-α
expression.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HIBD
|
hypoxic ischemic brain damage
|
|
VA
|
vitamin A
|
|
RAR-α
|
retinoic acid receptor α
|
|
ATRA
|
all-trans retinoic acid
|
|
MMP
|
mitochondrial transmembrane
potential
|
|
RNAi
|
RNA interference
|
|
OGD
|
oxygen-glucose deprivation
|
References
|
1
|
Xue BP: Advance in the treatment of
hypoxic-ischemic encephalopathy. Yi Xue Xin Xi. 24:25712011.
|
|
2
|
Fatemi A, Wilson MA and Johnston MV:
Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol.
36:835–858, vii. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XL, Zhao YS, Yang YJ, Xie M and Yu
XH: Therapeutic window of hyperbaric oxygen therapy for
hypoxic-ischemic brain damage in new-borne rats. Brain Res.
1222:87–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo P, Lin M, Lin M, Chen Y, Yang B and He
Q: Function of retinoic acid receptor alpha and p21 in
all-trans-retinoic acid-induced acute T-lymphoblastic leukemia
apoptosis. Leuk Lymphoma. 50:1183–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westerink RH and Ewing AG: The PC12 cell
as model for neurosecretion. Acta Physiol (Oxf). 192:273–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun B, Taing A, Liu H, Nie G, Wang J, Fang
Y, Liu L, Xue Y, Shi J, Liao YP, Ku J, Xia T and Liu Y: Nerve
growth factor-conjugated mesoporous silica nanoparticles promote
neuron-like PC12 Cell proliferation and neurite growth. J Nanosci
Nanotechnol. 16:2390–2393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhang J, Zhu X, Wang P, Wang X and
Li D: Progesterone reduces inflammation and apoptosis in neonatal
rats with hypoxic ischemic brain damage through the PI3K/Akt
pathway. Int J Clin Exp Med. 8:8197–8203. 2015.PubMed/NCBI
|
|
8
|
Xu RR and Li YD: Research progress of
interaction between Bcl-2 family and mitochondrial apoptosis
pathway. Journal of Zhong Guo Lao Nian Xue. 33:2977–2979. 2013.
|
|
9
|
Jiang W, Wen EY, Gong M, Shi Y, Chen L, Bi
Y, Zhang Y, Liu YF, Chen J, Qu P, et al: The pattern of retinoic
acid receptor expression and subcellular, anatomic and functional
area translocation during the postnatal development of the rat
cerebral cortex and white matter. Brain Res. 1382:77–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Yu Q, Jiang W, Bi Y, Zhang Y,
Gong M, Wei X, Li T and Chen J: All-trans retinoic acid suppresses
apoptosis in PC12 cells injured by oxygen and glucose deprivation
via the retinoic acid receptor α signaling pathway. Mol Med Rep.
10:2549–2555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang FF, Huang W, Li YF and Gao ZH:
Research status of siRNA non-viral delivery vector. Yao Xue Xue
Bao. 46:1436–1443. 2011.PubMed/NCBI
|
|
12
|
Fan JC, Wang H, Xu C, Luo Z and Wu J: The
influence of centrifugal speed and time on TEM sample preparation
of culture cell. J Chongqing Med Univ. 34:1008–1010. 2009.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henry-Mowatt J, Dive C, Martinou JC and
James D: Role of mitochondrial membrane permeabilization in
apoptosis and cancer. Oncogene. 23:2850–2860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinopoulos C, Tretter L and Adam-Vizi V:
Depolarization of in situ mitochondria due to hydrogen
peroxide-induced oxidative stress in nerve terminals: Inhibition of
alpha-ketoglutarate dehydrogenase. J Neurochem. 73:220–228. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maden M: Retinoic acid in the development,
regeneration and maintenance of the nervous system. Nat Rev
Neurosci. 8:755–765. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Doze F, Debruyne D, Albessard F, Barre
L and Defer GL: Pharmacokinetics of all-trans retinoic acid, 13-cis
retinoic acid, and fenretinide in plasma and brain of Rat. Drug
Metab Dispos. 28:205–208. 2000.PubMed/NCBI
|
|
18
|
di Masi A, Leboffe L, De Marinis E, Pagano
F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P and Nervi C:
Retinoic acid receptors: From molecular mechanisms to cancer
therapy. Mol Aspects Med. 41:1–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo T, Wagner E, Crandall JE and Dräger
UC: A retinoic-acid critical period in the early postnatal mouse
brain. Biol Psychiatry. 56:971–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thompson Haskell G, Maynard TM,
Shatzmiller RA and Lamantia AS: Retinoic acid signaling at sites of
plasticity in the mature central nervous system. J Comp Neurol.
452:228–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goncalves MB, Boyle J, Webber DJ, Hall S,
Minger SL and Corcoran JP: Timing of the retinoid-signalling
pathway determines the expression of neuronal markers in neural
progenitor cells. Dev Biol. 278:60–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gottlieb DI and Huettner JE: An in vitro
pathway from embryonic stem cells to neurons and glia. Cells
Tissues Organs. 165:165–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegenthaler JA, Ashique AM, Zarbalis K,
Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T,
et al: Retinoic acid from the meninges regulates cortical neuron
generation. Cell. 139:597–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao CT, Li TY, Qu P, Zhao Y, Wang R and
Liu YX: Effects of early intervention on learning and memory in
young rats of marginal vitamin A deficiency and it's mechanism.
Zhonghua Er Ke Za Zhi. 44:15–20. 2006.(In Chinese). PubMed/NCBI
|
|
25
|
Mao CT, Li TY, Liu YX and Qu P: Effects of
marginal vitamin A deficiency and intervention on learning and
memory in young rats. Zhonghua Er Ke Za Zhi. 43:526–530. 2005.(In
Chinese). PubMed/NCBI
|
|
26
|
Shinozaki Y, Sato Y, Koizumi S, Ohno Y,
Nagao T and Inoue K: Retinoic acids acting through retinoid
receptors protect hippocampal neurons from oxygen-glucose
deprivation-mediated cell death by inhibition of c-jun-N-terminal
kinase and p38 mitogen-activated protein kinase. Neuroscience.
147:153–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Agudo M, Yip P, Davies M, Bradbury E,
Doherty P, McMahon S, Maden M and Corcoran JP: Aretinoic acid
receptorbeta agonist (CD2019 overcomes inhibition of axonal
outgrowth via phosphoinositide 3-kinase signalling in the injured
adult spinal cord. Neurobiol Dis. 37:147–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomes MJ, Martins S and Sarmento B: siRNA
as a tool to improve the treatment of brain diseases: Mechanism,
targets and delivery. Ageing Res Rev. 21:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katsuki H, Kurimoto E, Takemori S,
Kurauchi Y, Hisatsune A, Isohama Y, Izumi Y, Kume T, Shudo K and
Akaike A: Retinoic acid receptor stimulation protects midbrain
dopaminergic neurons from inflammatory degeneration via
BDNF-mediated signaling. J Neurochem. 110:707–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cotten CM and Shankaran S: Hypothermia for
hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol.
5:227–239. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang ZQ and Yu H: Clinical virology. BJ:
China Medical Science Press. China; pp. 1–153. 2005
|
|
32
|
Vento M, Asensi M, Sastre J, Lloret A,
García-Sala F and Viña J: Oxidative stress in asphyxiated term
infants resuscitated with 100% oxygen. J Pediatr. 142:240–246.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JR, Robertson K, Hickstein DD, Tsai
S, Hockenbery DM and Collins SJ: Dysregulated bcl-2 expression
inhibits apoptosis but not differentiation of retinoic acid-induced
HL-60 granulocytes. Blood. 84:440–445. 1994.PubMed/NCBI
|
|
34
|
Karmakar S, Banik NL and Ray SK:
Combination of all-traps retinoic acid and pacfitaxel-induced
differentiation and apoptosis in human glioblastoma U87MG
xenografts in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Keedwell RG, Zhao Y, Hammond LA, Qin S,
Tsang KY, Reitmair A, Molina Y, Okawa Y, Atangan LI, Shurland DL,
et al: A retinoid-related molecule that does not bind to classical
retinoid receptors potently induces apoptosis in human prostate
cancer cells through rapid caspase activation. Cancer Res.
64:3302–3312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thin TH, Li L, Chung TK, Sun H and Taneja
R: Stra13 is induced by genotoxic stress and regulates
ionizing-radiation-induced apoptosis. EMBO Rep. 8:401–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Orr B, White K, Belogortseva N,
Niles R, Boskovic C, Nguyen H, Dykes A and Park M: Chmp 1 A is
amediator of the anti-proliferative effects of All-traps retinoic
acid in human pancreatic cancer cells. Mol Cancer. 8:72009.
View Article : Google Scholar : PubMed/NCBI
|