Introduction
Aconitum carmichaelii Debeaux is widely used
in traditional Chinese medicine, which has been used in China and
other countries for >2,000 years due to its antipyretic,
antirheumatic and analgesic activities (1–3). There
are 32 prescriptions containing aconitum in the Chinese
Pharmacopoeia (4). However, the use
of aconitum has been associated with severe cardiovascular
toxicities, including tachyarrhythmia and hypotension (5). Aconitine is a key active component of
aconitum plants. A recent study has reported that when combined
with quercetin, aconitine synergistically inhibited the
proliferation of HeLa cells at a wide range of concentrations
(6). Identifying the toxic effects
of ACO is important for the safe clinical application of aconitum
species. The arrhythmogenic effects of ACO include the induction of
ventricular tachycardia (VT) and ventricular fibrillation (VF),
which result in a high mortality in affected patients (7,8). In
isolated sheep heart Purkinje fibers, ACO has been demonstrated to
act as a cardiac Na+ channel agonist that opens the
Na+ channels during the depolarization/repolarization
phase of an action potential, leading to a delayed repolarization
and early after-depolarization (7,9). Similar
effects of ACO were also obtained with isolated ventricular
myocytes of mice, rats and guinea pigs (10). A previous study has reported that
L-type calcium channel (LTCC) inhibition is a major mechanism of
the arrhythmogenic action of ACO on human cardiomyocytes (5). While the cardiotoxic effect of ACO has
been comprehensively documented in animal cardiomyocytes, the
effects of ACO in human cardiomyocytes and the underlying
mechanisms have remained to be assessed due to the lack of human
cardiomyocyte models and suitable methods.
Human induced pluripotent stem cell-derived
cardiomyocytes (hiPSC-CMs), which have a high similarity with
native human cardiac myocytes in their structure and in function,
have provided useful models to help elucidate cardiovascular
function and diseases (11,12). HiPSC-CMs have been successfully
adopted for modeling various cardiac diseases and for drug testing.
A recent scientific breakthrough, namely the development of the
Real-Time Cellular Analysis (RTCA) Cardio system, provides a
homogeneous population of relatively pure single cells in
vitro (13). The RTCA Cardio
system allows for the real-time, label-free and non-invasive
analysis of cardiomyocyte function. This platform has been used for
cardiovascular toxicity screening, drug-induced cardiac
contractility evaluation and estimating the risk of drug-induced
arrhythmia (14–17).
The present study aimed to monitor the cardiotoxic
effects of ACO in hiPSC-CMs by using the RTCA cardio system. It was
observed that ACO was capable of triggering arrhythmogenic effects
in hiPSC-CMs, as indicated by an increased beating frequency and a
decreased amplitude. Such changes were accompanied by dose- and
time-dependent intensive temporal profiling and gradually decreased
cell index (CI). The potential pro-apoptotic effects of ACO on
hiPSC-CMs were also evaluated. The resulting enhanced caspase-3 and
caspase-9 activities indicated that ACO-induced cell death was
mediated, at least in part, via caspase-dependent apoptotic
pathways.
Materials and methods
Reagents and materials
ACO was obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) and dissolved in dimethyl sulfoxide (DMSO).
Fetal bovine serum (FBS) for cell culture was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
PSCeasy® pluripotent stem cell culture medium (PSCM;
cat. no. CA1001500) was provided by Cellapybio (Beijing,
China).
Cell culture of hiPSC-CMs
HiPSC-CMs, provided by a domestic manufacturer (cat.
no. CA4024106; Cellapybio, Beijing, China), were cultured in
fibronectin-coated wells of 96-well plates according to the
manufacturer's protocols. In brief, the plates were prepared by
coating each well with 50 µl 0.1% gelatin overnight at 4°C. Frozen
vials of cells were thawed at 37°C and diluted with pre-warmed PSCM
at 500,000 cells/ml. After removing the excess coating solution,
100 µl of the cell suspension (containing 50,000 cells) was added
per well. The plates containing cells were kept at room temperature
for 30 min and then maintained at 37°C in a humidified incubator
containing air with 5% CO2. The culture medium was
refreshed daily without disturbing the attached cells.
MTT assay
The cell viability was determined by using the MTT
assay. In brief, hiPSC-CMs were seeded into 96-well plates at a
density of 50,000 cells/well. The cells were treated with ACO at
the concentration of 0.125, 0.25, 0.5, 1, 2, 4, 8 µM or DMSO as a
vehicle control (final concentration of DMSO, 0.3%). After
incubation for the indicated durations, 20 µl 0.5 mg/ml MTT
solution (Sigma-Aldrich; Merck KGaA) was added to each well,
followed by further incubation for 4 h. The medium was then removed
and the formazan crystals were dissolved in 100 µl DMSO. The
absorbance was measured at 570 nm on a microplate reader (Tecan
Infinite M1000; Tecan Group, Ltd, Maennedorf, Switzerland). The
relative cell viability was expressed as a percentage of the
control group.
Functional data recording and
analysis
The xCELLigence RTCA Cardio instrument (ACEA
Biosciences, Inc., San Diego, CA, USA) was used to monitor the
cardiomyocyte contractility. Impedance signals were recorded, from
which the CI value was calculated, a measure of relative changes in
electric impedance that represents the cell status. Hence, the
number of attached cells and their morphology were reflected by the
CI value. The E-Plate 96 (ACEA Biosciences, Inc.) was coated with
50 µl 0.1% gelatin overnight at 4°C. The solution was then replaced
by 150 µl pre-warmed PSCM and incubated at 37°C for 4 h. The
background impedance of the media was determined prior to seeding
the cells to ensure that all wells were functional. The software
automatically informs the researcher if any connection problems
arise. Following harvesting and counting, hiPSC-CMs were seeded
onto the plate at a density of 50,000 cells per well. The E-plate
was monitored every 15 min on the RTCA Cardio Instrument at 37°C in
a 5% CO2 incubator after incubation for 15 min at room
temperature for an initial cell adhesion at the bottom of each
well. The plate was then incubated in a 5% CO2 incubator
at 37°C. Typically, drug treatment was initiated 60–100 h after
cell seeding, when the contraction was continuous and stable.
ACO treatment of hiPSC-CMs
ACO was dissolved in dimethyl sulfoxide (DMSO). Once
the hiPSC-CMs generated robust and regular beating signals (usually
occurring on day 3 after seeding), drug treatment was initiated.
The culture medium was replaced with 90 µl fresh PSCM 4 h prior to
drug treatment. Subsequently, 90 µl drug solution at the two-fold
final concentration (dissolved in PSCM) was added to each well.
HiPSC-CMs were treated with ACO at the final concentrations of
0.25, 0.3, 0.5, 1, 1.5, 2, 2.5 and 3 µM or with a vehicle control.
The cellular response to the drug treatments was recorded over
20-sec every 5 min in the first 30 min and every 15 min thereafter.
After the data acquisition, the RTCA Cardio software1.0 was used to
calculate the parameters, including such as the normalized beating
rate and amplitude with statistical analysis. Experiments were
performed in 6 wells in parallel and repeated three times.
Measurement of the activities of
caspase-3 and caspase-9
Caspase-3 and caspase-9 activities in the lysates of
cells were determined using the CaspACE™ Assay System (Promega
Corp., Madison, WI, USA) and the Caspase-Glo® 9 Assay
kit (Promega Corp.) following the manufacturer's instructions. In
brief, control- or ACO-treated cells were lysed, incubated on ice
for 30 min and then centrifuged at 16,000 × g for 10 min at 4°C.
The supernatant fraction was collected. For the colorimetric
caspase-3 activity assay, the supernatant was incubated with 200 µM
DEVDpNA substrate at 37°C for 4 h and the absorbance was measured
at 405 nm using a microplate reader. Caspase-9 activity was
measured using a luminescent assay with the samples mixed with the
respective aliquot of Caspase-Glo® 9 reagent and
incubated for 3 h at room temperature. The luminescence was
measured with a plate-reading luminometer (GloMax®
Navigator; Promega Corporation, Madison, WI, USA) according to the
protocol of the luminometer manufacturer.
Statistical analysis
For data analysis, the cellular impedance index,
beating rate and amplitude were measured off-line using RTCA Cardio
software 1.0 and normalized for each well to the baseline
(pre-dose) values measured prior to the drug treatment. Statistical
analysis was performed using SPSS 21.0 software (IBM Corp., Armonk,
NY, USA). Values are expressed as the mean ± standard deviation.
Statistical significance of differences was estimated by one-way
analysis of variance with Tukey-Kramer corrections. Comparisons of
continuous variables between two groups were performed using
unpaired two-tailed Student's t-tests. Multiple group comparisons
were performed using Student-Newman-Keuls following analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cultured hiPSC-CMs exhibit a
functional cardiomyocyte phenotype on the xCELLigence Cardio
platform
Based on previous studies (18), cells were seeded on the E-Plate 96 at
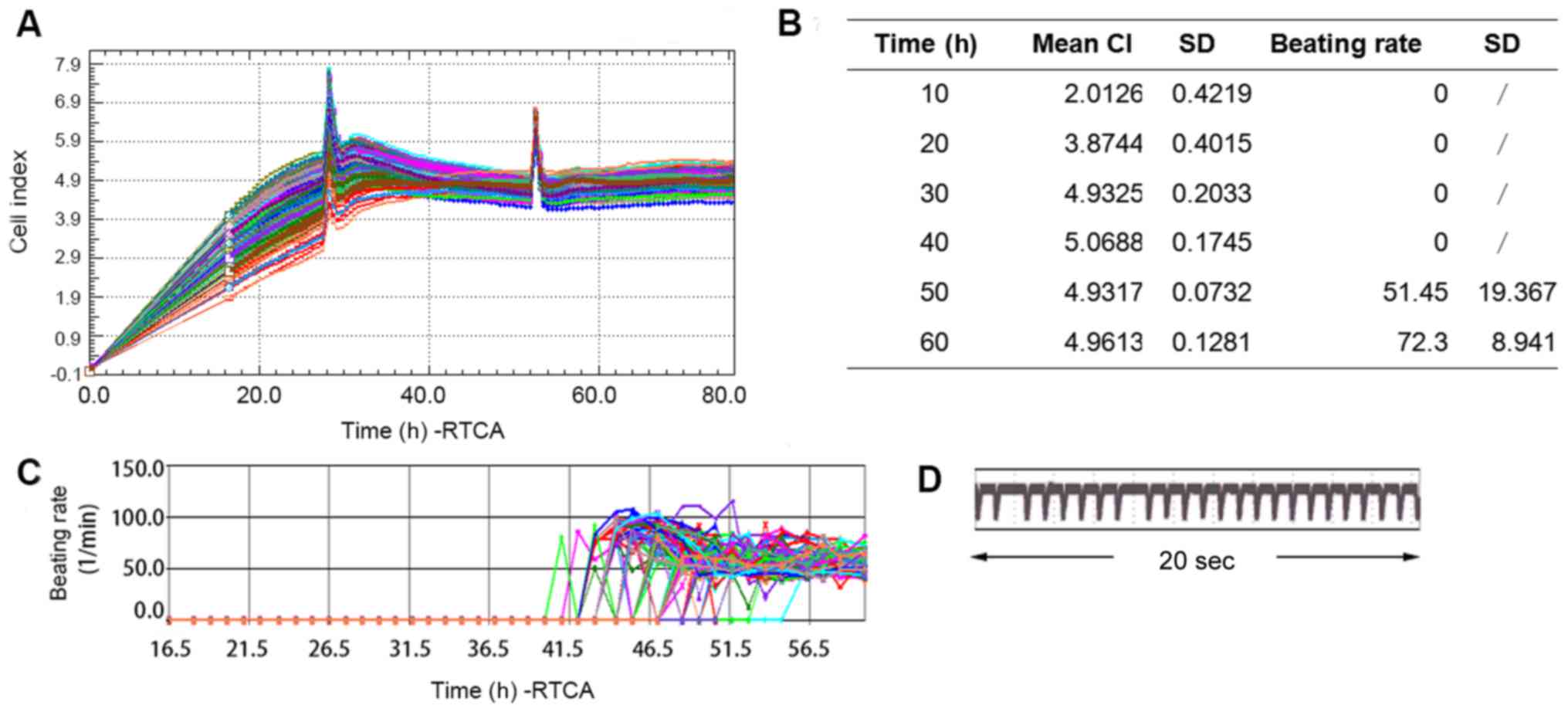
50,000/well. As presented in Fig. 1A and
B, after 40 h of culture, the CI reached a stable value of
5.0688±0.1745, which was representative of viable hiPSC-CMs.
Spontaneous contraction was observed at ~42 h after and stable
contraction was achieved at ~60 h with a beating rate of 72.3±8.941
bpm. Fig. 1C represents the beating
rates of different the wells of hiPSC-CMs from the beginning until
stable contraction. Typical transient pulse patterns of hiPSC-CMs
over a duration of 20 sec is presented in Fig. 1D. Based on these results, the
subsequent experiments were performed at 60 h after seeding.
Time- and dose-dependent effects of
ACO on hiPSC-CMs
In the clinical setting, ACO induces cell death of
cardiomyocyte in certain patients within hours of intravenous
administration (19,20). The present study investigated
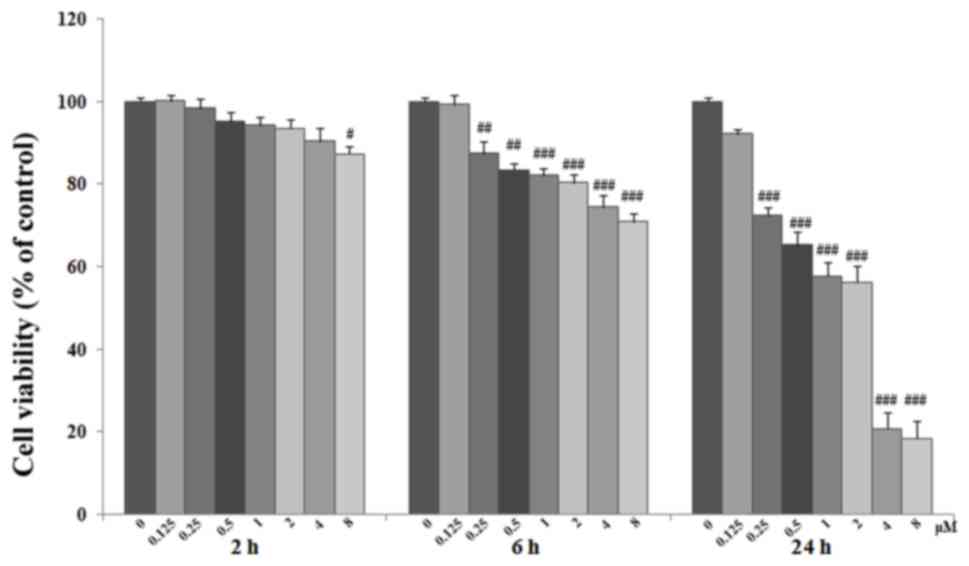
ACO-induced cytotoxicity by an MTT assay. Cells were grown on
fibronectin-coated plates and exposed to ACO at 0.125, 0.25, 0.5,
1, 2, 4 or 8 µM for 2, 6 or 24 h. As presented in Fig. 2, exposure to 0.125–4 µM ACO for 2 h
only had a marginal effect on the cell viability. However, exposure
for longer periods and/or to higher concentrations of ACO induced a
dose- and time-dependent decrease in viability compared with that
of the control cells (P<0.05 or P<0.01). ACO at 2–4 µM had a
significant but not excessive cytotoxic effect. Therefore, ACO was
used at the concentrations of 0.25, 0.3, 0.5, 1, 1.5, 2, 2.5 and 3
µM in the subsequent experiments.
Time- and dose-dependent
arrhythmogenic effects of ACO on hiPSC-CMs
The clinical use of ACO has been associated with
various arrhythmias by affecting the electrophysiological
characteristics of cardiomyocytes (5). To gain insight into the
electrophysiological effects of ACO, the beating properties of
hiPSC-CMs were assessed by using the xCELLigence Cardio platform.
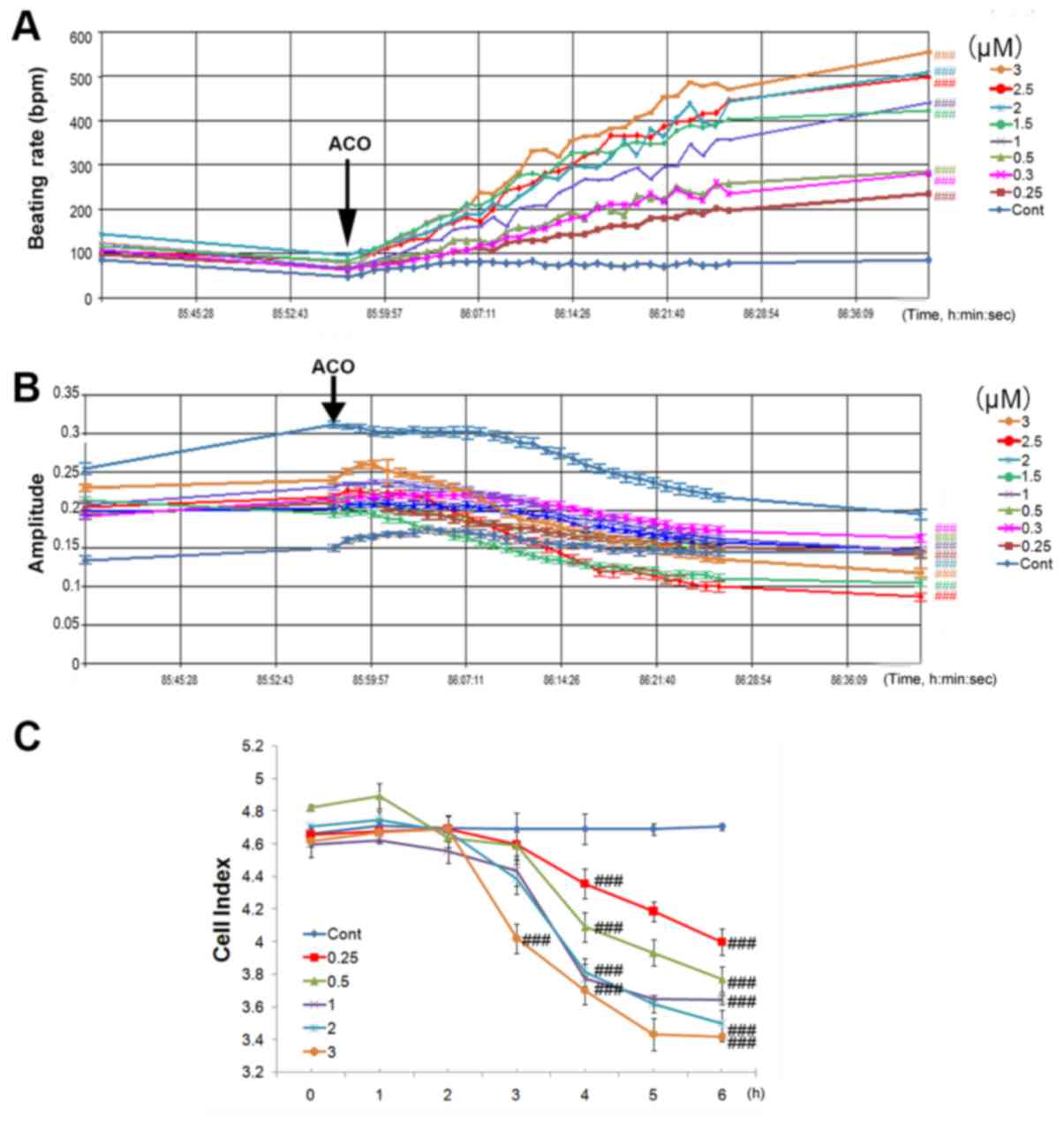
At 85 h after seeding, when the cardiomyocyte contraction was
continuous and stable, ACO at the concentration of 0.25, 0.3, 0.5,
1, 1.5, 2, 2.5 or 3 µM was added to the hiPSC-CMs. As presented in
Fig. 3A, the spontaneous beating
rate of hiPSC-CMs was rapidly increased by ACO. All concentrations
of ACO were capable of markedly enhancing the beating rate, and the
effect of ACO was time- and dose-dependent. ACO at 0.25 µM
increased the beating rate of hiPSC-CMs by 3.7-fold within 30 min,
while 3.0 µM ACO increased the beating rate by 7.3-fold. As
presented in Fig. 3B, amplitudes of
hiPSC-CMs were reduced in parallel with the increase of the beating
rates. A reduction of the CI was also observed within 6 h culture.
In comparison with the control group, ACO treatment induced a time-
and dose-dependent decrease in cell viability as shown by the cell
index (Fig. 3C). ACO at 3.0 µM
significantly decreased the CI after 3 h of incubation, while low
concentrations of ACO (0.25, 0.3, 0.5, 1.0, 1.5, and 2.0 µM) also
significantly decreased the CI within 4 h (P<0.001). Of note,
after 6 h of incubation, 3.0 µM ACO reduced the cell index by 27.2%
(P<0.001).
Effects of ACO on the typical temporal
profiling and beating rates of hiPSC-CMs
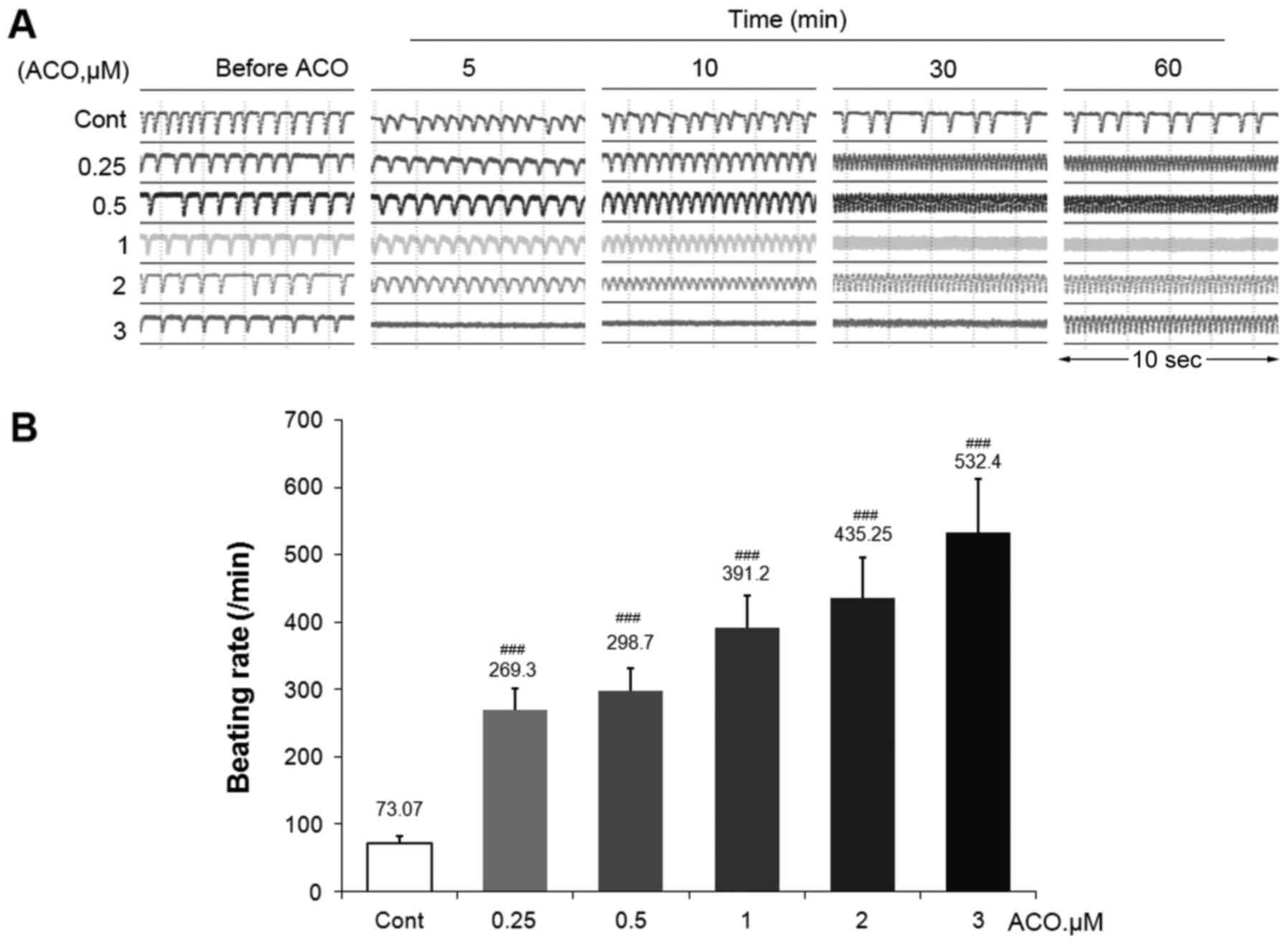
The transient pulse patterns of hiPSC-CMs treated
with various doses of ACO were then assessed. Fig. 4A presents typical temporal profiles
at 5 time-points prior to and after ACO treatment. ACO had time-
and dose-dependent stimulatory effects on the beating pattern of
hiPSC-CMs. Accelerated beating patterns were observed with all
concentrations of ACO immediately after addition of the drug. Of
note, 3.0 µM ACO caused a serious disorder within 5 min, followed
by an intense temporal profiling and relative low amplitude, which
may be associated with lethal cardiac arrhythmias. Furthermore, the
intensive beating patterns were gradually further compressed as
time went on. The beating rate of hiPSC-CMs after 30 min of ACO
treatment is presented in Fig.
4B.
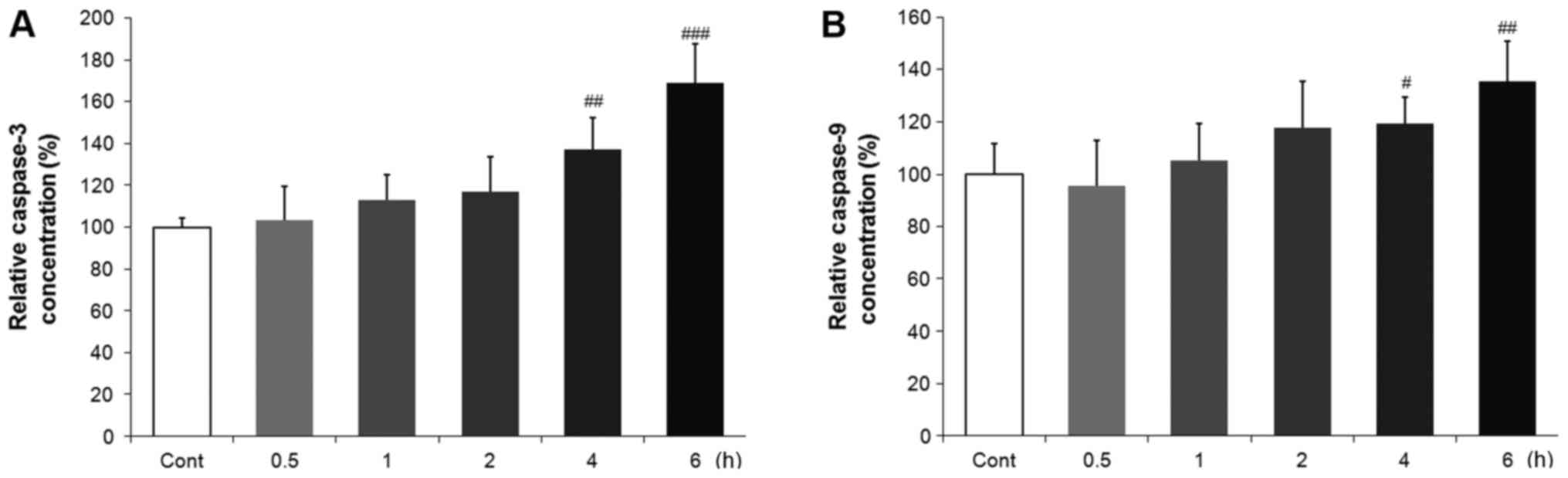
Effects of ACO on caspase-dependent
apoptosis of hiPSC-CMs
The activation of caspase is a unique feature of
apoptotic cell death. In order to investigate whether ACO-induced
cytotoxicity is associated with caspase-dependent apoptosis, the
activities of caspase-3 and caspase-9 were analyzed using assay
kits. hiPSC-CMs were grown onto fibronectin-coated plates and
exposed to 3.0 µM ACO, which was selected according to the
aforementioned results. The activities of caspase-3 and caspase-9
were detected at different time-points. As presented in Fig. 5, ACO treatment resulted in a
time-dependent increase in caspase-3 and caspase-9 activities after
4–6 h of treatment when compared with those in the control
group.
Discussion
Along with the extensive use of aconitum, the
cardiotoxic effect of ACO has received increasing attention
(7). Studies have reported that ACO
is capable of inducing VT and VF by opening the Na+
channels of isolated cardiac myocytes from mice, rats, guinea pigs
and rabbits (21). In addition,
ACO-induced LTCC inhibition has been reported (5,22). In
the present study, the RTCA Cardio system was applied, which is
able to monitor the contractility of cardiomyocyte in real-time, to
evaluate the arrhythmogenic effects of ACO in hiPSC-CMs. The
results indicate that ACO is capable of triggering arrhythmogenic
effects in hiPSC-derived cardiomyocytes in a dose- and
time-dependent manner. Furthermore, the results suggested that
ACO-induced cardiomyocyte death is mediated, at least in part, by
inducing apoptosis. Overall, these results are in agreement with
those of previous studies from cellular and human biopsy studies
demonstrating ACO-induced cardiotoxicity (7,9,22).
Due to the high incidence of drug-induced heart
failure and irreversible arrhythmia, the pre-clinical evaluation of
the cardiotoxicity of drugs is important (23). However, efficient approaches to
evaluate the cardiotoxicity of traditional or novel treatments have
been lacking. The development of drug evaluation systems remains
difficult due to the lack of human cardiomyocyte models and
suitable methods. HiPSC-CMs, which have predefined contractile
characteristics of native cardiomyocytes, a genetically relevant
background and the scope of serving as a model of relevant cardiac
disease phenotypes, have provided an opportunity for pre-clinical
drug evaluation (14,24). More importantly, hiPSC-CMs also have
stable electrophysiological and contractile characteristics, which
allows for a wide range of applications, including drug discovery,
toxicity testing and cardiac disease research (12,25). A
sensitive tool with accurate recording capacity is also required,
since most of the previous techniques only defined end-point
measurements, for example MTT assays and flow cytometry, which can
be performed at specific time points but not continuous. The
impedance-based system RTCA has provided an opportunity to reassign
a variety of assays, including pre-clinical drug evaluation,
compound validation, monitoring of compound effects and exploration
of cardiac disease models (13,15). The
RTCA Cardio system has provided a more sensitive tool to detect
potential cardiac side effects when compared to cytotoxicity assays
using the H9C2 cell line. Furthermore, the sensitive label-free
assay made it possible to detect the regular beating pattern of
cardiomyocytes under physiological or pathological conditions. The
RTCA Cardio system uses regular beating patterns to reflect the
detailed beating status and to assess the beating activities of
cardiomyocytes, which are diverse and comprehensive (16,26). To
analyze the detailed beating status, multiple parameters require
assessment. Thus, in the present study, using the RTCA Cardio
system, the dose and time responses to ACO were characterized by
four parameters, including the CI, beating rate, amplitude and
beating pattern in hiPSC-CMs. Compared to the H9C2 cell line, the
spontaneous rhythm of the hiPSC-CMs tended to be more consistent
and less irregular after cultivation for a certain duration.
The present results illustrated that ACO has time-
and dose-dependent stimulatory effects on the beating pattern of
hiPSC-CMs. The spontaneous beating rates of hiPSC-CMs were rapidly
increased by ACO. At all concentrations (0.25, 0.3, 0.5, 1, 1.5, 2,
2.5 and 3.0 µM), ACO was capable of markedly enhancing the beating
rate. Accelerated beating patterns were observed immediately upon
addition of the drug. Of note, 3.0 µM ACO caused an intensive
temporal profiling and relative low amplitude, which may be
associated with lethal cardiac arrhythmias. Furthermore, the
intensive beating patterns were gradually further compressed as
time went on. ACO at 0.25 µM increased the beating rate of
hiPSC-CMs by 3.7-fold within 30 min, while 3.0 µM of ACO increased
the beating rate by 7.3-fold when compared with the control group.
The resulting rapid collapse of the beating pattern due to
excessive acceleration indicated that ACO exerts a significant
stimulatory effect on cardiac contraction. The amplitudes of the
beating of the hiPSC-CMs were reduced in parallel with the increase
of the beating rates. A reduction of the CI and cell viability was
also observed within 6 h of incubation with ACO. In comparison with
the control group, ACO treatment induced a time- and dose-dependent
decrease in cell viability and CI. Treatment with ACO at 3.0 µM
significantly decreased the CI at 3 h, while low concentrations of
ACO (0.25, 0.5, 1 and 2.0 µM) also significantly decreased the CI
within 4 h.
Irreversible cell death, which includes apoptosis
and necrosis, is another aspect of ACO-induced cardiotoxicity.
Apoptosis (programmed cell death), which may be activated by a
caspase-dependent or -independent pathway, is one of the crucial
factors for cardiomyocyte loss. In order to investigate whether the
ACO-induced cytotoxicity is associated with caspase-dependent cell
apoptosis, the activities of caspase-3 and caspase-9 were analyzed
with specific assay kits. ACO caused a significant increase in
caspase-3 and caspase-9 activities after 4 and 6 h of treatment
when compared with those in the control group. These results
suggest that the ACO-induced cell death is mediated, at least in
part, by caspase-dependent cardiomyocyte apoptosis. However,
necrosis, involving swelling of mitochondria, irreversible damage
to cellular membranes, potentially includes in ACO-induced cell
death (27). The cytotoxicity of ACO
on the hiPSC-CMs was evident at dose as low as 0.25 µM. The present
results support the use of the hiPSC-derived cardiomyocytes as a
human cellular model to assess the potential cardiotoxicity of
pharmaceutical agents.
In conclusion, the present study suggested that ACO
dose- and time-dependently induced arrhythmia and cardiotoxicity.
An experimental in vitro cell model to mimic regular cardiac
contraction was explored as a tool to provide the novel insight
into the cardiac safety of ACO in vitro. More importantly,
the present study introduced an efficient and effective approach to
evaluate the potential cardiac risk of the tested compounds.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ and LC conducted the study. JZ and FZ designed
the experiments. XQ, FZ and CL performed experiments, and collected
and analyzed the data. All authors commented on the study and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CMs
|
cardiomyocytes
|
|
RTCA
|
real-time cell analysis system
|
|
hiPSC-CMs
|
human induced pluripotent stem
cell-derived cardiomyocytes
|
|
CI
|
cell index
|
References
|
1
|
Park G, Kim KM, Choi S and Oh DS: Aconitum
carmichaelii protects against acetaminophen-induced hepatotoxicity
via B-cell lymphoma-2 protein-mediated inhibition of mitochondrial
dysfunction. Environ Toxicol Pharmacol. 42:218–225. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Gu L, Yang L, Zhang D and Shen J:
Aconitine: A potential novel treatment for systemic lupus
erythematosus. J Pharmacol Sci. 133:115–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo JX, Zhang Y, Hu XY, Chen G, Liu XY,
Nie HM, Liu JL and Wen DC: Aqueous extract from Aconitum
carmichaelii Debeaux reduces liver injury in rats via regulation of
HMGB1/TLR4/NF-KB/caspase-3 and PCNA signaling pathways. J
Ethnopharmacol. 183:187–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Pharmacopoeia Committee, .
Pharmacopoeia of People's Republic of China [M]. Part 2Beijing:
Chemical Industry Press; 2015
|
|
5
|
Zhou YH, Piao XM, Liu X, Liang HH, Wang
LM, Xiong XH, Wang L, Lu YJ and Shan HL: Arrhythmogenesis toxicity
of aconitine is related to intracellular ca(2+) signals. Int J Med
Sci. 10:1242–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li XM, Liu J, Pan FF, Shi DD, Wen ZG and
Yang PL: Quercetin and aconitine synergistically induces the human
cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum
(ER) stress pathway. PLoS One. 13:e01910622018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oredipe OA and Stohs SJ: Effects of
lithium on time to onset of aconitine-induced initial arrhythmia
and ventricular tachycardia. Arch Int Pharmacodyn Ther.
240:249–256. 1979.PubMed/NCBI
|
|
8
|
Tak S, Lakhotia M, Gupta A, Sagar A, Bohra
G and Bajari R: Aconite poisoning with arrhythmia and shock. Indian
Heart J. 68 Suppl 2:S207–S209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imanaga I: Effects of some ions and drugs
on the aconitine-induced fibrillation of the Purkinje fibers. Jpn
Circ J. 31:1819–1831. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wangchuk P, Navarro S, Shepherd C, Keller
PA, Pyne SG and Loukas A: Diterpenoid alkaloids of Aconitum
laciniatum and mitigation of inflammation by 14-O-acetylneoline in
a murine model of ulcerative colitis. Sci Rep. 5:128452015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo L, Eldridge S, Furniss M, Mussio J and
Davis M: Use of human induced pluripotent stem cell-derived
cardiomyocytes (hiPSC-CMs) to monitor compound effects on cardiac
myocyte signaling pathways. Curr Protoc Chem Biol. 7:141–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scott CW, Zhang X, Abi-Gerges N, Lamore
SD, Abassi YA and Peters MF: An impedance-based cellular assay
using human iPSC-derived cardiomyocytes to quantify modulators of
cardiac contractility. Toxicol Sci. 142:331–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Hu N, Cao J, Wu J, Su K and Wang
P: A cardiomyocyte-based biosensor for antiarrhythmic drug
evaluation by simultaneously monitoring cell growth and beating.
Biosens Bioelectron. 49:9–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Sun S, Wang S, Zhang Q, Li M, Lan F,
Li S and Liu C: Liensinine- and Neferine-induced cardiotoxicity in
primary neonatal rat cardiomyocytes and Human-induced pluripotent
stem Cell-derived cardiomyocytes. Int J Mol Sci. 17(pii): E1862016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peters MF, Scott CW, Ochalski R and Dragan
YP: Evaluation of cellular impedance measures of cardiomyocyte
cultures for drug screening applications. Assay Drug Dev Technol.
10:525–532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abassi YA, Xi B, Li N, Ouyang W, Seiler A,
Watzele M, Kettenhofen R, Bohlen H, Ehlich A, Kolossov E, et al:
Dynamic monitoring of beating periodicity of stem cell-derived
cardiomyocytes as a predictive tool for preclinical safety
assessment. Br J Pharmacol. 165:1424–1441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gepstein L, Ding C, Rahmutula D, Wilson
EE, Yankelson L, Caspi O, Gepstein A, Huber I and Olgin JE: In vivo
assessment of the electrophysiological integration and
arrhythmogenic risk of myocardial cell transplantation strategies.
Stem Cells. 28:2151–2161. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kho D, MacDonald C, Johnson R, Unsworth
CP, O'Carroll SJ, du Mez E, Angel CE and Graham ES: Application of
xCELLigence RTCA biosensor technology for revealing the profile and
window of drug responsiveness in real time. Biosensors (Basel).
5:199–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mingxia Y: Analysis on the treatment of
ventricular arrhythmia caused by aconitine poisoning. China Health
Industry. 11:143–144. 2014.(In Chinese).
|
|
20
|
Ping W: To investigate the
quantity-effect-toxicity relationship of aconitine transdermal drug
delivery. Pharmacol Clinics Chin Materia Med. 29:53–56. 2013.(In
Chinese).
|
|
21
|
Chan TY: Aconitum alkaloid poisoning
because of contamination of herbs by aconite roots. Phytother Res.
30:3–8. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Wang X, Chung YY, Koh CH, Liu Z, Guo
H, Yuan Q, Wang C, Su S and Wei H: L-type calcium channel
inhibition contributes to the proarrhythmic effects of aconitine in
human cardiomyocytes. PLoS One. 12:e01684352017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao Q, Wang X, Wang S, Song Z, Wang J and
Ma J: Cardiotoxicity evaluation using human embryonic stem cells
and induced pluripotent stem cell-derived cardiomyocytes. Stem Cell
Res Ther. 8:542017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonsson MK, Wang QD and Becker B:
Impedance-based detection of beating rhythm and proarrhythmic
effects of compounds on stem cell-derived cardiomyocytes. Assay
Drug Dev Technol. 9:589–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eldridge S, Guo L, Mussio J, Furniss M,
Hamre J III and Davis M: Examining the protective role of ErbB2
modulation in human-induced pluripotent stem cell-derived
cardiomyocytes. Toxicol Sci. 141:547–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi B, Wang T, Li N, Ouyang W, Zhang W, Wu
J, Xu X, Wang X and Abassi YA: Functional cardiotoxicity profiling
and screening using the xCELLigence RTCA Cardio System. J Lab
Autom. 16:415–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S: Alternative cell death pathways
and cell metabolism. Int J Cell Biol. 2013:4636372013. View Article : Google Scholar : PubMed/NCBI
|