Introduction
Patients with spinal cord injury (SCI) suffer from
extreme pain while exercising (1).
This results in a notable decrease in quality of life, and a heavy
burden on family members and society (2). Progress has been made in understanding
the post-SCI pathophysiological changes (2). However, improving neurological
functional repair after damage still remains a challenge. Local
inflammatory response is a major factor that influences damage
repair (1). The inflammatory
response is regarded as a double-edged sword. On the one hand, it
can eliminate pathogens and necrotic tissue debris, thus
establishing the conditions for axon regeneration and tissue
remodeling (3). On the other hand,
excessive congregation of neurotoxic inflammatory factors and
mediators will also aggravate tissue damage (4). Thus, the role of inflammatory response
in SCI remains unclear.
SCI manifests as restricted movement and pain in the
corresponding damaged areas, including sphincter of Oddi
dysfunction, dystonia and pathological reflex (3). SCI is the primary cause of damaged
neurons and neurogliocyte death, so it has been a key focus of
medical research. In addition, SCI is associated with perpetual
spinal cord dysfunction (3).
In previous research, macrophages have been
demonstrated to exhibit diversity and plasticity in different
damaged micro-environments, known as macrophage polarization
(3). Furthermore, they can secrete
numerous proinflammatory mediators, including tumor necrosis factor
(TNF)-α, interleukin (IL)-12, IL-23 and nitric oxide (3). Therefore, it has proinflammatory
activity and aggravates tissue damage (5).
Toll-like receptor-4 (TLR4) is a pattern recognition
receptor, which is closely related to congenital immunity (6). TLR4 is a membrane receptor with
leucine-rich repeat. As is reported in previous study, injection of
lipopolysaccharide in the spinal cord can upregulate TLR4
expression and activate the inflammatory response (6). Thus, SCI has resulted in nerve injury
and functional defect, which reveals that TLR4 is distributed in
the spinal cord (3). SCI can destroy
the normal structure of the spinal cord (3). Meanwhile, it promotes extensive contact
of extracellular matrix (ECM) with inflammatory cells. ECM contains
numerous endogenous molecules, including laminin, collagen and
proteoglycan (3). It was suggested
in previous research that some endogenous substances will be
produced after SCI, including necrotic tissues and oxygen radicals.
These substances may stimulate microvascular endothelial cells
(3). Furthermore, NF-κB is activated
in nerve cells and gliocytes for nuclear translocation (3). In addition, the activated NF-κB can
elevate transcriptional activities of many inflammatory factor
genes, including intercellular adhesion molecule 1, IL-1, IL-6 and
TNF-α (3). There are binding sites
in enhancers and promoters of inflammatory factors corresponding to
those in NF-κB. In this way, the inflammatory response in damage
regions is upregulated and the severity of tissue damage is
increased (3). Zhong et al
(7) reported that the
TLR4/NF-κB/miRNA (miR)-146a pathway contributes to the assisted
reproductive technology-correlated preterm birth outcome. In the
present study, the neuroprotective effects of miR-146a in SCI, as
well as the possible underlying mechanisms of these effects, were
investigated.
Materials and methods
Animals and treatments
A total of 16 adult female Sprague-Dawley rats
(200–220 g; 6–8 weeks) were purchased from the Animal Laboratory of
Guangzhou Medical University (Guangzhou, China) and housed at
23±1°C with a humidity of 50–60%, in a 12-h light/dark cycle. The
study protocol was approved by the Institutional Animal Care and
Use Committee of Shandong University. All rats (n=16) were
randomized to a control group (n=6) or an SCI model group (n=10).
All rats were anesthetized with intraperitoneal 300 mg/kg chloral
hydrate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the
T8-T10 spinal column was exposed. The Impact One™ Stereotaxic
Impactor (Leica Microsystems GmbH, Wetzlar, Germany) was inserted
through the atlanto-occipital membrane at T9 and was an SCI model
was established as previously described (8). Rats of the sham group were anesthetized
with intraperitoneal 300 mg/kg chloral hydrate (Sigma-Aldrich;
Merck KGaA) without the induction of SCI.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from spinal cord
tissue or PC-12 cells transfected using the TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cDNA
synthesis was performed using the TaqMan MicroRNA RT kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed
using a Prism 7000 Real-Time PCR system with Power SYBR Green
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The conditions were as follows: 95°C for 5 min, followed by 40
cycles of three-step PCR (95°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec). The following primer sequences were utilized: miR-146a
sense, 5′-GCGAGGTCAAGTCACTAGTGGT-3′ and antisense,
5′-CGAGAAGCTTGCATCACCAGAGAACG-3′; U6 sense, 5′-CTCGCTTCGGCAGCACA-3′
and antisense, 5′-AACGCTTCACGAATTTGCGT-3′. miRNA expression was
calculated using the 2−ΔΔCq method (9).
Cell lines and cell culture
PC-12 cells were purchased from Shanghai library of
Chinese academy of sciences (Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. 100 ng of miR-146a mimics
(UGAGAACUGAAUUCCAUGGGUU), 100 ng of anti-miR-146a mimics
(AACCCAUGGAAUUCAGUUCUCA) and 100 ng of negative control mimics
(AAAAAAAAAA) were transfected into cells (1×106 cell/ml)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. After transfection
for 48 h, an SCI model was induced in PC-12 cells (1×106
cell/ml) using 100 ng/ml of LPS for 4 h at 37°C. Next, 2.5 nM of
TAK-242, a TLR4 inhibitor was added to the SCI in vitro
model (1×106 cell/ml) following anti-miR-146a for 48 h
at 37°C.
Measurement of inflammation
factors
PC-12 cells were collected following the induction
of LPS for 4 h by centrifugation at 1,000 × g for 10 min at 4°C.
TNF-α (ab100747), IL-1β (ab100704) and IL-6 (ab100712) content was
measured using ELISA kits (Abcam, Cambridge, MA, USA). Absorbance
was measured at 450 nm using a microplate reader (model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
PC-12 cells (2×106 cell/ml) were
homogenized in radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Nanjing, China) on ice and
protein was quantified using BCA buffer (Beyotime Institute of
Biotechnology). Protein separation (50 µg/per lane) was performed
using 8–10% SDS-PAGE, followed by transferring onto a
polyvinylidene difluoride membrane. The membrane was blocked with
5% skim milk for 1 h at 37°C, then incubated overnight at 4°C with
primary antibodies: Anti-inducible nitric oxide synthase (iNOS;
sc-649, 1:300), anti-prostaglandin E2 (PGE2; sc-20676, 1:500),
anti-TLR4 (sc-10741, 1:500), anti-myeloid differentiation primary
response 88 (MyD88; sc-11356, 1:500), anti-nuclear factor κB
(NF-κB; sc-109, 1:200) and anti-GADPH (sc-25778, 1:2,000) (all from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The membrane was
then incubated with peroxidase anti-rabbit IgG (sc-2004, 1:5,000,
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. The results were
detected using an enhanced chemiluminescence kit (Santa Cruz
Biotechnology, Inc.) and analyzed using sodium Image Lab 3.0
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Differences between
results were assessed using one-way analysis of variance followed
by Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
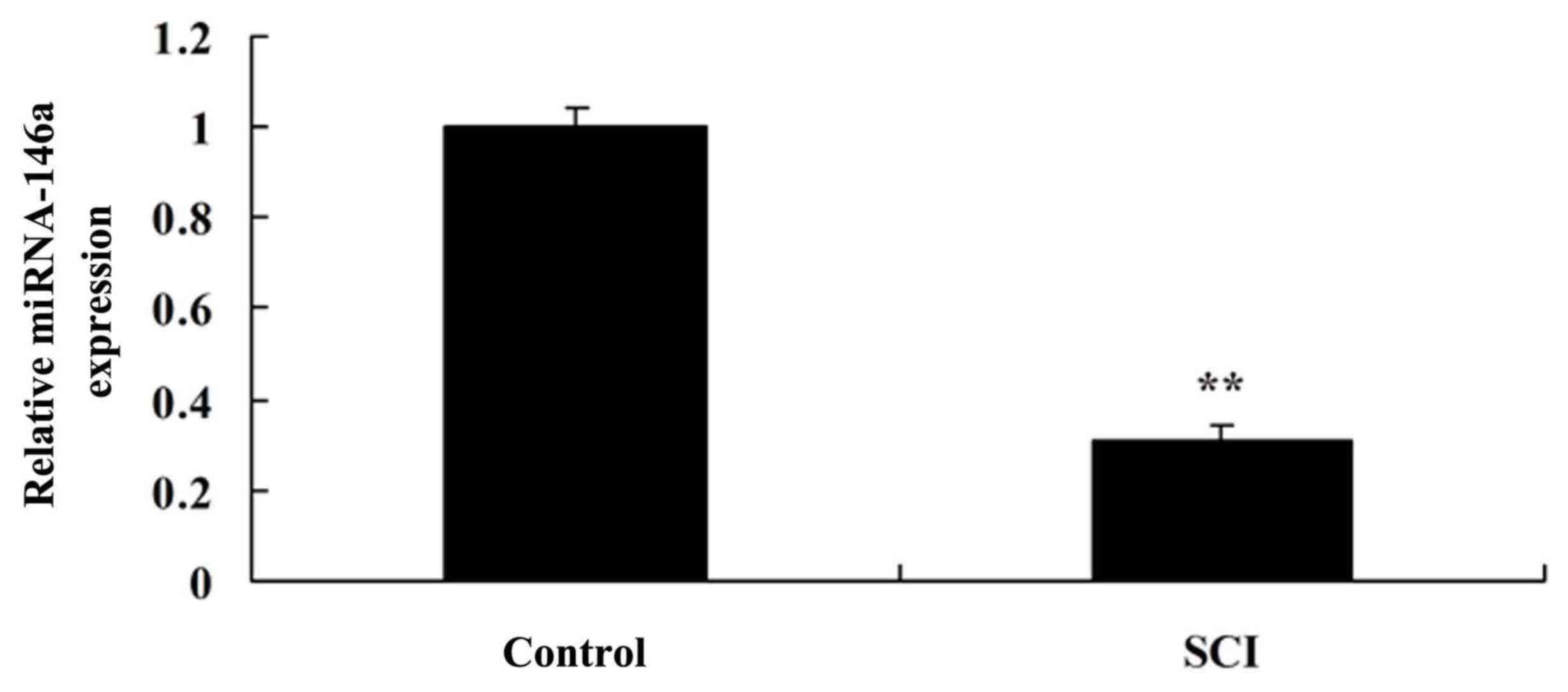
Expression level of miR-146a in an SCI
rat model
As indicated in Fig.
1, miR-146a expression was downregulated in the spinal cord
tissue of the SCI rat model compared with the control group. These
results suggested that miR-146a may be involved in the regulation
of inflammation in SCI.
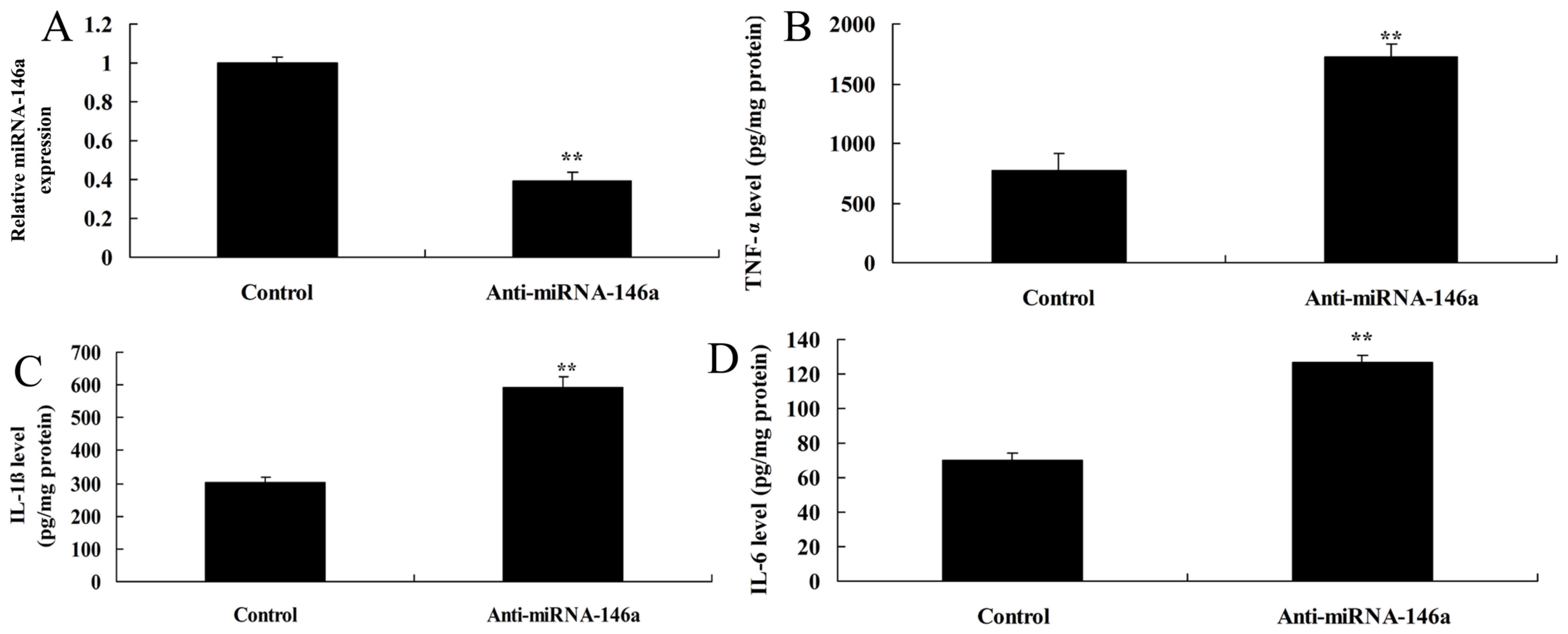
Downregulation of miR-146a increases
inflammation in an SCI model in vitro
miR-146a expression was downregulated in an in
vitro model of SCI using anti-miR-146a mimics. As indicated in
Fig. 2A, there was significant
inhibition of miR-146a expression in the in vitro model of
SCI transfected with anti-miR-146a compared with the control group.
Furthermore, TNF-α, IL-1β and IL-6 levels were significantly higher
in the miR-146a downregulation group compared with the control
group (Fig. 2B-D).
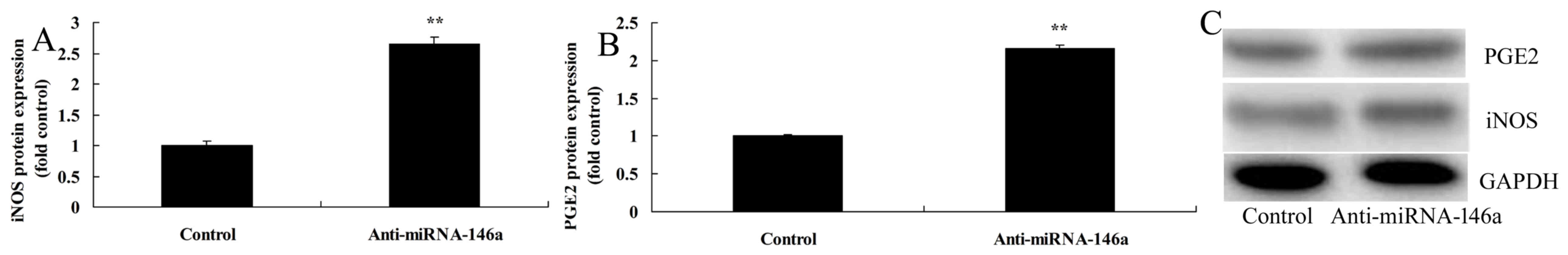
Downregulation of miR-146a enhances
iNOS and PGE2 protein expression in an SCI model in vitro
PGE2 and iNOS expression were evaluated in an SCI
model in vitro by western blot analysis. The results
indicated that iNOS and PGE2 protein expression were significantly
increased following miR-146a downregulation compared with the
control group (Fig. 3).
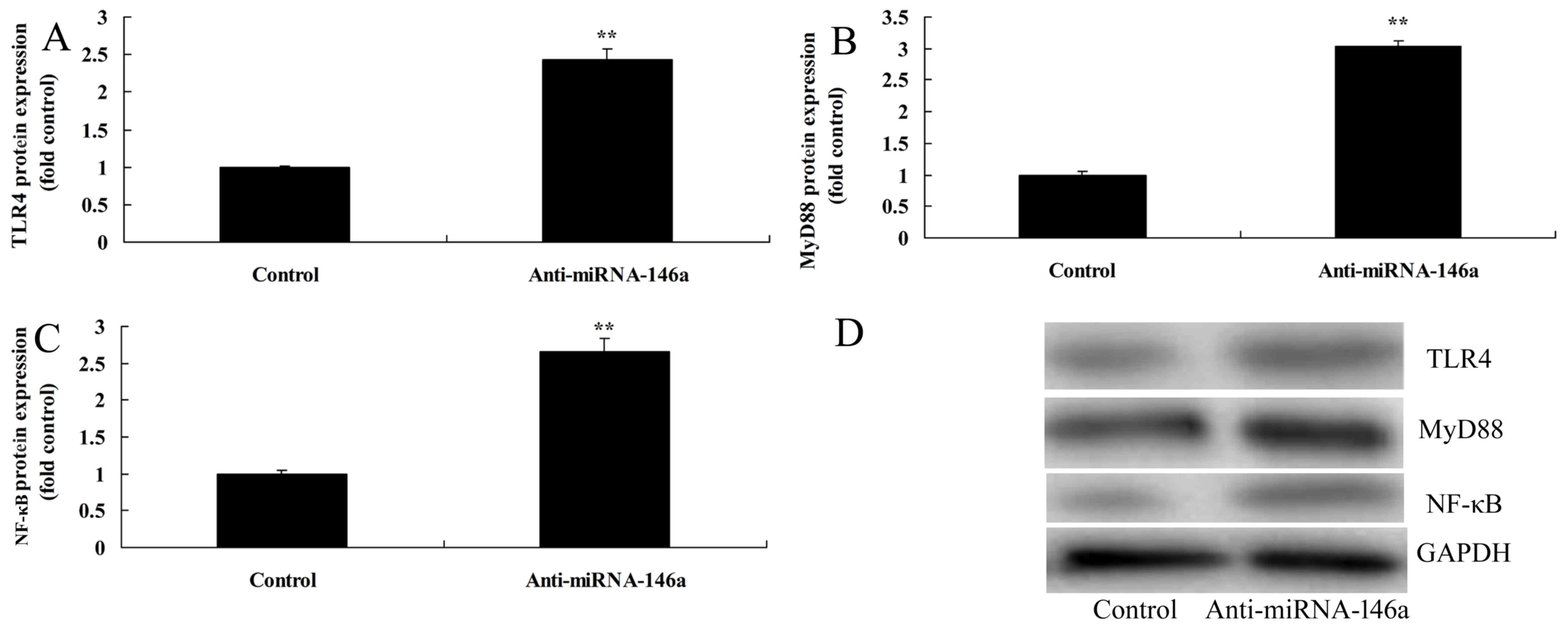
Downregulation of miR-146a increases
the level of TLR4, MyD88 and NF-κB expression in an SCI model in
vitro
In order to evaluate the mechanism of the
anti-inflammatory effect of miR-146a on SCI, the TLR4/MyD88/NF-κB
signaling pathway was examined in an SCI model following treatment
with anti-miR-146a. As indicated in Fig.
4, inhibition of miR-146a significantly promoted TLR4, MyD88
and NF-κB protein expression compared with the control group.
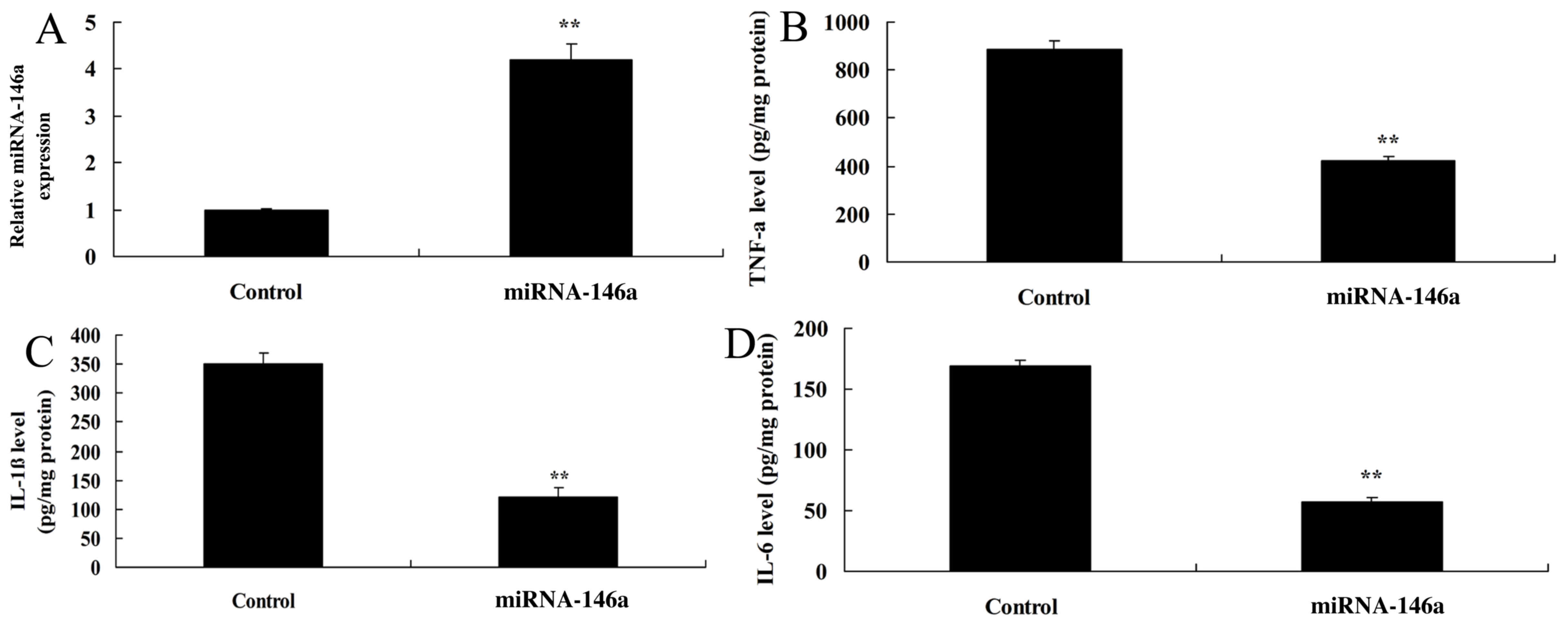
Upregulation of miR-146a decreases
inflammation in an SCI model in vitro
In order to evaluate whether upregulation of
miR-146a would affect inflammation in an SCI model in vitro,
cells were transfected with miR-146a mimics. As indicated in
Fig. 5A, miR-146a expression was
significantly upregulated in the SCI model transfected with
miR-146a mimics compared with the control group. The level of
expression of TNF-α, IL-1β and IL-6 was significantly lower in the
group with miR-146a upregulation compared with the control group
(Fig. 5B-D).
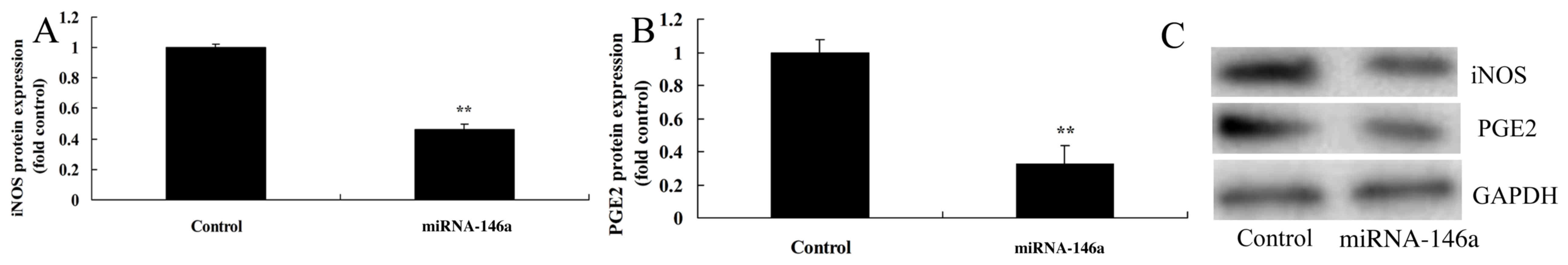
Upregulation of miR-146a inhibits iNOS
and PGE2 protein expression in an SCI model in vitro
Upregulation of miR-146a significantly inhibited
iNOS and PGE2 protein expression compared with the control group
(Fig. 6).
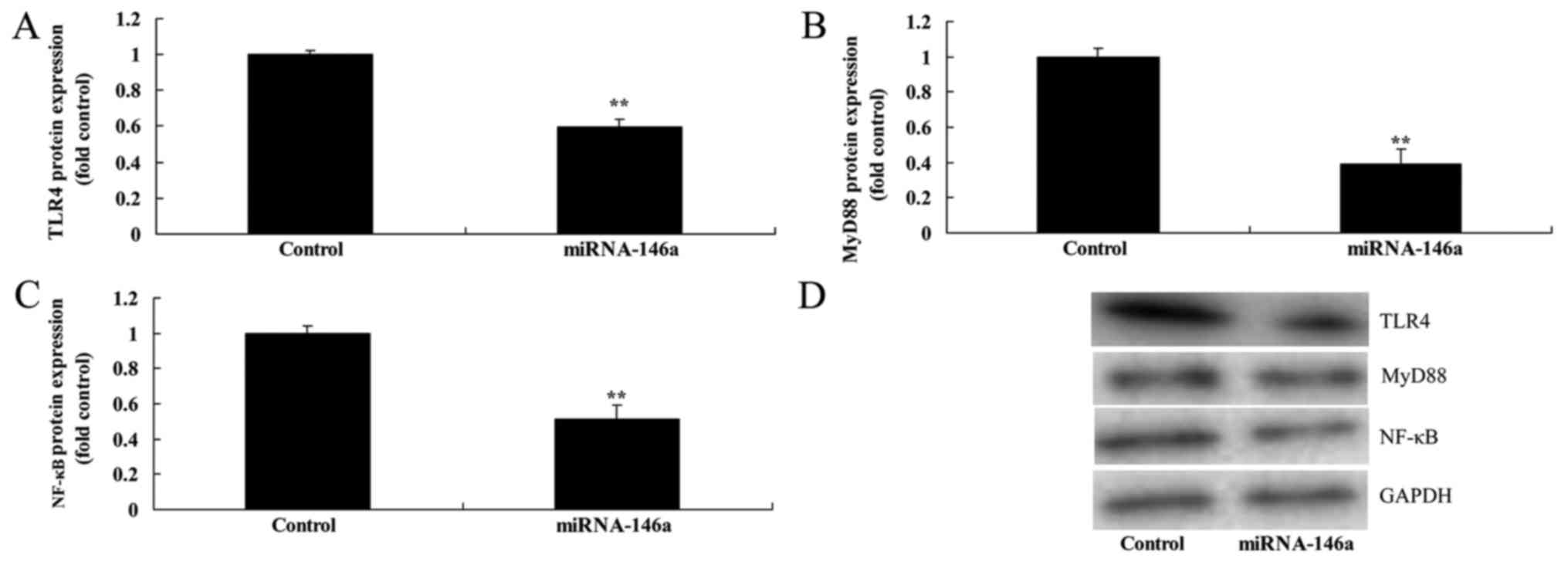
Upregulation of miR-146a suppresses
TLR4, MyD88 and NF-κB level expression in SCI model vitro
As expected, upregulation of miR-146a significantly
suppressed the levels of TLR4, MyD88 and NF-κB expression compared
with the control group (Fig. 7).
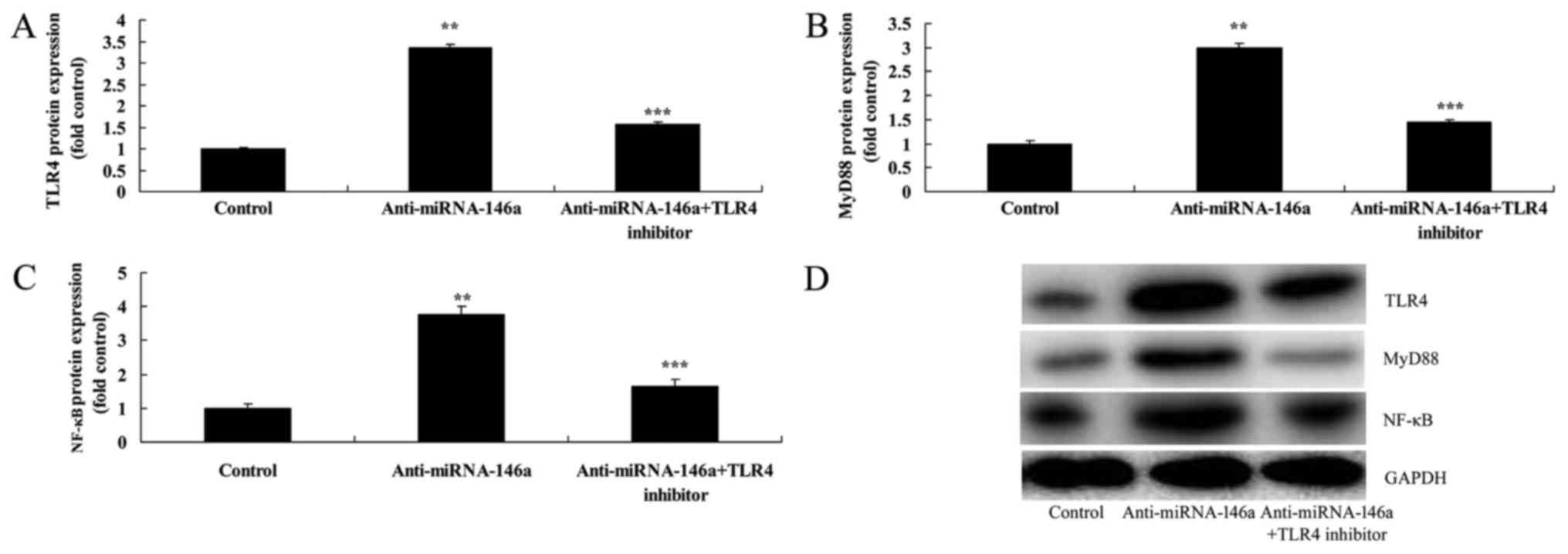
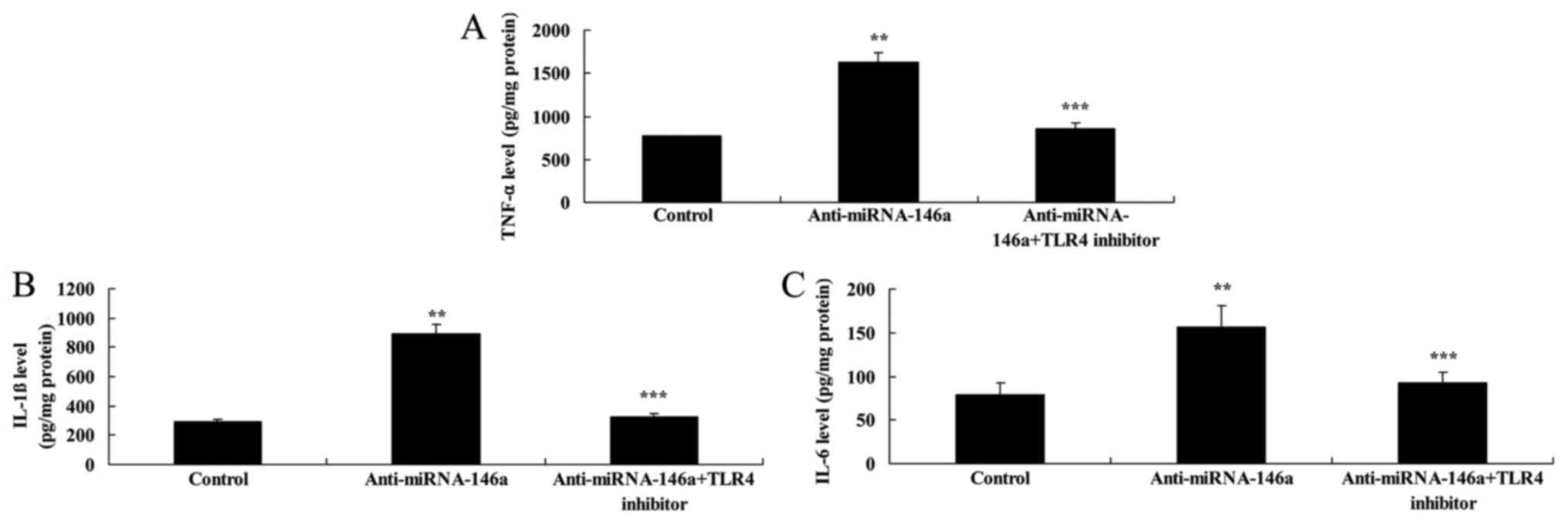
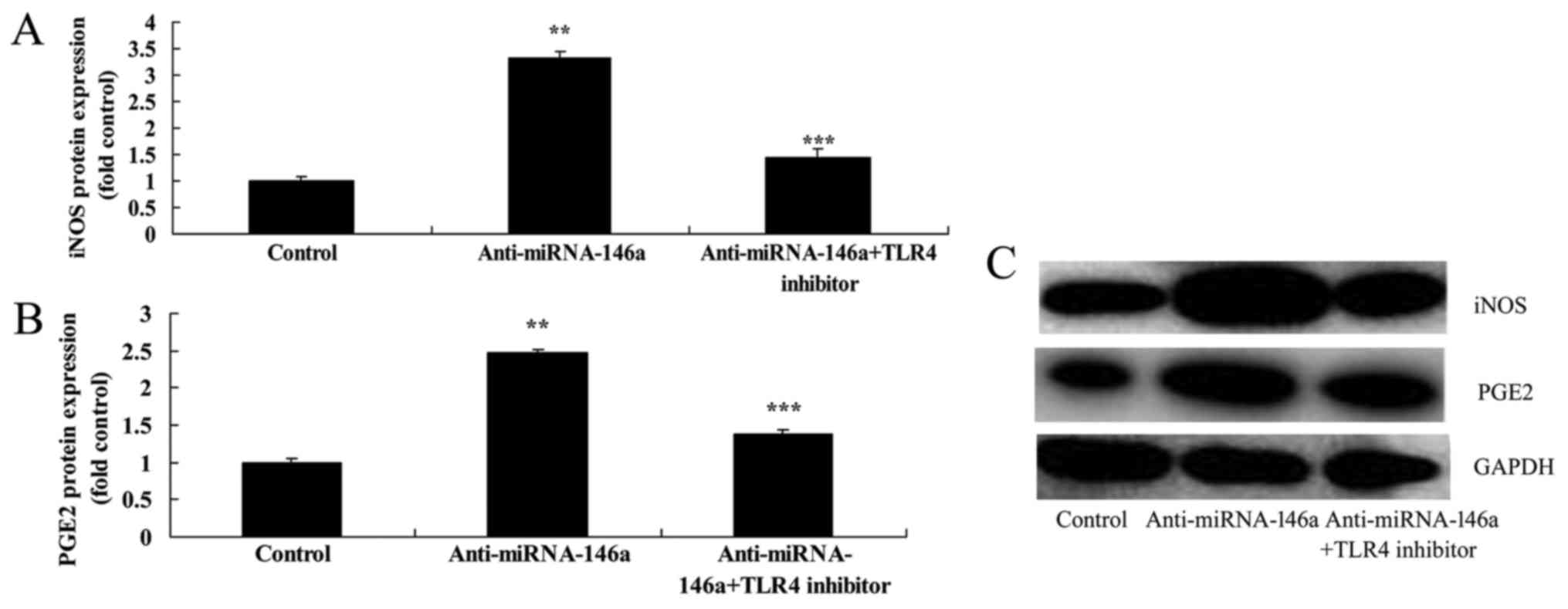
Inhibition of TLR4 attenuates the
proinflammatory effects of anti-miR-146a in an SCI model in
vitro
In order to evaluate the function of TLR4 in the
proinflammatory effects of anti-miR-146a in SCI, 2.5 nM of TAK-242,
a TLR4 inhibitor, was administered following transfection with
anti-miR-146a. As indicated in Fig.
8, inhibition of TLR4 significantly attenuated the
proinflammatory effects of anti-miR-146a on TLR4, MyD88 and NF-κB
expression compared with the anti-miR-146a group. In addition,
inhibition of TLR4 significantly attenuated the proinflammatory
effects of anti-miR-146a on TNF-α, IL-1β and IL-6 levels compared
with the anti-miR-146a group (Fig.
9). Furthermore, inhibition of TLR4 significantly attenuated
the proinflammatory effects of anti-miR-146a on iNOS and PGE2
protein expression compared with the anti-miR-146a group (Fig. 10).
Discussion
SCI refers to direct or indirect external trauma to
normal spine and spinal cord tissues, which impacts spinal cord
functions (3). For instance, this
may be caused by car accidents or falls. It was demonstrated in the
current study that miR-146a expression is downregulated in an SCI
rat model compared with the control group. Zhang et al
(10) reported that miR-146a could
inhibit the activities of inflammatory factors and NF-κB in lupus
nephritis. In the current study, only one SCI rat model was used,
but other SCI animal models exist, including an LPS-induced SCI
animal model of chronic cervical SCI (11). This LPS-induced SCI animal model is a
bacterial infection model, which simulates patients with menopause
SCI or uterine trauma SCI; by contrast the current study induced
damage at T9 for a classic SCI animal model. This immediately
destroys spinal cord to generate an acute SCI model.
The phenotype of macrophage polarity can be altered
under different inflammatory micro-environments (3). As key components of an inflammatory
micro-environment, inflammatory factors also serve key functions in
post-SCI nerve injury repair. This involves proinflammatory
factors, including IL-1β, TNF-α, IL-12, IL-6 and iNOS, as well as
inflammatory cytokines, including IL-4, IL-10 and IL-13 (3). Results from a previous study indicated
that overexpression of proinflammatory factors can promote
apoptosis and further aggravate nerve injury (3). Compared with the wild type, the
post-SCI damage area in IL-1β knockout mouse was markedly reduced.
Furthermore, IL-1β can enhance neuroplasticity and promote recovery
of motor function (3). TNF-α has
cytotoxic effects on nerve cells and oligodendroglia cells
(12). In addition, the TNF
superfamily receptor 1A (TNFR1) can neutralize damage induced by
TNF-α by preventing TNF-α receptor binding, so as to reduce TNF-α
receptor binding on nerve cells (3).
Applying TNFR1 in post-SCI local damage can notably reduce neuron
death (3). By contrast, inflammatory
cytokine IL-10 exerts important effects in regulating inflammatory
responses, including anti-inflammation, apoptosis inhibition and
neurotrophy (13). In the present
study, upregulated miR-146a could decrease inflammation and inhibit
expression of iNOS and PGE2 proteins in the SCI model in
vitro.
In recent research, nerve cells were identified to
be part of TLRs (3). It was
originally thought that TLRs were primarily involved in
immunity-related diseases. However, an increasing number of studies
have suggested that TLRs are not only involved in the
pathophysiological changes of neurodegenerative diseases, but also
serve key functions in disease occurrence (3). Notably, the majority of TLRs exert
their functions via the MyD88 pathway. Furthermore, TLR3 and TLR4
exert their functions in a MyD88 pathway-independent manner
(3). Expression of these
inflammatory factors may aggravate the secondary damage of spinal
cord. The TLR4 signaling pathway can be classified into the
MyD88-dependent and MyD88-independent pathways for cell
transmission (3). TLR4 can activate
IL-1 receptor-associated kinase through the MyD88-dependent
pathway, thus further activating TNF receptor-associated factor 6.
Subsequently, it activates the downstream transcription factors
NF-κB, activator protein 1 and interferon regulatory factor 5
(3). These transcription factors can
thus further induce expression of proinflammatory factors,
including IL-6, TNF-α and IL-12 (3).
As identified in the current study, upregulation of miR-146a can
significantly suppress the expression levels of TLR4, MyD88 and
NF-κB in an SCI model in vitro.
NF-κB is the generic term of the dimer transcription
factor family. Every monomer of the dimer contains the Rel region,
a sequence of 300 amino acids (3).
The Rel region can bind with DNA as well as inhibitory molecules.
Of these, the inhibitory molecule IκB contains 5–7 ankyrin repeat
domains (3), with 30 amino acids in
each domain. The domain is responsible for binding with the Rel
region of NF-κB (3). NF-κB can be
transferred into the nucleus following activation, so as to bind
with relevant genes of multiple inflammatory factors and induce
expression of proinflammatory factors (14). Activated TLRs can degrade IκB to
activate NF-κB, thus regulating the inflammatory response. Bao
et al (14) reported that
NF-κB can be activated within 30 min of SCI. Thus, NF-κB may also
be involved in the pathophysiology of secondary damage after SCI.
In addition, it was observed in the current study that TLR4
inhibition could attenuate the proinflammatory effects of
anti-miR-146a in an SCI model in vitro. The present study
suggests that miR-146a can suppress inflammation in SCI through the
TLR4/MyD88/NF-κB signaling pathway, which contributes to neural
regeneration. The present study was limited by only evaluating
miR-146a regulation of TLR4/MyD88/NF-κB signaling. Other
inflammation signaling pathways, including p-NF-κB and p-IκB
expression, will be investigated in future studies.
In summary, the present study demonstrated that
miR-146a exerts an anti-inflammatory effect in SCI, which can be,
at least in part, attributed to its modulation of the
TLR4/MyD88/NF-κB signaling pathway. These findings suggest that
miR-146a may be a promising therapeutic agent for SCI.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed MM, King KC, Pearce SM, Ramsey MA,
Miranpuri GS and Resnick DK: Novel targets for spinal cord injury
related neuropathic pain. Ann Neurosci. 18:162–167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hooshmand MJ, Galvan MD, Partida E and
Anderson AJ: Characterization of recovery, repair, and inflammatory
processes following contusion spinal cord injury in old female
rats: Is age a limitation? Immun Ageing. 11:152014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bank M, Stein A, Sison C, Glazer A, Jassal
N, McCarthy D, Shatzer M, Hahn B, Chugh R, Davies P and Bloom O:
Elevated circulating levels of the pro-inflammatory cytokine
macrophage migration inhibitory factor in individuals with acute
spinal cord injury. Arch Phys Med Rehabil. 96:633–644. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwan T, Floyd CL, Kim S and King PH: RNA
binding protein HuR is Translocated in astrocytes following spinal
cord injury and promotes the inflammatory response. J Neurotrauma.
34:1249–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang W, Li M, He F, Bian Z, Liu J, He Q,
Wang X, Sun T and Zhu L: Dopamine D1 receptor agonist A-68930
inhibits NLRP3 inflammasome activation and protects rats from
spinal cord injury-induced acute lung injury. Spinal Cord.
54:951–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia H, Xu S, Liu Q, Liu J, Xu J, Li W, Jin
Y and Ji Q: Effect of pioglitazone on neuropathic pain and spinal
expression of TLR-4 and cytokines. Exp Ther Med. 12:2644–2650.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng CZ, Shu YB, Luo YL and Luo J: The
role of miR-146a in modulating TRAF6-induced inflammation during
lupus nephritis. Eur Rev Med Pharmacol Sci. 21:1041–1048.
2017.PubMed/NCBI
|
|
8
|
Xin DQ, Hu ZM, Huo HJ, Yang XJ, Han D,
Xing WH, Zhao Y and Qiu QH: Schisandrin B attenuates the
inflammatory response, oxidative stress and apoptosis induced by
traumatic spinal cord injury via inhibition of p53 signaling in
adult rats. Mol Med Rep. 16:533–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K, Wu S, Li Z and Zhou J:
MicroRNA-211/BDNF axis regulates LPS-induced proliferation of
normal human astrocyte through PI3K/AKT pathway. Biosci Rep.
37:pii: BSR201707552017. View Article : Google Scholar
|
|
11
|
Suzuki H, Ahuja CS, Salewski RP, Li L,
Satkunendrarajah K, Nagoshi N, Shibata S and Fehlings MG: Neural
stem cell mediated recovery is enhanced by Chondroitinase ABC
pretreatment in chronic cervical spinal cord injury. PLoS One.
12:e01823392017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan W and Kastin AJ: Spinal cord injury
changes cytokine transport. CNS Neurol Disord Drug Targets.
15:1139–1150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paterniti I, Campolo M, Cordaro M,
Impellizzeri D, Siracusa R, Crupi R, Esposito E and Cuzzocrea S:
PPAR-α modulates the Anti-inflammatory effect of melatonin in the
secondary events of spinal cord injury. Mol Neurobiol.
54:5973–5987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao G, Li C, Qi L, Wang N and He B:
Tetrandrine protects against oxygen-glucose-serum
deprivation/reoxygenation-induced injury via PI3K/AKT/NF-κB
signaling pathway in rat spinal cord astrocytes. Biomed
Pharmacother. 84:925–930. 2016. View Article : Google Scholar : PubMed/NCBI
|