Introduction
Multiple trauma is defined as the simultaneous
injury of two or more anatomical sites of the body (1). Multiple trauma is often accompanied by
major bleeding, shock or severe physiological disorders, all of
which can be life threatening (1).
Patients with multiple trauma typically exhibit systemic
inflammatory response syndrome, sepsis or multiple organ
dysfunction syndrome (2). Sepsis is
a common complication of severe trauma, burns, shock and major
surgery, and one of the main causes of mortality in patients with
multiple trauma injuries (2,3).
Sepsis is a serious complication of infection with a
number of clinical manifestations and symptoms. Sepsis occurs when
there is excessive inflammation in response to an infection, which
subsequently damages the host (4).
It is commonly accepted that the activation of neutrophils,
lymphocytes and mononuclear macrophages and the release of
endogenous mediators serve key roles in the pathophysiology of
sepsis following trauma (5,6). In 1992, sepsis was defined by the
American Thoracic Society and the Society of Critical Care Medicine
as an infection and systemic inflammatory response syndrome
(7). Sepsis manifests as a systemic
inflammatory response syndrome caused by infection, which requires
rapid treatment (8).
Interleukin (IL)-6 is an important factor in immune
responses that is produced by activated monocytes and macrophages
(9). IL-6 promotes the growth and
differentiation of primary bone marrow-derived cells by
coordinating with colony stimulating factor, and enhances the
lysing ability of natural killer cells (10–12). A
previous study has reported that microRNA (miRNA or miR)-365 has a
negative regulatory effect on IL-6 expression in 293 and HeLa cells
(13). However, the regulatory
effect of miR-365 on IL-6 in blood monocytes from patients with
sepsis following multiple traumas is rarely reported. In the
present study, miR-365 expression was measured, as well as the
levels of IL-6 mRNA and protein in peripheral blood mononuclear
cells (PBMC) and serum from patients with sepsis following multiple
trauma, and the mechanism of action of miR-365 in sepsis was
investigated.
Patients and methods
Patients
A total of 26 patients with sepsis following
multiple trauma who received treatment at the Affiliated Hospital
of Nantong University (Nantong, China) between June 2012 and
January 2017 were included in the present study as the experimental
sepsis group. During the same time period 21 patients without
sepsis following multiple trauma were recruited as the negative
control (NC) group. Among the patients with sepsis, 11 were male
and 15 were female (age range, 18–60 years; median age, 41 years).
In the control group, 8 patients were male and 13 were female (age
range, 16–62 years; median age, 42 years). None of the participants
had diabetes, tumors or other immune diseases. The Ethics Committee
of Nantong University approved all procedures and written informed
consent was obtained from all patients or their families prior to
their inclusion in the present study.
Peripheral blood was collected from all participants
and stored at 4°C for 1–2 h. The serum was then separated,
centrifuged at a speed of 400 × g for 10 min at 4°C, and aliquoted
into Eppendorf tubes (100 µl in each tube) prior to storage at
−70°C. To collect PBMCs, anticoagulant venous blood was mixed with
an equal volume of serum-free Iscove's Modified Dulbecco's Medium
(cat. no. 12440053; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the mixture was added onto the surface of human lymphocyte
separation solution (5 ml; cat. no. 40503ES60; Shanghai Yeasen
Biotechnology Co., Ltd., Shanghai, China). Following centrifugation
at 400 × g for 30 min at 4°C the middle mist layer was gently
aspirated into tubes and mixed with 5× the volume of Hank's
balanced salt solution (cat. no. C0218; Beyotime Institute of
Biotechnology, Haimen, China), the mixture then underwent
centrifugation at 300 × g for 10 min. The cells were washed with
Hank's balanced salt solution twice, and then diluted to
3×106 cells/ml and seeded into culture dishes. Following
cultivation at 37°C in an atmosphere of 5% CO2 for 2 h,
the cells that adhered to the bottom of the culture dish were
identified as PBMCs.
Cells
THP-1 cells (Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) were cultured in RPMI-1640
complete medium (cat. no. 11875093; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (cat. no. SH30084.03;
Hyclone; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2 for 48 h. The cells were treated with 1 µg/ml
lipopolysaccharide (LPS) for 24 h to simulate a sepsis environment
(14) and then compared with THP-1
cells that were not treated with LPS. The expression of miR-365 and
IL-6 in the cells was measured.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 100 µl blood or
2×106 PBMCs (2×107/ml) using TRIzol reagent
(Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol (15). The same procedure
was used as for patient samples. The concentration and quality of
the RNA was measured using a Nanodrop ND2000 ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). Subsequently, complementary DNA was obtained by RT from 1 µg
RNA and stored at −20°C. RT of mRNA was performed using a
TIANScript II cDNA First Strand Synthesis kit, and RT of miRNA was
performed using an miRcute miRNA cDNA First Strand Synthesis kit
(both Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturers protocol.
PCR was performed using a SuperReal PreMix (SYBR
Green) RT-qPCR kit (Tiangen Biotech Co., Ltd.) to detect mRNA
expression of IL-6; GAPDH was used as an internal reference. The
PCR primer sequences used were as follows: IL-6 forward,
5′-GGCACTGGCAGAAAACAACC-3′ and reverse,
5′-GCAAGTCTCCTCATTGAATCC-3′; and GAPDH forward,
5′-GGGAAACTGCGGCGTGAT-3′ and reverse, 5′-AAAGGTGGAGGAGTGGGT-3′. The
reaction system (25 µl) was composed of 12.5 µl SYBR Premix EXTaq,
0.5 µl upstream primer, 0.5 µl downstream primer, 1 µl cDNA and
10.5 µl double distilled (dd) H2O. The PCR conditions
were an initial denaturation at 95°C for 30 sec, followed by 45
cycles of denaturation at 95°C for 5 sec and annealing at 57°C for
30 sec; with final extension at 72°C for 1 min (iQ5 Multicolor
Real-Time PCR Detection System; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The 2−ΔΔCq method (16) was used to calculate the relative
expression of IL-6 mRNA against GAPDH. Each sample had a minimum of
three repeats.
The expression of miR-365 was determined using U6 as
an internal reference. The PCR primer sequences used were as
follows: miR-365 forward, 5′-GCGTAATGCCCCTAAAAATCC-3′, the reverse
primer was universal and provided by the kit. The forward and
reverse PCR primer sequences used for U6 were
5′-AACGCTTCACGAATTTGCGT-3′ and 5′-CTCGCTTCGGCAGCACA-3′,
respectively. The reaction system (20 µl) contained 10 µl
RT-qPCR-Mix, 0.5 µl upstream primer, 0.5 µl downstream universal
primer, 2 µl cDNA and 7 µl ddH2O. The reaction protocol
was: Initial denaturation at 90°C for 30 sec; 40 cycles of
denaturation at 95°C for 5 sec and annealing at 60°C for 34 sec;
with final extension at 72°C for 1 min. The 2−ΔΔCq
method was used to calculate the relative expression of miR-365
against U6. Each sample had a minimum of three repeats.
Western blot analysis
A precooled radio-immunoprecipitation assay lysis
buffer (600 µl) composed of 50 mM Tris-base, 1 mM EDTA, 150 mM
NaCl, 0.1% sodium dodecyl sulfate, 1% Triton-X-100 and 1% sodium
deoxycholate (all Beyotime Institute of Biotechnology) was used to
lyse the samples (100 µl blood or 2×106 cells).
Following lysis for 50 min on ice, the mixture was centrifuged at
12,000 × g at 4°C for 5 min. The supernatant was used to determine
the protein concentration using a bicinchoninic acid protein
concentration determination kit [RTP7102, Real-Times (Beijing)
Biotechnology Co., Ltd., Beijing, China]. Protein samples (20 µg)
were mixed with a sodium dodecyl sulfate loading buffer (cat. no.
P0015; Beyotime, Institute of Biotechnology) prior to denaturation
in a boiling water bath for 5 min. The samples (20 µg/lane) were
then subjected to 10% SDS-PAGE gel electrophoresis. The resolved
proteins were transferred to polyvinylidene difluoride membranes on
ice (100 V, 2 h) and blocked with 5% skimmed milk at room
temperature for 1 h. The membranes were subsequently incubated with
rabbit anti-human primary antibodies against IL-6 (1:1,000; ab6672)
or β-actin (1:5,000; ab129348; both Abcam, Cambridge, UK) at 4°C
overnight. Following washing with PBS with Tween-20 three times for
15 min, the membranes were incubated with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:3,000;
ab6721; Abcam) for 1 h at room temperature. Following this they
were washed again with PBS with Tween-20 three times for 15 min.
The membranes were then developed using an ECL Western Blotting
Substrate kit (ab65623; Abcam) for imaging. Image Lab version 3.0
software (Bio-Rad Laboratories, Inc.) was used to acquire and
analyze the imaging signals. The relative content of IL-6 protein
was expressed as the IL-6/β-actin ratio.
ELISA
Serum levels of IL-6 were tested using a human IL-6
ELISA kit (ab178013; Abcam) according to the manufacturer's
protocol. In 96-well microplates, 50 µl samples (10 µl sample
liquid and 40 µl diluent) and blank (diluent) were set into
predefined wells. Horseradish peroxidase-labelled conjugates (100
µl) were added to all wells prior to sealing the plates for
incubation at 37°C for 1 h. Following washing of the plates five
times with washing liquid provided by the kit, substrates A (50 µl)
and B (50 µl; provided by the kit) were added into each well.
Following incubation at 37°C for 15 min, a stop solution (50 µl)
was added into each well, and the absorbance of each well was
measured at 450 nm within 15 min.
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool for the
study of the functions of miRNAs. To understand the regulatory
mechanism of IL-6, several different bioinformatics tools were used
to predict miRNA molecules that may regulate IL-6. These included
miRanda (microrna.org/microrna/home.do), TargetScan (targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and
PicTar (pictar.mdc-berlin.de). Wild-type (WT)
and mutant seed regions of miR-365 in the 3′-untranslated region
(UTR) of the IL-6 gene were chemically synthesized in vitro,
Spe-1 and HindIII restriction sites were added, and then cloned
into pMIR-REPORT luciferase reporter plasmids (Ambion; Thermo
Fisher Scientific, Inc.). Plasmids (0.8 µg) with WT or mutant
3′-UTR DNA sequences were co-transfected with agomiR-365 (100 nM;
Sangon Biotech Co., Ltd., Shanghai, China) into 293 T cells (Type
Culture Collection of the Chinese Academy of Sciences). Following
cultivation for 24 h the cells were lysed using Dual-luciferase
Reporter Assay System (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol, and the fluorescence
intensity was measured using a GloMax 20/20 luminometer (Promega
Corporation). Using Renilla fluorescence activity as the
internal reference, the fluorescence values of each group of cells
were measured.
Statistical analysis
The results were analyzed using SPSS version 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). The results
are expressed as the mean ± standard deviation. Data were tested
for normality. Multigroup measurement data were analyzed using
one-way analysis of variance. In case of homogeneity of variance,
least significant difference and Student-Newman-Keuls methods were
used; in case of heterogeneity of variance, Tamhane's T2 or
Dunnett's T3 method was used. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patients with sepsis following
multiple traumas exhibit higher IL-6 mRNA levels than patients
without sepsis
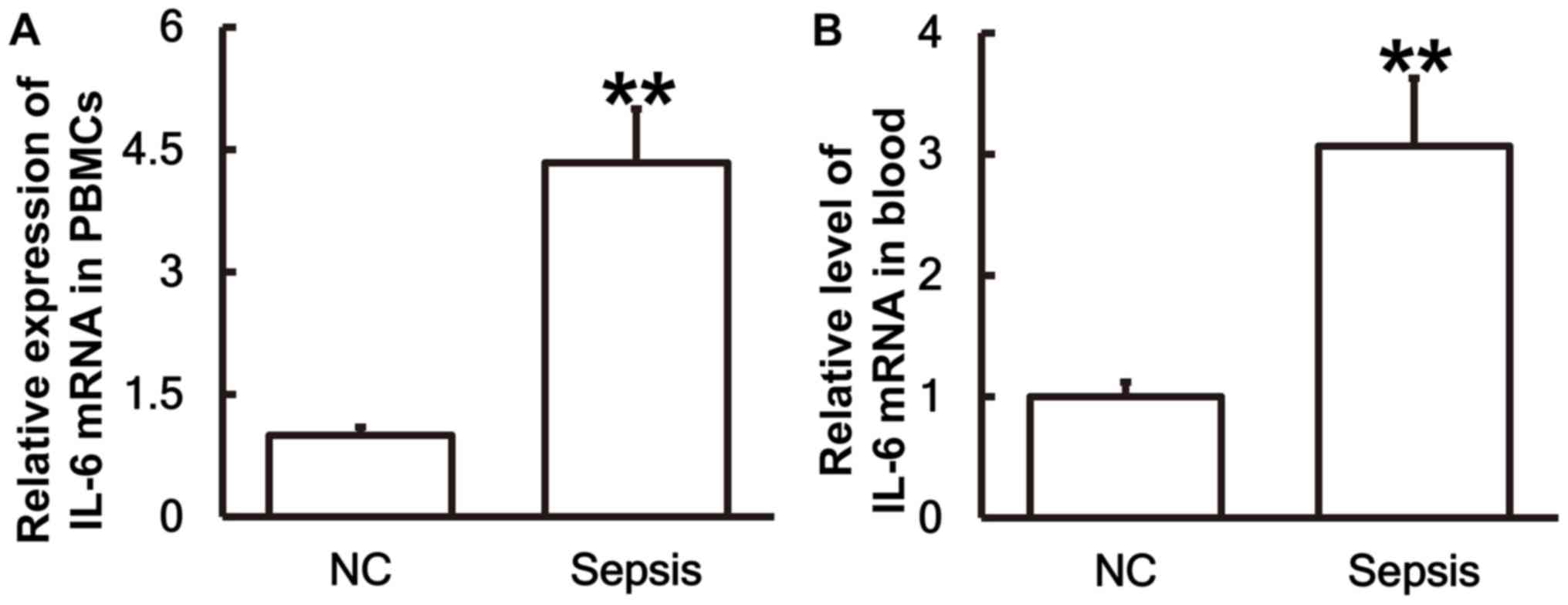
To measure the expression of IL-6 mRNA in different
samples, RT-qPCR was performed. The results demonstrated that the
levels of IL-6 mRNA in PBMCs (Fig.
1A) and blood (Fig. 1B) from
patients with sepsis were significantly higher than those in the
control group (P<0.01).
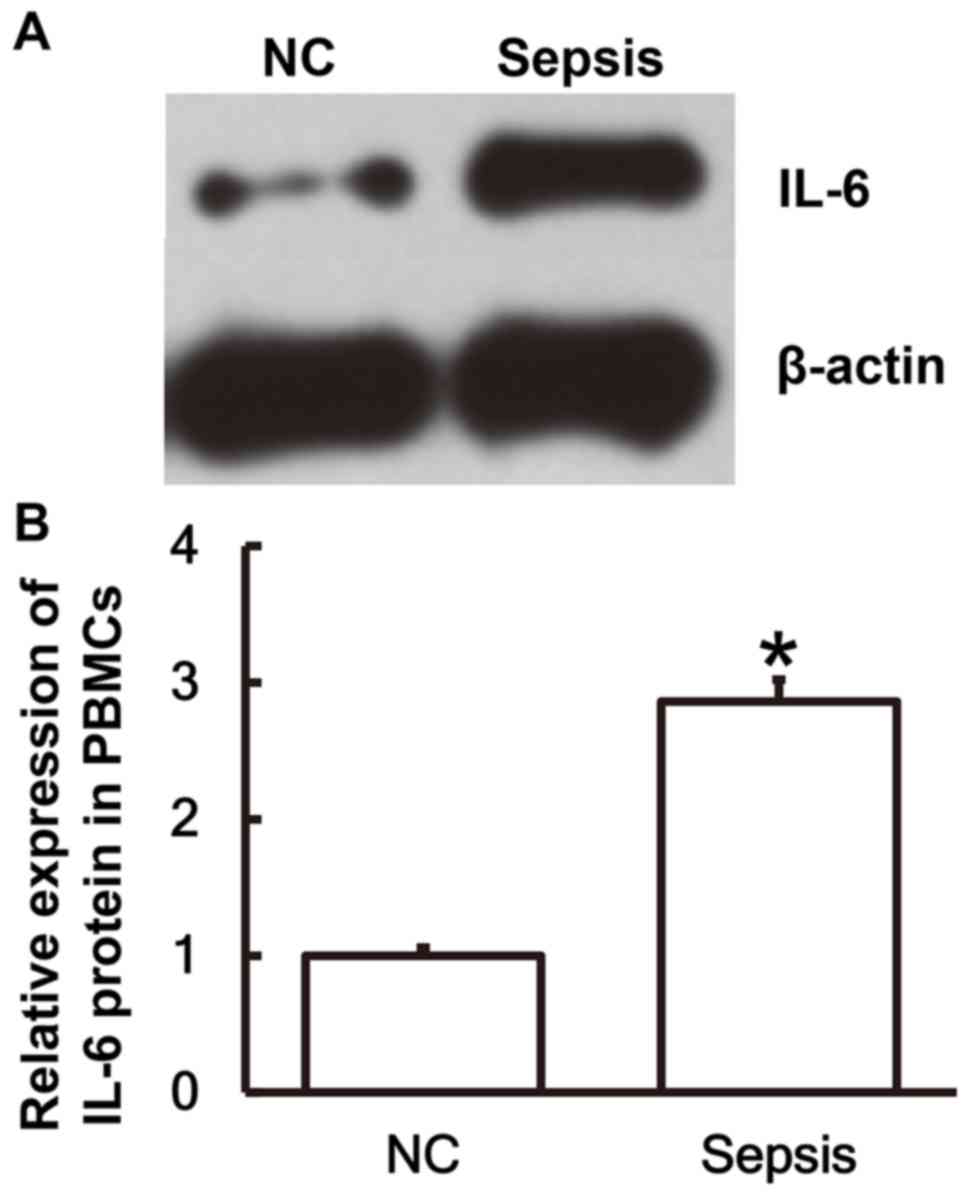
IL-6 protein expression in PBMCs is
upregulated in patients with sepsis following multiple trauma
To determine the expression of the IL-6 protein in
PBMCs, western blot analysis was performed. The results revealed
that IL-6 protein expression in PBMCs from patients with sepsis was
significantly elevated compared with the NC group (P<0.05;
Fig. 2A and B).
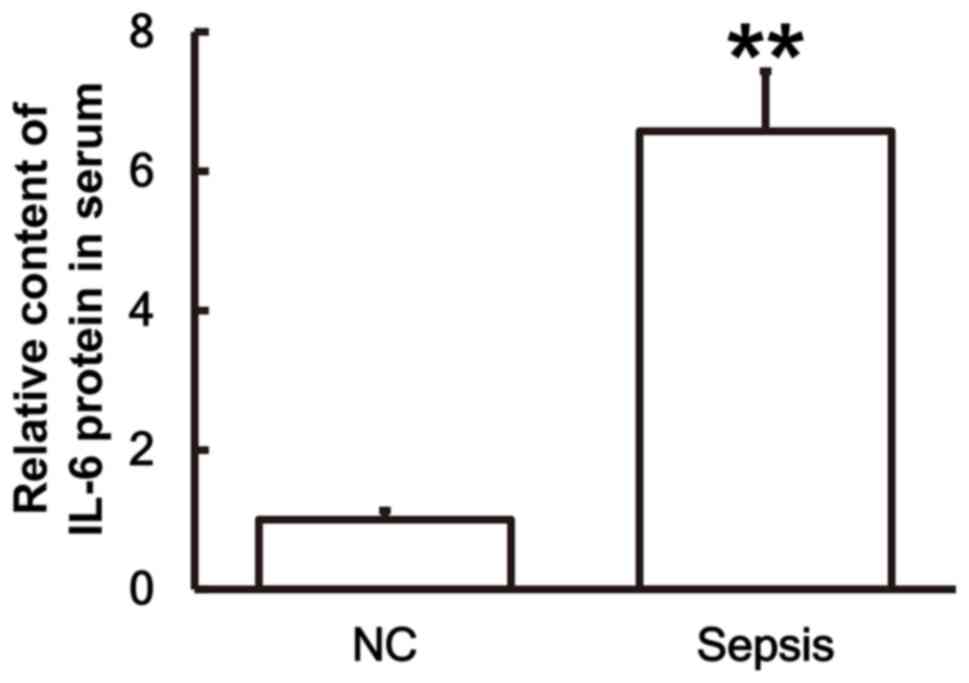
Serum from patients with sepsis
following multiple trauma contains a higher IL-6 content compared
with serum from patients without sepsis
To examine the secretion of IL-6 by PBMCs, ELISA was
performed. The results demonstrated that the IL-6 content in the
serum of patients with sepsis was significantly higher than in the
control group (P<0.01; Fig.
3).
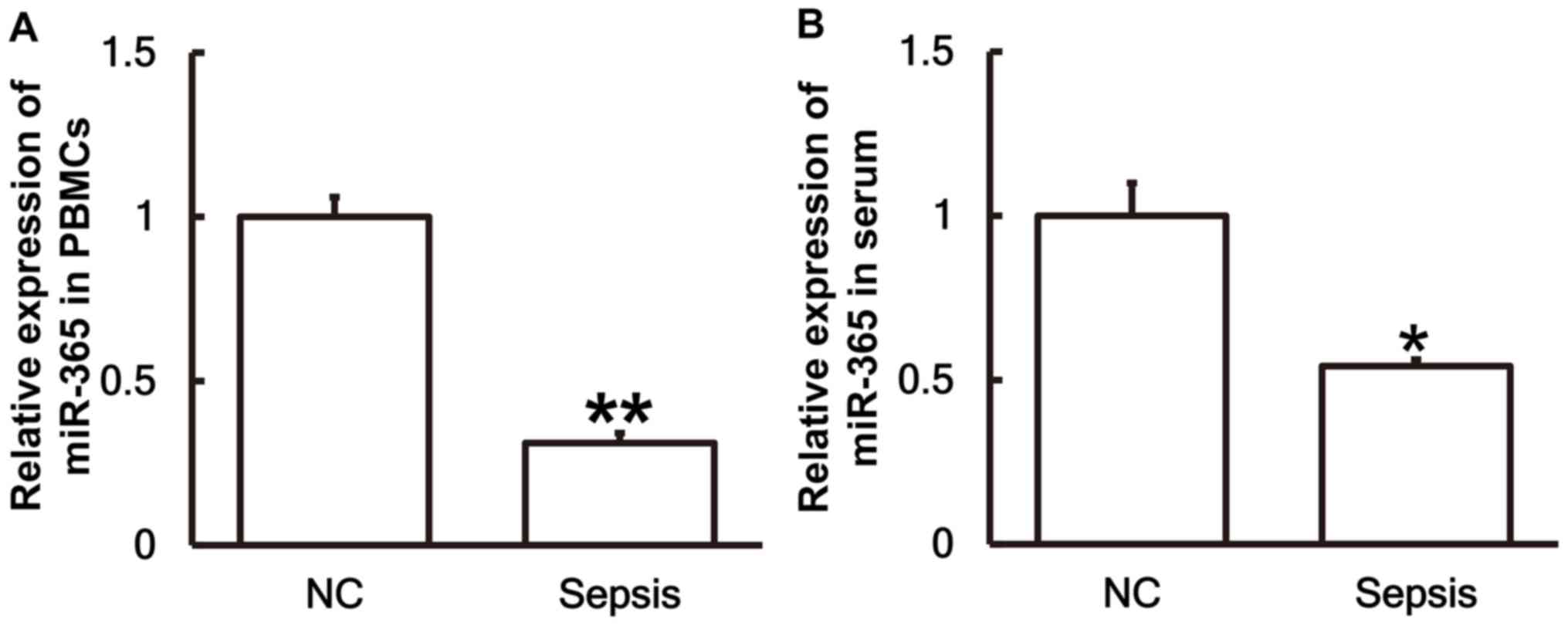
Patients with sepsis following
multiple trauma exhibit lower levels of miR-365 than patients
without sepsis
To investigate the levels of miR-365 in the serum
and PBMCs, RT-qPCR was performed. The results revealed that the
levels of miR-365 in PBMCs (Fig. 4A)
and serum (Fig. 4B) from patients
with sepsis were significantly lower than those in the control
group (P<0.01 and P<0.05, respectively).
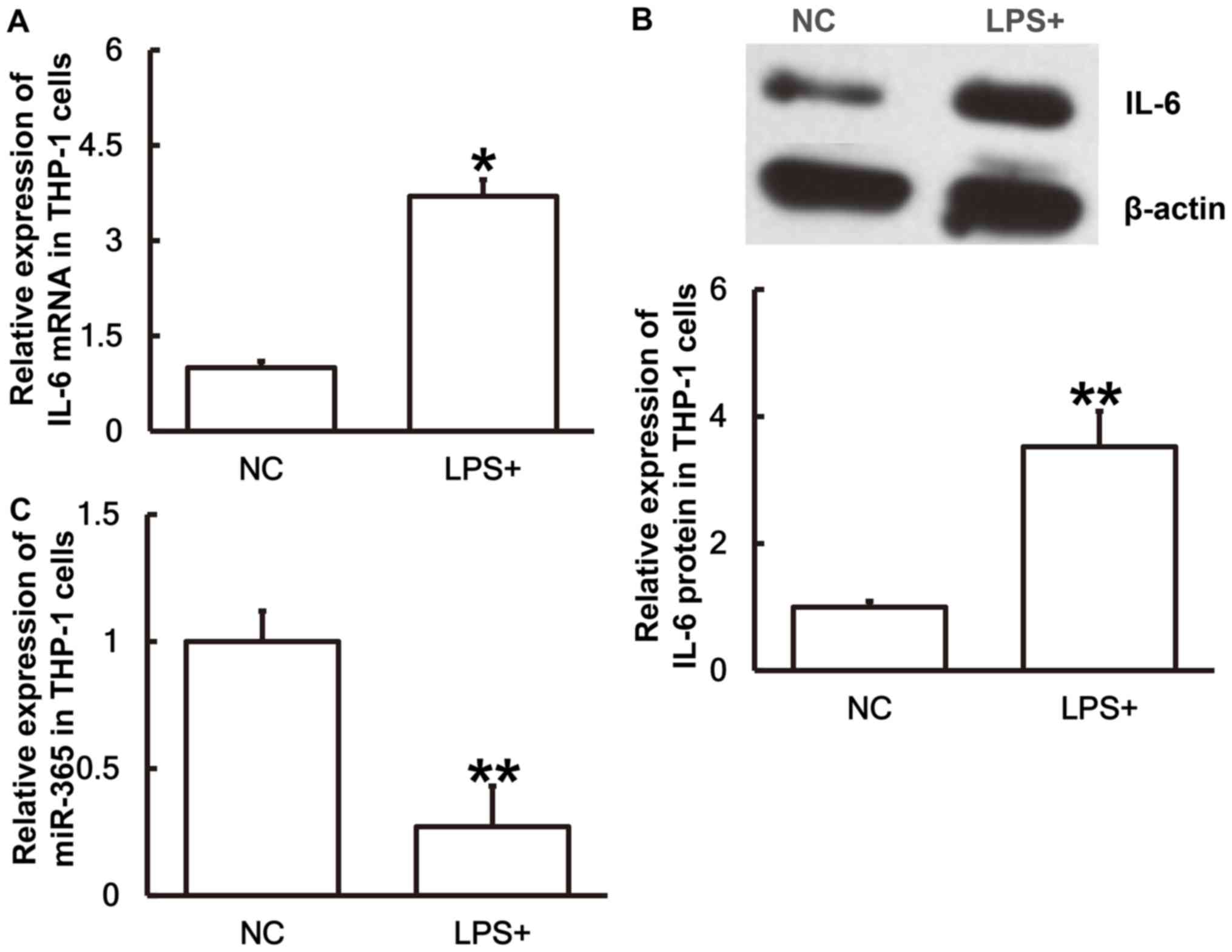
IL-6 expression is upregulated whereas
miR-365 expression is downregulated in THP-1 cells stimulated with
LPS
To simulate sepsis at a cellular level THP-1 cells
were treated with LPS for 24 h prior to the levels of miR-365 and
IL-6 being measured. The results revealed that IL-6 mRNA (Fig. 5A) and protein expression (Fig. 5B) in THP-1 cells treated with LPS
were significantly higher than those in the NC group (P<0.05 and
P<0.01, respectively). Conversely, the miR-365 expression in
cells treated with LPS was significantly lower than the negative
control group (P<0.01; Fig. 5C).
The results suggest that IL-6 expression is upregulated and miR-365
expression is downregulated in THP-1 cells stimulated with LPS.
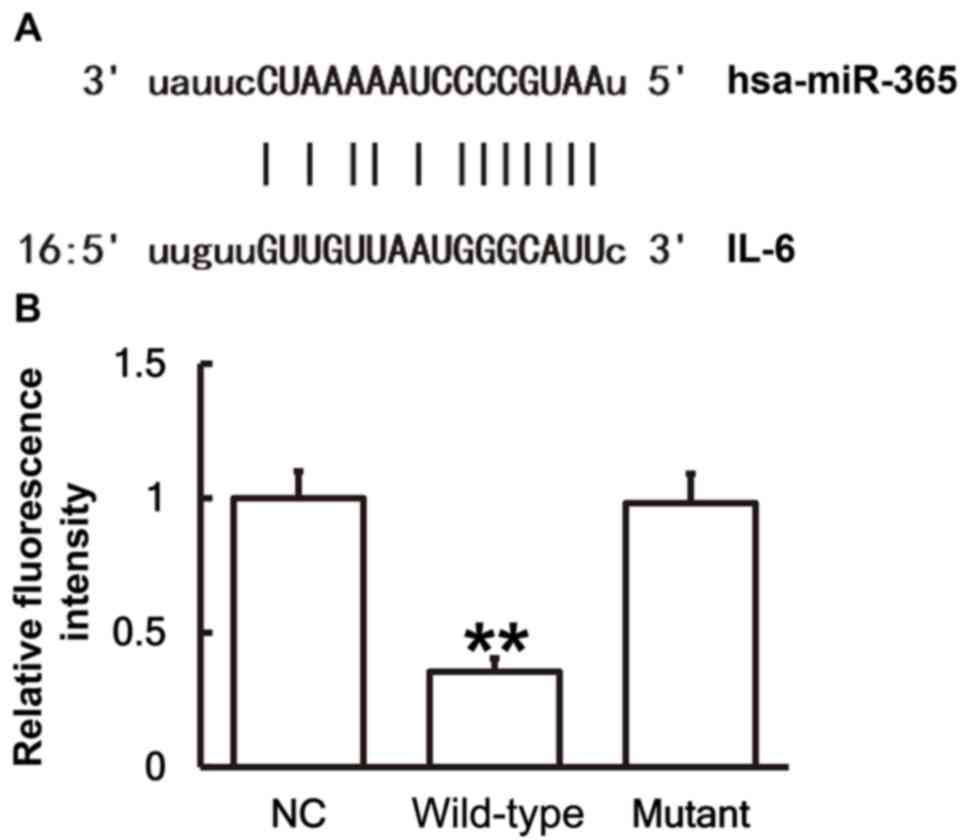
miR-365 binds with the 3′-UTR seed
region of IL-6 mRNA to regulate its expression
To identify interactions between miR-365 and the
3′-UTR of IL-6 mRNA, a dual luciferase reporter assay was
performed. Bioinformatics results identified that miR-365 was
potentially able to regulate IL-6 (Fig.
6A). The fluorescence value of cells co-transfected with
miR-365 mimics and WT luciferase reporter plasmids was
significantly lower than that in the NC group (P<0.01). By
contrast, the fluorescence value of cells co-transfected with
miR-365 mimics and mutant luciferase reporter plasmids was not
significantly different from that in the NC group (Fig. 6B). These results indicates that
miR-365 binds with the 3′-UTR seed region of IL-6 mRNA to regulate
its expression.
Discussion
Sepsis is a systemic inflammatory response syndrome
caused by infection, which can lead to acute organ dysfunction or
septic shock (17). In clinical
practice, septic shock caused by sepsis is the most common cause of
mortality in intensive care patients, and the mortality rate is
20–80% (18). Previous studies have
demonstrated that cytokines serve key roles in the pathophysiology
of post-traumatic sepsis, and the upregulation of tumor necrosis
factor (TNF)-α, IL-6 and IL-8 have been identified as associated
with post traumatic infection (19,20).
IL-6 is a pleiotropic cytokine synthesized by mononuclear cells,
macrophages, T cells, B cells, vascular endothelial cells and
fibroblasts in response to IL-1 and TNF-α (21). Under normal circumstances, IL-6
contents in the blood, serum and body are minimal, but it has a
high biological activity and exerts an effect via paracrine and
autocrine signaling pathways (22).
IL-6 induces the production of C-reactive protein and fibrinogen in
inflammation and promotes thrombosis (23). Increased levels of IL-6 in the body
can cause inflammatory diseases by binding to the IL-6 receptor
(24). The IL-6 receptor is
primarily expressed on the surface of liver cells, neutrophils,
macrophages or lymphocytes. In rheumatoid arthritis IL-6 stimulates
the secretion of inflammatory mediators by T lymphocytes and B
lymphocytes, and promotes the maturation and differentiation of B
lymphocytes (25). In inflammatory
responses IL-6 is chemotactic to other inflammatory cells,
including neutrophils and mononuclear macrophages (26). Riedemann et al (27) previously reported that IL-6 enhances
the proinflammatory activity of secondary inflammatory mediator
complement component 5a. Pritts et al (28) previously reported that nuclear
factor-κB and activator protein-1 are associated with the
regulation of IL-6 gene activation and synthesis in effector cells.
In the present study, it was identified that IL-6 mRNA and protein
expression are elevated in PBMCs and serum from patients with
sepsis following multiple trauma, which suggests that septic
infection following multiple trauma may activate mononuclear cells
and lymphocytes, which secrete abundant IL-6 to produce a large
number of antigen immune responses.
miRNA is widely associated with various
pathophysiological processes, including the proliferation, invasion
and migration of tumor cells, hypertension, diabetes mellitus and
atherosclerosis (29,30). It has been reported that the
expression of miR-365 in breast cancer and endometriosis tissues is
disordered (31). In NIH3T3 cells,
ultraviolet irradiation significantly increases the expression of
miR-365, which suggests that miR-365 has certain anti-tumor effects
(32). Overexpression of
tumor-suppressor gene NGX6 leads to the decreased expression of
miR-365, suggesting that miR-365 may also have oncogene activity
(33). The expression of miR-365 is
downregulated in senescent lung fibroblasts, which indicates that
miR-365 may be associated with the regulation of cell proliferation
(34). In addition, miR-365 has
negative regulation on IL-6 expression in 293 and HeLa cells
(13). In the present study it has
been identified that miR-365 is downregulated and IL-6 is
upregulated in PBMCs and serum from patients with sepsis following
multiple trauma. These results reveal that the levels of miR-365
and IL-6 in the serum reflect inflammatory responses and tissue
injuries. In addition, THP-1 cells treated with LPS to simulate a
sepsis environment achieved similar results. The dual luciferase
reporter assay demonstrated that IL-6 is a direct target gene of
miR-365.
In conclusion, the present study demonstrates that
increased expression of IL-6 in patients with sepsis following
multiple trauma is caused by decreased expression of miR-365. In
addition, miR-365 may regulate the occurrence and immune response
of sepsis following multiple trauma via IL-6. These results may
elucidate agents for clinical diagnosis and treatment of the
disease.
Acknowledgements
The authors would like to thank Dr Zhongwei Huang of
Department of Emergency Surgery, Affiliated Hospital of Nantong
University (Nantong, China) for his kind assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ conceived and designed the present study. HG
performed the experiments and wrote the manuscript. XS and JX
collected the samples. All authors collaborated to interpret
results and develop the manuscript. The final version of the
manuscript has been read and approved by all authors, and each
author believes that the manuscript represents honest work.
Ethics approval and consent to
participate
The Ethics Committee of Nantong University approved
all procedures and written informed consent was obtained from all
patients or their families prior to their inclusion in the present
study.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hilbert-Carius P, Wurmb T, Lier H, Fischer
M, Helm M, Lott C, Böttiger BW and Bernhard M: Care for severely
injured persons: Update of the 2016 S3 guideline for the treatment
of polytrauma and the severely injured. Anaesthesist. 66:195–206.
2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaukonen KM, Bailey M, Pilcher D, Cooper
DJ and Bellomo R: Systemic inflammatory response syndrome criteria
in defining severe sepsis. N Engl J Med. 372:1629–1638. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen KO, Held L, Kraus A, Hildebrand F,
Mommsen P, Mica L, Wanner GA, Steiger P, Moos RM, Simmen HP and
Sprengel K: The impact of mild induced hypothermia on the rate of
transfusion and the mortality in severely injured patients: A
retrospective multi-centre study. Eur J Med Res. 21:372016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Y, Yang Y, He X, Dong S, Wang W, Wang D
and Zhang P: Esmolol reduces apoptosis and inflammation in early
sepsis rats with abdominal infection. Am J Emerg Med. 35:1480–1484.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patrick AL, Grin PM, Kraus N, Gold M,
Berardocco M, Liaw PC and Fox-Robichaud AE; Canadian Critical Care
Translational Biology Group, : Resuscitation fluid composition
affects hepatic inflammation in a murine model of early sepsis.
Intensive Care Med Exp. 5:52017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin JB and Badeaux JE: Interpreting
laboratory tests in infection: Making sense of biomarkers in sepsis
and systemic inflammatory response syndrome for intensive care unit
patients. Crit Care Nurs Clin North Am. 29:119–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
American College of Chest
Physicians/Society of Critical Care Medicine Consensus Conference:
Definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. Crit Care Med. 20:864–874. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warren HS: Strategies for the treatment of
sepsis. N Engl J Med. 336:952–953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang M, Yang D, Xiang M and Wang J: Role
of interleukin-6 in regulation of immune responses to remodeling
after myocardial infarction. Heart Fail Rev. 20:25–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vila N, Reverter JC, Yague J and Chamorro
A: Interaction between interleukin-6 and the natural anticoagulant
system in acute stroke. J Interferon Cytokine Res. 20:325–329.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tezono K, Sarker KP, Kikuchi H, Nasu M,
Kitajima I and Maruyama I: Bioactivity of the vascular endothelial
growth factor trapped in fibrin clots: Production of IL-6 and IL-8
in monocytes by fibrin clots. Haemostasis. 31:71–79.
2001.PubMed/NCBI
|
|
12
|
Anderson AE, Pratt AG, Sedhom MA, Doran
JP, Routledge C, Hargreaves B, Brown PM, Lê Cao KA, Isaacs JD and
Thomas R: IL-6-driven STAT signalling in circulating CD4+
lymphocytes is a marker for early anticitrullinated peptide
antibody-negative rheumatoid arthritis. Ann Rheum Dis. 75:466–473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D,
Luo R, Zhong Y, Chen HC and Fang LR: miR-365, a novel negative
regulator of interleukin-6 gene expression, is cooperatively
regulated by Sp1 and NF-kappaB. J Biol Chem. 286:21401–21412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rump K, Brendt P, Frey UH, Schäfer ST,
Siffert W, Peters J and Adamzik M: Aquaporin 1 and 5 expression
evoked by the β2 adrenoreceptor agonist terbutaline and
lipopolysaccharide in mice and in the human monocytic cell line
THP-1 is differentially regulated. Shock. 40:430–436. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rio DC, Ares M Jr, Hannon GJ and Nilsen
TW: Purification of RNA using TRIzol (TRI reagent). Cold Spring
Harb Protoc. 2010:pdb.prot5439. 2010. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock, 2012. Intensive Care
Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heumann D, Glauser MP and Calandra T:
Molecular basis of host-pathogen interaction in septic shock. Curr
Opin Microbiol. 1:49–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J and Li C: Systemic inflammatory
response syndrome, compensatory anti-inflammatory response syndrome
and multiple organ dysfunction syndrome. Chin J Crit Care Med.
1–40. 2003.
|
|
20
|
Mimasaka S, Hashiyada M, Nata M and
Funayama M: Correlation between serum IL-6 levels and death:
Usefulness in diagnosis of ‘traumatic shock’? Tohoku J Exp Med.
193:319–324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeLong WG Jr and Born CT: Cytokines in
patients with polytrauma. Clin Orthop Relat Res. 1–65.
2004.PubMed/NCBI
|
|
22
|
Stover JF, Sakowitz OW, Schoning B,
Rupprecht S, Kroppenstedt SN, Thomale UW, Woiciechowsky C and
Unterberg AW: Norepinephrine infusion increases interleukin-6 in
plasma and cerebrospinal fluid of brain-injured rats. Med Sci
Monit. 9:BR382–BR388. 2003.PubMed/NCBI
|
|
23
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tone M, Powell MJ, Tone Y, Thompson SA and
Waldmann H: IL-10 gene expression is controlled by the
transcription factors Sp1 and Sp3. J Immunol. 165:286–291. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu F, Liu H, Xu L, Li Y, Liu X, Shi L, Su
Y, Qiu X, Zhang X, Yang Y, et al: Hypoxia-inducible factor-1α
perpetuates synovial fibroblast interactions with T cells and B
cells in rheumatoid arthritis. Eur J Immunol. 46:742–751. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley
M and Montaner LJ: Activation and repression of interleukin-12 p40
transcription by erythroid Kruppel-like factor in macrophages. J
Biol Chem. 279:18451–18456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riedemann NC, Neff TA, Guo RF, Bernacki
KD, Laudes IJ, Sarma JV, Lambris JD and Ward PA: Protective effects
of IL-6 blockade in sepsis are linked to reduced C5a receptor
expression. J Immunol. 170:503–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pritts T, Hungness E, Wang Q, Robb B,
Hershko D and Hasselgren PO: Mucosal and enterocyte IL-6 production
during sepsis and endotoxemia-role of transcription factors and
regulation by the stress response. Am J Surg. 183:372–383. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varshney J and Subramanian S: MicroRNAs as
potential target in human bone and soft tissue sarcoma
therapeutics. Front Mol Biosci. 2:312015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohlsson Teague EM, Van der Hoek KH, Van
der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG and
Hull LM: MicroRNA-regulated pathways associated with endometriosis.
Mol Endocrinol. 23:265–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo L, Huang ZX, Chen XW, Deng QK, Yan W,
Zhou MJ, Ou CS and Ding ZH: Differential expression profiles of
microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem
Photobiol. 85:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XY, Wu MH, Liu F, Li Y, Li N, Li GY
and Shen SR: Differential miRNA expression and their target genes
between NGX6-positive and negative colon cancer cells. Mol Cell
Biochem. 345:283–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maes OC, Sarojini H and Wang E: Stepwise
up-regulation of microRNA expression levels from replicating to
reversible and irreversible growth arrest states in WI-38 human
fibroblasts. J Cell Physiol. 221:109–119. 2009. View Article : Google Scholar : PubMed/NCBI
|