Introduction
Clopidogrel is a drug used for restraining the
function of platelets, and is widely used in thrombosis-related
diseases in clinical practice, such as coronary heart disease and
cerebral infarction (1). After
percutaneous coronary intervention (PCI), clopidogrel should be
used routinely to prevent thrombus, and it is recommended as the
first-line drug for acute coronary syndrome by Guidelines for
Treatment of Cardiovascular Diseases in China (2). Previous findings confirmed that
(3), approximately 4–44% patients
applying clopidogrel for a long time have no response to
clopidogrel treatment or cannot achieve the desired clinical
antiplatelet effect, which is clinically known as clopidogrel
resistance.
The incidence rates of clinical cardiovascular
events in patients with clopidogrel resistance are significantly
increased, leading to poor prognosis (4). Patients complicated with atrial
fibrillation after PCI are often treated with antiplatelet therapy
via clopidogrel to prevent stent thrombosis, thereby enhancing the
revascularization effect, and preventing myocardial ischemia,
thromboembolism and other serious complications (5). Although some patients apply clopidogrel
in antiplatelet therapy in clinical practice, myocardial ischemia
and even thromboembolism still occur, suggesting that it may be
related to the cytochrome P450 2C19 (CYP2C19) genotype (6).
To improve the treatment effect of clopidogrel on
patients complicated with atrial fibrillation after PCI, the
prognoses of patients with different CYP2C19 genotype after
applying clopidogrel were investigated in this study.
Materials and methods
General data
Eighty patients who were complicated with atrial
fibrillation and treated with clopidogrel antiplatelet therapy
within one year after PCI in Meizhou Hospital Affiliated to
Zhongshan University (Meizhou, China) from September 2015 to
January 2017 were selected. All the patients were diagnosed via
clinical manifestations and past medical history, and signed the
informed consent before enrollment. This study was approved by the
Ethics Committee of Meizhou Hospital Affiliated to Zhongshan
University. The CYP2C19 genotypes were detected before enrollment.
Subjects were divided into two groups according to the CYP2C19
genotype: extensive metabolism (EM) group (CYP2C19-1) and poor
metabolism (PM) group (CYP2C19-2 and CYP2C19-3). The EM group
comprised 30 males and 10 females aged 40–70 years, with an average
of 65.7±2.1 years. In terms of the New York Heart Association
(NYHA) cardiac function grading at enrollment, there were 32 cases
of grade II and below, and 8 cases of grade III and above. The PM
group was comprised of 29 males and 11 females aged 40–70 years,
with an average of 66.0±2.1 years. In terms of the NYHA cardiac
function grading at enrollment, there were 31 cases of grade II and
below, and 9 cases of grade III and above. There were no
statistically significant differences in the sex, age and NYHA
cardiac function grading at enrollment between the two groups
(P>0.05).
Instruments and reagents and genotype
detection method
DNA extraction kit (Qiagen), CYP2C19 hybridization
developing kit (BaiO, Shanghai, China), GTR22-1 high-speed freezing
centrifuge (Beijing Shidai Beili Co., Ltd., Beijing, China),
BaiOBE2.0 bio-chip reader (BaiO), DYY-6C electrophoresis apparatus
(Beijing Liuyi Instrument Plant, Beijing, China) and experimental
primers (Sangon Biotech, Shanghai, China) were used. CYP2C19
genotypes of all the subjects were hybridized using an e-Hyb
full-automatic hybridization instrument and read using BaiOBE 2.0
biochip reader.
Observation indexes
After operation, all subjects regularly took 75 mg
clopidogrel (national medicine permission no. J20080090, Sanofi
Pharmaceutical Co., Ltd.) orally to maintain the treatment. The
univariate and multivariate analyses were performed for the sex,
age, body mass index, blood platelet count, hemoglobin level, total
cholesterol level, number of lesions at the onset, length of
thrombus at the onset, history of smoking, drinking and
hypertension of patients. The related risk factors and independent
risk factors of clopidogrel resistance were determined, and the
platelet aggregation rate and clopidogrel resistance rate were
compared between the two groups during treatment. Finally, the
non-fatal myocardial infarction and serious life-threatening
complications in both groups were observed.
Evaluation criteria
Determination of platelet aggregation rate: Before
and at 1, 3 and 12 months after application of antiplatelet drugs,
the platelet aggregation rates in patients were determined using
the Annoron measuring equipment (Beijing, China). Adenosine
diphosphate (ADP) was used as the inducer, and all operations were
performed by laboratory physicians with more than 5 years of
experience in strict accordance with the instructions. Clopidogrel
resistance was detected via light turbidimetry using 5 µmol/l ADP
as the inducer. The difference between the actual platelet
aggregation rate and the maximum platelet aggregation rate after
application of clopidogrel of <10% indicated clopidogrel
resistance; the difference between 10 and 29% indicated clopidogrel
semi-resistance; the difference of >30% indicated normal
response to clopidogrel.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 (IBM Corp., Armonk, NY, USA) was used for the statistical
analysis. Measurement data were presented as mean ± standard
deviation (mean ± SD). The t-test was used for the comparison of
means between the two groups, and the Chi-square test was used for
the comparison of rates between the two groups. Univariate analyses
were first performed for the sex, age, body mass index, blood
platelet count, hemoglobin level, total cholesterol level, number
of lesions at the onset, length of thrombus at the onset, the
history of smoking, drinking and hypertension of patients.
Non-conditional multivariate logistic regression analyses were then
performed for items with statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Univariate analyses of clopidogrel
resistance
Univariate analyses showed that the increased total
cholesterol level, higher number of lesions at the onset, larger
length of thrombus at the onset, and the history of smoking and
drinking were the related risk factors of clopidogrel resistance in
patients complicated with atrial fibrillation after PCI (Tables I and II).
 | Table I.Univariate analyses of measurement
data (mean ± SD). |
Table I.
Univariate analyses of measurement
data (mean ± SD).
| Parameters | With atrial
fibrillation | Without atrial
fibrillation | t-test | P-value |
|---|
| Age (years) | 65.7±2.1 | 66.0±2.1 |
0.639 |
0.525 |
| Body mass index
(kg/m2) | 25.6±0.3 | 25.7±0.3 |
1.491 |
0.140 |
| Blood platelet count
(×109/l) | 205.6±12.1 | 206.5±12.0 |
0.334 |
0.739 |
| Hemoglobin level
(g/l) | 138.6±2.5 | 138.7±2.5 |
0.179 |
0.858 |
| Total cholesterol
level (mmom/l) | 4.2±0.2 | 3.7±0.2 | 11.180 | <0.001 |
| Number of lesions at
the onset (pcs) | 2.1±0.2 | 1.5±0.1 | 16.971 | <0.001 |
| Length of thrombus at
the onset (mm) | 25.6±2.3 | 16.5±1.5 | 20.960 | <0.001 |
 | Table II.Univariate analyses of enumeration
data (n). |
Table II.
Univariate analyses of enumeration
data (n).
| Characteristics | With atrial
fibrillation | Without atrial
fibrillation | χ2
test | P-value |
|---|
| Sex |
|
|
0.065 |
0.799 |
| Male | 30 | 29 |
|
|
|
Female | 10 | 11 |
|
|
| Smoking history |
|
| 15.622 | <0.001 |
| Yes | 20 | 3 |
|
|
| No | 20 | 37 |
|
|
| Drinking history |
|
| 18.119 | <0.001 |
| Yes | 20 | 2 |
|
|
| No | 20 | 38 |
|
|
| Hypertension
history |
|
| 2.813 |
0.094 |
| Yes | 11 | 5 |
|
|
| No | 29 | 35 |
|
|
Multivariable logistic regression
analyses of clopidogrel resistance
Multivariable logistic regression analyses with
atrial fibrillation as a dependent variable showed that the
increased total cholesterol level and the history of smoking and
drinking were the independent risk factors of atrial fibrillation
after PCI (Table III).
 | Table III.Multivariable logistic regression
analyses of clopidogrel resistance. |
Table III.
Multivariable logistic regression
analyses of clopidogrel resistance.
| Characteristics | β | SE | W | P-value | Odds ratio (OR) | 95% confidence
interval (CI) |
|---|
| Total cholesterol
level (mmom/l) | 1.873 | 0.834 | 5.050 | 0.025 | 6.503 | 1.272–33.273 |
| Number of lesions at
the onset (pcs) | 0.786 | 0.613 | 1.645 | 0.201 | 2.193 | 0.661–7.729 |
| Length of thrombus at
the onset (mm) | 0.035 | 0.044 | 0.601 | 0.439 | 1.035 | 0.951–1.127 |
| Smoking history | 1.835 | 0.777 | 5.595 | 0.018 | 0.159 | 0.035–0.731 |
| Drinking history | 1.738 | 0.707 | 6.086 | 0.041 | 0.553 | 1.505–36.589 |
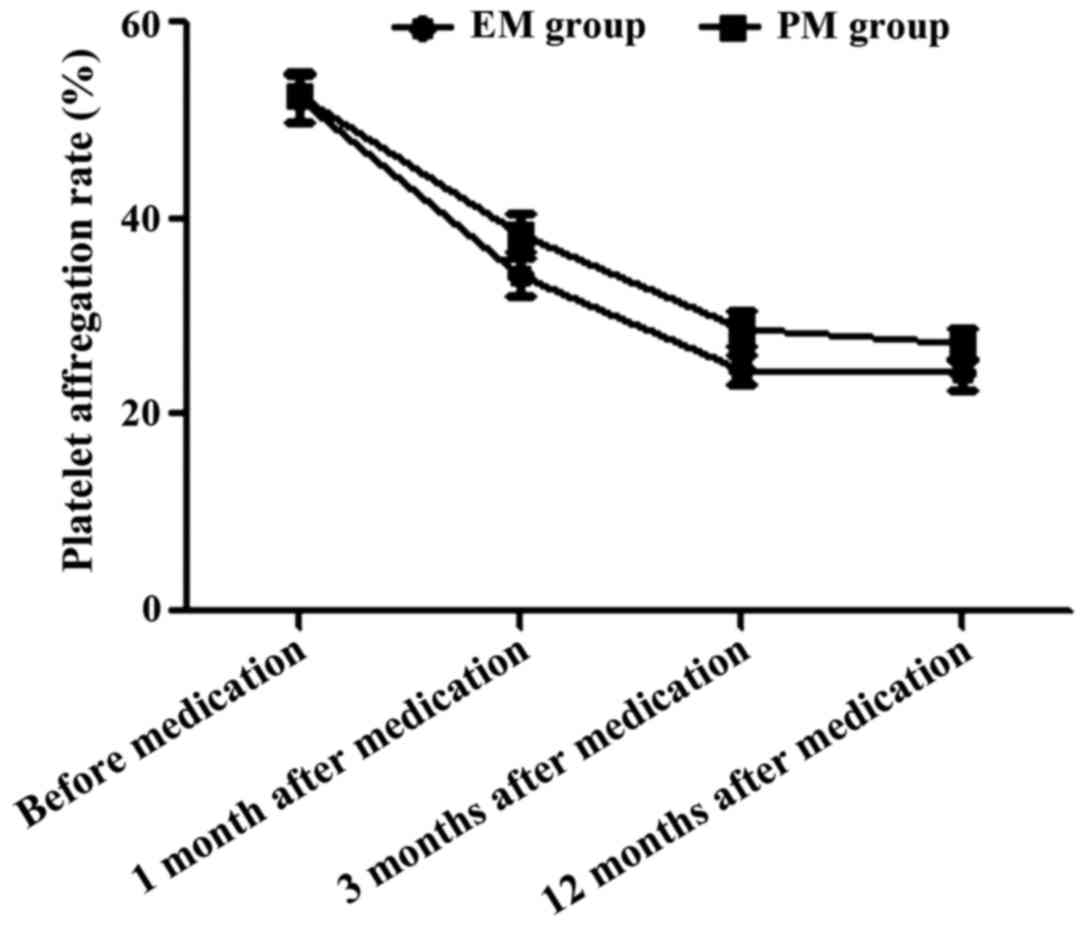
Comparison of platelet aggregation
rate between the two groups during treatment
Before medication and at 1, 3 and 12 months after
medication, the platelet aggregation rates in EM group were
52.3±2.5, 34.2±1.9, 24.5±1.5 and 24.3±1.6%, respectively; the rates
in PM group were 52.4±2.5, 38.6±2.1, 28.9±1.7 and 27.3±1.6%,
respectively. The platelet aggregation rate before application of
antiplatelet drugs had no statistically significant difference
between the two groups (t=0.179, P>0.05); the platelet
aggregation rates in EM group at 1, 3 and 12 months after
medication were significantly lower than those in PM group in the
same period (t=9.826, 12.274 and 8.386, P<0.05) (Fig. 1).
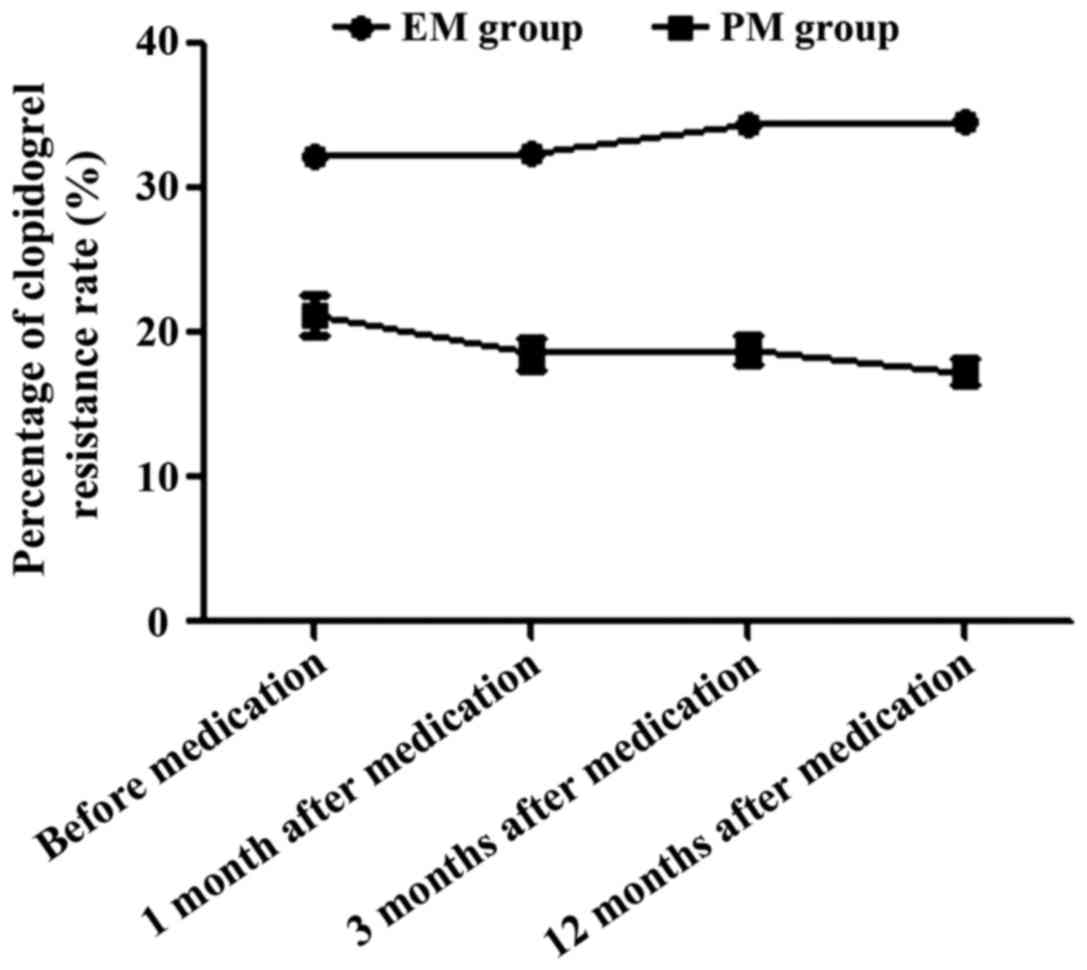
Comparison of clopidogrel resistance
rate between the two groups during treatment
Before medication and at 1, 3 and 12 months after
medication, the clopidogrel resistance rates in EM group were
32.3±0.5, 32.4±0.5, 34.5±0.5 and 34.6±0.6%, respectively; the rates
in PM group were 21.3±1.4, 18.6±1.1, 18.9±1.0 and 17.3±0.9%,
respectively. The clopidogrel resistance rate before application of
antiplatelet drugs had no statistically significant difference
between the two groups (t=46.797, P>0.05); the clopidogrel
resistance rates in EM group before medication and at 1, 3 and 12
months after medication were significantly higher than those in PM
group in the same period (t=72.233, 88.247 and 101.154, P<0.05)
(Fig. 2).
Comparison of non-fatal myocardial
infarction between two groups
The onset time of non-fatal myocardial infarction in
EM group was earlier than that in PM group (P<0.05), the infarct
area was larger than that in PM group (P<0.05), and the left
ventricular ejection fraction (EF) after onset was lower than that
in PM group (P<0.05) (Table
IV).
 | Table IV.Comparison of non-fatal myocardial
infarction between the two groups [n (%)]. |
Table IV.
Comparison of non-fatal myocardial
infarction between the two groups [n (%)].
| Group | Onset time
(month) | Infarct area (%) | EF% after onset
(%) |
|---|
| EM group | 5.9±0.5 | 15.6±1.3 | 40.1±2.1 |
| PM group | 9.1±1.1 | 10.5±1.8 | 45.6±2.5 |
| t-test | 16.750 | 14.527 | 10.654 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of serious life-threatening
complications between the two groups
The total proportion of angina relapse, stent
thrombosis, left heart failure, cardiac death and cerebral
hemorrhage in EM group was obviously lower than that in PM group
(P<0.05) (Table V).
 | Table V.Comparison of serious
life-threatening complications between two groups [n (%)]. |
Table V.
Comparison of serious
life-threatening complications between two groups [n (%)].
| Group | Angina relapse | Stent
thrombosis | Left heart
failure | Cardiac death | Cerebral
hemorrhage | Total incidence
rate |
|---|
| EM group | 8 | 1 | 5 | 1 | 1 | 16 (40.0%) |
| PM group | 1 | 1 | 0 | 0 | 0 | 2 (5.0%) |
| χ2
test |
|
| – |
|
| 12.115 |
| P-value |
|
| – |
|
|
0.001 |
Discussion
Patients complicated with atrial fibrillation after
PCI require continuous anticoagulation and antiplatelet therapy,
among which clopidogrel is the most commonly-used anti-platelet
drug (7). The research suggests that
CYP2C19 gene polymorphism is closely associated with the
pharmacokinetics of clopidogrel, and the risk of relapses of severe
cardiovascular events in patients with clopidogrel resistance is
significantly higher than that in patients with normal
susceptibility (8). It is suggested
that the usage amount of clopidogrel be increased for patients with
clopidogrel resistance (9).
CYP2C19-1, CYP2C19-2 and CYP2C19-3 are three major clopidogrel
genotypes in Chinese population, the first one of which belongs to
the EM type, namely the clopidogrel-sensitive type, and the last
two of which belong to the PM type, namely the high-risk
clopidogrel-resistant type (10).
In this study, the related factors of atrial
fibrillation in subjects with different CYP2C19 phenotypes taking
clopidogrel after PCI were analyzed, and it was found that the
increased total cholesterol level, higher number of lesions at the
onset, larger length of thrombus at the onset, and the history of
smoking and drinking were the related risk factors of atrial
fibrillation after PCI, and the increased total cholesterol level
and the history of smoking and drinking were the independent risk
factors of atrial fibrillation after PCI. In addition, the
comparisons of platelet aggregation rate and clopidogrel resistance
rate between the two groups during treatment showed that the
platelet aggregation rates in EM group at 1, 3 and 12 months after
medication were significantly lower than those in PM group in the
same period, and the clopidogrel resistance rates in EM group
before medication and at 1, 3 and 12 months after medication were
significantly higher than those in PM group in the same period,
suggesting that the platelet aggregation rate in EM group was
affected and the clopidogrel resistance rate was higher. At the
same time, the comparison of non-fatal myocardial infarction
between the two groups revealed that the onset time of non-fatal
myocardial infarction in EM group was earlier than that in PM
group, the infarct area was larger than that in PM group, and the
EF% after onset was lower than that in PM group. Finally, the
comparisons of serious life-threatening complications between the
two groups showed that the total proportion of angina relapse,
stent thrombosis, left heart failure, cardiac death and cerebral
hemorrhage in EM group was obviously lower than that in PM group,
indicating that the risk of fatal complications in EM group was
obviously lower than that in PM group.
Clopidogrel metabolizes mainly through the
cytochrome P450 enzyme system in liver (11), producing active metabolites, thus
inhibiting the platelet aggregation and achieving an anti-platelet
effect (12). Previous findigs have
confirmed that (13) CYP2C19 gene
polymorphism is a major factor affecting the anti-platelet effect
of clopidogrel. CYP2C19-2 and CYP2C19-3 are PM subtypes of CYP2C19
(14), which are mainly expressed in
Asian populations (15) with higher
mutation rates (16); thus, the
incidence rate of severe cardiovascular complications is increased
significantly in PM subjects (17).
The long-term application of clopidogrel in patients complicated
with atrial fibrillation after PCI should be paid attention to
(18); the anti-platelet therapy
regimen should be adjusted in time (19) to avoid stent thrombosis-induced
complications (20).
In conclusion, the increased total cholesterol level
and the history of smoking and drinking are the independent risk
factors of clopidogrel resistance in patients complicated with
atrial fibrillation after PCI. The incidence rates of fatal and
non-fatal cardiac complications are increased significantly in
patients with PM CYP2C19 genotype, and attention is required to
this in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and ZZ collected the general information of
patients. BL, ZL and PZ were responsible for observation index
analysis. ZY and XH contributed to the conception and design of the
study. HW, WC and JH analysed the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The patients were diagnosed via clinical
manifestations and past medical history, and signed the informed
consent before enrollment. This study was approved by the Ethics
Committee of Meizhou Hospital Affiliated to Zhongshan University
(Meizhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Danielak D, Karaźniewicz-Łada M, Komosa A,
Burchardt P, Lesiak M, Kruszyna Ł, Graczyk-Szuster A and Główka F:
Influence of genetic co-factors on the population pharmacokinetic
model for clopidogrel and its active thiol metabolite. Eur J Clin
Pharmacol. 73:1623–1632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guirgis M, Thompson P and Jansen S: Review
of aspirin and clopidogrel resistance in peripheral arterial
disease. J Vasc Surg. 66:1576–1586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kagami T, Yamade M, Suzuki T, Uotani T,
Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, Umemura K, Miyajima H,
et al: Comparative study of effects of vonoprazan and esomeprazole
on anti-platelet function of clopidogrel or prasugrel in relation
to CYP2C19 genotype. Clin Pharmacol Ther. Sep 5–2017.(Epub ahead of
print). PubMed/NCBI
|
|
4
|
Larson EA and Miller NJ:
Point-Counterpoint: CYP2C19 Genotyping for Clopidogrel. S D Med.
70:13–15. 2017.PubMed/NCBI
|
|
5
|
Mirabbasi SA, Khalighi K, Wu Y, Walker S,
Khalighi B, Fan W, Kodali A and Cheng G: CYP2C19 genetic variation
and individualized clopidogrel prescription in a cardiology clinic.
J Community Hosp Intern Med Perspect. 7:151–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amin AM, Sheau Chin L, Mohamed Noor DA,
Mostafa H, Abdul Kader MASK, Kah Hay Y and Ibrahim B: The effect of
CYP2C19 genetic polymorphism and non-genetic factors on clopidogrel
platelets inhibition in East Asian coronary artery disease
patients. Thromb Res. 158:22–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berinstein E and Levy A: Recent
developments and future directions for the use of pharmacogenomics
in cardiovascular disease treatments. Expert Opin Drug Metab
Toxicol. 13:973–983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao
Q, Wang H and Qi P: Effects of dual-dose clopidogrel, clopidogrel
combined with tongxinluo capsule, and ticagrelor on patients with
coronary heart disease and CYP2C19*2 gene mutation after
percutaneous coronary interventions (PCI). Med Sci Monit.
23:3824–3830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Zhang J, Sun H, Ming T, Liu X,
Cong Y, Li F and Li Z: Association between platelet function and
recurrent ischemic vascular events after TIA and minor stroke. Int
J Clin Pharmacol Ther. 55:789–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erathi HV, Durgaprasad R, Velam V, Sarma
PV, Rodda M, Kapil C and Kanavath SN: Evaluation of On-Clopidogrel
platelet reactivity overtime, SYNTAX SCORE, genetic polymorphisms
and their relationship to one year clinical outcomes in STEMI
patients undergoing PCI. Minerva Cardioangiol. 66:16–25.
2018.PubMed/NCBI
|
|
11
|
Borse MS, Dong OM, Polasek MJ, Farley JF,
Stouffer GA and Lee CR: CYP2C19-guided antiplatelet therapy: A
cost-effectiveness analysis of 30-day and 1-year outcomes following
percutaneous coronary intervention. Pharmacogenomics. 18:1155–1166.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan SSN, Fong AYY, Mejin M, Gerunsin J,
Kong KL, Chin FYY, Tiong LL, Lim MSH, Asri S, Khiew NZ, et al:
Association of CYP2C19*2 polymorphism with clopidogrel response and
1-year major adverse cardiovascular events in a multiethnic
population with drug-eluting stents. Pharmacogenomics.
18:1225–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palacharla RC, Nirogi R, Uthukam V,
Manoharan A, Ponnamaneni RK and Kalaikadhiban I: Quantitative in
vitro phenotyping and prediction of drug interaction potential of
CYP2B6 substrates as victims. Xenobiotica. 28:1–13. 2017.
|
|
14
|
Peng L, Liu J, Qin L, Liu J, Xi S, Lu C
and Yin T: Interaction between platelet-derived microRNAs and
CYP2C19*2 genotype on clopidogrel antiplatelet responsiveness in
patients with ACS. Thromb Res. 157:97–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cavallari LH: Personalizing antiplatelet
prescribing using genetics for patients undergoing percutaneous
coronary intervention. Expert Rev Cardiovasc Ther. 15:581–589.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosafio F, Lelli N, Mimmi S, Vandelli L,
Bigliardi G, Dell'Acqua ML, Picchetto L, Pentore R, Ferraro D,
Trenti T, et al: Platelet function testing in patients with acute
ischemic stroke: An observational study. J Stroke Cerebrovasc Dis.
26:1864–1873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wang Y and Wang H: Distribution of
CYP2C19 polymorphisms in Mongolian and Han nationals and the choice
of specific antiplatelet drugs. Int J Clin Pharm. 39:791–797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tatarunas V, Kupstyte N, Zaliunas R,
Giedraitiene A and Lesauskaite V: The impact of clinical and
genetic factors on ticagrelor and clopidogrel antiplatelet therapy.
Pharmacogenomics. 18:969–979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YJ, Kim N, Jang IJ, Cho JY, Nam RH,
Park JH, Jo HJ, Yoon H, Shin CM, Park YS, et al: Pantoprazole does
not reduce the antiplatelet effect of clopidogrel: A randomized
controlled trial in Korea. Gut Liver. 11:504–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cavallari LH, Weitzel KW, Elsey AR, Liu X,
Mosley SA, Smith DM, Staley BJ, Winterstein AG, Mathews CA, Franchi
F, et al: Institutional profile: University of Florida Health
Personalized Medicine Program. Pharmacogenomics. 18:421–426. 2017.
View Article : Google Scholar : PubMed/NCBI
|