Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of liver cancer. It is the fourth most commonly
occurring cancer and the third most common cause of
cancer-associated cases of mortality in China (1). HCC is usually caused by the hepatitis C
virus, the hepatitis B virus, alcohol abuse and other causes of
cirrhosis (2). The main treatment
for patients with hepatocellular carcinoma include surgical
resection, liver transplantation, interventional therapy,
radiotherapy, chemotherapy and immunotherapy (3). Another type of liver cancer is
hepatoblastoma, which is specifically formed by immature liver
cells (4). Hepatoblastoma is the
most common liver cancer of early childhood (5). The treatment of hepatoblastoma is
mainly based on platinum-based chemotherapy combined with complete
surgical resection of the masses (6). Approximately 15% of the cases require
liver transplantation (7). The main
treatments for HCC and hepatoblastoma include resection,
transplantation, radiofrequency ablation, chemoembolization and
sorafenib (8,9). Unfortunately, current treatment
approaches for HCC and hepatoblastoma are far from satisfactory as
the cancers continue to have a high recurrence and metastasis rate
(10,11), and there is an urgent need for novel
therapeutic strategies and targets.
Resolvins are active substances with specific lipid
structures (12). They are derived
from ω-3 unsaturated fatty acids, primarily eicosapentaenoic acid
and docosahexaenoic acid (DHA) (13). Functionally, resolvins promote the
resolution of the inflammatory response back to a non-inflamed
state (12,14). Recent studies using various different
models have demonstrated that resolvin D1 (RvD1) may exert a
liver-protective effect (15–17).
Furthermore, evidence indicates that RvD1 is able to regulate
Toll-like receptor 4-mediated inflammatory responses (18,19) in
numerous diseases, including HCC. The present study aimed to
determine whether RvD1 could inhibit the proliferation of
lipopolysaccharide (LPS)-treated liver cancer cells, and to
elucidate the possible underlying mechanism of its effect. To the
best of our knowledge, no other reports on this topic have been
published to date. If the hypothesis is verified, RvD1 may have
potential for clinical use in liver cancer therapy.
Mitogen-activated protein kinase (MAPK) is a
serine/threonine-specific protein kinase that is stimulated by
extracellular molecules, including cytokines, neurotransmitters,
hormones and tumor-promoting substances. There are three types of
MAPK signal transduction pathway in mammalian organisms: The
extracellular signal-regulated kinase (ERK) signaling pathway
regulates cell growth and differentiation (20); while the c-Jun N-terminal kinase
(JNK) (21) and p38 MAPK (22,23)
signaling pathways serve key roles in inflammation and apoptosis in
response to stress. MAPK pathways are typically activated following
an inflammatory response; however, resolvins, particularly RvD1,
are able to inhibit this signaling transduction (24). Therefore, it was speculated that RvD1
may inhibit cell proliferation and the inflammatory response in
LPS-treated liver cancer cells by targeting MAPK. In the present
study, the effect of RvD1 on inflammatory cytokine production and
MAPK signaling was evaluated in LPS-treated liver cancer cells.
Materials and methods
Cell culture and treatment
The liver cancer cell lines HepG2 (hepatoblastoma)
and PLC/PRF/5 (hepatocellular carcinoma) were purchased from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The liver cancer cells were cultured in minimum
essential medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml
penicillin and 100 µg/ml streptomycin), and incubated in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cells were untreated or treated with 100 ng/ml LPS (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 24 h (25) at 37°C with or without various
concentrations (0, 0.025, 0.05, 0.1 and 0.2%) of RvD1 (Cayman
Chemical Company, Ann Arbor, MI, USA) administered as a
pretreatment for 30 min (26) at
37°C. RvD1 was prepared in PBS before use. Untreated cells were
used as controls.
ELISA assay
Cells were collected and centrifuged at 500 × g for
5 min at room temperature. The protein levels of inflammatory
cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β and
IL-6] in the culture media of the liver cancer cells were detected
with corresponding Human ELISA kits (cat. nos. PT518, PI305 and
PI330; Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocols.
MTT assay
Cell proliferation were determined by MTT assay at
12, 24, 48 and 72 h. Following the various treatments, cells
(5×104 cells/well) in 96-well plates were incubated with
MTT solution (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) for 4 h at
37°C. The resultant formazan crystals were dissolved in dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA), and the absorbance in each
well was measured with a microplate reader (Molecular Devices LLC,
Sunnyvale, CA, USA) at a wavelength of 570 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the cells in
different groups. cDNA was synthesized using RNA PCR kit (AMV;
version 3.0; Takara Biotechnology Co., Ltd., Dalian, China)
following the manufacturer's protocols. For cDNA synthesis, samples
were incubated at 42°C for 60 min, 70°C for 5 min and 4°C for 60
min. qPCR was performed using an Applied Biosystems
StepOne™ Real-Time PCR system (Thermo Fisher Scientific,
Inc.) with the SYBR Premix Ex Taq kit (Takara Biotechnology Co.,
Ltd.). The PCR conditions were as follows: 95°C for 10 min,
followed by 40 cycles of initiation at 95°C for 30 sec, annealing
at 55°C for 30 sec and elongation at 72°C for 30 sec, and then 4°C
for 60 min. The expression levels of TNF-α, IL-1β and IL-6 mRNA
were defined based on the threshold cycle (Cq), and the relative
expression levels were calculated as 2−ΔΔCq (27) following normalization by GAPDH. The
sequences of the PCR primers were as follows: GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; TNF-α forward,
5′-GAGGCCAAGCCCTGGTATG-3′ and reverse, 5′-CGGGCCGATTGATCTCAGC-3′;
IL-1β forward, 5′-AGCTACGAATCTCCGACCAC-3′ and reverse,
5′-CGTTATCCCATGTGTCGAAGAA-3′; IL-6 forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′.
Western blot analysis
Cells (1×106) were washed with cold PBS
and lysed in radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). The concentration of protein was
measured by NanoDrop™ 2000 (Thermo Fisher Scientific,
Inc.). Equal amounts of protein (40 µg/lane) were separated by 10%
SDS-PAGE and electrotransferred to nitrocellulose membranes. The
membranes were then blocked in PBS containing 0.1% Tween-20 and 5%
(w/v) non-fat dried milk at room temperature for 30 min, and
incubated overnight at 4°C with primary antibodies against TNF-α,
IL-1β, IL-6, p-ERK, p-JNK and p-p38 (cat. no. 3707, cat. no. 12703,
cat. no. 12153, cat. no. 4370, cat. no. 9251 and cat. no. 9211,
respectively; dilution, 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA). The blots were incubated with HRP-conjugated
secondary antibodies (cat. no. A0208; dilution, 1:1,000; Beyotime
Institute of Biotechnology) at room temperature for 2 h, then bands
were detected by BeyoECL Plus kit (Beyotime Institute of
Biotechnology). GAPDH (cat. no. 5174; dilution, 1:2,000; Cell
Signaling Technology, Inc.) was used as an internal control. The
density of the western blot bands was quantified with ImageJ 1.43
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analyses were conducted using SPSS 22.0
software (IBM Corp., Armonk, NY, USA). Data were analyzed by
one-way analysis of variance followed by Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RvD1 inhibits the upregulation of
TNF-α, IL-1β and IL-6 protein levels in the culture medium of
LPS-treated liver cancer cells
ELISA was performed to identify the protein levels
of inflammatory cytokines in the supernatant of treated cells. As
shown in Fig. 1, compared with the
control group, 100 ng/ml LPS significantly increased the protein
levels of TNF-α, IL-1β and IL-6 in the culture medium.
Pre-treatment of the cells with RvD1 (0.05, 0.1 and 0.2%)
significantly repressed the LPS-induced increases in TNF-α, IL-1β
and IL-6 levels. This effect appeared to be in a
concentration-dependent manner.
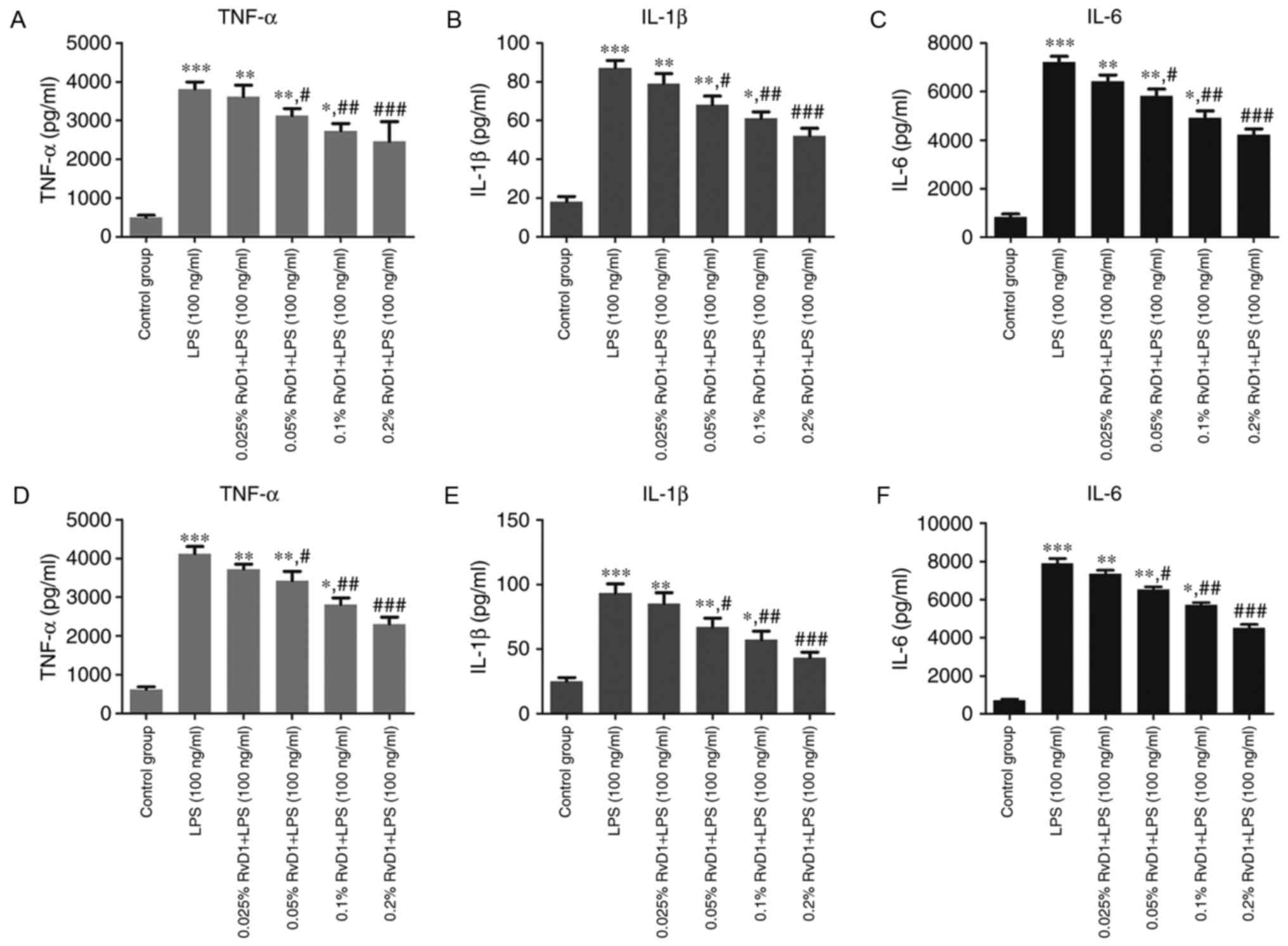 | Figure 1.Effect of RvD1 on TNF-α, IL-1β and
IL-6 protein levels in the culture medium of liver cancer cells
treated with LPS. ELISA was performed to measure the protein levels
of TNF-α, IL-1β and IL-6 in (A-C) HepG2 and (D-F) PLC/PRF/5 cells.
*P<0.05, **P<0.01, ***P<0.001 vs. control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. RvD1, resolvin D1; LPS,
lipopolysaccharide; TNF, tumor necrosis factor; IL,
interleukin. |
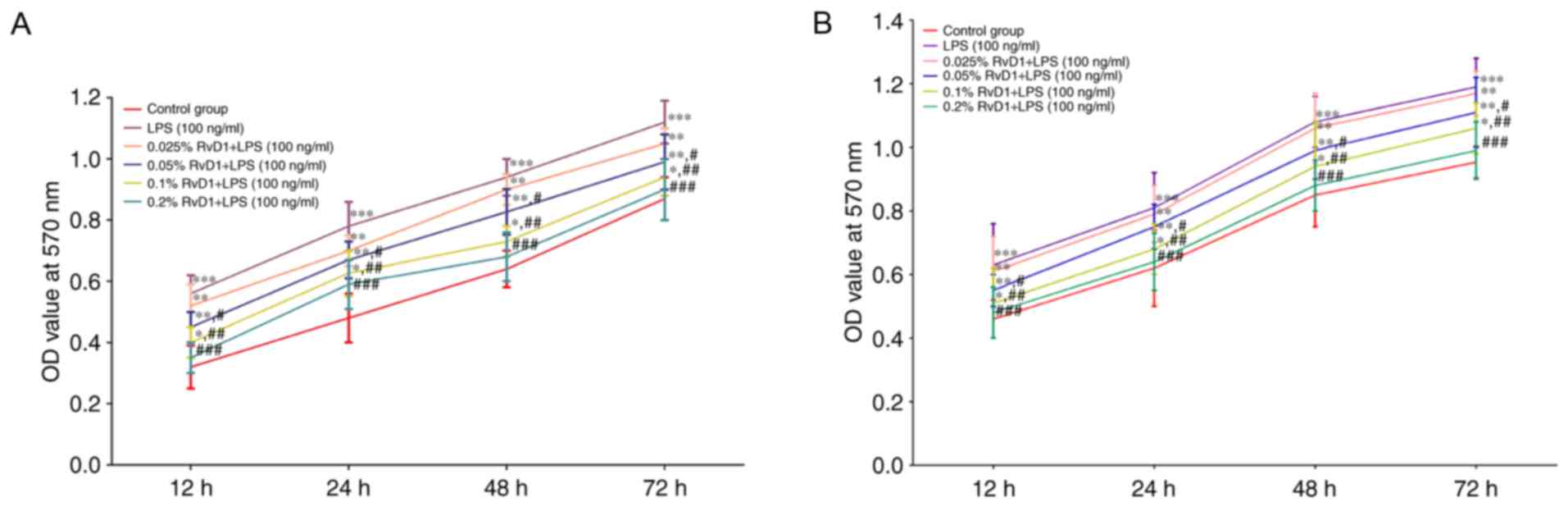
RvD1 reduces the proliferation of
LPS-treated liver cancer cells
To clarify the role of RvD1 in liver cancer, cells
were treated with LPS and an MTT assay was performed to determine
the effects of RvD1 on cell proliferation. Compared with the
control group, 100 ng/ml LPS significantly promoted cell viability
and proliferation (Fig. 2). Compared
with the 100 ng/ml LPS group, RvD1 pre-treatment (0.05, 0.1 and
0.2%) significantly reduced cell proliferation. This effect
appeared to be in a concentration-dependent manner.
RvD1 inhibits the mRNA and protein
expression of TNF-α, IL-1β and IL-6 in LPS-treated liver cancer
cells
RT-qPCR and western blot analyses were performed to
evaluate the effects of RvD1 on the levels of inflammatory factors
in liver cancer cells. The results indicated that 100 ng/ml LPS
significantly upregulated the mRNA and protein expression levels of
the inflammatory cytokines TNF-α, IL-1β and IL-6 compared with the
control group (Figs. 3–5). The addition of RvD1 (0.05, 0.1 and
0.2%) significantly attenuated the LPS-induced upregulation. This
effect appeared to be in a concentration-dependent manner.
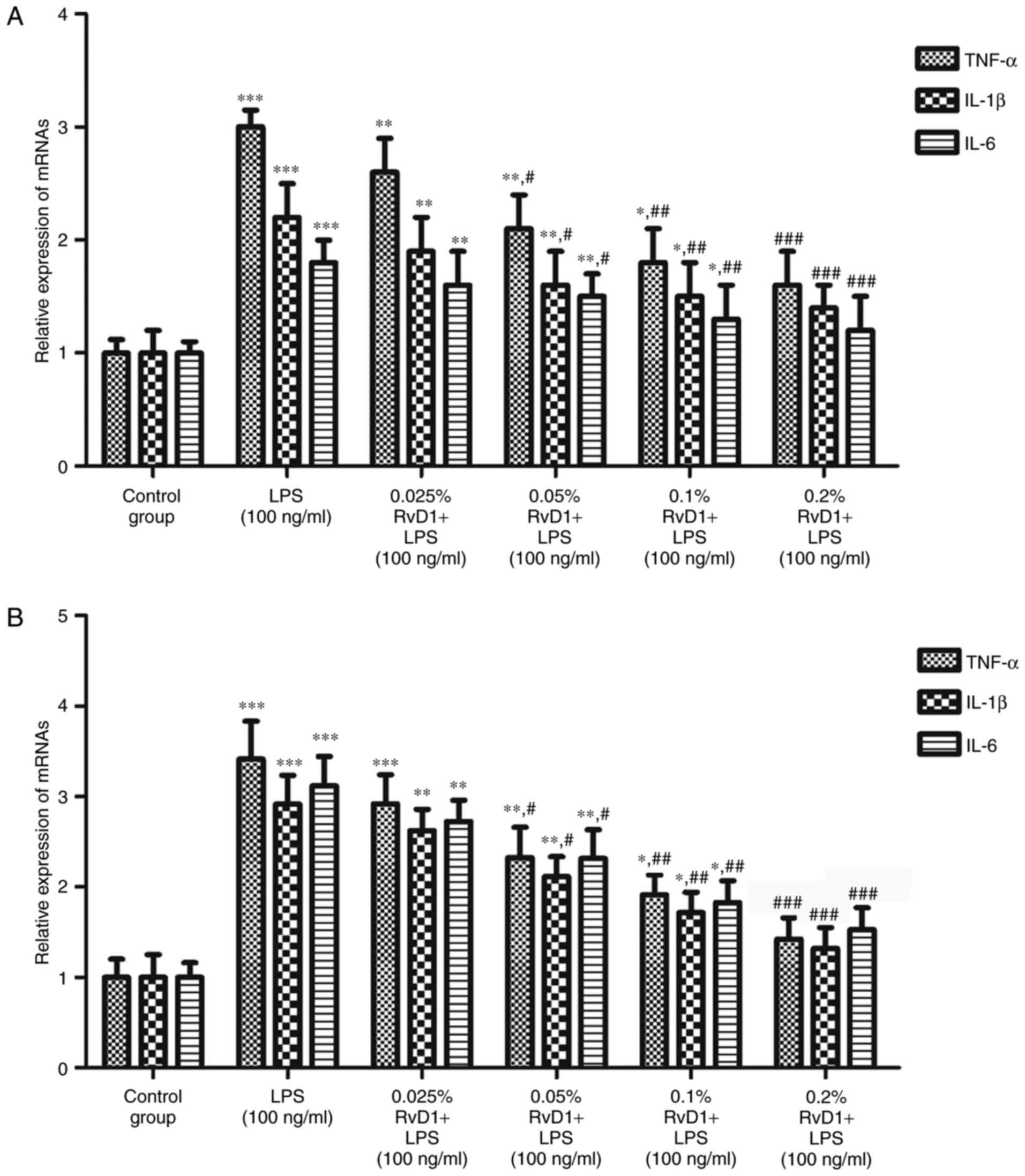 | Figure 3.Effect of RvD1 on the expression of
TNF-α, IL-1β and IL-6 mRNA in LPS-treated liver cancer cells. The
expression levels of TNF-α, IL-1β and IL-6 mRNA in (A) HepG2 and
(B) PLC/PRF/5 cells were determined by reverse
transcription-quantitative polymerase chain reaction. *P<0.05,
**P<0.01, ***P<0.001 vs. control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. RvD1, resolvin D1; LPS,
lipopolysaccharide; TNF, tumor necrosis factor; IL,
interleukin. |
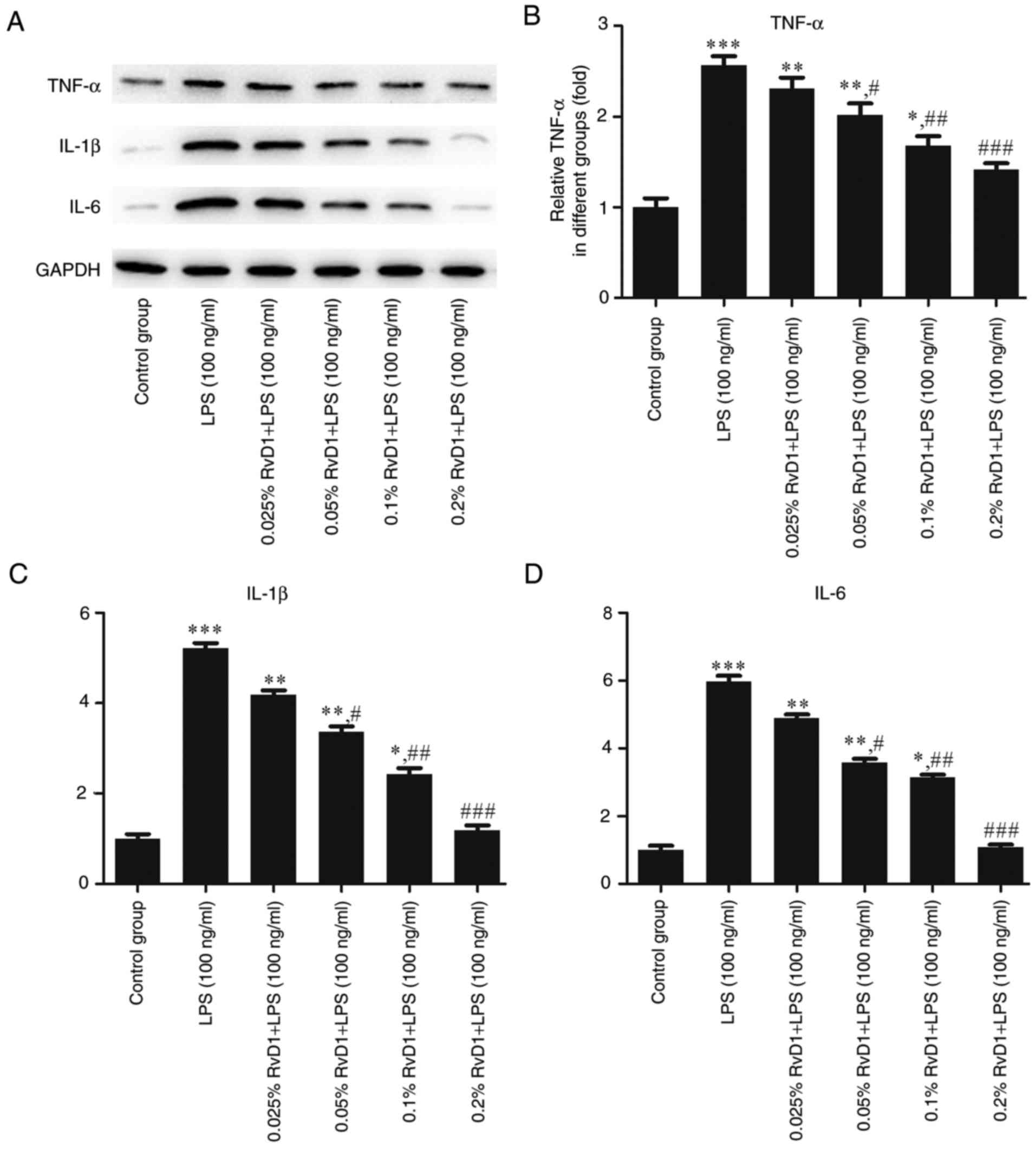 | Figure 5.Effects of RvD1 on the expression of
TNF-α, IL-1β and IL-6 protein in LPS-treated PLC/PRF/5
hepatocellular carcinoma cells. (A) Western blot bands representing
TNF-α, IL-1β and IL-6 protein. Relative protein levels of (B)
TNF-α, (C) IL-1β and (D) IL-6 were calculated. *P<0.05,
**P<0.01, ***P<0.001 vs. control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. RvD1, resolvin D1; LPS,
lipopolysaccharide; TNF, tumor necrosis factor; IL,
interleukin. |
RvD1 inhibits the expression of p-ERK,
p-JNK and p-p38 protein in LPS-treated liver cancer cells
To verify whether RvD1 plays a role in the MAPK
signaling pathway, western blotting was performed to measure the
expression levels of p-ERK, p-JNK and p-p38 proteins in liver
cancer cells. As indicated in Figs.
6 and 7, 100 ng/ml LPS
significantly enhanced the expression of p-ERK, p-JNK and p-p38
protein compared with the control group. The addition of RvD1
(0.05, 0.1 and 0.2%) significantly attenuated the LPS-induced
upregulation. This effect appeared to be in a
concentration-dependent manner.
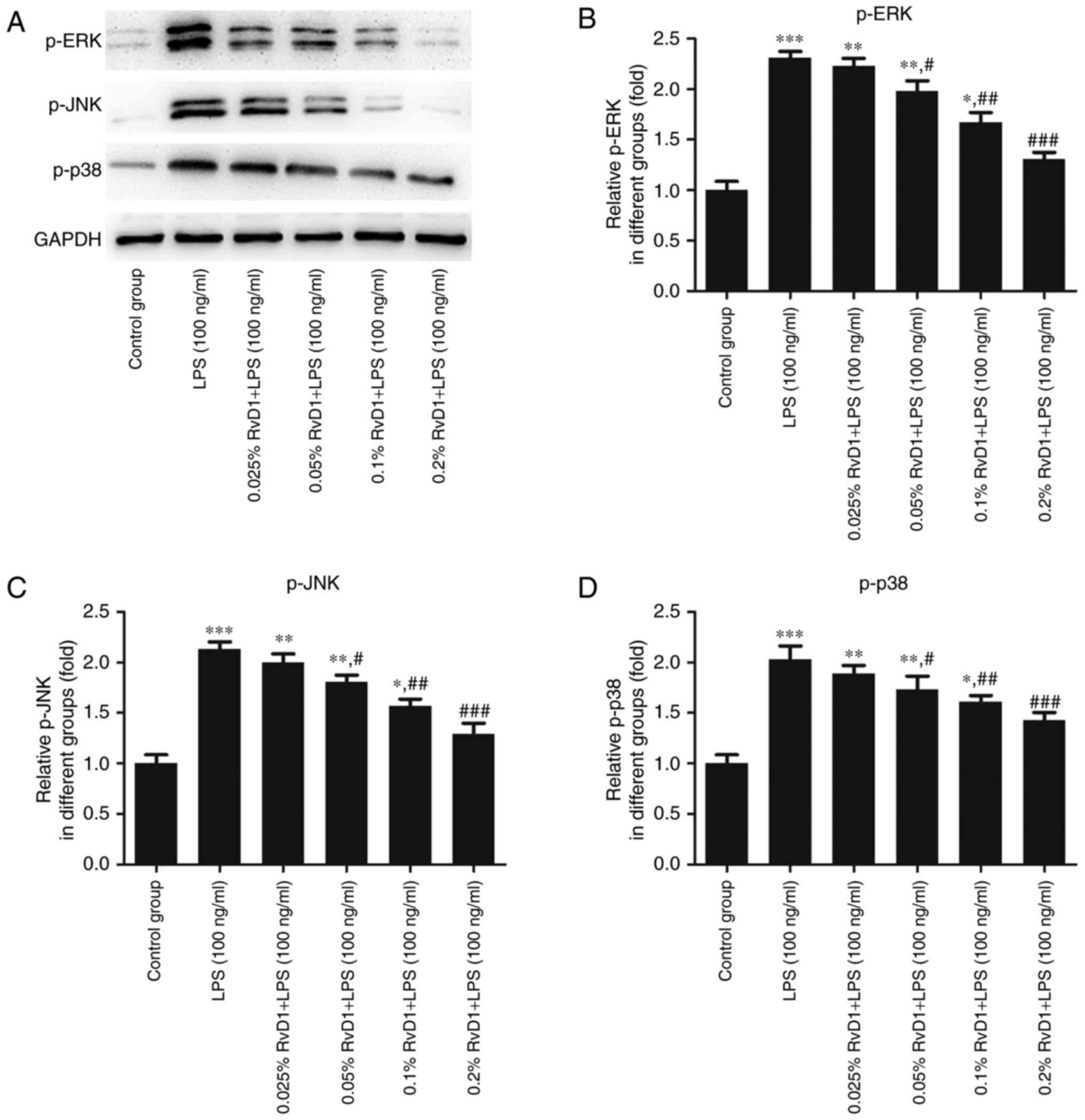 | Figure 6.Effects of RvD1 on the expression of
p-ERK, p-JNK and p-p38 protein in LPS-treated HepG2 hepatoblastoma
cells. (A) Western blot bands representing p-ERK, p-JNK and p-p38
protein. Relative protein levels of (B) p-ERK, (C) p-JNK and (D)
p-p38 were calculated. *P<0.05, **P<0.01, ***P<0.001 vs.
control group. #P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. RvD1, resolvin D1; LPS,
lipopolysaccharide; p-, phosphorylated; ERK, extracellular
signal-related kinase; JNK, c-Jun N-terminal kinase. |
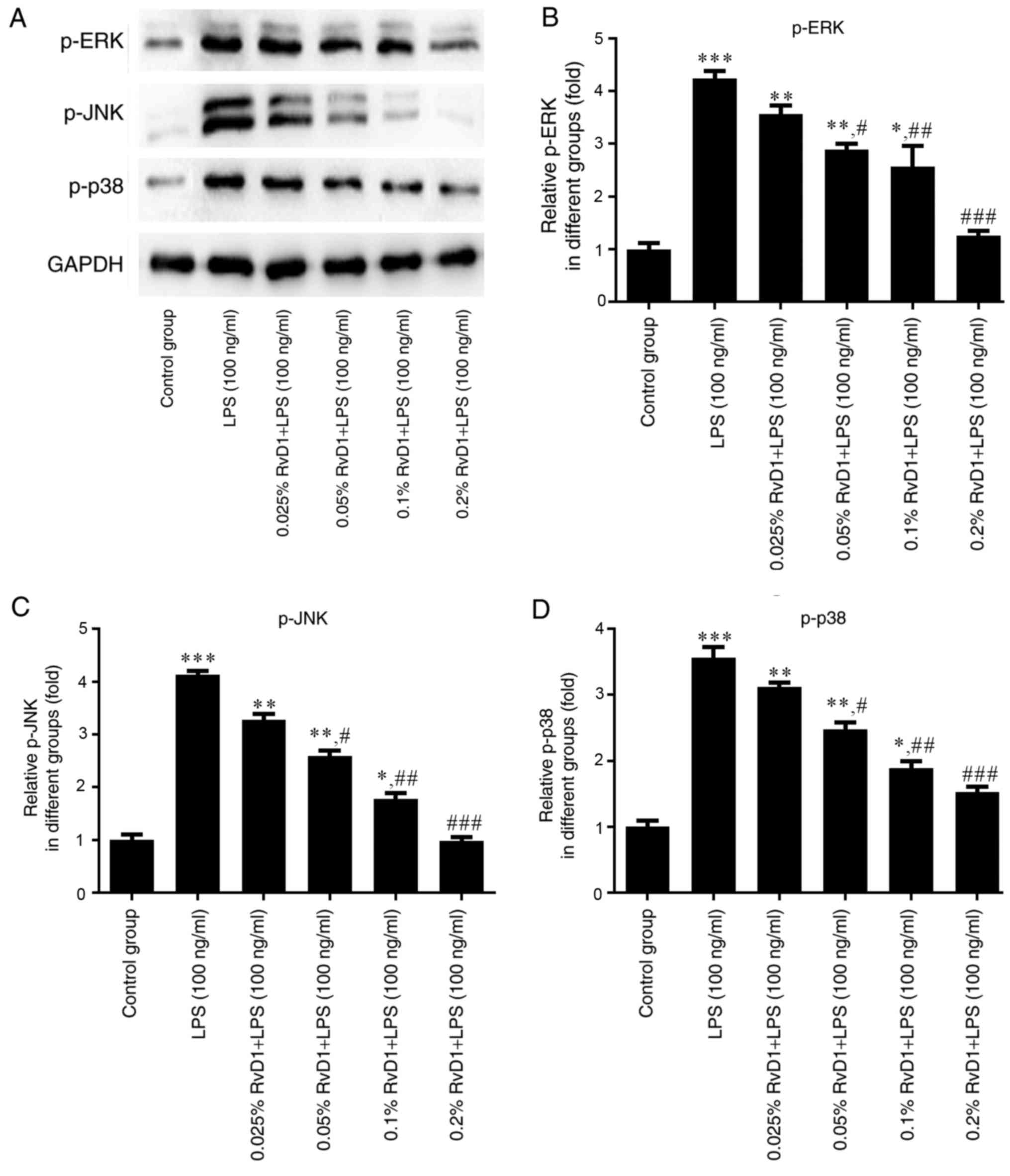 | Figure 7.Effects of RvD1 on the expression of
p-ERK, p-JNK and p-p38 protein in LPS-treated PLC/PRF/5
hepatocellular carcinoma cells. (A) Western blot bands representing
p-ERK, p-JNK and p-p38 protein. Relative protein levels of (B)
p-ERK, (C) p-JNK and (D) p-p38 were calculated. *P<0.05,
**P<0.01, ***P<0.001 vs. control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. RvD1, resolvin D1; LPS,
lipopolysaccharide; p-, phosphorylated; ERK, extracellular
signal-related kinase; JNK, c-Jun N-terminal kinase. |
Discussion
RvD1 is a type of resolvin that is derived from ω-3
polyunsaturated fatty acids, including DHA. It has been
demonstrated to serve a key function in acute inflammation in
certain animal disease models (28).
In the present study, an MTT assay indicated that LPS (100 ng/ml)
significantly increased the proliferation of liver cancer cells,
while RvD1 inhibited this increase, suggesting that RvD1 may
inhibit the proliferation of LPS-treated liver cancer cells.
Therefore, RvD1 may be useful in therapies against liver
cancer.
Recent studies have demonstrated that inflammation
is associated with the development and progression of multiple
cancer types (29,30). Tumor-associated inflammation can
promote angiogenesis and metastasis, and a persistent inflammatory
microenvironment can trigger tumorigenesis by inducing certain
genetic mutations (31).
TNF-α, IL-1β, IL-6 are pro-inflammatory cytokines,
which may serve key functions in the pathological mechanisms of
cachexia in cancer (32). These
cytokines are involved in cell proliferation, differentiation and
apoptosis (33). TNF-α has been
implicated in a number of cancer cachexia-associated processes,
including anorexia and body weight loss, metabolic alterations and
systemic inflammation (34). IL-1β
and IL-6 are members of the interleukin family of cytokines and are
activated through proteolytic processing. They play critical roles
in many chronic diseases, including atherosclerosis, diabetes and
various cancer types (35,36).
In the present study, the effect of RvD1 on the
aforementioned inflammatory cytokines was evaluated by ELISA,
RT-qPCR and western blotting. The results identified that RvD1
treatment markedly decreased the mRNA and protein levels of these
inflammatory cytokines in LPS-treated liver cancer cells, and
restrained their LPS-induced release. Collectively, these results
demonstrate that RvD1 treatment can significantly suppress the
LPS-induced expression and release of TNF-α, IL-1β and IL-6, as
representative inflammatory cytokines, in liver cancer cells.
MAPK signaling, including the ERK, JNK and p38
pathways, is responsible for transducing extracellular signals to
influence numerous cellular processes, including cell
proliferation, survival and death (37). In order to investigate whether RvD1
exerted its effects via MAPK, western blotting experiments were
performed. RvD1 significantly suppressed the LPS-induced increases
in p-ERK, p-JNK and p-p38 in liver cancer cells.
In summary, the present study demonstrated that RvD1
inhibits the proliferation of LPS-treated liver cancer cells, and
reduces the expression of TNF-α, IL-1β and IL-6 at the protein and
mRNA levels, as well as their release, in LPS-treated liver cancer
cells. In addition, RvD1 decreases p-ERK, p-JNK and p-p38 levels in
LPS-treated liver cancer cells. Therefore, RvD1 may suppress liver
cancer cell proliferation by targeting MAPK signaling. However,
further research is required to confirm this hypothesis. In
conclusion, RvD1 may be a novel and effective anti-inflammatory
mediator, and could form the basis of a novel therapy for
hepatoblastoma or hepatocellular carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YL was a major contributor in writing the manuscript
and interpreting the data. QX analyzed the data and revised the
manuscript. GY collected the data. WX helped analyse the data. HJ
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desai JR, Ochoa S, Prins PA and He AR:
Systemic therapy for advanced hepatocellular carcinoma: An update.
J Gastrointest Oncol. 8:243–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pascual S, Herrera I and Irurzun J: New
advances in hepatocellular carcinoma. World J Hepatol. 8:421–438.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed I and Lobo DN: Malignant tumours of
the liver. Surgery (Oxford). 25:34–41. 2007. View Article : Google Scholar
|
|
5
|
Hiyama E: Pediatric hepatoblastoma:
Diagnosis and treatment. Transl Pediatr. 3:293–299. 2014.PubMed/NCBI
|
|
6
|
Trobaugh-Lotrario AD and Katzenstein HM:
Chemotherapeutic approaches for newly diagnosed hepatoblastoma:
Past, present, and future strategies. Pediatr Blood Cancer.
59:809–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khaderi S, Guiteau J, Cotton RT, O'Mahony
C, Rana A and Goss JA: Role of liver transplantation in the
management of hepatoblastoma in the pediatric population. World J
Transplant. 4:294–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villanueva A, Hernandez-Gea V and Llovet
JM: Medical therapies for hepatocellular carcinoma: A critical view
of the evidence. Nat Rev Gastroenterol Hepatol. 10:34–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trobaugh-Lotrario AD, Meyers RL, O'Neill
AF and Feusner JH: Unresectable hepatoblastoma: Current
perspectives. Hepat Med. 9:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bober J and Samek P: Surgery of the
tumours of the liver. Bratisl Lek Listy. 103:403–407.
2002.PubMed/NCBI
|
|
11
|
Horiike N, Iuchi H, Ninomiya T, Kawai K,
Kumagi T, Michitaka K, Masumoto T and Onji M: Influencing factors
for recurrence of hepatocellular carcinoma treated with
radiofrequency ablation. Oncol Rep. 9:1059–1062. 2002.PubMed/NCBI
|
|
12
|
Shinohara M, Mirakaj V and Serhan CN:
Functional metabolomics reveals novel active products in the DHA
metabolome. Front Immunol. 3:812012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duvall MG and Levy BD: DHA- and
EPA-derived resolvins, protectins, and maresins in airway
inflammation. Eur J Pharmacol. 785:144–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serhan CN, Chiang N, Dalli J and Levy BD:
Lipid mediators in the resolution of inflammation. Cold Spring Harb
Perspect Biol. 7:a0163112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Gong X, Jiang R, Wang B, Kuang G,
Li K and Wan J: Resolvin D1 attenuates CCl4-induced acute liver
injury involving up-regulation of HO-1 in mice. Immunopharmacol
Immunotoxicol. 38:61–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuang H, Hua X, Zhou J and Yang R:
Resolvin D1 and E1 alleviate the progress of hepatitis toward liver
cancer in long-term concanavalin A-induced mice through inhibition
of NF-κB activity. Oncol Rep. 35:307–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang J and Lee S: Resolvin D1 protects the
liver from ischemia/reperfusion injury by enhancing M2 macrophage
polarization and efferocytosis. Biochim Biophys Acta.
1861:1025–1035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palmer CD, Mancuso CJ, Weiss JP, Serhan
CN, Guinan EC and Levy O: 17(R)-Resolvin D1 differentially
regulates TLR4-mediated responses of primary human macrophages to
purified LPS and live E. coli. J Leukoc Biol. 90:459–470. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Q, Wu J, Lin Z, Hua Q, Zhang W, Ye L,
Wu G, Du J, Xia J, Chu M and Hu X: Resolvin D1 alleviates the lung
ischemia reperfusion injury via complement, immunoglobulin, TLR4
and inflammatory factors in rats. Inflammation. 39:1319–1333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang S and Liu G: Targeting the
Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett.
13:1041–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao J, Yang J, Zhang Y, Li T, Wang C, Xu
L, Hu Q, Wang X, Jiang S, Nie X and Chen G: Arsenic trioxide
mediates HAPI microglia inflammatory response and subsequent neuron
apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol Appl
Pharmacol. 303:79–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baig MS, Liu D, Muthu K, Roy A, Saqib U,
Naim A, Faisal SM, Srivastava M and Saluja R: Heterotrimeric
complex of p38 MAPK, PKCδ, and TIRAP is required for AP1 mediated
inflammatory response. Int Immunopharmacol. 48:211–218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Liu X, Wu Y, Liao Y, Huang Y, Wei
X and Ma M: P38 MAPK pathway mediates cognitive damage in
pentylenetetrazole-induced epilepsy via apoptosis cascade. Epilepsy
Res. 133:89–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelmoaty S, Wigerblad G, Bas DB,
Codeluppi S, Fernandez-Zafra T, El-Awady el-S, Moustafa Y,
Abdelhamid Ael-D, Brodin E and Svensson CI: Spinal actions of
lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced
mechanical hypersensitivity and spinal TNF release. PLoS One.
8:e755432013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hossein R, Reza A, Mehdi H and Akram M:
The effect of extracted bacterial LPS from Salmonella enteritidis
on COX-2 in hepg2 cell line in induction and inhibition conditions.
Sci Res Essays. 6:5771–5775. 2011.
|
|
26
|
Xie W, Wang H, Liu Q, Li Y, Wang J, Yao S
and Wu Q: ResolvinD1 reduces apoptosis and inflammation in primary
human alveolar epithelial type 2 cells. Lab Invest. 96:526–536.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Recchiuti A: Resolvin D1 and its GPCRs in
resolution circuits of inflammation. Prostaglandins Other Lipid
Mediat. 107:64–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casper C and Fitzmaurice C:
Infection-related cancers: Prioritising an important and eliminable
contributor to the global cancer burden. Lancet Glob Health.
4:e580–e581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang F, Wang Y, Hemmings BA, Rüegg C and
Xue G: PKB/Akt-dependent regulation of inflammation in cancer.
Semin Cancer Biol. May 2–2017.(Epub ahead of print).
|
|
31
|
Kumar S, Chan CJ and Coussens LM:
Inflammation and cancer. Encyclopedia of Immunobiology.
420:406–415. 2016. View Article : Google Scholar
|
|
32
|
Suganuma M, Okabe S, Kurusu M, Iida N,
Ohshima S, Saeki Y, Kishimoto T and Fujiki H: Discrete roles of
cytokines, TNF-alpha, IL-1, IL-6 in tumor promotion and cell
transformation. Int J Oncol. 20:131–136. 2002.PubMed/NCBI
|
|
33
|
Kuninaka S, Yano T, Yokoyama H, Fukuyama
Y, Terazaki Y, Uehara T, Kanematsu T, Asoh H and Ichinose Y: Direct
influences of pro-inflammatory cytokines (IL-1beta, TNF-alpha,
IL-6) on the proliferation and cell-surface antigen expression of
cancer cells. Cytokine. 12:8–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patel HJ and Patel BM: TNF-α and cancer
cachexia: Molecular insights and clinical implications. Life Sci.
170:56–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piccioli P and Rubartelli A: The secretion
of IL-1β and options for release. Semin Immunol. 25:425–429. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feigerlová E and Battaglia-Hsu SF: IL-6
signaling in diabetic nephropathy: From pathophysiology to
therapeutic perspectives. Cytokine Growth Factor Rev. 37:57–65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zanke BW, Boudreau K, Rubie E, Winnett E,
Tibbles LA, Zon L, Kyriakis J, Liu FF and Woodgett JR: The
stress-activated protein kinase pathway mediates cell death
following injury induced by cis-platinum, UV irradiation or heat.
Curr Biol. 6:606–613. 1996. View Article : Google Scholar : PubMed/NCBI
|