Introduction
Although percutaneous coronary intervention (PCI)
has become the standard treatment for stenotic coronary artery
diseases, the incidence of restenosis can reach 10–60% after the
balloon catheter and stent are placed (1). Even when a drug-eluting stent (DES) is
used, restenosis still occurs, with an incidence of 10–20%.
Furthermore, because the drug that is intended to inhibit vascular
smooth muscle cells delays the re-endothelialization of the
vascular intima, the risk of stent thrombosis formation has also
become another dilemma in intervention therapy (2,3).
Coronary smooth muscle cell proliferation and
migration induce hyperplasia of the vascular intima, which is the
main reason for post-PCI restenosis. Delayed vascular
endothelialization caused by coating drugs that are used to inhibit
the proliferation of smooth muscle cells is the key factor
responsible for delayed thrombosis (4,5). Based
on previous research and the recent advancements in modern
pharmacology, the current team optimized the anti-proliferation
drug loading technique via vacuum coating and selected paclitaxel
and hirudin for the coronary balloon catheter to prepare paclitaxel
+ hirudin-eluting balloons, with the aim to explore the feasibility
of compound drug-eluting balloons for the treatment of coronary
artery diseases. As a microtubular inhibitor, paclitaxel is highly
liposoluble (10,000 times more liposoluble than rapamycin); because
of this advantage, paclitaxel has been widely applied in
stent/balloon elution as an anti-cell proliferation drug (6). In contrast, hirudin is a direct
thrombin inhibitor that possesses high water solubility. The
current team applied paclitaxel and hirudin to the balloon in a
specific ratio. Hirudin serves as the carrier of paclitaxel. Their
combination may inhibit the proliferation of vascular smooth muscle
cells maximally but that of vascular endothelial cells minimally,
thereby preventing restenosis and delayed thrombosis after PCI.
In a previous study conducted by our team, a cell
co-culture system was used to stimulate the action mode of the
paclitaxel + hirudin compound on vascular smooth muscle cells and
vascular endothelial cells in rabbits, and the half maximal
effective concentration of the paclitaxel + hirudin compound on the
inhibition of proliferation and migration of rabbit smooth muscle
cells and endothelial cells was determined (7). Furthermore, we explored the effect of
the paclitaxel + hirudin compound on rabbit vascular endothelial
and smooth muscle cells using a cell co-culture technique to
determine the satisfactory ratio of paclitaxel to hirudin (to
maximally inhibit smooth muscle cells and to minimally inhibit
endothelial cells simultaneously) (8). The results showed that paclitaxel
combined with a small dosage of hirudin (3.13 µg/ml) did not have a
noticeable inhibitory effect on the growth of rabbit vascular
endothelial cells in vitro, but it had a significant
inhibitory effect on the growth of rabbit smooth muscle cells.
Considering the differences between animal and human cells in
tolerance and response to drug stimulation, the current team used
human coronary smooth muscle and endothelial cells to determine the
optimal ratio of paclitaxel to hirudin for human cells, and the
results showed that the optimal concentrations were 1 µmol/l
paclitaxel and 0.39 mg/ml hirudin, which can be used to develop
drug-eluting balloons (9–11).
Based on the previous studies, in this study, we
prepared paclitaxel + hirudin-eluting balloons according to the
predetermined optimal ratio. Using the SeQuent Please
paclitaxel-coated balloon (B. Braun Melsungen AG, Melsungen,
Germany) as the control, which is already on the market, this study
investigated the drug release from the paclitaxel + hirudin
compound-coated balloon in the coronary arteries of healthy white
pigs within 180 days and evaluated the interventional effect of the
experimental balloon on vascular reactivity. The results of this
study can provide data to support clinical experiments in the
related field and may shed new light on the development of
compound-eluting balloons.
Materials and methods
Materials
Domesticated pigs were provided by Nanhui Huaxin
Special Animal Farm (Shanghai, China) and the animals met the
SOP315 quarantine criteria. The drug-eluting balloon catheters
included 23 Lepu balloons (3.0×15 mm; batch no. DB1509001; Lepu
Medical, Beijing, China) and 12 B. Braun balloons (3.0×16 mm; batch
no. 15126809; B. Braun Melsungen AG). Paclitaxel was purchased from
the Jiangsu Yew Pharmaceutical Co., Ltd. (purity, 97.7%; batch no.
20131001; Wuxi, China). Terfenadine (article no. T9652-25G; batch
no. 078k1345; purity, 100%), buspirone hydrochloride (article no.
B7148-1G; batch no. BCBL7606V; purity, 100%), methanol (article no.
34860-4L-R; batch no. WXBB7001V), and acetonitrile (article no.
34851-4L; batch no. WXBC0095V) were purchased from Sigma-Aldrich:
Merck KGaA (St. Louis, MO, USA). Formic acid (article no. 50144;
batch no. 1203436) was from DikmaPure (Beijing, China).
The laboratory and surgical equipment included a
vertical chamber, ultra-cold freezer (DW-86L628; Qingdao Haier Co.,
Ltd., Qingdao, China), a Heraeus Multifuge X3R centrifuge (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), an API 4000
spectrometer (Shanghai AB SCIEX Analytical Instrument Trading Co.,
Shanghai, China), an LC-20AD high-performance liquid chromatograph
(Shimadzu Corp., Kyoto, Japan), an autosampler (SIL-20AC/HT;
Shimadzu Corp.), a pressure pump (batch no. 201501011; KDL,
Zhejiang, China), an Innova 2100–IQ digital imaging system, and
standard interventional and auxiliary consumable materials [i.e.,
7-F puncture sheath set (batch no. 201511038), 0.035″ × 150 cm
guidewire (batch no. 201510033) (both from KDL), 6-F guide catheter
(batch no. 201506033; Lepu Medical), and guidewire (batch no.
201510033; Beijing Tiandi Hexie Technology Co., Ltd., Beijing,
China)].
Methods
In each animal, each of the coronary arteries was
treated with one balloon. The pressure holding time was 50 sec. An
upstream 17-mm-long vascular section located 5 mm away from the
balloon inflation site was sampled as the control sample, and the
17-mm-long section at the inflation site was used as the
experimental sample for drug content analysis.
All treatment procedures performed in this study
were approved by the Institute for Animal Ethics at Dongzhimen
Hospital Affiliated to Beijing University of Chinese Medicine
(Beijing, China) [approval no. Hui Zhi Ying Hua (2015) 1230 Gateway
(2015/12)].
Animal grouping
The pigs were divided into the following time-point
groups: instant, day 7, 30, and 180 groups. In each group, one pig
for the control balloon and two pigs for the experimental balloons
were used.
Angiography and balloon treatment
The balloon treatment was performed aseptically. The
pigs were subjected to general anaesthesia. The day before surgery,
a one-time dose of aspirin [325 mg, per os (PO)] and
clopidogrel (150 mg, PO) was given to each pig. During the
postoperative observational period, 100 mg of aspirin and 75 mg
clopidogrel were administered PO each day until the experiment
ended. Before the last follow-up after balloon treatment, two blood
samples were taken from each pig. One sample was anti-coagulated
with EDTA for drug content analysis and the other was not
anti-coagulated. In addition, blood samples were taken for analysis
instantly after inflation of each balloon during surgery. Before
surgery, heparin sodium solution at 150 U/kg was administered via
vein, and the activated clotting time (ACT) was monitored to ensure
the value was >300 during the operation. The surgical procedures
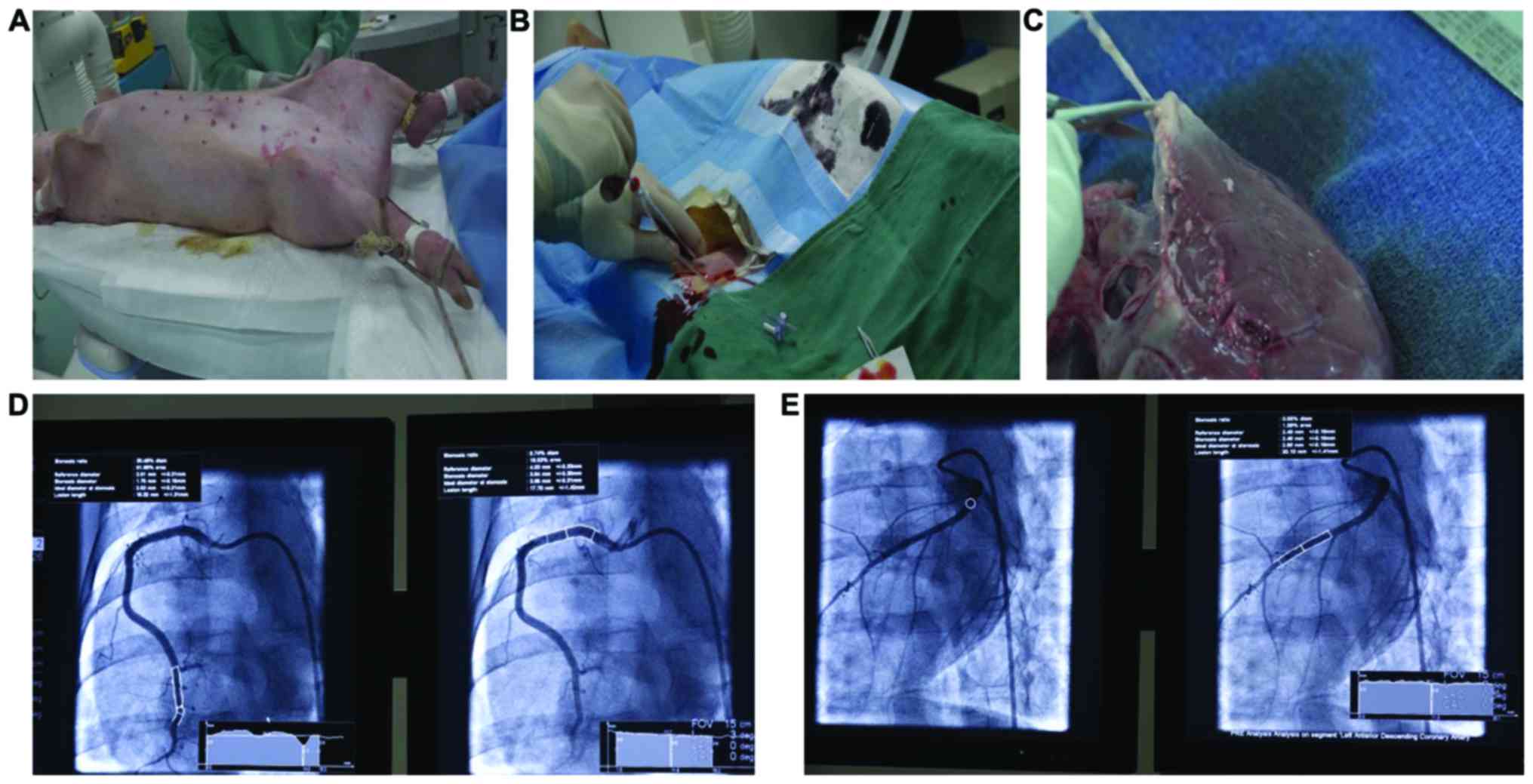
are shown in Fig. 1A-C. During the
operation, real-time monitoring of haemodynamics,
electrocardiography (ECG) and oxygen saturation levels
(SpO2) were performed. The balloon was guided into the
coronary artery to reach the target site with the aid of a guide
catheter and 0.014″ guidewire (Fig. 1D
and E). The balloon was inflated with the pressure pump to
ensure that the balloon sufficiently attached to the vessel wall.
After 50 sec of pressure holding, the pressure was removed and the
guides were withdrawn. After the treatment, angiography was
performed to assess the condition of the treated vascular section.
After surgery, all apparatus and equipment were removed. After
anaesthesia recovery, the pigs were observed until the designated
survival time-points. After the observation was completed, the pigs
were subjected to anaesthesia again for blood sampling.
Approximately 12,500 U heparin sodium injection was intravenously
administered, and then angiography was performed to observe the
treated vascular section. After re-examination, the pigs were
sacrificed by intravenous infusion of potassium chloride (20 mg
equivalent/head) after general anaesthesia with 2–2.5% sevoflurane
inhalation. Autopsy was immediately performed.
Paclitaxel content determination
A homogenate of the vessel wall was prepared with
water in a 1:9 ratio (w/v). Approximately 50 µl of the vessel wall
homogenate or whole-blood sample was mixed well with 200 µl of
precipitant (1:1 methanol to acetonitrile containing 5 ng/ml
buspirone as the internal standard). The sample was centrifuged at
2,504 × g and 4°C for 15 min. The supernatant was collected and
diluted with 0.1% formic acid solution to twice the volume. Liquid
chromatography-electrospray ionization-tandem mass spectrometry
(LC-ESI-MS/MS) was then performed.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and were processed with SPSS version 20.0 software (IBM Corp.,
Armonk, NY, USA). One-way analyses of variance were used for
comparisons among groups, and t-tests were used for comparisons
between groups. P<0.05 was considered statistically
significant.
Results
General health condition
During the experiment, none of the groups showed
adverse clinical signs except for one pig in the day-180
experimental group that died at an early stage (the reason for the
death might be acute failure of the respiratory system, which had
no relation with the experimental balloon). Autopsies showed no
abnormalities in any of the groups.
TIMI grade blood flow
The pigs underwent angiography immediately and at
day 7, 30, and 180 after balloon treatment. Some from both the
control and experimental groups exhibited violent spasms but
recovered without intervention shortly thereafter. Noticeable
stenosis was not observed in the lumens with a TIMI (Thrombolysis
in Myocardial Infarction study group) blood flow grade 3. No
significant differences were observed between the control and
experimental groups.
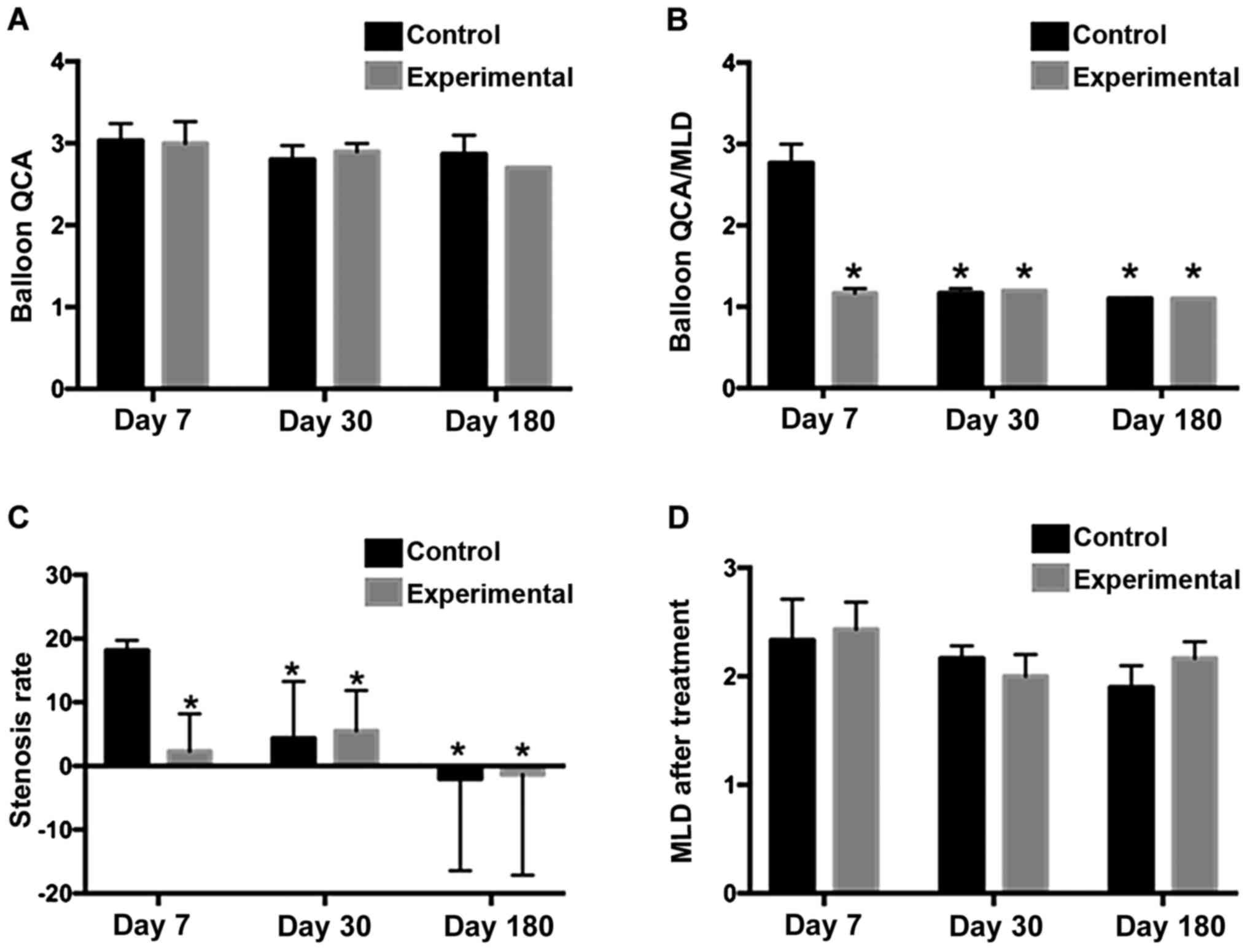
Outcome of quantitative coronary
angiography (QCA)
Coronary angiography of the experimental and control
groups at different observational time-points showed open lumens of
the blood vessels without dissection, angiomas, angiographic
filling defects, or excess stenosis. Furthermore, the observation
indices of some of the treated vascular segments in both the
control and experimental groups presented a negative increase with
a prolonged of the follow-up time. No significant differences were
observed between the control and experimental groups at different
time-points, except for day 30 (Table
I, Fig. 2).
 | Table I.QCA outcomes at different
time-points. |
Table I.
QCA outcomes at different
time-points.
| Groups |
|
|
|
|
|
|---|
|
|
|
|
|
|
|---|
| Balloon | Post-operative time
(days) | Balloon QCA | Balloon/artery
ratio | MLD before balloon
treatment (mm) | MLD after balloon
treatment (mm) | MLD at the time of
follow-up (mm) | Stenosis rate
(%) |
|---|
| Control |
7 |
3±0.2 |
1.1±0.0 |
2.8±0.2 |
2.3±0.4 |
2.3±0.2 |
18.2±1.6 |
| Experimental |
7 |
3±0.2 |
1.2±0.0a |
2.6±0.1 |
2.4±0.2 |
2.3±0.3 |
11.1±10.7a |
| Control | 30 |
2.8±0.2a |
1.2±0.1a |
2.4±0.3 |
2.2±0.1 |
2.3±0.4 |
4.4±8.9a |
| Experimental | 30 |
2.8±0.1a |
1.2±0.0a |
2.4±0.2 |
1.9±0.2 |
2.1±0.3 |
9.9±12.4a,b |
| Control | 180 |
2.8±0.2a |
1.1±0.0 |
2.5±0.2 |
2.0±0.2 |
2.5±0.3 |
−1.7±13.5a |
| Experimental | 180 |
2.7±0.0a |
1.1±0.0 |
2.5±0.2 |
2.0±0.2 |
2.5±0.3 |
−1.7±13.5a |
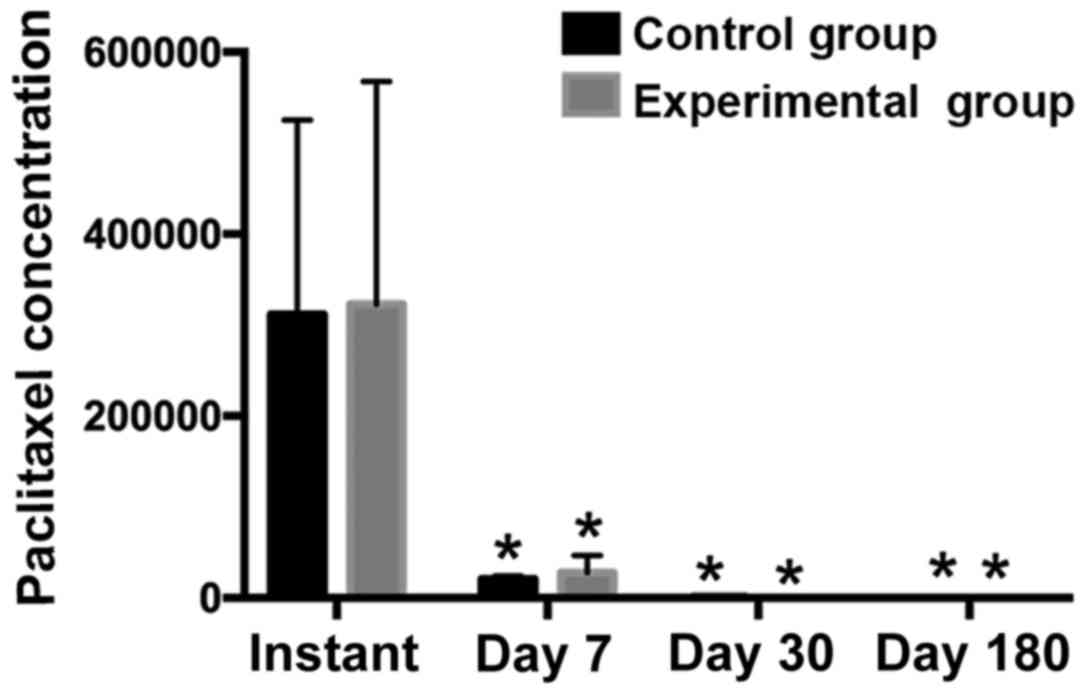
Paclitaxel content in the coronary
wall
The paclitaxel content in the vascular wall in the
experimental group was higher than that in the control group at
different time-points, although the differences were not
significant (P>0.05). The experimental balloon group was not
worse in paclitaxel adsorption than the B. Braun control
paclitaxel-coated balloon group, but the experimental balloon was
slightly better than the control balloon in transient paclitaxel
release and the anchoring effect of paclitaxel on the vascular wall
(Table II, Fig. 3).
 | Table II.Paclitaxel content in the coronary
wall. |
Table II.
Paclitaxel content in the coronary
wall.
| Groups | Postoperative
time-point (days) | Sample section | Paclitaxel
concentration (ng/g) | Total content of
paclitaxel (ng) |
|---|
| Control | Instant | Control | 6,250 | 65,000 | 8,360 | 338 | 2,509 | 487 |
|
|
| Treated | 534,000 | 293,000 | 108,000 | 19,544 | 7,735 | 3,629 |
| Experimental | Instant | Control | 47,600 | 28,300 | 21,000 | 2,675 | 849 | 903 |
|
|
| Treated | 605,000 | 167,000 | 196,000 | 2,509 | 487 | 31,823 |
| Experimental | Instant | Control | 10,400 | 13,300 | 6,060 | 924 | 871 | 496 |
|
|
| Treated | 56,300 | 410,000 | 137,000 | 2,871 | 14,883 | 6,713 |
| Control | 7 | Control | 56.7 | 52.0 | 61.8 | 2.98 | 1.46 | 2.04 |
|
|
| Treated | 23,000 | 16,400 | 22,200 | 736 | 531 | 571 |
| Experimental | 7 | Control | 259 | 399 | 64.8 | 8.88 | 11.6 | 1.83 |
|
|
| Treated | 36,900 | 38,000 | 5,510 | 1,524 | 1,041 | 147 |
| Experimental | 7 | Control | 35.2 | 1,150 | 201 | 0.901 | 15 | 4.82 |
|
|
| Treated | 14,600 | 40,100 | 5,810 | 283 | 1,528 | 97.6 |
| Control | 30 | Control | 0 | 103 | 0 | 0 | 2 | 6.73 |
|
|
| Treated | 0 | 2,820 | 1,400 | 0 | 69.7 | 51.1 |
| Experimental | 30 | Control | 0 | 0 | 0 | 0 | 0 | 0 |
|
|
| Treated | 0 | 161 | 588 | 2 | 6.73 | 13.8 |
| Experimental | 30 | Control | 0 | 913 | 0 | 0 | 26.3 | 0 |
|
|
| Treated | 291 | 31.5 | 264 | 10.2 | 0.488 | 5.65 |
| Control | 180 | Control | 0 | 0 | 0 | 0 | 0 | 0 |
|
|
| Treated | 21.8 | 0 | 39.0 | 0.953 | 0 | 1.53 |
| Experimental | 180 | Control | 0 | 0 | 0 | 0 | 0 | 0 |
|
|
| Treated | 38 | 27.2 | 0 | 0 | 0 | 0 |
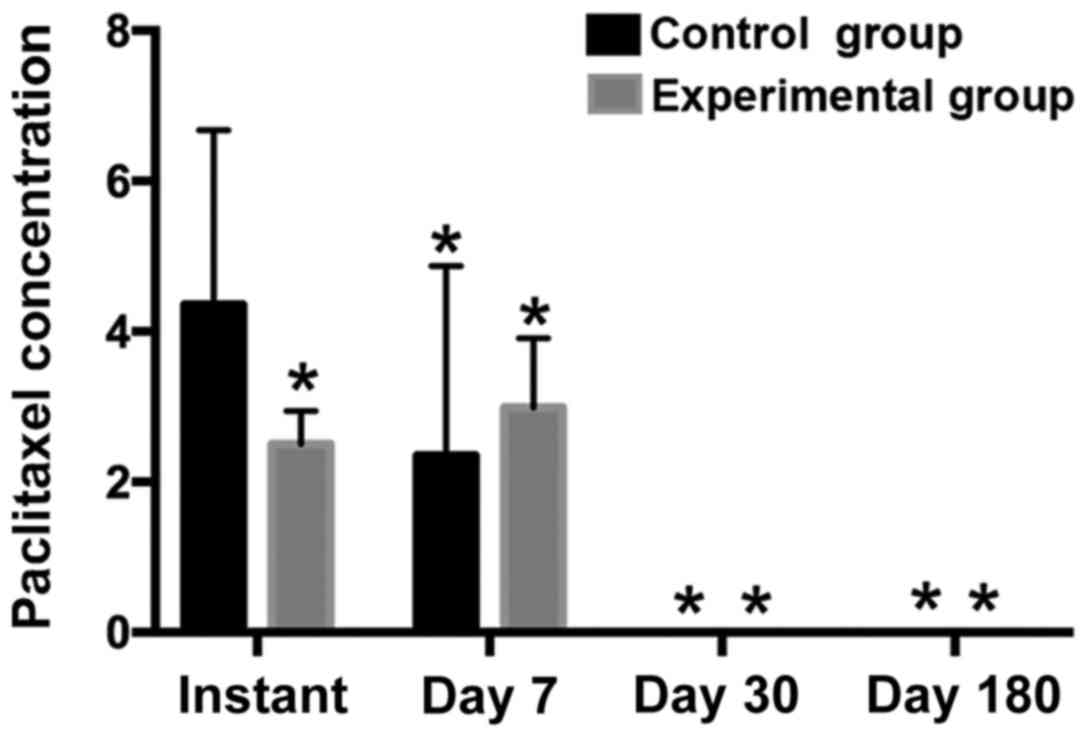
Paclitaxel content in blood
The paclitaxel content in blood in the experimental
group was lower than that in the control group, particularly in the
instant time-point group (P<0.05). The expulsion rate of the
experimental balloon group was lower than that of the control
balloon group, implying better safety and drug stability (Table III, Fig.
4).
 | Table III.Paclitaxel content in blood. |
Table III.
Paclitaxel content in blood.
|
|
|
| Paclitaxel
concentration (ng/ml) |
|---|
|
|
|
|
|
|---|
| Groups | Postoperative time
(days) | Blood-sampling
time | RCA | LCA | LAD |
|---|
| Control | Instant | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 2.28 | 6.86 | 3.92 |
| Experimental | Instant | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 0 | 0 | 2.91 |
| Experimental | Instant | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 2.04 | 3.99 | 2.57 |
| Control | 7 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 2.05 | 0 | 5.01 |
| Experimental | 7 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 7.97 | 3.93 | 2.95 |
| Experimental | 7 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 4.74 | 3.77 | 2.09 |
| Control | 30 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 0 | 0 | 0 |
| Experimental | 30 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 0 | 0 | 0 |
| Experimental | 30 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 3.86 | 4.56 | 4.04 |
| Control | 180 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 0 | 0 | 0 |
| Experimental | 180 | Before
treatment | 0 | 0 | 0 |
|
|
| After
treatment | 0 | 0 | 0 |
Discussion
Currently, the incidence of thrombosis after
treatment with new-generation DESs has decreased to 0.2% (12). Although the incidence of thrombosis
after PCI is low, the consequences of thrombosis are severe,
including a high mortality, and it is the main reason for death
after interventional surgery. Therefore, decreasing the risk of
thrombosis has become a focus for the research and development of
balloons and stents (13).
The development of peri-PCI thrombus primarily
involves two steps: thrombin activation and platelet activation, in
which clot retraction serves as a connecting link in the process.
Apart from transforming fibrinogen into fibrin to participate in
the clotting process, thrombin induces direct and indirect
thrombin-mediated interactions between molecules and cells by
activating thrombin receptors (14).
With the cascade of reactions in the clotting process, thrombin
increases the permeability of the endothelium to cause a series of
vascular injuries. Furthermore, thrombin promotes a proliferative
response after vascular injury by exerting the function of growth
hormones. In addition, thrombin forms a complex network with
platelets, protein kinases and adhesion molecules. Therefore,
thrombin not only initiates restenosis, but it is active through
the whole process. Effective inhibition of thrombin is of
significance in the treatment of anti-cell proliferation and
restenosis. Theoretically, effective anti-coagulation and
anti-platelet treatments will help decrease the incidence of
thrombosis. However, a study was conducted among 8,402 patients
with stenotic coronary artery diseases in whom Cypher and Taxus
stents were implanted (15). Among
these patients, confirmed stent thrombosis was found in 83 patients
and potential stent thrombosis was observed in 127 patients.
Furthermore, ~66% of the patients with confirmed/potential stent
thrombosis reported good compliance with clopidogrel. These
observations indicate that even patients with good compliance with
clopidogrel are not exempt from the risk of stent thrombosis. These
data pose the question of how ticagrelor, a newly developed
powerful P2Y12 receptor antagonist, will perform in preventing
stent thrombosis. For patients who underwent PCI, pre-hospital
ticagrelor administration at a loading dose or in-hospital
ticagrelor administration did not prevent thrombosis; the
proportion of the patients who received pre-hospital ticagrelor was
0.2% and that of the patients who received in-hospital ticagrelor
was 1.2% at day 30 after surgery (P=0.02). In addition, ticagrelor
failed to noticeably improve the TIMI blood flow condition or
ST-segment resolution before PIC (13). Therefore, even the use of a new type
of anti-platelet drugs cannot prevent thrombosis completely, and
more effective anti-thrombosis and anti-cell proliferation
strategies are warranted.
Currently, stents and balloons coated with
paclitaxel have been widely applied in clinical practice with
satisfactory safety and effectiveness. As a microtubule inhibitor,
paclitaxel primarily inhibits the G0/G1 and G2/M stages of the cell
cycle, and it inhibits spindle formation, transmembrane signal
transduction and gene expression related to cell division to
inhibit microtubule-dependent cellular physiological processes,
such as proliferation, migration and secretion (16,17).
Paclitaxel-eluting stents significantly decrease the incidence of
in-stent restenosis, as well as the incidence of adverse cardiac
events and target lesion revascularization (6,18). It is
worth mentioning that paclitaxel-coated balloons also present
unequalled advantages compared with other drug-coated balloons.
Presently, all drug-coated balloons on the market use paclitaxel as
the anti-proliferative drug. Paclitaxel is highly liposoluble,
which allows it to be absorbed rapidly by cells through the cell
membrane. Moreover, the one-time embedding technique of balloons
can produce a long-lasting anti-hyperplasia effect, which is
suitable for local transport of drugs. Furthermore, paclitaxel,
even at micromolar concentrations, can effectively inhibit the
proliferation and migration of vascular smooth muscle cells.
Therefore, paclitaxel-coated balloons have a broad prospect in
anti-restenosis applications (18).
B. Braun SeQuent Please balloons arrived on the market in 2004. In
the product, the dosage of paclitaxel is 3 µg/mm2, the
matrix is Ultravist 370, and the drug release time during balloon
inflation is 30–60 sec. However, numerous studies have shown that
the incidence of restenosis after implantation of the
paclitaxel-eluting stent can reach up to 10–20%. The delayed
thrombosis has greatly limited the clinical application of
paclitaxel-eluting stents, and the occurrence of delayed thrombosis
and restenosis is primarily associated with the inhibitory effect
of paclitaxel on the proliferation and migration of intimal smooth
muscle cells (19–26).
Hirudin is a direct thrombin inhibitor. The no. 63
tyrosine in natural hirudin is sulphated and can act on the
non-active substrate-recognition site and enzyme active centre of
thrombin to play an anti-coagulation role (27). Different from heparin, the inhibitory
and deactivation effect of hirudin on thrombin does not rely on
anti-thrombin III and II, protein C or tissue factor pathway
inhibitors, and it does not combine with platelets which dampens
activity. Hirudin inhibits thrombin-induced platelet aggregation;
therefore, it possesses good anti-coagulation and anti-thrombotic
effects (28). Bivalirudin, a
recombinant, new-generation derivative of hirudin, displays an
excellent anti-coagulation effect and clinical curative effect
(29). Even more, the Chinese
guidelines for PCI (2016) recommended intravenous injection of
bivalirudin 3–4 h after PCI to reduce preoperative complications
(30). Studies on rabbit models of
arterial injury showed that short-term treatment with hirudin
reduced the neointimal area by 44–59% (31,32).
More than 10 years ago, an ‘endothelial-friendly’ drug stent was
developed, in which recombinant hirudin and iloprost were combined
(33). This stent was tested in pig
and goat animal experiments, and the results showed that hirudin +
iloprost-coated stents decreased the risk of thrombosis and
effectively reduced the restenotic area of the coronary artery.
This type of stent provided the current team with a new idea for a
combined drug loading technique: that a combination of two monomers
for balloon/stent coating may effectively decrease post-PCI
restenosis incidence and prevent thrombosis. Natural hirudin has
biological immunogenicity, and it can work as a satisfactory
template to guide the restoration of vascular tissue. The
combination of paclitaxel and hirudin for eluting has
anti-proliferation and thrombosis-preventing effects and does not
influence the restoration of vascular tissue. The paclitaxel +
hirudin combination may be the best design concept of drug-eluting
balloons.
The results of this study showed that paclitaxel +
hirudin-eluting balloons effectively inhibited intimal
proliferation and significantly decreased the incidence of
restenosis at different time-points within 180 days follow-ups. In
protecting residual vessel lumens and decreasing restenosis rate,
the effects of the experimental balloon were not worse or better
than those of the on-sale paclitaxel-eluting balloon. Hirudin,
serving as the carrier for paclitaxel, mediates the slow release of
paclitaxel, which might be one of the main mechanisms underlying
the effects of this combination on inhibiting intimal proliferation
and preventing/curing restenosis. Before balloon inflation, the
compound-eluting balloon catheter was easy to manipulate. After
surgery, angiography showed clear images of the balloon-treated
vascular sections. Observations of the local anatomy did not show
vascular necrosis, vascular thinning or inflammatory exudation.
Determination of paclitaxel contents in the vascular wall and blood
showed that the concentrations were both within the non-toxic
range, which indicates that the local concentration of
hirudin-mediated paclitaxel release is safe, and without noticeable
toxic effects or side-effects. According to LC-ESI-MS/MS, the
instant release of paclitaxel and the instant absorption of
paclitaxel by the vascular wall in the experimental group was
better than those parameters in the control group. The follow-up at
day 180 showed that the paclitaxel content in the vascular wall was
stable and robust in the experimental group, which effectively
prevented the incidence of restenosis caused by the proliferation
of vascular smooth muscle cells. The determination of paclitaxel
content in the blood samples showed that the expulsion rate of
paclitaxel in the experimental group was lower than that in the
control group, which corresponds to better safety and drug
stability with an improved long-term effect of the experimental
balloon compared with that of the control balloon.
In conclusion, the new type of paclitaxel +
hirudin-eluting balloons utilized in this study possesses excellent
safety and histocompatibility, which can effectively prevent late
thrombosis, preserve the residual lumen area, and decrease
restenosis incidence. However, the drug release and absorption
processes of the studied balloon in vivo may require a
longer observational time. The preventive effect of the paclitaxel
+ hirudin-eluting balloon on post-PCI restenosis needs a larger
sample size for validation. Furthermore, the working
micro-mechanism remains to be explored.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 81273913) and Scientific Grants of
Beijing University of Chinese Medicine (no. 2013-ZDXKKF-27).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and XianW conceived and designed the study, and
wrote the article. HL, XiaohangW and GW conducted the experiments
and collected the data. XS conducted the analysis, and XianW
supervised the whole process and gave constructive advice. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Dongzhimen Hospital Affiliated to Beijing University of Chinese
Medicine (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dietz U, Dauer C and Lambertz H: Combining
short stent implantation and drug-eluting stenting for routine use
yields a low restenosis rate. Exp Clin Cardiol. 11:294–297.
2006.PubMed/NCBI
|
|
2
|
Gurvitch R, Lefkovits J, Warren RJ, Duffy
SJ, Clark DJ, Eccleston D, Yan BP, Reid C, Brennan A,
Andrianopoulos N, et al: Clinical outcomes of drug-eluting stent
use in patients with ST elevation myocardial infarction. Int J
Cardiol. 143:283–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghattas A, Shantsila E and Lip GY:
Antithrombotic therapy after percutaneous coronary intervention in
anticoagulated patients: A fine balance between thrombosis and
bleeding. Ther Adv Cardiovasc Dis. 5:5–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stone GW, Lansky AJ, Pocock SJ, Gersh BJ,
Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie
BR, et al: HORIZONS-AMI Trial Investigators: Paclitaxel-eluting
stents versus bare-metal stents in acute myocardial infarction. N
Engl J Med. 360:1946–1959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oduncu V, Erkol A, Tanboğa IH and Kırma C:
Late bare metal stent thrombosis. Türk Kardiyol Dern Arş - Arch
Turk Soc Cardiol. 38:422–425. 2010.
|
|
6
|
Heldman AW, Cheng L, Jenkins GM, Heller
PF, Kim DW, Ware M Jr, Nater C, Hruban RH, Rezai B, Abella BS, et
al: Paclitaxel stent coating inhibits neointimal hyperplasia at 4
weeks in a porcine model of coronary restenosis. Circulation.
103:2289–2295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X and Zhao HB: Effects of paclitaxel
hirudin complex on proliferation and migration of vascular smooth
muscle cells and endothelial cells of rabbits. Chin J Evid Based
Cardiovasc Med. 1:65–69. 2009.
|
|
8
|
Wang X and Zhao HB: Effects of paclitaxel
hirudin complex on the growth of rabbit vascular endothelial cells
and smooth muscle cells (unpublished PhD thesis). Shanxi Medical
University. 2011.
|
|
9
|
Li HM and Wang X: Influence of hirudin on
growth of in vitro human coronary artery smooth muscle cells and
human coronary artery endothelial cells. Chin J Evid Based
Cardiovasc Med. 7:792–794,797. 2015.
|
|
10
|
Li HM and Wang X: Influence of
lipopolysaccharide on growth of in vitro human coronary artery
smooth muscle cells: An investigation on establishing in-stent
restenosis inflammatory cells model. Chin J Evid Based Cardiovasc
Med. 8:29–33. 2016.
|
|
11
|
Li HM and Wang X: Influence of
lipoposaccharide (LPS) on expressions of inflammatory factors of in
vitro human coronary artery smooth muscle cells (HCASMC): An
investigation on establishing in-stent restenosis inflammation cell
model. Chin J Evid Based Cardiovasc Med. 8:297–301. 2016.
|
|
12
|
Jensen LO, Thayssen P, Christiansen EH,
Maeng M, Ravkilde J, Hansen KN, Hansen HS, Krusell L, Kaltoft A,
Tilsted HH, et al: SORT OUT IV Investigators: Safety and efficacy
of everolimus-versus sirolimus-eluting stents: 5-year results from
SORT OUT IV. J Am Coll Cardiol. 67:751–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montone RA, Hoole SP and West NE:
Prehospital ticagrelor in ST-segment elevation myocardial
infarction. N Engl J Med. 371:2338–2339. 2014.PubMed/NCBI
|
|
14
|
van Hinsbergh VW: Endothelium - role in
regulation of coagulation and inflammation. Semin Immunopathol.
34:93–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puricel S, Cuculi F, Weissner M,
Schmermund A, Jamshidi P, Nyffenegger T, Binder H, Eggebrecht H,
Münzel T, Cook S, et al: Bioresorbable coronary scaffold
thrombosis: Multicenter comprehensive analysis of clinical
presentation, mechanisms, and predictors. J Am Coll Cardiol.
67:921–931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yvon AM, Wadsworth P and Jordan MA: Taxol
suppresses dynamics of individual microtubules in living human
tumor cells. Mol Biol Cell. 10:947–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xin S, Yu F, Yang C and Hao X: Inhibition
of paclitaxel against neuroglioma cells u251 growth and its
mechanism. Afr J Tradit Complement Altern Med. 14:174–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellis SG, Kereiakes DJ, Metzger DC, Caputo
RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, et
al: ABSORB III Investigators: Everolimus-eluting bioresorbable
scaffolds for coronary artery disease. N Engl J Med. 373:1905–1915.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerber RT, Latib A, Ielasi A, Cosgrave J,
Qasim A, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I,
et al: Defining a new standard for IVUS optimized drug eluting
stent implantation: The PRAVIO study. Catheter Cardiovasc Interv.
74:348–356. 2009.PubMed/NCBI
|
|
20
|
Hong MK, Mintz GS, Lee CW, Park DW, Choi
BR, Park KH, Kim YH, Cheong SS, Song JK, Kim JJ, et al:
Intravascular ultrasound predictors of angiographic restenosis
after sirolimus-eluting stent implantation. Eur Heart J.
27:1305–1310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joner M, Finn AV, Farb A, Mont EK,
Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK and Virmani R:
Pathology of drug-eluting stents in humans: Delayed healing and
late thrombotic risk. J Am Coll Cardiol. 48:193–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CW, Kang SJ, Park DW, Lee SH, Kim YH,
Kim JJ, Park SW, Mintz GS and Park SJ: Intravascular ultrasound
findings in patients with very late stent thrombosis after either
drug-eluting or bare-metal stent implantation. J Am Coll Cardiol.
55:1936–1942. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morice MC, Serruys PW, Sousa JE, Fajadet
J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P,
Guagliumi G, et al: RAVEL Study Group. Randomized study with the
sirolimus-coated Bx Velocity Balloon-Expandable Stent in the
treatment of patients with de novo native coronary artery lesions:
A randomized comparison of a sirolimus-eluting stent with a
standard stent for coronary revascularization. N Engl J Med.
346:1773–1780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moses JW, Leon MB, Popma JJ, Fitzgerald
PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams
DO, Teirstein PS, et al: SIRIUS Investigators: Sirolimus-eluting
stents versus standard stents in patients with stenosis in a native
coronary artery. N Engl J Med. 349:1315–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moses JW, Dangas G, Mehran R and Mintz GS:
Drug-eluting stents in the real world: How intravascular ultrasound
can improve clinical outcome. Am J Cardiol. 102 Suppl:24J–28J.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pfisterer M, Brunner-La Rocca HP, Buser
PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F,
Osswald S and Kaiser C; BASKET-LATE Investigators, : Late clinical
events after clopidogrel discontinuation may limit the benefit of
drug-eluting stents: An observational study of drug-eluting versus
bare-metal stents. J Am Coll Cardiol. 48:2584–2591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warkentin TE: Bivalent direct thrombin
inhibitors: Hirudin and bivalirudin. Best Pract Res Clin Haematol.
17:105–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He L, Vicente CP, Westrick RJ, Eitzman DT
and Tollefsen DM: Heparin cofactor II inhibits arterial thrombosis
after endothelial injury. J Clin Invest. 109:213–219. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lincoff AM, Bittl JA, Kleiman NS,
Sarembock IJ, Jackman JD, Mehta S, Tannenbaum MA, Niederman AL,
Bachinsky WB, Tift-Mann J III, et al: REPLACE-1 Investigators:
Comparison of bivalirudin versus heparin during percutaneous
coronary intervention (the Randomized Evaluation of PCI Linking
Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J
Cardiol. 93:1092–1096. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Section of Interventional Cardiology of
Chinese Society of Cardiology of Chinese Medical Association;
Specialty Committee on Prevention and Treatment of Thrombosis of
Chinese College of Cardiovascular Physicians; Editorial Board of
Chinese Journal of Cardiology: Chinese guidelines for percutaneous
coronary intervention (2016). Zhonghua Xin Xue Guan Bing Za Zhi.
44:382–400. 2016.(In Chinese). PubMed/NCBI
|
|
31
|
Thome LM, Gimple LW, Bachhuber BG,
McNamara CA, Ragosta M, Gertz SD, Powers ER, Owens GK, Humphries JE
and Sarembock IJ: Early plus delayed hirudin reduces restenosis in
the atherosclerotic rabbit more than early administration alone:
Potential implications for dosing of antithrombin agents.
Circulation. 98:2301–2306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerdes C, Faber-Steinfeld V, Yalkinoglu O
and Wohlfeil S: Comparison of the effects of the thrombin inhibitor
r-hirudin in four animal models of neointima formation after
arterial injury. Arterioscler Thromb Vasc Biol. 16:1306–1311. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alt E, Haehnel I, Beilharz C, Prietzel K,
Preter D, Stemberger A, Fliedner T, Erhardt W and Schömig A:
Inhibition of neointima formation after experimental coronary
artery stenting: A new biodegradable stent coating releasing
hirudin and the prostacyclin analogue iloprost. Circulation.
101:1453–1458. 2000. View Article : Google Scholar : PubMed/NCBI
|