Introduction
Breast cancer is the most common invasive cancer and
the leading cause of cancer-related death among women worldwide
(1,2). Based on their patterns of gene
expression (3,4), breast cancer can be classified into
four major subtypes. Two of these are derived from estrogen
receptor (ER)-positive tumours (luminal A and B), and two are
derived from ER-negative tumours (basal-like and HER2 positive)
(4–6). Triple negative breast cancer (TNBC) is
a basal-like subtype of breast cancer that accounts for
approximately 20% of all breast cancers (7,8).
Compared with other breast cancer subtypes, TNBC is a heterogeneous
subgroup of tumours with a higher metastasis rate, worse prognosis,
and higher relapse risk (8).
Therefore, TNBC has attracted increasing amounts of attention in
oncology research. At the St. Gallen International Breast Cancer
Conference, it was confirmed that the major therapies for breast
cancer include surgery, radiation therapy, endocrine therapy,
targeted therapy and chemotherapy (9,10).
Surgery and radiotherapy are local therapy methods for the
treatment of cancer. Because of the high metastasis and relapse
rates of TNBC, other therapeutic approaches also need to be applied
in TNBC treatment. However, TNBC cannot be treated by endocrine
therapy because it lacks the expression of ER, progesterone
receptor (PgR), and human epidermal growth factor receptor 2 (HER2)
(11,12). Although a number of possible targeted
therapies have been studied, there are currently no ideal targeted
agents for treating TNBC in the adjuvant, neoadjuvant, or
metastatic setting (13). Standard
chemotherapy, especially anthracycline-based agents, remains the
backbone of current TNBC treatment strategies (8), but there is still a high risk for
recurrence and disease progression (14,15).
Therefore, it is urgent to identify highly efficient and low-toxic
treatment strategies for this specific subtype of breast
cancer.
Naphthalimides, an important class of DNA
intercalators, have shown high anti-cancer activity against a range
of human cancer cell lines (16).
One naphthalimide drug, amonafide, which is a DNA intercalator and
a topoisomerase II poison (16), has
good activity against advanced breast cancer (17,18).
Although amonafide has reached the phase 2 clinical trial stage,
further application of amonafide is unlikely because of unexpected
dose-limiting bone marrow toxicity (17–22). To
minimize the toxicity, additional novel naphthalimide derivatives
have been designed such as UNBS5162, which has anti-cancer effects
without haematological toxicity (23,24).
Mijatovic et al (23),
reported that UNBS5162 displays significant anti-cancer activity in
human prostate cancer orthotopic models, namely, PC-3 and DU-145.
In vitro, UNBS5162 induces late apoptosis in DU-145 cells
through inducing proautophagic effects or senescence, but not in
PC-3 cells. In addition, UNBS5162 can induce a pRb-mediated growth
delay, with cell arrest in the G2 phase of the cycle. Moreover,
UNBS5162 has also been found to markedly decrease the expression of
pro-angiogenic CXCL chemokines, which are potent autocrines and/or
paracrine inducers of cancer cell growth (25,26). In
light of this, UNBS5162 may have multiple different effects that
suppress the growth of cancer. UNBS5162 further more showed
reliable anticancer properties and lower toxic side effects in
Phase I clinical trials (24).
Hence, UNBS5162 can be regarded as a promising anticancer drug.
However, the effects and mechanism of UNBS5162 on TNBC are still
unknown. We therefore performed a preliminary study to clarify this
issue.
In the present study, we found UNBS5162 was
effective in inducing growth inhibition and apoptosis of TNBC
MDA-MB-231 cells. Moreover, we determined the effect of UNBS5162 on
the PI3K/AKT/mTOR (PAM) pathway, because PAM represents the main
signalling pathway responsible for cell metabolism, survival,
proliferation and motility regulation (27) and is often activated in TNBC
(28). We discovered that UNBS5162
could effectively suppress the growth of cells via inhibition of
PAM pathway.
Materials and methods
Drugs and reagents
UNBS5162
(N-{2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl}urea)
was purchased from MedChemExpress (Middlesex, NJ, USA). Paclitaxel
was obtained from Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany).
RPMI-1640 medium was obtained from HyClone (Logan, Utah, USA).
Fetal bovine serum (FBS), LDS Sample buffer and pre-stain protein
marker were obtained from Thermo Fisher Scientific, Inc., (Waltham,
MA, USA). Penicillin/streptomycin was purchased from Sigma-Aldrich;
Merck KGaA. 0.25% trypsin with EDTA and CCK8-kit were purchased
from Beijing Solarbio Science & Technology Co., Ltd., (Beijing,
China). DMSO was purchased from Ameresco, Inc., (Framingham, MA,
USA). RIPA Lysis Buffer, BCA Protein Assay kit, and Protease
Inhibitor Cocktail were obtained from Beijing ComWin Biotech Co.,
Ltd., (Beijing, China). ECL developer was purchased from PTG
(Chicago, IL, USA). Transwell was obtained from EMD Millipore
(Billerica, MA, USA). Matrigel was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). Annexin V-FITC/PI Apoptosis Detection
kit was purchased from 4A Biotech Co., Ltd., (Beijing, China).
Primary antibodies against AKT (cat no. 9272), phosphorylated
(p)-AKT (cat no. 13038), mTOR (cat no. 2972), p-mTOR (cat no.
2971), p-P70S6K (cat no. 9204) and P-4EBP1 (cat no. 9451) were
purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Anti-rabbit Immunoglobulin G (IgG) secondary antibodies (cat no.
SA00001-2, HRP-conjugated goat anti-rabbit), anti-mouse
Immunoglobulin G (IgG) secondary antibodies (cat no. SA00001-1,
HRP-conjugated goat anti-mouse), primary antibodies against BCL-2
(cat no. 12789-1-AP), BAX (cat no. 60267-1-Ig), Caspase-3 (cat no.
19677-1-AP) and GAPDH (cat no. 10494-1-AP) were purchased from
Proteintech Group, Inc., (Rosemont, IL, USA).
Cells culture
MDA-MB-231 cells and HFF-1 fibroblasts were
purchased from Shanghai Institutes for Biological Sciences. Cells
were incubated in RPMI 1640 containing 10% serum, 100 U/ml
penicillin, and 0.1 mg/ml streptomycin, and were maintained at 37°C
in 5% CO2. Cells were washed three times by PBS after
the logarithmic growth phase and then were digested by trypsin.
When the cells changed their morphology from a spindle shape to a
circular shape, culture medium was added to the flask to inhibit
the digestion. After centrifugation, the cells were resuspended in
the culture medium and seeded in 6-well plates in preparation for
subsequent experiments. In the meantime, UNBS5162 was diluted in
0.1% DMSO. When grown to 80% confluency, 10 µM UNBS5162 or 0.1%
DMSO, respectively, was added to the cells as the experimental and
negative control group (NC). Then, the cells were cultured for 24
h.
Cell CCK-8 proliferation assay
MDA-MB-231 cells and HFF-1 fibroblasts were seeded
in 96-well plates at a density of 1,000 cells/well in 100 µl of
culture medium and were allowed to attach overnight in a
CO2 incubator. In the first group, MDA-MB-231 cells and
HFF-1 fibroblasts were treated with UNBS5162 at concentrations of
0, 0.1, 1, 10, and 100 µM for a period of 72 h. In the second
group, MDA-MB-231 cells were treated with 10 µM UNBS5162, 10 µM
paclitaxel and 0.1% DMSO respectively for 24, 48, and 72 h. The
selection of drug concentration was referred to the previous
literatures (23,29). After the treatment, 10 µl CCK-8
solution was added to each well of the plate. Cells were incubated
for 1.5 h in the incubator at 37°C. Then, the optical density of
the cells was measured using a microplate reader at an absorbance
of 450 nm. Each sample was evaluated in triplicate.
Flow cytometric analysis of apoptosis
with annexin V/PI double staining
After MDA-MB-231 cells were treated with 10 µm
UNBS5162 or 0.1% DMSO respectively for 24 h, the cells were
digested by trypsin without EDTA, centrifuged for 5 min,
re-suspended in pre-cooled PBS at 4°C, and centrifuged again. After
centrifugation the supernatant was discarded. The cells were
re-suspended in binding buffer solution. The density of cell
suspension was 1–5×106 cells /ml. FITC-Annexin V (5 µl)
and PI (5 µl) were added to 100 µl of cell suspension in a 5 ml
polystyrene tube. The mixture was incubated in the dark at room
temperature for 15 min, and then mixed with 400 µl of binding
buffer. Subsequently, the stained cells were analysed by flow
cytometry using a FACScan flow cytometer. Each sample was evaluated
in triplicate.
Cell migration and invasion
assays
Cell migration and invasion assays were carried out
in 24-well plates using transwell polycarbonate membrane filter
inserts (8 µm pore size; BD Biosciences.
After MDA-MB-231 cells were incubated in culture
medium with UNBS5162 or DMSO respectively for 24 h, the cells were
digested by trypsin, washed once with culture medium, centrifuged
and re-suspended in serum-free medium.
For the migration assay, the cells were suspended in
serum-free medium (100 µl, 1×105 cells/well) and placed
directly in the upper chamber of a transwell.
For the invasion assay, matrigel was diluted in
serum-free medium (100 µl, at a ratio of 1:6), added to the upper
chamber of a transwell, incubated in the incubator for 4 h, and
dried in serum-free medium. Then, cells suspended in serum-free
medium (100 µl, 1×105 cells/well) were placed in the
matrigel-coated upper chamber of a transwell.
The lower chamber contained medium with 10% FBS as a
chemoattractant. Following incubation of the cells at 37°C in 5%
CO2 for 24 h, the filters were removed. Non-migrating
cells were wiped off the upper side of the filter with cotton
swabs. Migratory cells on the lower side of the filter were fixed
in 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet
for 20 min, and then rinsed in PBS. After the filters were
subjected to microscopic inspection (magnification, ×200), the
number of the migratory cells was determined by counting five
random microscopic fields per filter. Each sample was evaluated for
three independent experiments.
Western blot analysis
After treatment with drugs for 24 h, cells were
washed with PBS twice and lysed in RIPA buffer containing protease
inhibitors (15 mM NaCl, 1 mM MgCl2, 1 mM
MnCl2, 2 mM CaCl2, 2 mM phenylmethylsulfonyl
fluoride, and protease inhibitor mixture) on ice for 30 min. After
centrifugation at 13,000 × g for 20 min at 4°C, the supernatant
containing the protein lysates was collected. Then, the protein
concentration was estimated using the BCA method. The protein
lysates were heated at 95°C for 5 min. Equal amounts of protein (20
µg) were separated by SDS-PAGE and transferred onto a PVDF
membrane. Membranes were blocked with 5% nonfat milk for 1 h and
then incubated with the primary antibody overnight at 4°C with
antibodies against AKT (1:1,000 dilution), p-AKT (1:1,000
dilution), mTOR (1:1,000 dilution), p-mTOR (1:1,000 dilution),
p-P70S6K (1:1,000 dilution), P-4EBP1 (1:1,000 dilution), BCL-2
(1:1,000 dilution), BAX (1:1,000 dilution), active caspase-3
(1:1,000 dilution), and GAPDH (1:5,000 dilution) separately. After
being washed with TBST buffer three times for 15 min, the membranes
were incubated with the secondary antibody (diluted 1:5,000) for 1
h at room temperature. Membranes were washed again three times for
15 min with blocking solution and then photographed using the ECL
detection system. Each sample was evaluated for five independent
experiments. The intensity of signal on each membrane was analysed
by using the Quantity One software. GAPDH was used as a loading
control. Each membrane was normalized to GAPDH.
Statistical analysis
Each experimental value was expressed as the means ±
standard (SD) and analyzed using Student's t test or One-way ANOVA
with Duncan's multiple comparison post hoc test. All analyses were
performed with SPSS 18.0 software. P<0.05 was considered to
indicate a statistically significant difference.
Results
UNBS5162 inhibits the proliferation of
MDA-MB-231 cells
To determine the effects of UNBS5162 on TNBC cells,
the viability of MDA-MB-231 cells treated with UNBS5162 was
analysed using CCK-8 assay. MDA-MB-231 cells and HFF-1 fibroblasts
were treated with UNBS5162 at concentrations of 0, 0.1, 1, 10, and
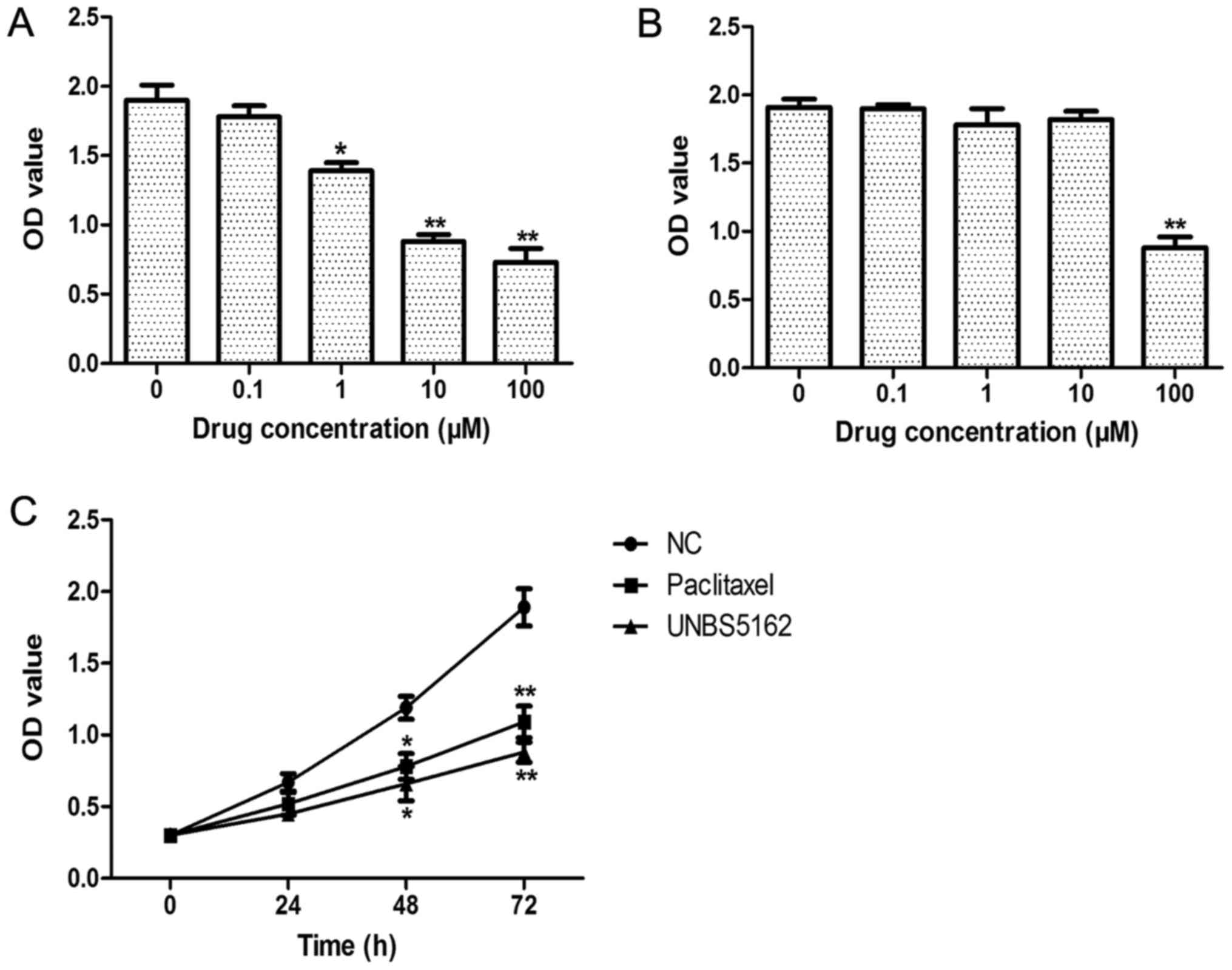
100 µM for a period of 72 h. As shown in Fig. 1A, we found 1, 10, and 100 µM UNBS5162
treatment could decrease the viability of MDA-MB-231 cells in a
dose-dependent manner. Moreover, for fibroblasts treated with
UNBS5162 at different doses, their viability was inhibited only in
the 100 µM UNBS5162 treated group (Fig.
1B). Therefore, in the following experiments, 10 µM UNBS5162, a
highly effective and low toxicity dose, was used (Fig. 1C). We also observed the
time-dependent roles of UNBS5162 in MDA-MB-231 cells. Paclitaxel at
10 µM, a dose that shows anticancer roles in MDA-MB-231 cells, was
selected as the positive control. Similar to paclitaxel, UNBS5162
significantly suppressed the proliferation of MDA-MB-231 cells at
48 and 72 h (P<0.05). Thus, these results indicated that
UNBS5162 suppressed the proliferation of MDA-MB-231 cells.
UNBS5162 induces apoptosis of
MDA-MB-231 cells
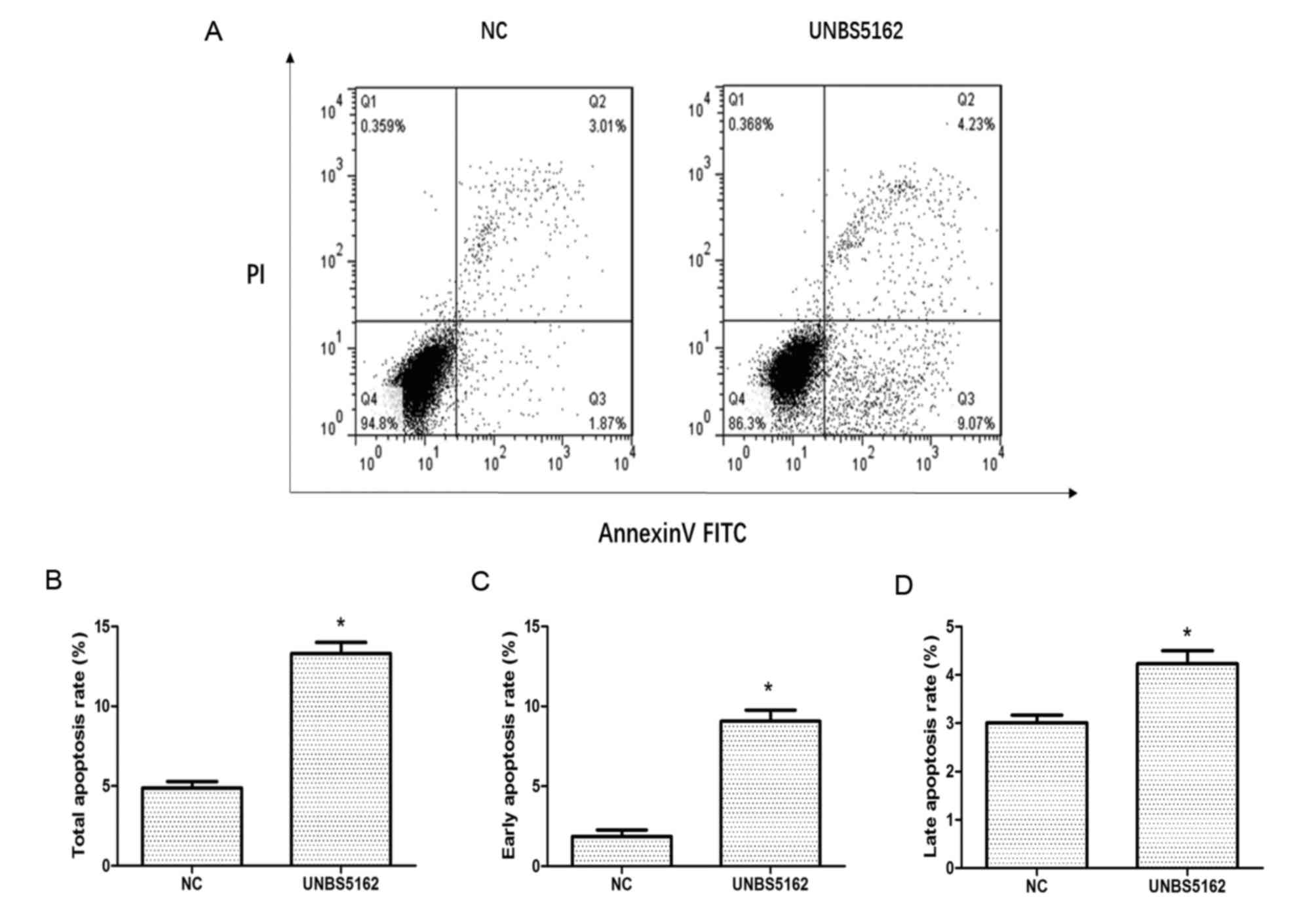
Apoptosis of MDA-MB-231 cells was measured
quantitatively by flow cytometry after staining with Annexin V-FITC
and PI. As shown in Fig. 2, a
quadrant diagram of flow cytometry shows the discrimination between
live cells (annexin V-FITC−/PI−, lower left
quadrant), early apoptosis cells (annexin
V-FITC+/PI−, lower right quadrant), late
apoptotic cells (annexin V-FITC+/PI+, upper
right quadrant) and necrotic cells (annexin
V-FITC−/PI+, upper left quadrant). Overall,
4.88±0.4% of the cells were undergoing apoptosis in the negative
control group, which included 1.87% early apoptotic cells and 3.01%
late apoptotic cells. Meanwhile, the proportion of apoptotic cells
rose to 13.3±0.7% in the UNBS5162 treated group, which included
9.07% early apoptotic cells and 4.23% late apoptotic cells.
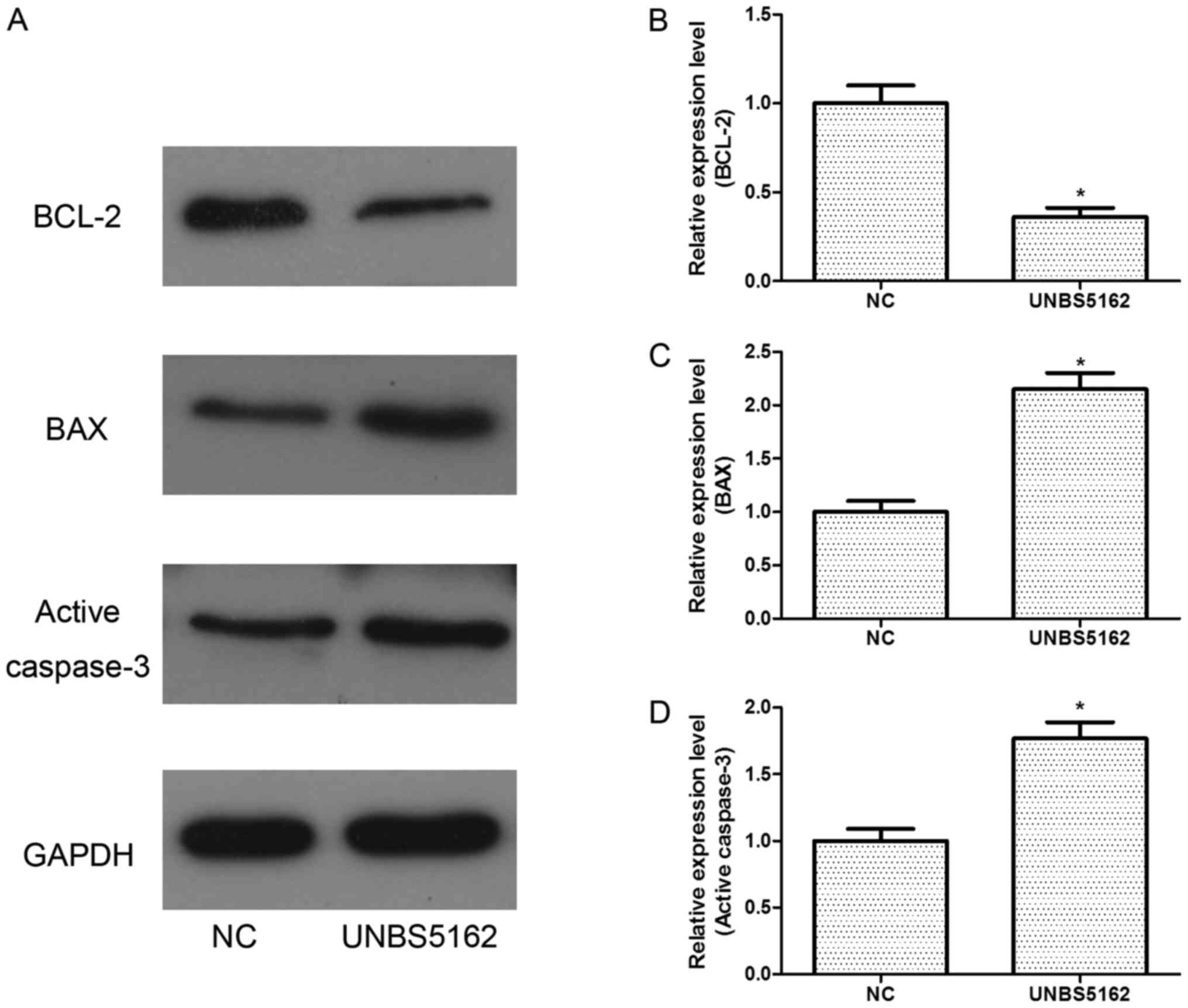
To further study the effects of UNBS5162 on cell
apoptosis, western blot assays were also performed. The expression
of BCL-2, an anti-apoptosis protein, was decreased (Fig. 3). Meanwhile, the expression levels of
pro-apoptosis proteins BAX and active caspase-3 were increased
remarkably (Fig. 3).
All these results indicated UNBS5162 could
effectively induce the apoptosis of MDA-MB-231 cells.
UNBS5162 inhibits the migration and
invasion of MDA-MB-231 cells
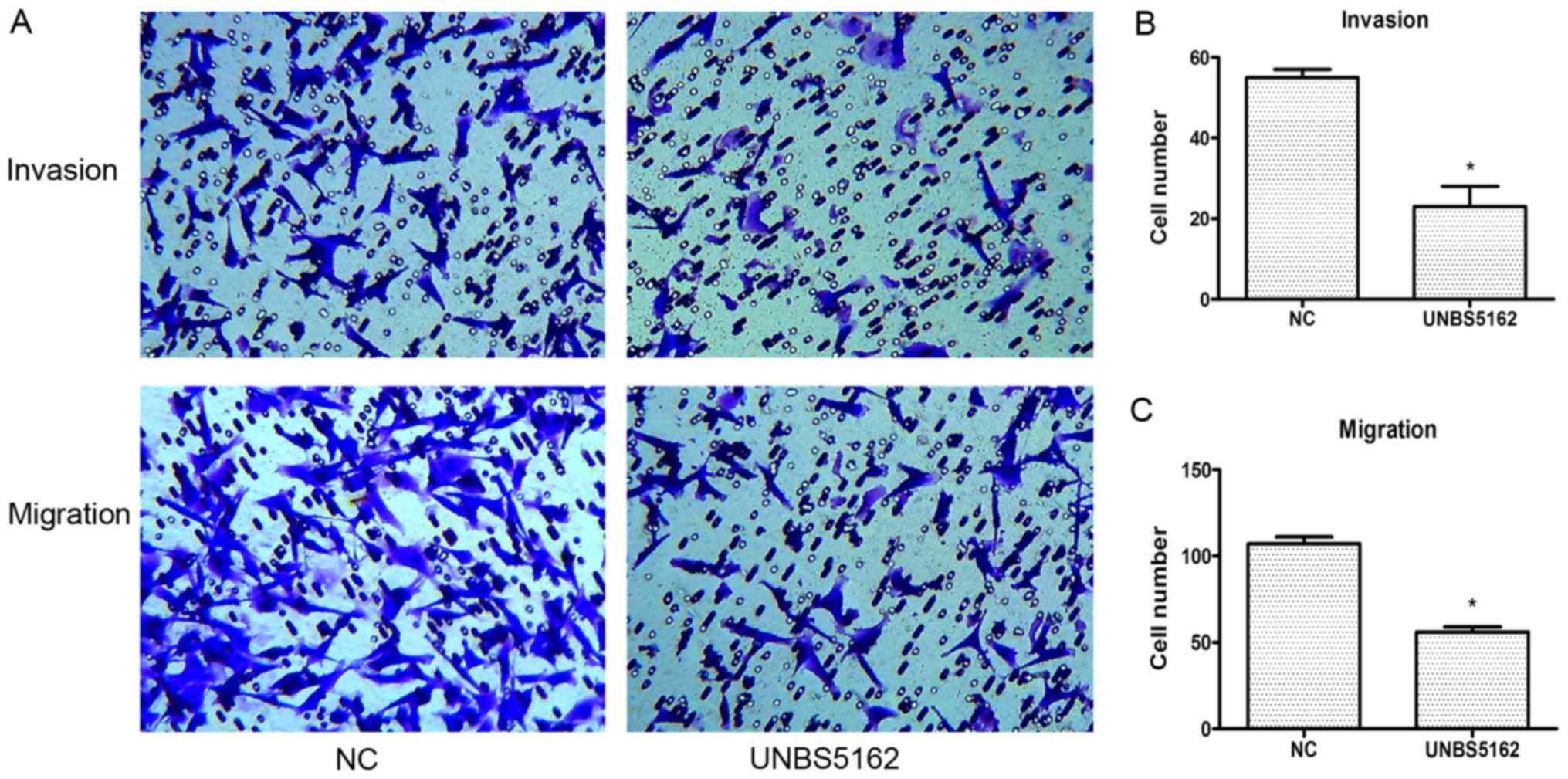
To address whether UNBS5162 affects cell invasion
and migration, we subsequently conducted transwell assays as
described in the Methods section. In these assays, the cells were
pretreated with UNBS5162 and DMSO respectively for 24 h. According
to the results of CCK-8 proliferation assay, the viability of cells
had no significant difference between UNBS5162 treated group and
negative control group after treated with drugs for 24 h. Besides,
cells added into the chamber are the same between UNBS5162 treated
group and negative control group. Therefore, during the early
stages of the transwell assays, the viability and numbers of cells
in the upper side of the membrane in these two groups are similar.
These preconditions can confirm the effectiveness and accuracy of
the experimental results.
In the invasion assay, compared to the NC group, the
number of the invading cells in the UNBS5162 treated group was
decreased significantly (P<0.05; Fig.
4A and B). In the migration assay, the number of crystal violet
positive cells in the UNBS5162 treated group was less than that in
the NC group (P<0.05; Fig. 4A and
C). These results suggested that UNBS5162 could inhibit the
migration and invasion of MDA-MB-231 cells effectively.
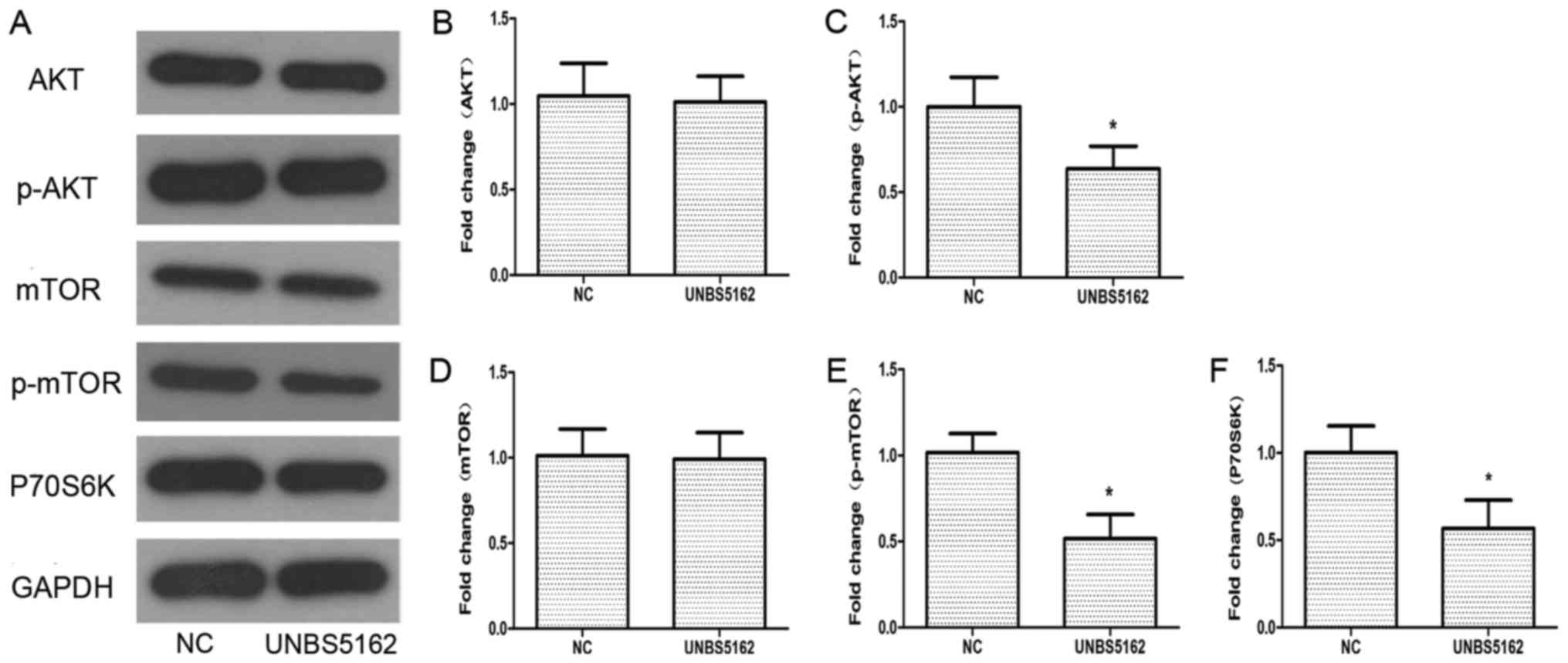
UNBS5162 inhibits the activation of
PI3K/AKT/mTOR (PAM) pathway
To understand how UNBS5162 affects the viability of
MDA-MB-231 cells, western blotting was applied to detect the
protein expression of components in the PAM pathway, which plays a
critical role in cellular growth and apoptosis (27). The expression of some key proteins in
the PAM pathway was detected, including AKT, phosphorylated-AKT
(p-AKT), mTOR, phosphorylated-mTOR (p-mTOR), p-P70S6K and P-4EBP1.
As shown in Fig. 5, the levels of
p-AKT and p-mTOR were decreased significantly compared with the NC
group. The phosphorylation levels of P70S6K and 4EBP1, as
downstream targets of mTOR, were also decreased after treatment
with UNBS5162. According to these experimental data, UNBS5162 might
inhibit the viability of MDA-MB-231 cells via the PAM pathway.
Discussion
This research revealed that UNBS5162 can inhibit
TNBC cell proliferation, migration and invasion as well as promote
cell apoptosis via blocking the PAM pathway. Therefore, UNBS5162
has a good anti-cancer effect in vitro on TNBC cells.
To date, UNBS5162, as an anti-cancer naphthalimide,
has been studied for use for various cancers using basic research
and clinical experiments (23,24).
Mijatovic et al (23),
performed a profound and comprehensive study on the effects and
mechanism of UNBS5162 in human prostate cancers and found that
UNBS5162 could inhibit the growth of human prostate cancer through
different action ways. For example, UNBS5162 was able to act as a
pan-antagonist of CXCL chemokine expression to inhibit cancer
growth. In addition, Mijatovic et al (23), found that UNBS5162 could inhibit the
proliferation of MCF-7 when they used the MTT colorimetric assay to
determine the antiproliferative activity of UNBS5162 against nine
human cancer cell lines. Because MCF-7 is an ER-positive breast
cancer, we thus sought to ascertain whether UNBS5162 can also
inhibit the growth of ER-negative breast cancer. TNBC is a type of
ER-negative breast cancer, and the MDA-MB-231 cell line is a
typical TNBC that possesses stronger drug resistance and has higher
rates of recurrence and metastasis. Therefore, we selected the
MDA-MB-231 cell to confirm our thinking. In this study, our results
showed that UNBS5162 indeed effectively suppressed the
proliferation, migration and invasion of MDA-MB-231 cells. Hence,
UNBS5162 might a possible therapeutic drug for TNBC treatment in
the future.
Apoptosis is a complex process of programmed cell
death that is regulated by a range of cell signals. Apoptosis is
initiated and executed through two major pathways, namely, the
extrinsic and intrinsic pathways (30). The extrinsic pathway is triggered by
extracellular ligands binding to cell surface death recptors. The
intrinsic pathway is initiated by a variety of intracellular
factors generated when cells are stressed. The BCL-2 family plays a
key role in regulating the process of the intrinsic pathway. BCL-2
and BCL-xL protect the cell against apoptosis, but BAX and BCL-2
homologous antagonist/killer (BAK) induce cellular apoptosis
(31). Both pathways have a final
common pathway, which involves activation of the effector caspases
(caspase-3, caspase-6, and caspase-7) by initiator caspases
(32). Therefore, the expression of
BCL-2, active caspase-3, BAX becomes one of the celluar apoptosis
signs. In our research, the BAX and active caspase-3 levels were
increased but BCL-2 level was decreased in UNBS5162-treated cells,
which demonstrated UNBS5162 accelerated apoptosis of MDA-MB-231
cells.
The PAM pathway regulates many cell functions,
mainly associated with cell growth, proliferation and motility
regulation (27). Activation of PI3K
can phosphorylate and activate AKT, localizing it in the plasma
membrane (33). After the activation
of AKT, there is a series of downstream effects, such as activating
PtdIns-3 ps (34), inhibiting p27
(35), and activating mTOR (35), which can affect transcription of
P70S6K and 4EBP1 (35). In addition,
the mTOR complexes, mTORC1 and mTORC2, play a critical role in the
PAM pathway. The activation of mTORC1 promotes the phosphorylation
of P70S6K and 4EBP1 and leads to an increase in protein synthesis
and cell growth (36,37). While mTORC1 relays signals following
PI3K-AKT activation, mTORC2 contributes to complete AKT activation
(37). According to the literatures,
the PAM pathway is overactive in TNBC (28), thus allowing cell proliferation and
reducing apoptosis. Hence, blocking the PAM pathway by the PI3K
inhibitor NVP-BKM120 has been studied for TNBC treatment, and it
effectively induced TNBC growth inhibition and apoptosis (15). Ayub et al (38) used PI3K and mTORC inhibitors, namely,
NVP-BKM120 and KU0063794, respectively, to regulate the PAM
signalling pathway in MDA-MB-231 cells. Their study showed that
these inhibitors might suppress cell proliferation and induce
apoptosis through the PAM pathway (38). Thus, blocking the PAM pathway is
effective in TNBC treatment. In our study, the expression levels of
the key PAM pathway proteins that include p-AKT, p-mTOR, p-P70S6K
and P-4EBP1 were obviously decreased after TNBC cells were treated
with UNBS5162. Based on the changes of p-AKT, p-P70S6K and P-4EBP1,
both mTORC1 and mTORC2 could effectively involve in the roles of
UNBS5162 and influence the cell growth. Therefore, UNBS5162 might
inhibit TNBC cell proliferation and metastasis and induce apoptosis
via inhibiting the PAM pathway. However, one phenomenon cannot be
ignored: A negative feedback loop during monotherapy with UNBS5162
might play a role in PAM pathway, which would obviously reduce the
effect of UNBS5162. To prevent this phenomenon, combined UNBS5162
with other drugs to cure TNBC is one of our future research
directions.
According to the above mentioned work, UNBS5162 as a
potential anti-cancer drug could be applied to treat TNBC in the
future. Certainly, this is just a preliminary study into the use of
UNBS5162 for TNBC treatment. The pharmacodynamics, toxicology and
detailed mechanism for the inhibition of UNBS5162 on TNBC remain to
be elucidated by animal experiments and clinical tests in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XY performed the experiments and wrote the
manuscript. ML and ZX investigated the relevant literature and
revised the manuscript. DC collected and analyzed the experimental
data. SS designed the experiments and approved the final version
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loi S, Pommey S, Haibe-Kains B, Beavis PA,
Darcy PK, Smyth MJ and Stagg J: CD73 promotes anthracycline
resistance and poor prognosis in triple negative breast cancer.
Proc Natl Acad Sci USA. 110:11091–11096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel
Members: Tailoring therapies-improving the management of early
breast cancer: St gallen international expert consensus on the
primary therapy of early breast cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: 10th St. Gallen conference: Progress
and promise: Highlights of the international expert consensus on
the primary therapy of early breast cancer 2007. Ann Oncol.
18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banda M, Speyer CL, Semma SN, Osual KO,
Kounalakis N, Torres Torres KE, Barnard NJ, Kim HJ, Sloane BF,
Miller FR, et al: Metabotropic glutamate receptor-1 contributes to
progression in triple negative breast cancer. PLoS One.
9:e811262014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pal SK, Childs BH and Pegram M: Triple
negative breast cancer: Unmet medical needs. Breast Cancer Res
Treat. 125:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lehmann BD and Pietenpol JA: Clinical
implications of molecular heterogeneity in triple negative breast
cancer. Breast. 24 Suppl 2:S36–S40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perou CM: Molecular stratification of
triple-negative breast cancers. Oncologist. 16 Suppl 1:S61–S70.
2011. View Article : Google Scholar
|
|
15
|
Juvekar A, Burga LN, Hu H, Lunsford EP,
Ibrahim YH, Balmañà J, Rajendran A, Papa A, Spencer K, Lyssiotis
CA, et al: Combining a PI3K inhibitor with a PARP inhibitor
provides an effective therapy for BRCA1-related breast cancer.
Cancer Discov. 2:1048–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braña MF and Ramos A: Naphthalimides as
anti-cancer agents: Synthesis and biological activity. Curr Med
Chem Anticancer Agents. 1:237–255. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costanza ME, Berry D, Henderson IC, Ratain
MJ, Wu K, Shapiro C, Duggan D, Kalra J, Berkowitz I and Lyss AP:
Amonafide: An active agent in the treatment of previously untreated
advanced breast cancer-a cancer and leukemia group B study (CALGB
8642). Clin Cancer Res. 1:699–704. 1995.PubMed/NCBI
|
|
18
|
Scheithauer W, Dittrich C, Kornek G,
Haider K, Linkesch W, Gisslinger H and Depisch D: Phase II study of
amonafide in advanced breast cancer. Breast Cancer Res Treat.
20:63–67. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuhn JG, Burris HA III, Jones SF, Hein DE,
Willcutt NT, Greco FA, Thompson DS, Meluch AA, Schwartz RS and
Brown DM: Phase I/II dose-escalation trial of amonafide for
treatment of advanced solid tumors: Genotyping to optimize dose
based on polymorphic metabolism. J Clin Oncol. 25:25032007.
|
|
20
|
Ratain MJ, Mick R, Berezin F, Janisch L,
Schilsky RL, Williams SF and Smiddy J: Paradoxical relationship
between acetylator phenotype and amonafide toxicity. Clin Pharmacol
Ther. 50:573–579. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ingrassia L, Lefranc F, Kiss R and
Mijatovic T: Naphthalimides and azonafides as promising anti-cancer
agents. Curr Med Chem. 16:1192–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv M and Xu H: Overview of naphthalimide
analogs as anticancer agents. Curr Med Chem. 16:4797–4813. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mijatovic T, Mahieu T, Bruyère C, De Nève
N, Dewelle J, Simon G, Dehoux MJ, van der Aar E, Haibe-Kains B,
Bontempi G, et al: UNBS5162, a novel naphthalimide that decreases
CXCL chemokine expression in experimental prostate cancers.
Neoplasia. 10:573–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahadevan D, Northfelt DW, Chalasani P,
Rensvold D, Kurtin S, Von Hoff DD, Borad MJ and Tibes R: Phase I
trial of UNBS5162, a novel naphthalimide in patients with advanced
solid tumors or lymphoma. Int J Clin Oncol. 18:934–941. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernandez EJ and Lolis E: Structure,
function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol.
42:469–499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Massihnia D, Galvano A, Fanale D, Perez A,
Castiglia M, Incorvaia L, Listì A, Rizzo S, Cicero G, Bazan V, et
al: Triple negative breast cancer: shedding light onto the role of
pi3k/akt/mtor pathway. Oncotarget. 7:60712–60722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blanchard Z, Paul BT, Craft B and Elshamy
WM: BRCA1-IRIS inactivation overcomes paclitaxel resistance in
triple negative breast cancers. Breast Cancer Res. 17:52015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krammer PH: CD95's deadly mission in the
immune system. Nature. 407:789–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lo AC, Woo TT, Wong RL and Wong D:
Apoptosis and other cell death mechanisms after retinal detachment:
Implications for photoreceptor rescue. Ophthalmologica. 226 Suppl
1:S10–S17. 2011. View Article : Google Scholar
|
|
32
|
Krammer PH, Arnold R and Lavrik IN: Life
and death in peripheral T cells. Nat Rev Immunol. 7:532–542. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
King D, Yeomanson D and Bryant HE: PI3King
the lock: Targeting the PI3K/Akt/mTOR pathway as a novel
therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol.
37:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Man HY, Wang Q, Lu WY, Ju W, Ahmadian G,
Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, et al: Activation
of PI3-kinase is required for AMPA receptor insertion during LTP of
mEPSCs in cultured hippocampal neurons. Neuron. 38:611–624. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rafalski VA and Brunet A: Energy
metabolism in adult neural stem cell fate. Prog Neurobiol.
93:182–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rozengurt E, Soares HP and Sinnet-Smith J:
Suppression of feedback loops mediated by PI3K/mTOR induces
multiple overactivation of compensatory pathways: An unintended
consequence leading to drug resistance. Mol Cancer Ther.
13:2477–2488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ayub A, Yip WK and Seow HF: Dual
treatments targeting IGF-1R, PI3K, mTORC or MEK synergize to
inhibit cell growth, induce apoptosis, and arrest cell cycle at G1
phase in MDA-MB-231 cell line. Biomed Pharmacother. 75:40–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|