Introduction
Hodgkin's lymphoma (HL), a B cell-derived lymphoma,
is a potentially curable lymphoid malignancy (1). Classical HL (CHL), which accounts for
95% of all HL, is a lymphatic hematopoietic systemic disease
characterized by the occurrence of Hodgkin-Reed-Sternberg (HRS)
cells in affected reactive lymphadenopathy (2). CHL is classified into 4 types,
including nodular sclerosis HL, lymphocyte-depleted classical HL,
lymphocyte-rich HL and mixed cellularity HL (3). At present, the most popular therapeutic
regimen consists of adriamycin, bleomycin, vinblastine and
dacarbazine, and remains the first-line therapy for patients with
CHL (4). CHL is curable in the
majority of cases (70–80% all stages) treated by conventional
chemotherapy and/or combined radiotherapy; peripheral stem-cell
transplantation can improve the outcome of patients with relapse
following first-line chemotherapy, but with an increased rate of
inevitable risks of lung and heart disease, or even other secondary
cancers (5). Therefore, novel
therapeutics are required for patients with CHL.
Histone deacetylase (HDAC) is a protein deacetylase
that causes genetic changes, altering chromatin structure and
modulating transcriptional and translational processes (6,7). HDACs
are expressed in various malignant tumors, downregulating relevant
tumor suppressor genes (8). HDACs
serve an important role in regulating carcinogenesis in various
tumors, including CHL (9,10). HDAC inhibitors (HDACIs), a class of
therapeutic anticancer drugs, have been widely investigated
(7,11). HDACIs induce a series of changes,
including chromatin remodeling, regulation of transcription
factors, cell cycle arrest and apoptosis induction (12–14). The
class I HDAC-selective inhibitor MGCD0103, a specific benzamide
histone deacetylase inhibitor, has been effective in controlling a
number of cancers such as follicular lymphoma, myelogenous leukemia
and Hodgkin's lymphoma in clinical trials (15–17).
Previous studies suggested that HDACIs are a target for specific
epigenetic changes associated with cancer and other diseases, and
many HDACIs have entered clinical studies (18). A better understanding of gene
expression and phenotype, homeostasis and neoplastic development
that is altered by HDACs would help gain more knowledge about CHL,
and may represent efficient tools for enhancing treatment in
patients with CHL.
Nuclear factor (NF)-κB has been recently
investigated and demonstrated to have an important role in CHL
(19,20). Programmed death-ligand 1 (PD-L1)
inhibitors are used in treating patients with relapsed CHL
(21,22). Academic researches indicated that
PD-L1 inhibitors, including nivolumab and pembrolizumab demonstrate
remarkable activity in relapsed CHL (23,24).
MGCD0103 effects on B cell lymphoma-2 (Bcl-2), NF-κB and PD-L1
levels require further study. In the present study, the expression
levels of HDAC1, 2, 3 and 11 in CHL tissues were examined, and the
effects of MGCD0103 on NF-κB and PD-L1 levels in CHL were assessed,
to explore the potential therapeutic value of the class-I HDAC
inhibitor MGCD0103 in combined relative target drugs for patients
with CHL.
Materials and methods
Reagents
The anchorage-dependent cell line L1236 and the
suspension-cultured cell line L428 used in the present study were
obtained from Chinese Academy of Sciences (Shanghai, China). The
HDACI MGCD0103 was supplied from MethylGene, Inc. (Toronto,
Canada). Antibodies against Bcl-2 (cat. no. ab32124) were provided
by the Department of Pathology, Shanghai Cancer Center, Fudan
University (Shanghai, China). Antibody against α-tubulin (cat. no.
ab52866) was obtained from Abcam (Cambridge, MA, USA). Antibodies
against NF-κB (cat. no. 8242) and PD-L1 (cat. nos. 13684 and 25048)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin G (IgG) antibody (cat. no. 10285-1-AP) was acquired
from ProteinTech Group, Inc. (Chicago, IL, USA). Annexin V FITC
Apoptosis Detection kit was acquired from BD Biosciences (Franklin
Lakes, NJ, USA). Fluorescent-dye conjugated secondary antibodies
(Alexa Fluor® 488-conjugated; cat. no. ab150077) were
obtained from Abcam (Cambridge, UK).
Cell culture and group design
The L1236 and L428 cell lines, maintained at an
atmosphere of 5% CO2 and 37°C, were cultured in RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplied with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). Cells in the exponential phase were harvested
and used in subsequent experiments. Cells in the MGCD0103 0.5, 1
and 2 µM groups were treated for 24 h with HDACI MGCD0103 at 0.5, 1
and 2 µM, respectively. An equal volume of dimethyl sulfoxide
(DMSO) was added in the control group.
Protein extraction and western blot
analysis
Radioimmunoprecipitation assay extraction reagents
with 1% phenylmethanesulfonyl fluoride and 1% DL-dithiothreitol
were applied to extract the total protein of the L236 cells. L428
cells were harvested and dissolved in 9.8 M urea (S1961; Beyotime
Institute of Biotechnology, Haimen, China), 15 mM EDTA (P1045;
Beyotime Institute of Biotechnology) and 30 mM Tris medium (ST774;
Beyotime Institute of Biotechnology), and treated with a cell
disruption step using the ultrasonic technique. Disrupted cells
were then centrifuged (1,000 × g; 5 min; 4°C), insoluble compounds
were removed and the supernatant was collected. A bicinchoninic
protein assay (Pierce; Thermo Fisher Scientific, Inc.) was employed
to measure the concentrations of the lysate protein of the two cell
types. Equal amounts of protein (20 µg) in each group were
separated by 12% SDS-PAGE, and then the proteins were transferred
onto polyvinylidene difluoride membranes (PVDF). Subsequently, 5%
non-fat dry milk dissolved in TBST (20 mM Tris-HCl, 150 mM NaCl,
0.1% Tween 20; pH 7.40) was used to block non-specific antigens on
the PVDF membranes at room temperature for 1 h. Subsequently, the
membranes were incubated with primary antibodies at 4°C overnight
(anti-Bcl-2, 1:1,000; anti-NF-κB, 1:1,000; anti-PD-L1, 1:1,000).
Subsequently, the membranes were washed with TBST three times for 5
min and incubated with goat anti-rabbit IgG secondary antibody
(1:1,000) at room temperature for 1 h. α-tubulin was used as a
loading control (α-tubulin antibody, 1:1,000). The images of
western blotting were captured using an Omega Lum G imaging system
(Gel Company, Inc., San Francisco, CA, USA) and the intensity of
bands was determined using AlphaEase FC software 4.1.0 (Alpha
Innotech Corporation; ProteinSimple, San Jose, CA, USA).
Cell apoptosis and cycle analyzed by
flow cytometry
According to the manufacturer's protocol, cell
apoptosis and cycle analysis were measured using propidium iodide
and Annexin-V staining. Initially, L1236 and L428 cells were
treated with MGCD0103 or DMSO for 24 h at 37°C as described above.
L1236 cells were seeded onto a 6-well plate with RPMI 1640 medium
at a density of 1×106 cells/ml and L428 cells were
seeded onto a 6-well plate and suspended at a density of
1×106 cells/ml in RPMI-1640 medium per well 4°C,
following treatment. Subsequently, L1236 and L428 cells were
harvested and washed with PBS twice. For the analysis of the cell
cycle, cells were treated with RNase (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 0.2 mg/ml, and
stained with propidium iodide (FITC Annexin V Apoptosis Detection
kit I; BD Biosciences, Franklin Lakes, NJ, USA) at a final
concentration of 10 µg/ml in the dark at 4°C for 20 min,
subsequently. Finally, cells were detected by Immunocytometer
Systems (FACSCalibur; BD Biosciences) and data was analyzed using
Flowing software (version 2.5.1; http://flowingsoftware.btk.fi/). For the analysis of
cell apoptosis, both types of cells were suspended in binding
buffer from the kit at a density of 1×105/well,
respectively. Subsequently, the cells were stained with 5 µl
propidium iodide and 5 µl Annexin V-fluorescein isothiocyanate for
30 min in darkness at 4°C, and then detected using Immunocytometer
Systems.
Fluorescence staining and confocal
laser scanning techniques
Coverslips were kept flat on the bottom of a 6-well
plate following cleaning, disinfection and 24-h ultraviolet
irradiation. Subsequently, the L1236 cells were seeded on
coverslips at a density of 1×106/well and cultured in an
incubator at 37°C for 12 h. Cells were treated with MGCD0103 (0.5,
1 and 2 µm) or with DMSO in the control cells for 24 h at 37°C.
L1236 and L428 cells were rinsed with PBS three times for 5 min and
fixed with 4% paraformaldehyde at 25°C for 15 min, followed by
permeabilization of the cells in 0.2% Triton X-100 at 25°C for a
further 20 min. Subsequently, the coverslips were rinsed with PBS
again three times for 5 min and blocked by incubating the
L1236-attached cells in 5% bovine serum albumin (BSA; A8010;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) at 25°C for 60 min. L428 cells were suspended and blocked in
5% BSA at 25°C for 60 min. Cells were then incubated with rabbit
anti-human Bcl-2 (1:100), NF-κB (1:400) and PD-L1 (1:50) antibodies
for 12 h at 4°C. Following washing with PBS three times for 5 min,
the cells were incubated with Alexa 488-coupled goat anti-rabbit
IgG secondary antibodies (1:1,000) for 1 h at 4°C in the dark.
Finally, DAPI was used as a counterstain to label the nuclei at
25°C for 15 min. The stained L1236-attached cells and
L428-suspension cells were the acquired and images were captured
under fluorescent and laser confocal microscopy (magnification,
×600; Lexel Laser, Fremont, CA, USA).
Statistical analysis
Data are presented as the mean + standard error of
the mean, and experiments were performed and repeated three times
independently. Statistical analysis data of the total Annexin-V
positive cells (% DMSO), data of the cell cycle distribution (% of
DMSO), and the data of expressions of Bcl-2, NF-κB and PD-L1
(integrated optical density at the wavelength of 520 nm/area;
compared with DMSO) were analyzed for significant differences using
Student's t-test. Bcl-2, NF-κB and PD-L1 protein expression
(relevant to DMSO) were analyzed for significant differences using
one-way analysis of variance and post hoc Turkey's tests. SPSS 20.0
(IBM Corp., Armonk, NY, USA) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
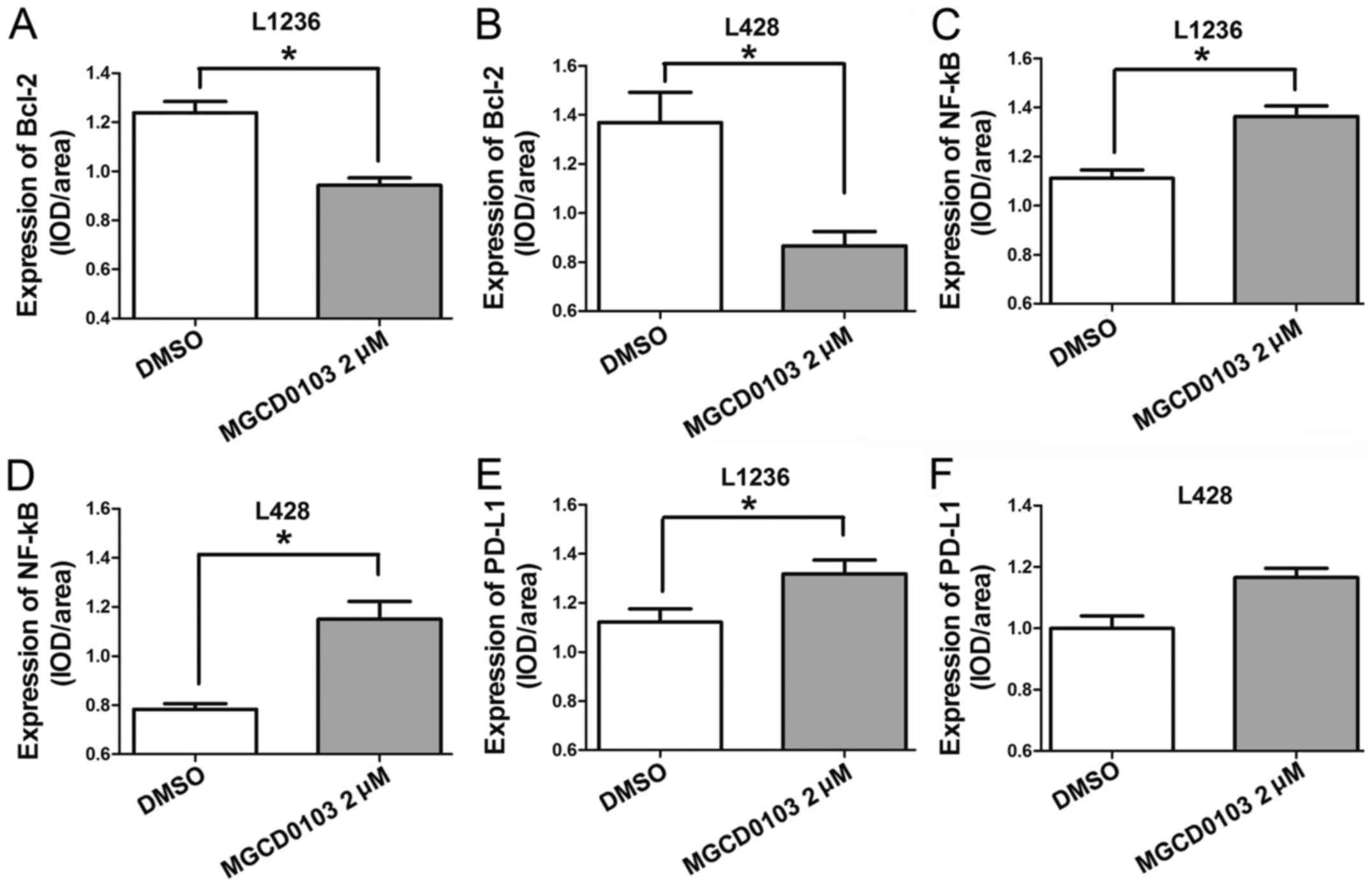
MGCD0103 downregulates Bcl-2, and
increases NF-κB and PD-L1 expression levels
Two HL L1236 and L428 cell lines were treated with
varying concentrations of MGCD0103 (0, 0.5, 1 or 2 µM) for 24 h,
and protein levels of Bcl-2, NF-κB and PD-L1 were measured by
western blotting (Fig. 1A). No
statistically significant differences were identified in protein
expression levels following treatment of the 2 cell lines with
MGCD0103 at a concentration of 0.5 µM (Fig. 1B-G). In the L1236 cell line, MGCD0103
significantly inhibited Bcl-2 expression at a concentration of 1
(P<0.01) and 2 µM (P<0.001; Fig.
1B), and upregulated NF-κB at 2 µM (P<0.05, Fig. 1D) and PD-L1 at 1 (P<0.05) and 2 µM
(P<0.001; Fig. 1F). Similarly, in
the L428 cell line, MGCD0103 inhibited Bcl-2 expression at 1
(P<0.001) and 2 µM (P<0.001; Fig.
1C), and upregulated NF-κB at 2 µM (P<0.01; Fig. 1E) and PD-L1 at 1 (P<0.05) and 2 µM
(P<0.001; Fig. 1G).
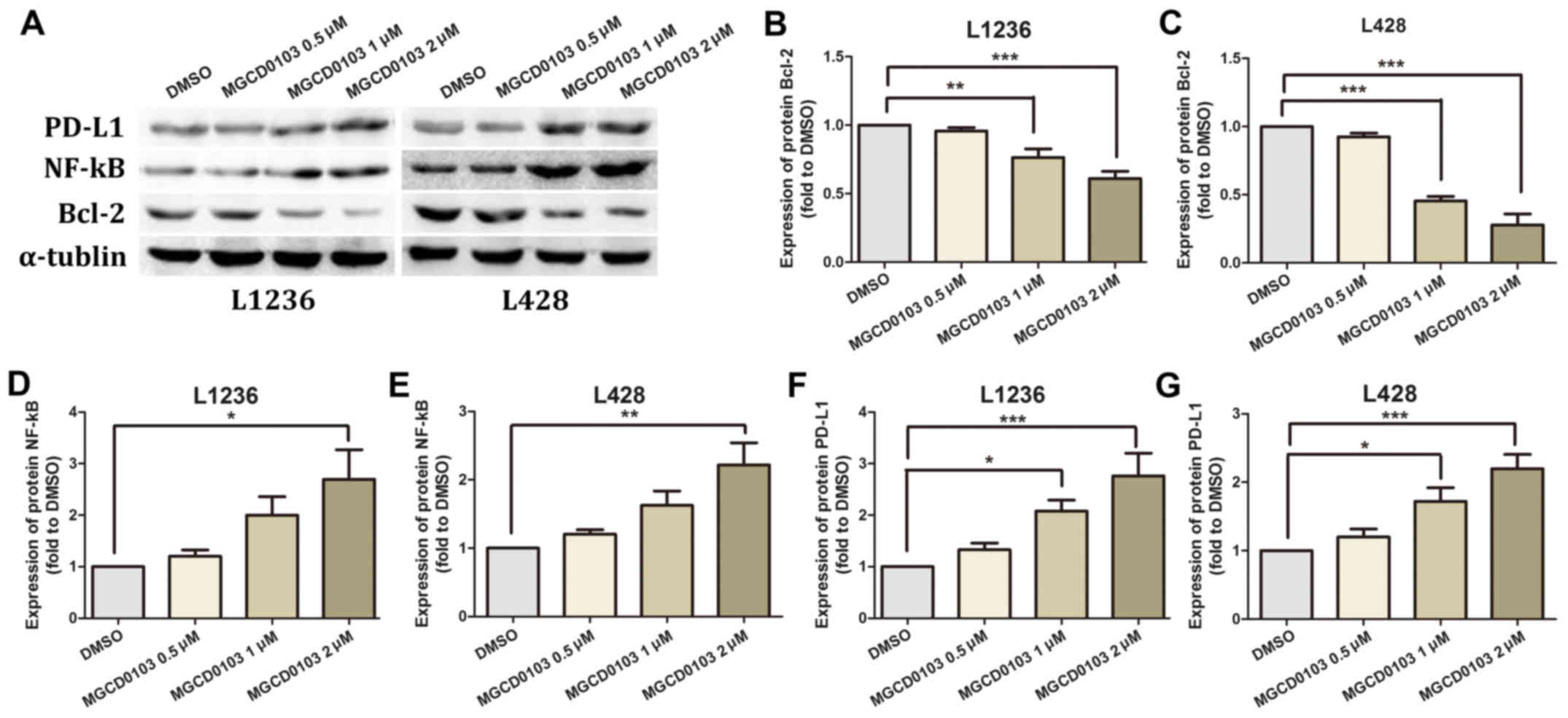 | Figure 1.(A) Western blotting was employed to
assess Bcl-2, NF-κB and PD-L1 protein levels, with α-tubulin as a
loading control. In MGCD0103 groups, cells were treated for 24 h
with MGCD0103 at 0.5, 1 and 2 µM, with the DMSO group considered as
control cells. (B and C) Bcl-2 levels decreased in a dose-dependent
manner in the MGCD0103 groups compared with the control group. (D
and E) NF-κB expression was higher in the MGCD0103 groups than in
controls. (F and G) Similarly, PD-L1 expression was higher in the
MGCD0103 group compared with control cells. Data are presented as
the mean + the standard error of the mean. *P<0.05, **P<0.01,
and ***P<0.001. Bcl-2, B cell lymphoma-2; NF, nuclear factor;
PD-L1, programmed death-ligand 1; DMSO, dimethyl sulfoxide. |
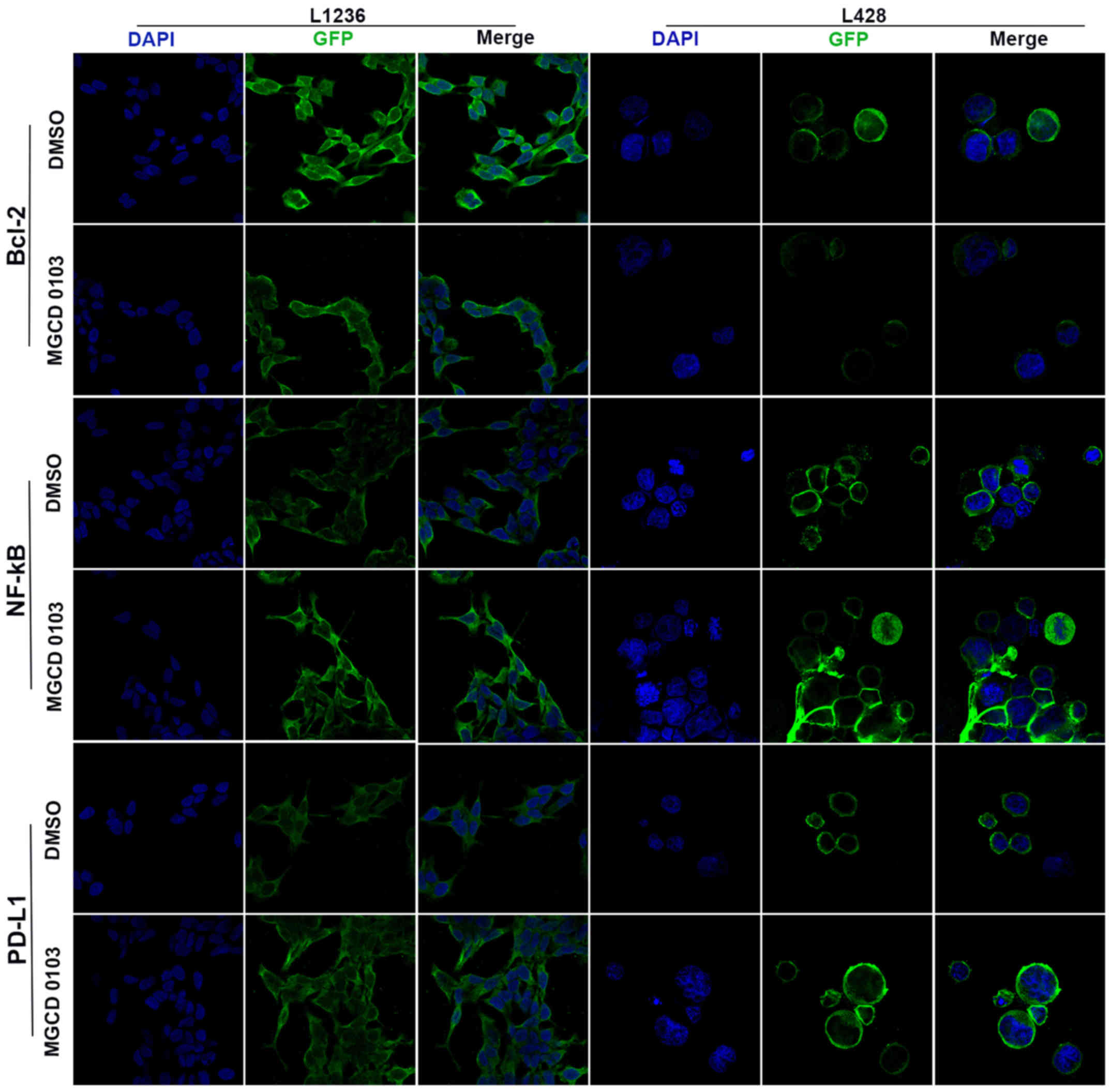
Laser confocal microscopy was also applied to
examine the protein expression levels following treatment with
MGCD0103 at 2 µM (Figs. 2 and
3). The expression levels of various
proteins were assessed using the IOD/area ratio. These findings
suggested that Bcl-2 expression was decreased whereas NF-κB and
PD-L1 were upregulated in the MGCD0103 2 µM group compared with the
DMSO group in the L1236 cell line; all differences were
statistically significant (P<0.05; Fig. 3A, C and E). In the L428 cell line,
Bcl-2 was also downregulated and NF-κB upregulated following
treatment with 2 µM MGCD0103 (P<0.05; Fig. 3B and D); and although there was no
significant difference, PD-L1 was markedly increased in the
MGCD0103 2 µM group compared with the DMSO group (Fig. 3F).
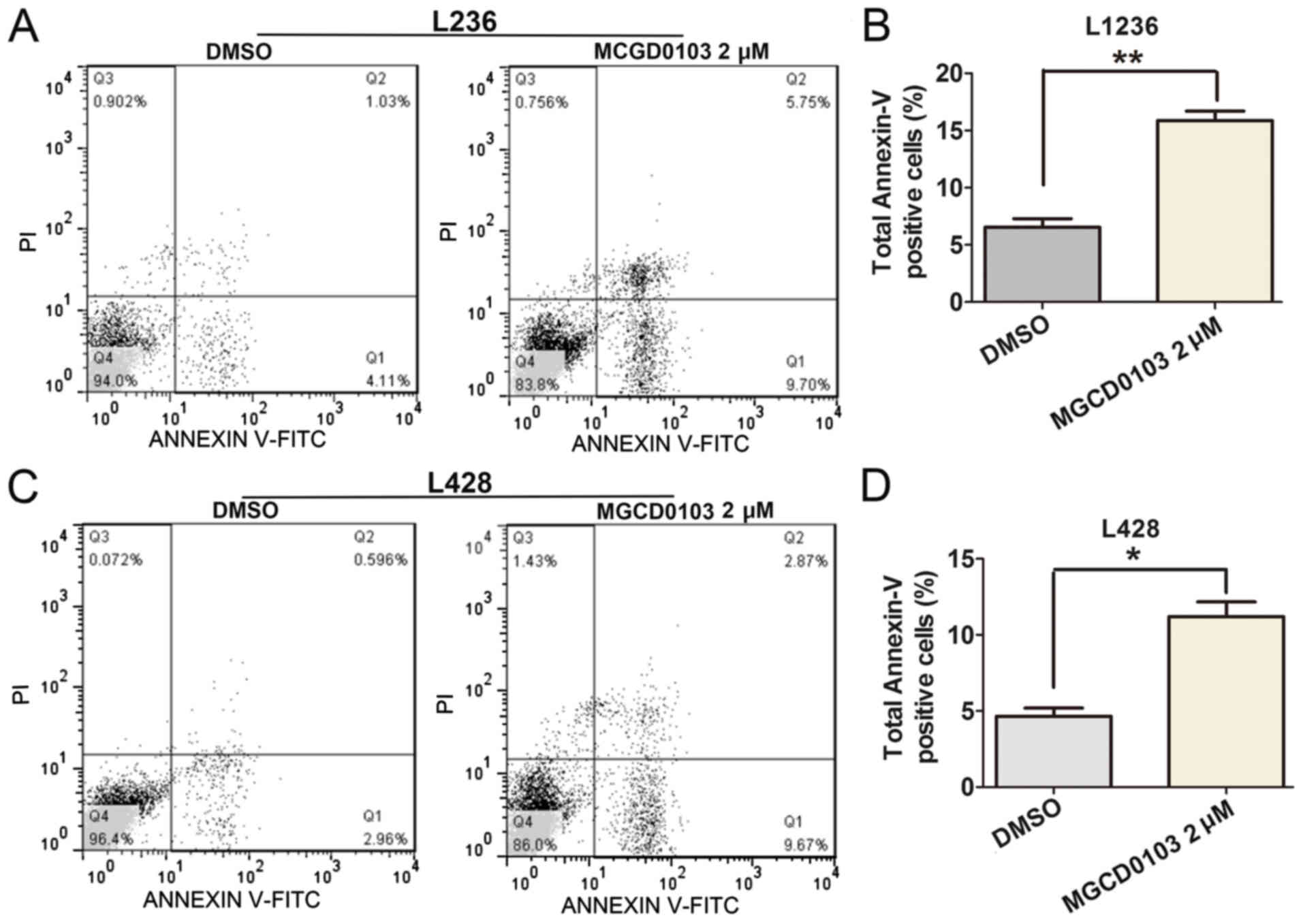
MGCD0103 induces cell apoptosis and
cell cycle arrest in L1236 and L428 cells
To explore the role of MGCD0103 on cell apoptosis,
flow cytometry was applied (Fig. 4).
Following treatment with MGCD0103 at a final concentration of 2 µM
for 24 h, the apoptosis rate was significantly increased in the
MGCD0103 group compared with the DMSO group, as revealed by the
increased proportion of total Annexin-V positive-stained cells
(Fig. 4A and C). Quantitative
analysis of total Annexin-V positive-stained cells was performed,
and total rates of Annexin-V positive cells were significantly
increased following treatment with MGCD0103 in the L1236
(P<0.01; Fig. 4B) and L428
(P<0.05; Fig. 4D) cell lines.
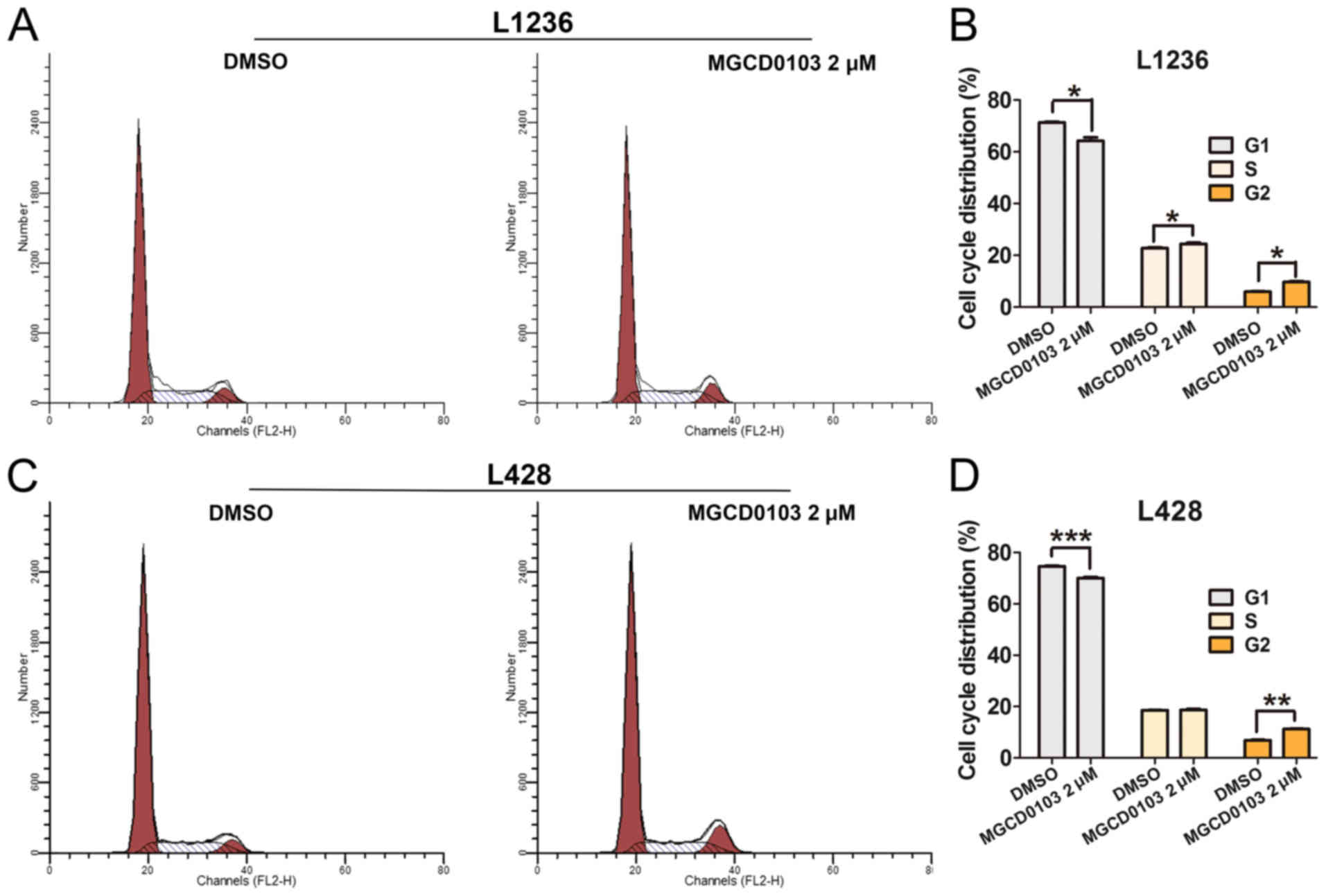
Furthermore, flow cytometry was employed to assess the effects of
MGCD0103 on cell cycle in L1234 and L428 cells (Fig. 5). The results demonstrated that
MGCD0103 treatment resulted in significantly decreased numbers of
cells in the G1 phase in L1236 (P<0.05; Fig. 5A and B) and L428 (P<0.001;
Fig. 5C and D) cells, with
significantly increased cells in the G2 phase in L1236 (P<0.05;
Fig. 5B) and L428 (P<0.01;
Fig. 5D) cells compared with control
groups. These findings demonstrated that MGCD0103 could induce cell
apoptosis and cell cycle arrest.
Discussion
No consensus is currently available regarding the
treatment of CHL following relapse. Immune checkpoint inhibitors
are a potential avenue for such patients; however, the complete
remission rate is ~17–21% (25). A
previous study demonstrated that expression levels of certain HDACs
are associated with clinicopathological characteristics in CHL
(26). The results suggested that
HDAC1, 3 and 11 are expressed at increased levels in CHL, whereas
HDAC2 is decreased (26). In
addition, increased expression of HDAC1 predicts shorter
progression-free and overall survival (OS), while an increased
expression of HDAC11 predicts lower OS (26). The current findings provided insights
into the effects on Bcl-2, NF-κB and PD-L1 levels by the treatment
of the class I HDACI MGCD0103 in an experimental system; namely,
that MGCD0103 enhanced the expression levels of PD-L1 and NF-κB,
and reduced the expression of Bcl-2 in CHL.
Bcl-2, a regulatory protein of the Bcl-2 family,
serves an important role in promoting cell survival and inhibiting
pro-apoptotic proteins (27). It has
been demonstrated that Bcl-2 overstimulation and overexpression,
and upregulation of the oncogene myc may induce aggressive B-cell
malignancies (28). The present
findings demonstrated that MGCD0103 had a direct dose-dependent
effect in inducing Bcl-2 expression, an apoptosis-related protein,
and arresting cell cycle in CHL cell lines.
NF-κB is a protein complex associated with DNA
transcription, cytokine regulation and cell survival in multiple
cell types (29). As a nuclear
transcription factor, NF-κB promotes cell proliferation in acute
myelogenous leukemia cells (30).
VvpE, an elastase mediated by NF-κB, is associated with cell death
and the inflammatory response in human intestinal epithelial cells
(31). It was demonstrated that
NF-κB is widely expressed in malignant lymphoma, and activation of
NF-κB subunits may be associated with the biological functions of
HL (32). Buglio et al
(33) identified that MGCD0103 is
able to induce tumor necrosis factor-α expression and secretion, in
association with NF-κB activation. They demonstrated that MGCD0103
may synergize with proteasome inhibitors by HDAC6-independent
mechanisms, providing mechanistic rationale for exploring this
potentially less-toxic combination for the treatment of lymphoma.
Thus, HDACIs combined with NF-κB inhibitor may yield synergistic
anti-tumor effects, in accordance with the present findings.
PD-L1, also known as B7 homolog 1 or cluster of
differentiation 274 (CD274), is a transmembrane protein encoded by
the CD274 gene. PD-L1 has been demonstrated to serve an important
role in suppressing the immune system in multiple processes,
including pregnancy, inflammation and autoimmune diseases (34–36).
Notably, antibodies specifically targeting PD-L1 ligands have
provided novel treatments of multiple types of cancer (37). In metastatic renal cell carcinoma,
McDermott et al (38)
demonstrated that immune-oncology monotherapy can be regarded as
ideal second-in-line treatment option. Increased expression of
PD-L1 predicts a poor prognosis in colon carcinoma and PD-L1 may
describe a future treatment target (39). Previous studies further demonstrated
the efficacy of PD-1-targeted therapy in patients with metastatic
gastric cancer (40). Previous
studies have indicated that PD-1 is associated with inducing T cell
tolerance, and can limit T cell responses that may prevent
immune-medicated tissue damage (41–43).
PD-L1 is correlated with antitumor immunity (44). PD-L1 expressed on the cell surface
may help identify immune checkpoint blockade therapies for patients
with non-Hodgkin's lymphoma (45).
It has been suggested that MGCD0103 may directly inhibit CHL cell
growth and survival (46). The
present study demonstrated that MGCD0103 may enhance the protein
expression levels of NF-κB and PD-L1; these findings indicated that
MGCD0103 may regulate cell-mediated immunity of CHL. To a certain
extent, this effect of MCD0103 is detrimental to anti-tumor immune
function in the microenvironment in which HRS cells reside.
Therefore, whether MGCD0103 and PD-1 inhibitors have synergistic
effects in the treatment of CHL requires further investigation.
Previous studies have indicated that HDACIs may
regulate PD-L1 expression; however these findings have been
inconsistent. Booth et al (47) recently demonstrated that HDACIs are
capable of reducing HDAC protein expression levels as well as PD-L1
amounts in melanoma cells; meanwhile, Woods et al (48) revealed that class I HDACIs upregulate
PD-L1 in melanoma. Therefore, these studies indicated that HDACs
have dual-regulation functions and mechanisms in regulating
multiple physiological and biochemical processes. The present
findings indicated that HDACIs may upregulate PD-L1. This may
depend on tumor type and specific molecular biological
characteristics in the specific tumor microenvironment.
Briere et al (49) demonstrated that MGCD0103 upregulated
PD-L1 and antigen presentation genes including class I and II human
leukocyte antigen family members in a panel of non-small cell lung
cancer cell lines in vitro. It was concluded that the
combination of MGCD0103 and PD-L1 inhibitor demonstrated increased
anti-tumor activity compared with either therapy alone in two
syngeneic tumor models. In addition, MGCD0103 decreased
T-regulatory cell numbers in the tumor microenvironment.
The present results demonstrate that the type I
HDACI MGCD0103 decreases Bcl-2 levels and upregulates PD-L1, which
indicates the decreased immune ability of CD4+ in the
microenvironment of CHL. The combined use of HDACIs and a PD-L1
inhibitor theoretically may improve treatment outcome in patients
with CHL. Furthermore, the type I HDACI MGCD0103 may also
upregulate NF-κB, which seems to induce resistance towards
anti-apoptotic drugs. It seems, therefore, necessary to use
anti-NF-κB drugs in combination with HDACIs. Clinical trials
combining HDACIs with NF-κB and/or PD-L1 inhibitors should be
designed to further improve treatment outcomes for patients with
CHL.
The present study had some limitations. The
molecular mechanisms by which HDACIs affect CHL have not been
deeply investigated in this primary study. A previous study
demonstrated that blockage of PD-L1/PD-L2 on 9p24.1 may prolong
progression-free survival in patients with CHL (50). However, the effects of HDACIs on
9p24.1 amplification in CHL have not yet been reported. Based on
the present data, the effects of HDACIs on 9p24.1 amplification
deserve further assessment. The current study focused on exploring
the possibility of combining HDACIs and other targeted drugs such
as NF-κB and/or PD-L1 inhibitors. Therefore, the effects of HDACIs
on CHL were assessed in terms of Bcl-2, NF-κB and PD-L1 expression
levels.
Acknowledgements
The authors would like to thank Professor Allen
Cusack for his valuable suggestions and language editing
services.
Funding
The present study was supported by the Science
Foundation of Shanghai Municipal Commission of Science and
Technology (grant no. 15ZR1437500) and Science Foundation of
Xinjiang Commission of Science and Technology (grant no.
2018D01C243).
Availability of data and materials
All data generated or analyzed during the study are
included in this article.
Authors' contributions
XL, SY, ASS and ZM conceived and designed the
present study. RH and XZ performed the experiments. RH XZ, ASS, ZM
and XL collected the data. RH, XZ, SY, XL, ASS and ZM performed the
data analysis and interpretation. RH, XL, SY, ASS and ZM were
responsible for literature search. RH was involved in the
preparation of manuscript. All the authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gobbi PG, Ferreri AJ, Ponzoni M and Levis
A: Hodgkin lymphoma. Crit Rev Oncol Hematol. 85:216–237. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venkataraman G, Mirza MK, Eichenauer DA
and Diehl V: Current status of prognostication in classical Hodgkin
lymphoma. Br J Haematol. 165:287–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Küppers R: The biology of Hodgkin's
lymphoma. Nat Rev Cancer. 9:15–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jalali A, Ha FJ, Chong G, Grigg A,
Mckendrick J, Schwarer AP, Doig R, Hamid A and Hawkes EA: Hodgkin
lymphoma: An Australian experience of ABVD chemotherapy in the
modern era. Ann Hematol. 95:809–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer RM, Gospodarowicz MK, Connors JM,
Pearcey RG, Wells WA, Winter JN, Horning SJ, Dar AR, Shustik C,
Stewart DA, et al: ABVD alone versus radiation-based therapy in
limited-stage Hodgkin's lymphoma. N Engl J Med. 366:399–408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morales CR, Li DL, Pedrozo Z, May HI,
Jiang N, Kyrychenko V, Cho GW, Kim SY, Wang ZV, Rotter D, et al:
Inhibition of class I histone deacetylases blunts cardiac
hypertrophy through TSC2-dependent mTOR repression. Sci Signal.
9:ra342016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falkenberg KJ and Johnstone RW: Histone
deacetylases and their inhibitors in cancer, neurological diseases
and immune disorders. Nat Rev Drug Discov. 13:673–691. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phelps MP, Bailey JN, Vleeshouwer-Neumann
T and Chen EY: CRISPR screen identifies the NCOR/HDAC3 complex as a
major suppressor of differentiation in rhabdomyosarcoma. Proc Natl
Acad Sci USA. 113:15090–15095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Locatelli SL, Cleris L, Stirparo GG,
Tartari S, Saba E, Pierdominici M, Malorni W, Carbone A, Anichini A
and Carlo-Stella C: BIM upregulation and ROS-dependent necroptosis
mediate the antitumor effects of the HDACi Givinostat and Sorafenib
in Hodgkin lymphoma cell line xenografts. Leukemia. 28:1861–1871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolden JE, Peart MJ and Johnstone RW:
Anticancer activities of histone deacetylase inhibitors. Nat Rev
Drug Discov. 5:769–784. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minucci S and Pelicci PG: Histone
deacetylase inhibitors and the promise of epigenetic (and more)
treatments for cancer. Nat Rev Cancer. 6:38–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagni C, Floresta G, Monciino G and
Rescifina A: The search for potent, small-molecule HDACIs in cancer
treatment: A decade after vorinostat. Med Res Rev. 37:1373–1428.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin TY, Fenger J, Murahari S, Bear MD,
Kulp SK, Wang D, Chen CS, Kisseberth WC and London CA: AR-42, a
novel HDAC inhibitor, exhibits biologic activity against malignant
mast cell lines via down-regulation of constitutively activated
Kit. Blood. 115:4217–4225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peart MJ, Smyth GK, van Laar RK, Bowtell
DD, Richon VM, Marks PA, Holloway AJ and Johnstone RW:
Identification and functional significance of genes regulated by
structurally different histone deacetylase inhibitors. Proc Natl
Acad Sci USA. 102:3697–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batlevi CL, Crump M, Andreadis C, Rizzieri
D, Assouline SE, Fox S, van der Jagt RHC, Copeland A, Potvin D,
Chao R and Younes A: A phase 2 study of mocetinostat, a histone
deacetylase inhibitor, in relapsed or refractory lymphoma. Br J
Haematol. 178:434–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jude JG, Spencer GJ, Huang X, Somerville
TDD, Jones DR, Divecha N and Somervaille TCP: A targeted knockdown
screen of genes coding for phosphoinositide modulators identifies
PIP4K2A as required for acute myeloid leukemia cell proliferation
and survival. Oncogene. 34:1253–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kirschbaum MH: Histone deacetylase
inhibitors and Hodgkin's lymphoma. Lancet Oncol. 12:1178–1179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zagni C, Floresta G, Monciino G and
Rescifina A: The search for potent, small-molecule HDACIs in cancer
treatment: A decade after vorinostat. Med Res Rev. 37:1373–1428.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenquist R and Stamatopoulos K: B-cell
malignancies: All roads lead to NF-κB activation. Semin Cancer
Biol. 39:1–2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weniger MA and Küppers R: NF-κB
deregulation in Hodgkin lymphoma. Semin Cancer Biol. 39:32–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roemer MGM, Redd R and Cader F: Major
histocompatibility complex class II and programmed death ligand 1
expression predict outcome after programmed death 1 blockade in
classic hodgkin lymphoma. J Clin Oncol. 36:942–950. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka Y, Maeshima AM, Nomoto J, Makita S,
Fukuhara S, Munakata W, Maruyama D, Tobinai K and Kobayashi Y:
Expression pattern of PD-L1 and PD-L2 in classical Hodgkin
lymphoma, primary mediastinal large B-cell lymphoma, and gray zone
lymphoma. Eur J Haematol. 100:511–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pianko MJ, Moskowitz AJ and Lesokhin AM:
Immunotherapy of lymphoma and myeloma: Facts and hopes. Clin Cancer
Res. 24:1002–1010. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alinari L and Blum K: How I treat relapsed
classical Hodgkin lymphoma after autologous stem cell transplant.
Blood. 127:287–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Villasboas JC and Ansell S: Checkpoint
inhibition: programmed cell death 1 and programmed cell death 1
ligand inhibitors in Hodgkin lymphoma. Cancer J. 22:17–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang R, Zhang X, Sophia S, Min Z and Liu
X: Clinicopathological features and prediction values of HDAC1,
HDAC2, HDAC3, and HDAC11 in classical Hodgkin lymphoma. Anticancer
Drugs. 29:364–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delbridge A, Grabow S, Strasser A and Vaux
DL: Thirty years of BCL-2: Translating cell death discoveries into
novel cancer therapies. Nat Rev Cancer. 16:99–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karube K and Campo E: MYC alterations in
diffuse large B-cell lymphomas. Semin Hematol. 52:97–106. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richmond A: Nf-kappa B, chemokine gene
transcription and tumour growth. Nat Rev Immunol. 2:664–674. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Tang P, Chen Y, Chen J, Ma R and
Sun L: Overexpression of microRNA-125b inhibits human acute myeloid
leukemia cells invasion, proliferation and promotes cells apoptosis
by targeting NF-κB signaling pathway. Biochem Biophys Res Commun.
488:60–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SJ, Jung YH, Song EJ, Jang KK, Choi SH
and Han HJ: Vibrio vulnificus VvpE stimulates IL-1β production by
the hypomethylation of the IL-1β promoter and NF-κB activation via
lipid raft-dependent ANXA2 recruitment and reactive oxygen species
signaling in intestinal epithelial cells. J Immunol. 195:2282–2293.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Oliveira KA, Kaergel E, Heinig M,
Fontaine JF, Patone G, Muro EM, Mathas S, Hummel M, Andrade-Navarro
MA, Hübner N and Scheidereit C: A roadmap of constitutive NF-κB
activity in Hodgkin lymphoma: Dominant roles of p50 and p52
revealed by genome-wide analyses. Genome Med. 8:282016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buglio D, Mamidipudi V, Khaskhely NM,
Brady H, Heise C, Besterman J, Martell RE, MacBeth K and Younes A:
The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin
lymphoma cell lines and synergizes with proteasome inhibitors by an
HDAC6-independent mechanism. Br J Haematol. 151:387–396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YH, Tian M, Tang MX, Liu ZZ and Liao
AH: Recent insight into the role of the PD-1/PD-L1 pathway in
feto-maternal tolerance and pregnancy. Am J Reprod Immunol.
74:201–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Le Burel S, Champiat S, Routier E,
Aspeslagh S, Albiges L, Szwebel TA, Michot JM, Chretien P, Mariette
X, Voisin AL and Lambotte O: Onset of connective tissue disease
following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis.
77:468–470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seko Y, Yagita H, Okumura K, Azuma M and
Nagai R: Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in
the development of murine acute myocarditis caused by
coxsackievirus B3. Cardiovasc Res. 75:158–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McDermott DF, Huseni MA, Atkins MB, Motzer
RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et
al: Clinical activity and molecular correlates of response to
atezolizumab alone or in combination with bevacizumab versus
sunitinib in renal cell carcinoma. Nat Med. 24:749–757. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen XY, Zhang J, Hou LD, Zhang R, Chen W,
Fan HN, Huang YX, Liu H and Zhu JS: Upregulation of PD-L1 predicts
poor prognosis and is associated with miR-191-5p dysregulation in
colon adenocarcinoma. Int J Immunopathol Pharmacol.
32:20587384187903182018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ravi R, Noonan KA, Pham V, Bedi R,
Zhavoronkov A, Ozerov IV, Makarev E, Artemov V A, Wysocki PT, Mehra
R, et al: Bifunctional immune checkpoint-targeted antibody-ligand
traps that simultaneously disable TGFβ enhance the efficacy of
cancer immunotherapy. Nat Commun. 9:7412018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fife BT, Pauken KE, Eagar TN, Obu T, Wu J,
Tang Q, Azuma M, Krummel MF and Bluestone JA: Interactions between
PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop
signal. Nat Immunol. 10:1185–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lau J, Cheung J, Navarro A, Lianoglou S,
Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et
al: Tumour and host cell PD-L1 is required to mediate suppression
of anti-tumour immunity in mice. Nat Commun. 8:145722017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gravelle P, Burroni B, Péricart S, Rossi
C, Bezombes C, Tosolini M, Damotte D, Brousset P, Fournié JJ and
Laurent C: Mechanisms of PD-1/PD-L1 expression and prognostic
relevance in non-Hodgkin lymphoma: A summary of immunohistochemical
studies. Oncotarget. 8:44960–44975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buglio D, Mamidipudi V, Khaskhely NM,
Brady H, Heise C, Besterman J, Martell RE, MacBeth K and Younes A:
The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin
lymphoma cell lines and synergizes with proteasome inhibitors by an
HDAC6-independent mechanism. Br J Haematol. 151:387–396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Booth L, Roberts J, Poklepovic A, Kirkwood
J and Dent P: HDAC inhibitors enhance the immunotherapy response of
melanoma cells. Oncotarget. 8:83155–83170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Woods DM, Sodré AL, Villagra A, Sarnaik A,
Sotomayor EM and Weber J: HDAC inhibition upregulates PD-1 ligands
in melanoma and augments immunotherapy with PD-1 blockade. Cancer
Immunol Res. 3:1375–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Briere D, Sudhakar N, Woods DM, Hallin J,
Engstrom LD, Aranda R, Chiang H, Sodré AL, Olson P, Weber JS and
Christensen JG: The class I/IV HDAC inhibitor mocetinostat
increases tumor antigen presentation, decreases immune suppressive
cell types and augments checkpoint inhibitor therapy. Cancer
Immunol Immunother. 67:381–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Roemer MG, Advani RH, Ligon AH, Natkunam
Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et
al: PD-L1 and PD-L2 genetic alterations define classical Hodgkin
lymphoma and predict outcome. J Clin Oncol. 34:2690–2697. 2016.
View Article : Google Scholar : PubMed/NCBI
|