Introduction
Sepsis appears when an infection defeats the immune
system, which IS usually able to fight against germs, and reaches
the bloodstream. This term frequently used is septicaemia, which
denotes a severe infection of the bloodstream.
Bacteraemia indicates the presence of bacteria in
the blood. It is a medical emergency and that must be promptly
controlled with appropriate antibiotics (1). Bacteraemia is a main cause of morbidity
and mortality in hospitals, that can evolve into bloodstream
infections (BSI). BSI are a major healthcare concern and are
associated with a disease burden in hospitals comparable with
myocardial infarction, major stroke and trauma, holding the 8th
position in intra-hospital mortality causes (2). Bacteraemia can be classified according
to the site of acquisition, either as community-onset or
nosocomial-acquired. Bacteraemia is of particular concern due to
the high 30-day mortality rate of 16–29% (3). Consequently, there are local and
national surveillance programs that monitor the occurrence of
bacteraemia in hospitals in an effort to improve prevention by
empirical therapy and the control of invasive infections. Studies
reporting data from hospital cohorts have described overall
incidences, risk factors and mortality (4,5);
however, at least to the best of our knowledge, few studies to date
have investigated the timing (daily incidence) of bacteraemia
(6,7).
Advances in medical pathogen detection technology
and the demographic changes have altered the epidemiology of
bacteraemia in recent decades, resulting in a shift in the pathogen
spectrum. Although between the years 1987–2000, the aetiology of
bacteraemia was dominated by Gram-positive bacteria, the prevalence
of Gram-negative agents has increased and Escherichia coli
has re-emerged as the most prevalent pathogen (8).
The major cause of morbidity and mortality worldwide
is septicaemia and this type of infection can lead to longer
periods of hospitalization and increased costs. It is estimated
that the incidence rate of cases treated for sepsis in hospitals is
427 cases and that of cases of severe sepsis is 331 cases per
100,000 person-years (9).
The prevalent pathogen implicated in septicaemia is
Staphylococcus aureus (SA). This bacterium can also lead to
the development of infective endocarditis, as well as
osteoarticular, skin and soft tissue infections, such as surgical
site infections (10),
pleuropulmonary manifestations (parapneumonic pleurisy) (11) and device-related infections (12). SA is implicated in 26% of cases of
native endocarditis in adults, and the number of cases is
increasing (13). This bacteria is
often associated with severe fungal infections caused by fungi,
such as Aspergillus or Fusarium (14). This clinical situation occurs
particularly in immunosuppressed patients, or in patients with
liver cirrhosis (15). With time,
treatment becomes difficult due to the increased antibiotic
resistance among the species isolated (16). As septicaemia is the most common
bacterial infection, particularly in immunocompromised patients, it
is important to determine the sensitivity and resistance of
bacterial strains to antibiotics. Certain geographical variations
in resistance have been described and in such cases, one must
select the adequate antibiotic for each bacterium isolated
(17). Blood cultures are essential
for the diagnosis of BSI when there is a suspicion of severe focal
infection that presents a risk of sepsis, as pyelonephritis,
bronchopneumonia or infectious endocarditis. Blood cultures are
also recommended for patients with severe septic syndrome,
prolonged fever, prosthetic valves and metabolic disorders, such as
diabetes.
The present study aimed to assess the epidemiology,
aetiology, and temporal changes of bacteraemia episodes in patients
from the County Clinical Emergency Hospital of Craiova, Romania, in
an aim to determine the incidence and prevalence rates of these
bacteria among patients with severe invasive infections.
Materials and methods
Study population
For the purpose of this study, from the hospital
electronic database, we extracted all blood cultures collected
between September 1, 2016 and July 31, 2017 from 505 patients (216
females and 289 males) hospitalized at the Clinical Emergency
Hospital of Craiova, Romania. The patients were hospitalized in
either the Intensive Care Unit (ICU; 272 patients), surgical wards
(General Surgery, Plastic Surgery, Orthopaedic, Paediatric Surgery,
Ophthalmology, Neurosurgery, Urology and Gynaecology wards; 47
patients), medical wards (Internal Medicine, Cardiology, Diabetes,
Nephrology and Neurology wards, 129 patients), paediatric wards (45
patients) and oncological wards (12 patients). A total of 974 blood
cultures were included in the study. The hospitalization time till
the collection of the first blood culture was between 1 and 30
days, with a mean of 7.18 days.
A community-acquired (CA) bloodstream infection was
defined as a positive blood culture in the first 48 h of admission,
excluding re-admissions when the final discharge was >48 h. A
hospital-acquired (HA) BSI was defined as a positive blood culture
collected after at least 48 h from admission to the hospital, or
within 48 h from the hospital discharge.
Microbiological methods
For collecting the blood cultures, we used
specialized bottles provided together with the automated system
Bactalert 3D® (bioMérieux SA, Marcy-l′Étoile, France),
which contain the special formulation of culture media that allows
the detection of both patients who did not receive antibiotic
therapy and of those treated with antibiotics. For each patient, a
set comprised of two culture bottles was collected, one for aerobic
bacteria and one for anaerobic bacteria. The system incubated the
bottles for up to 7 days and alerted the detection of growth.
Immediately following the detection, the positive bottle was
removed from the Bactalert 3D system and bacterial smears were
examined following Gram staining which shows the shape of the
bacteria (cocci or bacilli) and Gram grouping (Gram-positive and
Gram-negative). According to the aspect indicated, the bacteria
were inoculated on a Columbia blood agar plate, MacConkey and
Sabouraud media (all from bioMérieux SA) with incubation at 37°C
for 24 h.
The identification of isolated microorganisms was
performed using the system Vitek 2 Compact® (bioMérieux
SA) from the 24-h cultures on appropriate media based on the
biochemical properties of bacteria assessed in specially designed
cards with reagents that will change the colour after the enzymatic
reaction. The cards are scanned every 15 min inside the system for
colour change and once an identification is made, the card is
ejected. The time for identification ranges from 4 to 14 h. We used
the identification cards, GP (for Gram-positive bacteria) and GN
(for Gram-negative bacteria).
The Vitek 2 Compact® system was also used
for antimicrobial susceptibility testing (AST), according to the
guidelines of the current Clinical Laboratory Standards Institute
(CLSI) (18), using the cards
AST-N204 for Gram-negative bacteria of the genus Escherichia
coli, Klebsiella spp. and Enterobacter spp., AST-N222
for Gram-negative glucose non-fermenters bacteria, AST-P592 for
Gram-positive bacteria of the genus Staphylococcus spp., and
AST-GP67 for Enterococcus spp., Streptococcus spp.
and other Gram-positive bacteria. The base for automated bacterial
antibiotic susceptibility is the antibiotic microdilution method:
The bacterial strain is cultured in each of the cells from the card
that contain various concentrations of antibiotics. Each cell is
read 3 times once in 15 min, and each reading uses 16 points to
determine the absorbance of the cell, that is proportional with the
concentration of bacteria. From these data for each strain, the
minimum inhibitory concentration (MIC) for each antibiotic was
determined, defined as the minimal concentration of antibiotic that
stops bacterial growth in liquid culture.
Following the primary reading of the AST results,
the software delivered with the Vitek 2 Compact®
analysed the resistance data using an advanced expert system (AES),
that uses an exhaustive database of strains and MICs to validate
the results and to interpret them, according to current CLSI
guidelines. The final result includes both the MIC value obtained
for each antibiotic and the interpretation as susceptible,
intermediate resistant or resistant, that is of clinical value.
Moreover, AES can classify the microorganisms in resistance
phenotypes and detect the resistance mechanisms, as for example
Extended Spectrum Beta-Lactamase (ESBLs) production.
Data and statistical analysis
The computerised hospital records of all eligible
patients were retrieved and reviewed from the hospital electronic
database, which contained clinical and laboratory data of all
patients admitted to our hospital. The access to the database for
the purpose of this study was approved by the Ethics Committee of
Clinical County Emergency Hospital of Craiova, Romania. The patient
specific data was depersonalised (e.g., names were not recorded,
ages were approximated as greater or lower than 70 years).
Candidate variables included demographic data, hospitalization time
and diagnostics. Patients whose age was <18 years were
considered children, and patients whose age was >18 years were
considered adults.
Additionally, data related to blood culture,
including the number of blood specimens used for culture, the
number of positive cultures (if any), and the identification of all
cultured isolates and their antimicrobial susceptibility, were also
retrieved. The data were entered into STATA statistical software
version 13.1 (StataCorp LP, College Station, TX, USA).
These patient groups were compared in terms of their
demographic characteristics, underlying diseases, pathogens
isolated and outcomes. The comparisons between the prevalence of
infections in patient groups included the calculation of risk
ratios and the significant difference between the groups was
assessed using the Chi-squared test. In addition, the Chi-square
test was used for the comparison of the prevalence of various
pathogens in CA and HA invasive infections, or the prevalence of
bacteraemia in groups defined by the presence or absence of the
risk factors. The trend of increasing or decreasing prevalence with
various infectious agents in consecutive months was tested using
the Chi-square test for trend. The monthly relative prevalence
rates were calculated in each month by dividing the number of
patients with blood cultures positive for a certain bacteria by the
number of patients with positive blood cultures in that month.
For the calculation of the risk of invasive
infection and the mortality risk, and for the comparison of those
between the groups we used Kaplan-Meyer survival analysis. The
specialized procedures of STATA for survival analysis were used to
calculate the incidence rates and the incidence rates ratio.
Briefly, for each patient, we counted the number of days that
patient remained in the hospital till the development of infection
or death. All the days for all the patients were counted and then
summed up. In a similar manner, the total number of patient/days
that all patients spent in the hospital was calculated. The ratio
between the two numbers is the incidence rate. The comparisons
between patient groups defined by binary risk factors (e.g.,
diabetes) were performed using the log-rank test, which compares
the equality of survivor functions in the 2 groups.
Following the initial Kaplan-Meyer survival
analysis, we used a Cox regression model to quantify the effect of
various risk factors on the risk of invasive infection and death,
measured by the Hazard Ratio (HR). The performance of the Cox
regression model was assessed by the calculation of sensitivity and
specificity, followed by the construction of the receiver operator
characteristic (ROC) curve and the calculation of the area under
the curve (AUC) as the main measure of model fit and
performance.
The multiple antibiotic resistance index (MAR) was
calculated as the ratio between the number of resistant antibiotics
and the total number of antibiotics tested for a given strain. The
mean MARs in patients from different wards were compared by the
analysis of variance (ANOVA) test, with the Bonferroni correction
(19). When we had only 2 types of
wards, as the patients were only from 2 different types of wards,
we used the Student's t-test instead.
We calculated the average MAR per ward type by
adding the MAR values for all patients hospitalised in wards
belonging to a certain ward type and then divided by that the
number of patients. We used multivariate logistic regression to
analyse the risk factors for invasive infection or death, expressed
as the odds ratio (OR). We used a logistic model with the dependent
variable infection or death, in which we entered all the
independent variables together: sex, age, ward type, diagnosis.
Continuous data are expressed as the mean ± standard deviation. All
the statistical tests, comparisons and regression coefficients were
considered significant if the probability to reject the null was
P<0.05.
Results
A total of 140 from the 505 patients analysed had
positive blood cultures. In total, we collected 974 blood cultures,
from which we isolated 170 bacterial strains. The prevalence rate
of invasive bloodstream infections was 140/505, 27.72%. The
incidence rate of invasive infections was 5.75 per 100
patient-days. The incidence rate did not differ significantly
between males and females (P=0.185) or between patients <70
years of age and those >70 years (P=0.491), but was
significantly higher in patients with diabetes (incidence rate
ratio, 1.49; P=0.072) (Table I). The
time from admission until the collection of a positive blood
culture varied between 0 and 30 days, with a mean of 5.27 days. A
total of 26 patients of the 140 (18.57%) had a positive blood
culture in the first day, 42 patients (30.00%) in the first 2 days
and 62 patients (44.28%) in the first 5 days. The hazard function
from the survival analysis model revealed that the cumulative risk
of infection was high in the first 2 days from admission, as these
were patients with CA invasive infections. This then exhibited a
decrease in day 3 and began to mildly increase beginning at day 4.
The invasive infections that are diagnosed from day 3 of
hospitalization are considered HA and their risk increase
proportionally with the number of days spent in the hospital. In
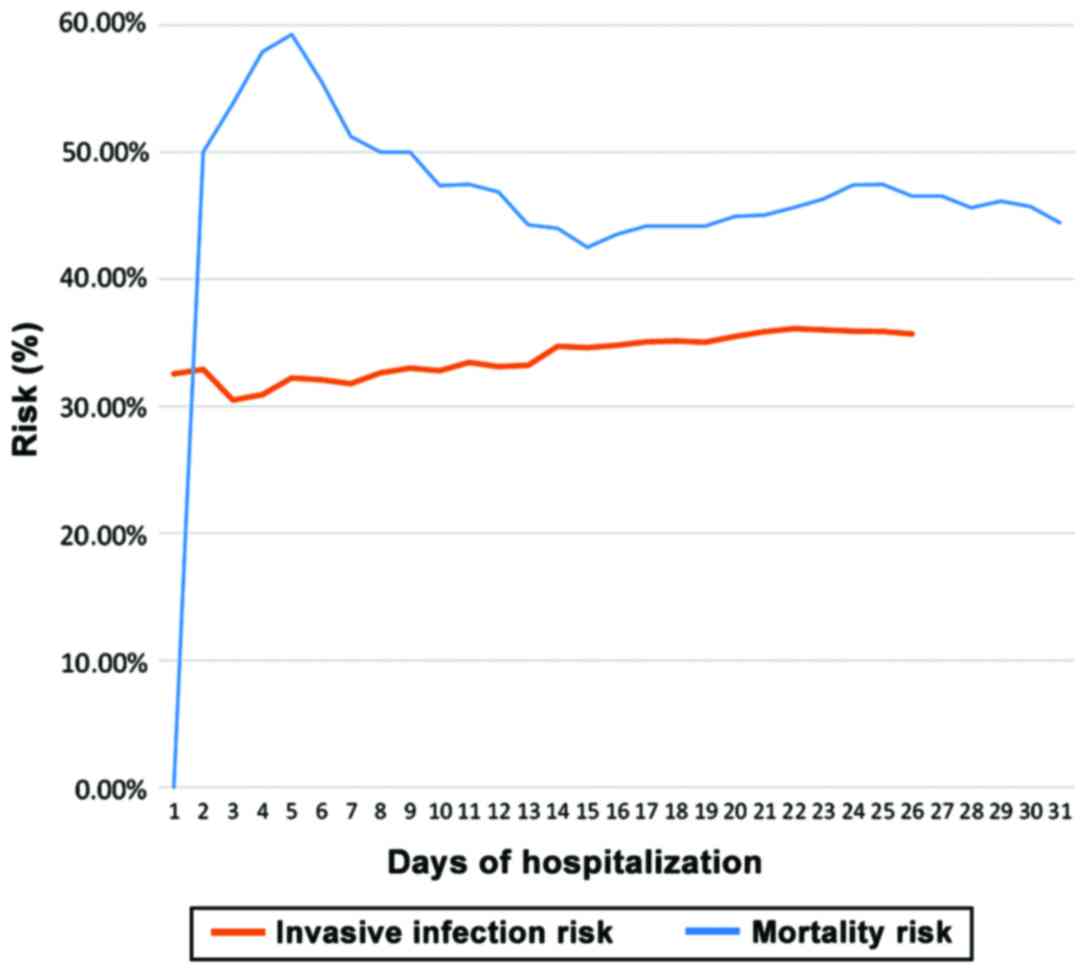
this study, the infection risk remained steady beginning at day 14
of hospitalization (Fig. 1).
 | Table I.Incidence rates of the invasive
infection and death broken down by risk factors. |
Table I.
Incidence rates of the invasive
infection and death broken down by risk factors.
|
| Invasive infection
risk | Mortality risk |
|---|
|
|
|
|
|---|
|
| Incidence rate (per
100 patient-days) | Incidence rate
ratio |
P-valuea | Incidence rate (per
100 patient-days) | Incidence rate
ratio |
P-valuea |
|---|
| Global incidence
rate | 5.75 |
|
| 5.40 |
|
|
| Risk factor |
| Diabetes |
|
Present | 6.81 | 1.49 | 0.072 | 3.19 | 0.49 | 0.028b |
|
Absent | 4.56 |
|
| 6.51 |
|
|
| Sex |
|
Female | 0.65 | 1.22 | 0.185 | 6.09 | 1.52 | 0.332 |
|
Male | 0.53 |
|
| 4.00 |
|
|
| Age |
| ≥70
years | 5.45 | 1.10 | 0.491 | 7.48 | 1.68 | 0.037b |
| <70
years | 6.02 |
|
| 4.45 |
|
|
| ICU admission |
|
Yes | 6.31 | 0.96 | 0.798 | 7.57 | 4.88 |
<0.001b |
| No | 6.56 |
|
| 1.55 |
|
|
In total, 140 patients developed bacteraemia, 35
(25,00%) in the first 2 days from admission, 88 (62,85%) in the
first seven days from admission and 132 (94,29%) in the first 5
days. The incidence rate of death was 5.40 per 100 patient-days.
The infection risk was not markedly related with sex, age,
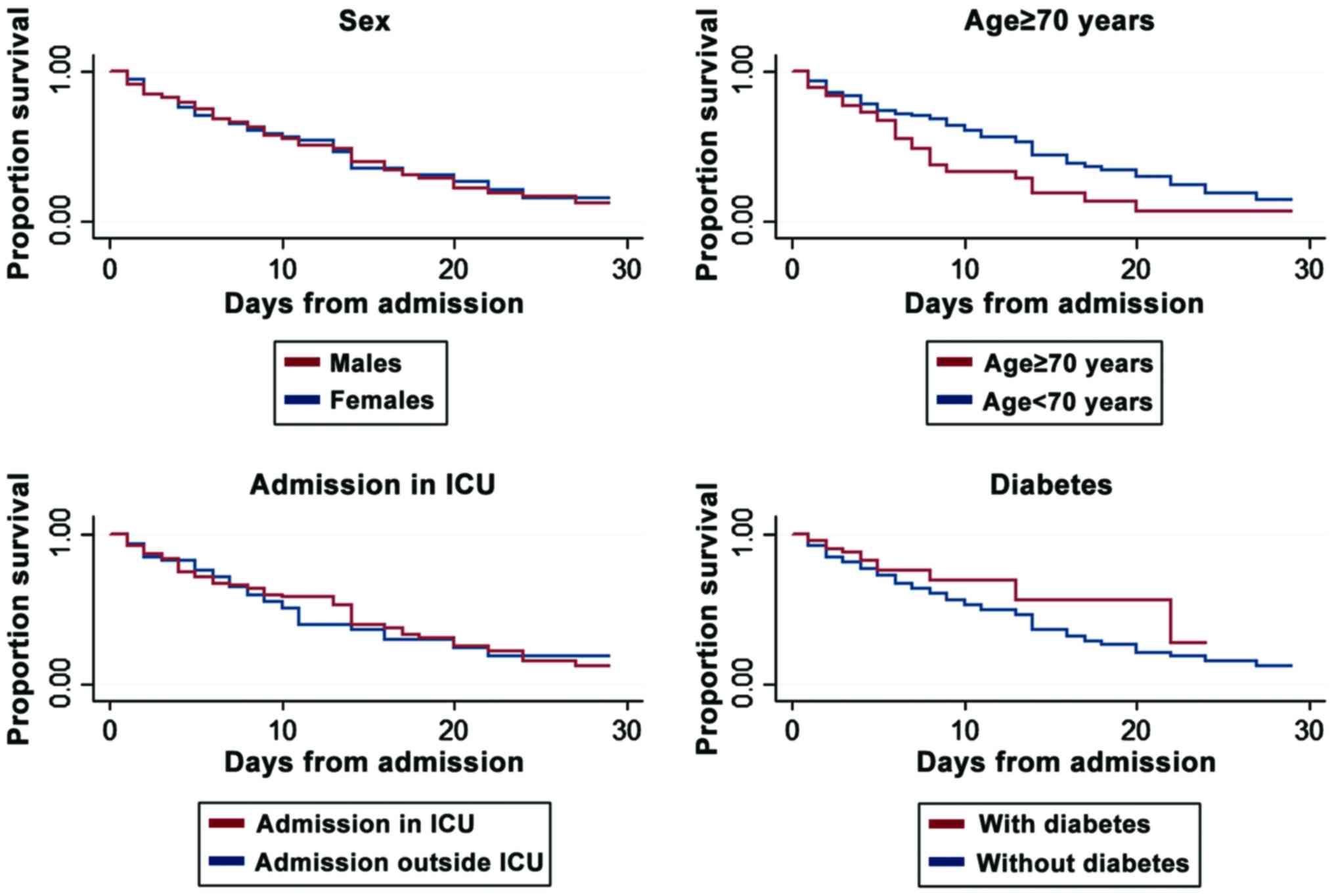
admission to the ICU or diabetes. Nevertheless, The Kaplan-Meyer
survival curves revealed a weak association of infection risk with
age ≥70 years and diabetes (Fig.
2).
In total, 21 patients of the 140 (15.00%) died, 5
(3.57%) in the first day, 7 (5.00%) in the first 2 days and 15
(10.71%) in the first 5 days. The death risk was significantly
higher for patients aged ≥70 years [incidence rate ratio (IRR),
1.68; P=0.037] and for patients admitted to the ICU (IRR, 4.88;
P<0.001), and lower for diabetic patients (IRR, 0.49; P=0.028),
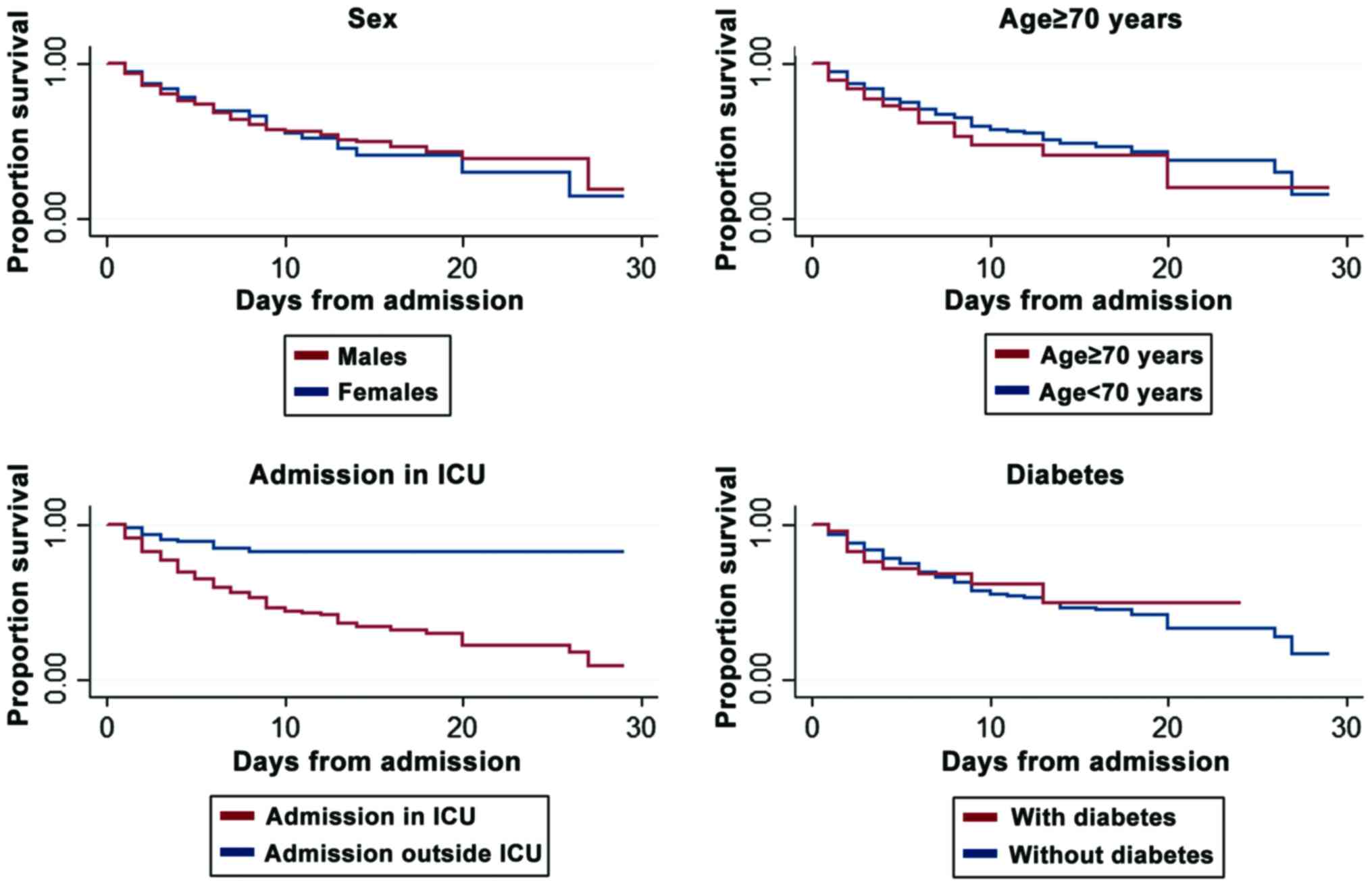
but not for females vs. males (P=0.332) (Table I). The Kaplan-Meyer survival curves
revealed an association of 30 days mortality risk with age,
admission to the ICU and diabetes (Fig.
3). The death risk was 0 in the first day, as none of the
patients died on the day of admission. The death risk then
increased over the first 5 days due to mortality associated with
the cause of hospitalization and the rapid evolution of CA invasive
infections. The majority of the patients for which blood cultures
were collected during the first days of hospitalization were
admitted for serious life-threatening conditions, such as traffic
accidents and other types of trauma injuries and fulminant
endocarditis, that explain the high mortality risk during the first
days from admission. After day 5, the death risk exhibited a marked
decrease until day 15, then it remained relatively steady till day
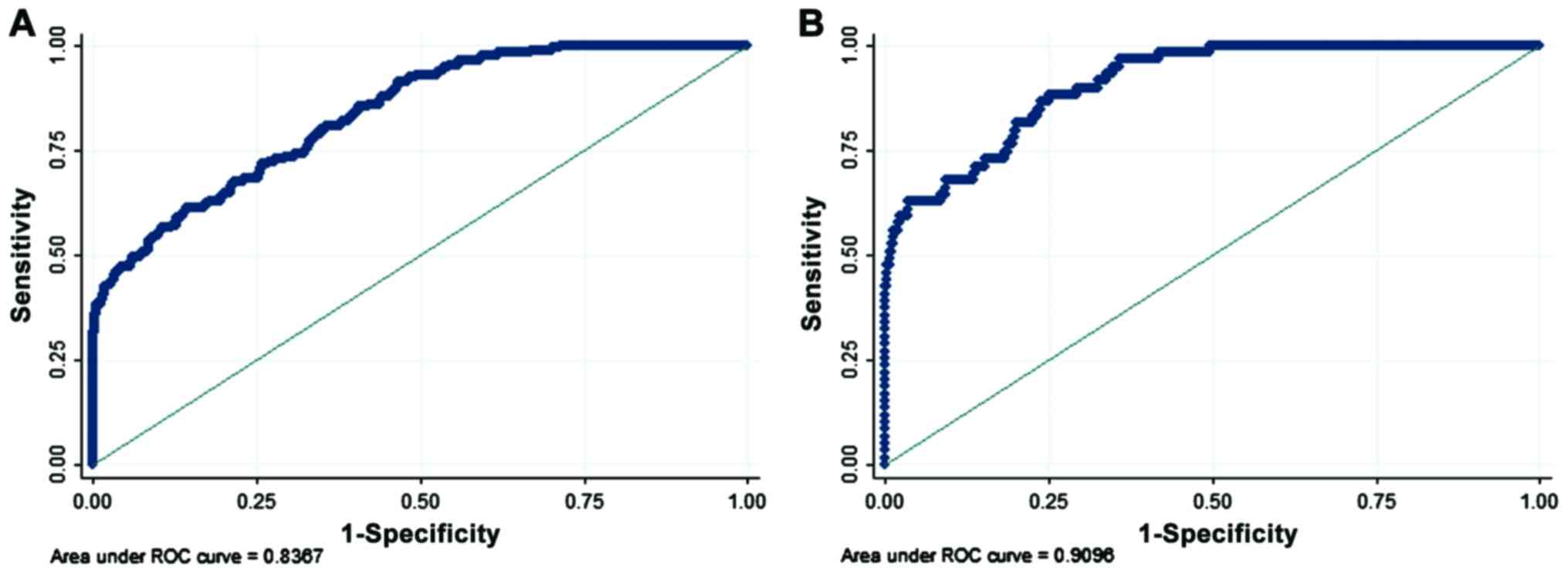
31 (Fig. 1). The survival analysis
Cox regression model had a good fit, and the AUC was 0.8367 for the
infection risk and 0.9096 for the mortality risk (Fig. 4).
We isolated 170 bacterial strains, and the most
frequent were SA (63 strains, 37.06%), Klebsiella spp. (27
strains, 15.88%), coagulase-negative staphylococci (CoNS) that
included Staphylococcus capitis, Staphylococcus hominis and
Staphylococcus lungdunensis (18 strains, 10.59%),
Enterococcus spp. (17 strains, 10.00%), Escherichia
coli (12 strains, 7.06%), Streptococcus spp., that
included Streptococcus pneumoniae, Streptococcus agalactiae
(11 strains, 6.47%), non-fermenters such as Burkholderia
cepacia (8 strains, 4.71%), Acinetobacter baumannii (9
strains, 5,29%) and other bacterial species (Table II).
 | Table II.Aetiology of invasive bloodstream
infections by hospital ward. |
Table II.
Aetiology of invasive bloodstream
infections by hospital ward.
| Ward | E. coli | Klebsiella
spp. | Burkholderia
cepacia | SA | Enterobacter
cloacae |
Streptococcus spp. | CoNS | Proteus
mirabilis | Enterococcus
spp. | Acinetobacter
baumannii | Bacillus
subtilis | Clostridium
subterminale | Total positive by
ward |
|---|
| ICU | 4 | 13 | 3 | 29 | 1 | 5 | 7 | 2 | 6 | 3 | 1 | 1 | 75 |
|
| (5.33%) | (17.33%) | (4.00%) | (38.67%) | (1.33%) | (6.67%) | (9.33%) | (2.67%) | (8.00%) | (4.00%) | (1.33%) | (1.33%) | (100%) |
| Plastic
σurgery | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
|
| (0%) | (0%) | (0%) | (100%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Medical wards | 1 | 5 | 0 | 6 | 0 | 0 | 1 | 0 | 3 | 2 | 0 | 0 | 18 |
|
| (5.56%) | (27.78%) | (0%) | (33.33%) | (0%) | (0%) | (5.56%) | (0%) | (16.67%) | (11.11%) | (0%) | (0%) | (100%) |
| Paediatric
wards | 0 | 0 | 2 | 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 10 |
|
| (0%) | (0%) | (20.00%) | (70.00%) | (0%) | (0%) | (10.00%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Cardiology | 1 | 6 | 0 | 4 | 0 | 4 | 3 | 0 | 2 | 3 | 0 | 0 | 23 |
|
| (4.35%) | (26.09%) | (0%) | (17.39%) | (0%) | (17.39%) | (13.04%) | (0%) | (8.70%) | (13.04%) | (0%) | (0%) | (100%) |
| Diabetes | 3 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 7 |
|
| (42.86%) | (14.29%) | (0%) | (14.29%) | (0%) | (14.29%) | (14.29%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Gynaecology | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
|
| (0%) | (0%) | (50%) | (50.00%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Nephrology | 3 | 2 | 0 | 9 | 0 | 0 | 3 | 0 | 5 | 1 | 0 | 0 | 23 |
|
| (13.04%) | (8.70%) | (0%) | (39.13%) | (0%) | (0%) | (13.04%) | (0%) | (21.74%) | (4.35%) | (0%) | (0%) | (100%) |
| Oncology | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
|
| (0%) | (0%) | (0%) | (33.33%) | (0%) | (33.33%) | (33.33%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Neurology | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
|
| (0%) | (0%) | (100%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (100%) |
| Urology | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 7 |
|
| (0%) | (0%) | (14.29%) | (57.14%) | (0%) | (0%) | (14.29%) | (0%) | (14.29%) | (0%) | (0%) | (0%) | (100%) |
| Total positive | 12 | 27 | 8 | 63 | 1 | 11 | 18 | 2 | 17 | 9 | 1 | 1 | 170 |
| by species | (7.06%) | (15.88%) | (4.71%) | (37.06%) | (0.59%) | (6.47%) | (10.59%) | (1.18%) | (10.00%) | (5.29%) | (0.59%) | (0.59%) | (100%) |
SA strains had a prevalence of 6.67% in the medical
wards, slightly lower than the prevalence of 9.23% in the surgical
wards, but without statistical significance (P=0.478). The
prevalence of SA was slightly lower in the ICU (5.64%) than in the
other wards (7.71%), but without reaching statistical significance
(P=0.198). The prevalence of SA invasive infections did not differ
significantly between adults and children (P=0.406), or between
males and females (P=0.085) (Table
III).
 | Table III.Prevalence rates of bacteraemia with
Staphylococcus aureus and MRSA by age, hospitalization
status (inpatient/outpatient), ward type and sex. |
Table III.
Prevalence rates of bacteraemia with
Staphylococcus aureus and MRSA by age, hospitalization
status (inpatient/outpatient), ward type and sex.
|
| S. aureus
infection |
| MRSA infection |
|
|---|
|
|
|
|
|
|
|---|
| Variable/or risk
factor | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| All 974
samples | 63 (6.47%) | 911 (93.53%) | – | 23
(36.51/2.36%)a | 951
(62.49/97.64%) | – |
| Sex |
| Male
(548 samples) | 42 (7.66%) | 506 (92.34%) | 0.085 | 15
(36.51/2.74%)a | 533
(64.29/97.26%) | 0.853 |
| Female
(426 samples) | 21 (4.93%) | 405 (95.07%) |
| 8
(38.10/1.88%)a | 418
(61.90/98.12%) |
|
| Age |
| Adults
(893 samples) | 56 (6.27%) | 837 (93.73%) | 0.406 | 21
(37.50/2.35%)a | 872
(62.50/97.65%)a | 0.047b |
|
Children (81 samples) | 7
(8.64%) | 74
(91.36%) |
| 2
(28.57/2.47%)a | 79
(71.43/97.53%)a |
|
| Type of ward |
| Medical
(240 samples) | 16 (6.67%) | 224 (93.33%) | 0.478 | 5
(31.25/1.88%)a | 235
(68.75/98.12%) | 0.967 |
|
Surgical (65 samples) | 6
(9.23%) | 59
(90.77%) |
| 2
(33.33/3.08%)a | 63
(66.67/96.92%)a |
|
| ICU admission |
| ICU
(585 samples) | 33 (5.64%) | 552 (94.36%) | 0.198 | 14
(42.42/2.39%)a | 571
(57.58/97.61%)a | 0.018b |
| Non-ICU
(389 samples) | 30 (7.71%) | 359 (92.29%) |
| 9
(30.00/2.31%)a | 380
(70.00/97.69%)a |
|
The methicillin-resistant SA (MRSA) prevalence in
patients from our study was 36.51% from the SA strains or 2.36%
from the total samples analysed. There were significant differences
in MRSA prevalence between adults and children (37.50 vs. 28.57%,
P=0.047) and between ICU and non-ICU patients (42.42 vs. 30.00%,
P=0.018) (Table III).
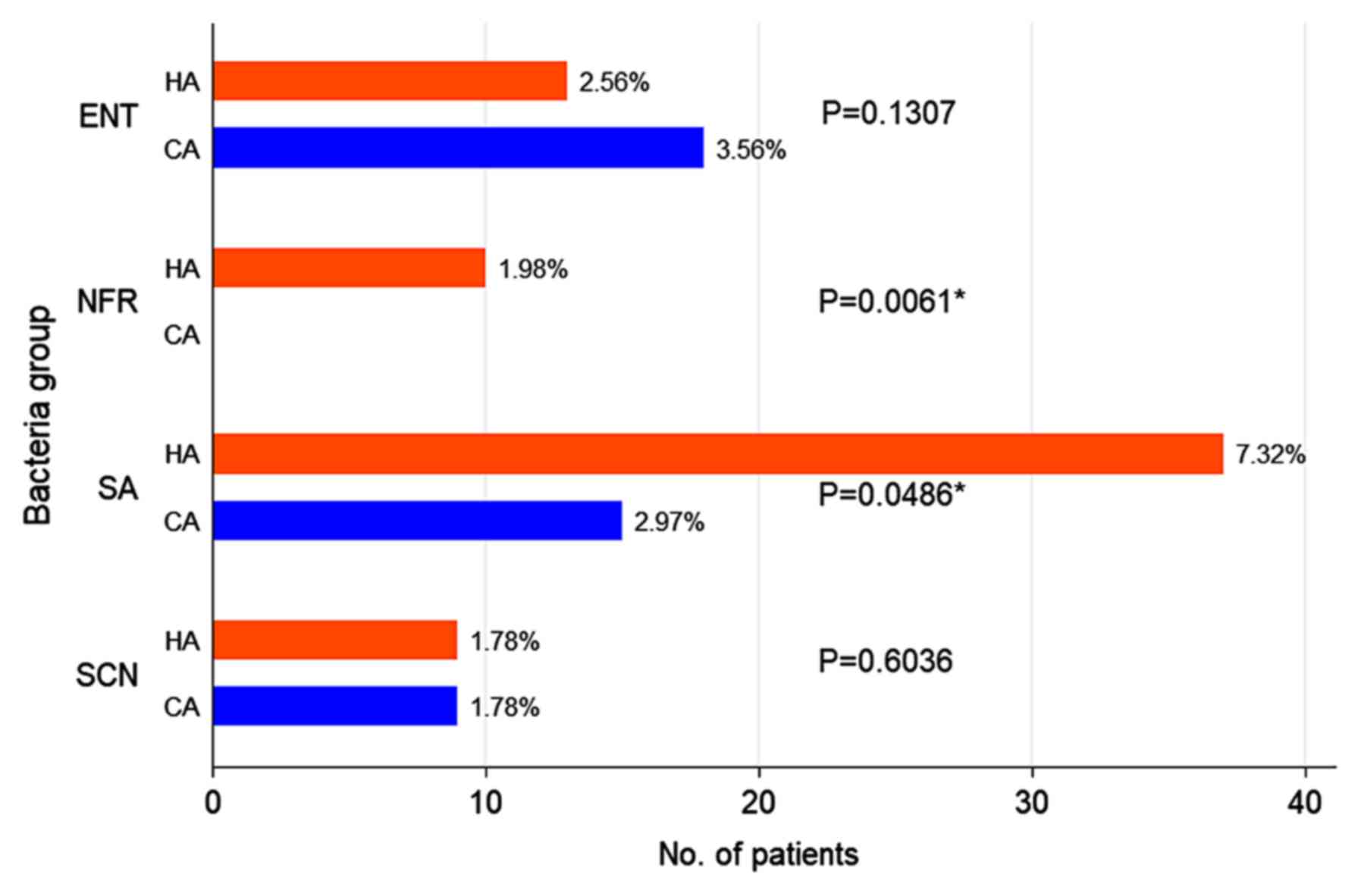
The CA infections had a different aetiology compared
with the HA infections. The number of HA infections with SA was
more than double compared with the CA SA infections (P=0.049). The
non-fermenters Gram-negative rods produced only HA infections
(P=0.006). The prevalence of CA and HA infections with
Enterobacteriaceae and coagulase-negative staphylococci did not
differ significantly (Fig. 5).
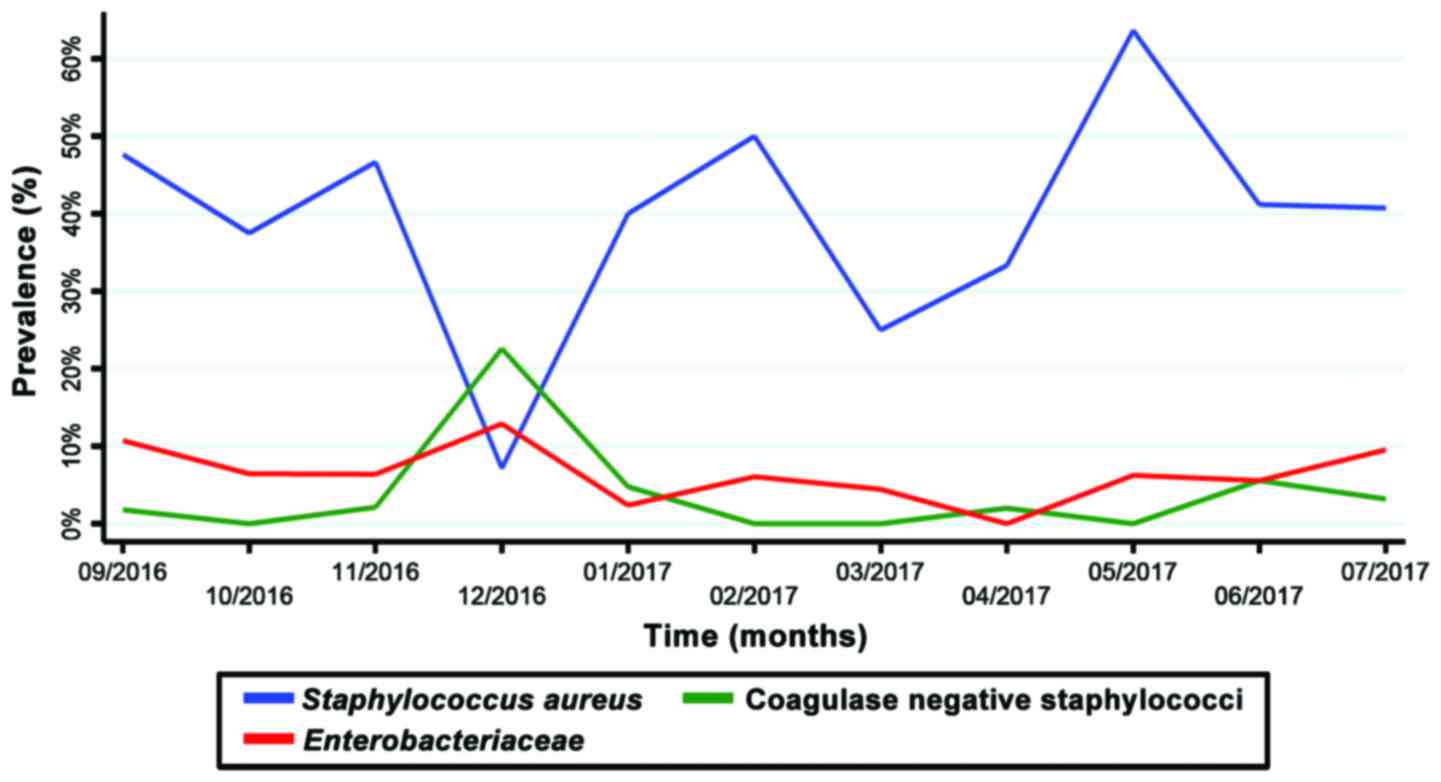
The relative prevalence of SA strains displayed a
seasonal variation (Chi-square test for trend slope, 0.0121;
P=0.006) and the highest prevalence was recorded in May, 2017
(Fig. 6). The prevalence of
infections with Enterobacteriaceae and coagulase negative
staphylococci was generally under 10%, without a clear trend
(Fig. 6).
Using the associated medical conditions of the
patients, we aimed to identify risk factors for invasive BSI. In
this regard, we analysed neurological, cardiac and renal
conditions, trauma, surgical procedures and various confirmed
infection sites that may be the point of origin of sepsis. The
positive blood culture rates differed by diagnosis, the highest
rate being in the surgical patients (71.87%) and patients with
central nervous system diseases (55.84%). Lower rates (under 50%)
were recorded for patients with trauma, chronic kidney disease,
cancer and other chronic diseases. The data are presented in
Table IV. We found the greatest
risk for invasive infections in patients subjected to surgery in
the last 7 days [risk ratio (RR), 2.29; P<0.001), who had
neurological conditions (RR, 1.87; P<0.0001) or who suffered
from trauma (RR, 1.29; P<0.001).
 | Table IV.The prevalence of bacteraemia in
different clinical conditions. |
Table IV.
The prevalence of bacteraemia in
different clinical conditions.
| Diagnostic | Samples | Culture positive
(n=170) | Culture negative
(n=804) | Risk ratio | P-value |
|---|
| Neurological
conditions | 77 | 43 (55.84%) | 34 (44.16%) | 1.87 |
<0.001a |
| Cardiac
conditions | 168 | 62 (36.90%) | 106 (63.10%) | 1.14 | 0.329 |
| Trauma | 35 | 15 (42.86%) | 20 (67.14%) | 1.29 |
<0.001a |
| Chronic renal
failure | 107 | 43 (40.19%) | 64 (59.81%) | 1.24 | 0.127 |
| Cancer | 39 | 15 (38.46%) | 24 (61.54%) | 1.14 | 0.542 |
| Chronic
diseases | 105 | 34 (32.38%) | 71 (67.62%) | 0.94 | 0.688 |
| Surgery <7
days | 32 | 23 (71.87%) | 9 (28.13%) | 2.29 |
<0.001a |
As part of the survival analysis, we also performed
a Cox proportional hazards regression which yielded the risk of
infection associated with each risk factor (Table V) and also the risk of mortality in
30 days (Table VI). The most
important risk factors for invasive infections were surgery in the
prior 7 days (HR, 5.06; P<0.001), neurological conditions (HR,
4.29; P<0.001), chronic renal failure (HR, 3.43; P<0.001),
cardiac conditions (HR, 1.92; P=0.002) and an age >70 years (HR,
1.76, P=0.018) (Table V). The most
important risk factors for mortality were admission into the ICU
(HR, 4.08; P<0.001), neurological conditions (HR, 4.05;
P<0.001), chronic renal failure (HR, 3.26; P=0.003), surgery in
the prior 7 days (HR, 2.95, P=0.011) and cancer (HR, 2.42; P=0.032)
(Table VI).
 | Table V.Proportional hazards Cox regression
model of the risk factors for invasive infections. |
Table V.
Proportional hazards Cox regression
model of the risk factors for invasive infections.
| Risk factor | Hazard ratio | Sth. Err. | Z | P>z | 95% CI |
|---|
| Female sex | 0.94 | 0.20 | −0.29 | 0.771 | 0.62 | 1.42 |
| Age ≥70 years | 1.76 | 0.42 | 2.37 | 0.018a | 1.10 | 2.82 |
| Diabetes | 0.57 | 0.16 | −1.99 | 0.046a | 0.32 | 0.99 |
| ICU admission | 1.02 | 0.20 | 0.12 | 0.907 | 0.70 | 1.50 |
| Neurological
conditions | 4.29 | 1.01 | 6.17 |
<0.001a | 2.70 | 6.82 |
| Cardiac
conditions | 1.92 | 0.40 | 3.09 | 0.002a | 1.27 | 2.90 |
| Trauma | 1.11 | 0.37 | 0.31 | 0.758 | 0.58 | 2.12 |
| Chronic renal
failure | 3.43 | 1.03 | 4.11 |
<0.001a | 1.90 | 6.16 |
| Cancer | 1.12 | 0.35 | 0.36 | 0.719 | 0.60 | 2.09 |
| Other chronic
diseases | 0.39 | 0.13 | −2.9 | 0.004a | 0.21 | 0.73 |
| Surgery <7
days | 5.06 | 1.39 | 5.88 |
<0.001a | 2.95 | 8.68 |
 | Table VI.Proportional hazards Cox regression
of the risk factors for 30 days mortality. |
Table VI.
Proportional hazards Cox regression
of the risk factors for 30 days mortality.
| Factor | Hazard ratio | Sth. Err. | Z | P>z | 95% CI |
|---|
| Female sex | 1.32 | 0.38 | −0.98 | 0.328 | 0.430561 | 1.325547 |
| Age ≥70 years | 1.26 | 0.37 | 0.79 | 0.429 | 0.708051 | 2.253894 |
| Diabetes | 0.38 | 0.23 | −1.60 | 0.109 | 0.115269 | 1.241342 |
| ICU admission | 4.08 | 1.49 | 3.85 |
<0.001a | 1.993626 | 8.356247 |
| Neurological
conditions | 4.05 | 1.28 | 4.43 |
<0.001a | 2.179642 | 7.513619 |
| Cardiac
conditions | 1.13 | 0.37 | 0.38 | 0.705 | 0.599142 | 2.131884 |
| Trauma | 1.24 | 0.59 | 0.46 | 0.646 | 0.491327 | 3.146228 |
| Chronic renal
failure | 3.26 | 1.30 | 2.96 | 0.003a | 1.491949 | 7.136156 |
| Cancer | 2.42 | 0.99 | 2.15 | 0.032a | 1.079461 | 5.408272 |
| Other chronic
diseases | 0.29 | 0.15 | −2.41 | 0.016a | 0.104955 | 0.791273 |
| Surgery <7
days | 2.95 | 1.26 | 2.53 | 0.011a | 1.27457 | 6.811629 |
The multiple antibiotic resistance (MAR) index is an
accurate measure of antibiotic resistance of a given strain. We
calculated the mean MAR for each species, broken down by ward type.
The data are presented in Table
VII. There were significant differences in resistance between
the wards only for Klebsiella spp., that had a higher
resistance in the ICU compared with the medical wards (P=0.008),
due to its high ability to acquire resistance plasmids by
horizontal gene transfer and for SA that is easily acquired from
the hospital environment and has a high capacity to modify the
resistance profile during antibiotic treatment administered in the
hospital. The resistance of SA was significantly higher in the ICU
than in the medical wards (P=0.020).
 | Table VII.The multiple antibiotic resistance
index (MAR) of isolated bacterial species, broken down by ward
type. |
Table VII.
The multiple antibiotic resistance
index (MAR) of isolated bacterial species, broken down by ward
type.
| Bacterial
species | All wards | ICU (2 wards) | Surgical (12 wards)
(P-valuea) | Medical (6
wards) | Paediatric (2
wards) | Oncologic (2
wards) |
P-valueb |
|---|
| E. coli | 28.90±15.99% | 26.77±19.70% | – | 30.43±14.27% | – | – | 0.7156 |
|
|
|
|
| (1.000) |
|
|
|
| Klebsiella
spp. | 53.87±27.13% | 63.68±23.48% | – | 30.58±20.89% | – | – | 0.0020c |
|
|
|
|
| (0.008) |
|
|
|
|
Burkholderia | 72.22±28.79% | 78.57±22.85% | 47.62±53.87% | 94.44±0.00% | 76.19±0.00% | – | 0.6344 |
| cepacia |
|
| (1.000) | (1.000) | (1.000) |
|
|
| SA | 52.83±20.67% | 58.03±17.38% | 57.91±19.29% | 49.70±23.87% | 33.53±17.48% | 35.71±0.00% | 0.0405c |
|
|
|
| (0.898) |
(0.020)d | (1.000) | (1.000) |
|
| Enterobacter
cloacae | 78.94±0.00% | 78.94±0.00% | – | – | – | – | – |
|
Streptococcus spp. | 26.11±25.82% | 24.96±27.58% | – | 21.43±0.00% | – | 40.00±0.00% | 0.8649 |
|
|
|
|
| (1.000) |
| (1.000) |
|
| CoNS | 38.20+18.69% | 40.36±14.89% | 28.57±0.00% | 36.11±25.58% | 13.33±0.00% | 61.54±0.00% | 0.4752 |
|
|
|
| (1.000) | (1.000) | (1.000) | (1.000) |
|
| Proteus
mirabilis | 70.09±0.00% | 70.09±0.00% | – | – | – | – | – |
| Enterococcus
spp. | 70.07±19.36% | 69.32±23.23% | 80.00±0.00% | 69.58±17.38% | – | – | 0.8837 |
|
|
|
| (1.000) | (1.000) |
|
|
|
|
Acinetobacter | 79.39±9.30% | 77.19±10.64% | – | 83.79±4.41% |
| – |
|
|
baumannii |
|
|
| (1.000) |
|
|
|
| Bacillus
subtilis | 27.78±0.00% | 27.78±0.00% | – | – | – | – | – |
| Clostridium
Subterminale | 0.00±0.00% | 0.00±0.00% | – | – | – | – | – |
The multivariate analysis of the risk of acquisition
an invasive infection with any bacteria (Table VIII) demonstrated a significant
effect of admission into the plastic surgery ward (OR=1.444,
P=0.082) and cardiology ward (OR, 2.313; P=0.001).
 | Table VIII.Results of the multivariate logistic
regression analysis on the risk of invasive infection with any
bacteria and in particular with MRSA. |
Table VIII.
Results of the multivariate logistic
regression analysis on the risk of invasive infection with any
bacteria and in particular with MRSA.
|
| Any invasive
infection | Invasive infection
with MRSA |
|---|
|
|
|
|
|---|
| Risk factor | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Male sex | 0.681
(0.42–0.99) | 0.075 | 1.684
(0.98–2.52) | 0.080a |
| Age >50
years | 1.003
(0.63–1.78) | 0.989 | 2.134
(0.99–5.73) | 0.055a |
| Ward type |
|
ICU | 0.687
(0.25–2.36) | 0.487 | 1.518
(0.96–3.02) | 0.058a |
| Plastic
surgery | 1.444
(0.98–1.98) | 0.082a | 3.299
(1.53–5.99) | 0.044a |
| Medical
wards | 1.134
(1.01–4.69) | 0.833 | 0.278
(0.12–1.06) | 0.075a |
|
Paediatric wards | 0.902
(0.24–3.57) | 0.875 | 0.805
(0.33–1.88) | 0.774 |
|
Cardiology | 2.313
(0.26–3.94) | 0.001a | 2.088
(1.34–5.65) | 0.009a |
|
Diabetes | 0.839
(0.18–3.16) | 0.806 | 0.153
(0.06–1.83) | 0.103 |
|
Gynaecology | 0.346
(0.06–2.63) | 0.257 | 0.704
(0.25–2.34) | 0.772 |
|
Nephrology | 1.265
(0.32–4.56) | 0.711 | 0.528
(0.02–2.21) | 0.348 |
|
Oncology | 0.462
(0.13–3.87) | 0.377 | 0.431
(0.11–4.32) | 0.467 |
|
Neurology | 0.1616
(0.01–3.01) | 0.195 | – | – |
| Diagnosis |
|
Neurological conditions | 4.543
(2.33–6.77) |
<0.001a | – | – |
| Heart
conditions | 1.446
(0.45–3.02) | 0.163 | – | – |
|
Trauma | 2.146
(0.95–3.01) | 0.088 | – | – |
| Renal
conditions | 1.492
(0.55–2.42) | 0.236 | – | – |
|
Neoplasms | 2.117
(0.99–5.66) | 0.054 | – | – |
| Chronic
conditions | 0.724
(0.44–3.33) | 0.378 | – | – |
| Constant | 0.672
(0.42–0.70) | <0.001 | 0.070
(0.02–0.11) | 0.001 |
| Area under the ROC
curve | 0.8367 | 0.9096 |
The multivariate analysis of the risk of acquisition
an invasive infection with MRSA (Table VIII) demonstrated a significant
effect of the male sex (OR, 1.684; P=0.080), an age >50 years
(OR, 2.134; P=0.050), admission into the ICU (OR, 1.518; P=0.058),
plastic surgery ward (OR, 3.299; P=0.044), medical wards (OR,
0.278; P=0.075) and cardiology (OR, 2.088; P=0.009).
Discussion
In our study, the highest prevalence of bacteraemia
was found in patients subjected to recent surgery (71.87%), which
had an increased risk for acquisition of bacterial strains during
or after surgery by inappropriate wound care. In neurological
patients, the bacteraemia rate was also high due to prolonged
immobilization, which is a risk factor for pulmonary or urinary
tract infections, both with invasive potential. The bacteraemia
prevalence was also relatively high in patients with chronic
debilitating diseases (chronic renal failure and cancer) which
lower the immunity that allows the invasion of blood by germs from
various sites. The prevalence of invasive infections was also high
in patients with neurological conditions (53.52%), mostly stroke,
patients that cannot move easily and have urinary catheters, so
they have a high risk of developing lung and kidney infections that
can reach the blood, on the background of lowered immunity due to
hospital stay and lack of activity. We found that the most
prevalent infectious agent was SA, similar with the study conducted
by Ungureanu et al (20).
Although there were minor differences between the prevalence of SA
between patients admitted to the ICU and those admitted to other
wards, the prevalence of MRSA was significantly higher in the
ICU.
The low resistance rate of SA isolated from children
is easily explained by the fact that these are mostly SA strains
carried by the patient, usually in the nose or on the skin, and
these strains were not exposed to long-term antibiotic treatments
due to the low age of the patient. By contrast, the higher
resistance of SA strains from adults can be explained by both the
higher resistance of a patient's own SA strain, but also by the
increased chance to acquire multi-resistant hospital SA strains,
particularly in the ICU. In general, patients admitted to the ICU
receive more antibiotics than other patients, which induces the
development of multi-resistant strains.
In addition, CoNS are usually saprophytic bacteria
in a patient's microbiota; thus, they do not have a high antibiotic
resistance that explains the minor differences observed in the MAR
between the wards. The lower MAR of SA in the medical wards
compared with the ICU can be explained by the lower exposure to
antibiotics, and the low MAR of CoNS in the paediatric wards is
easily explained by the overall low exposure time of children to
antibiotics, due to the low age (21,22).
In a cross-sectional study carried out on two
neonatal intensive care units in the Republic of Georgia, it was
shown that the number of cases with confirmed bacteraemia was 126
(63%) and from these, the most prevalent was Klebsiella
pneumoniae, accounting for 36 (29%) of positive isolates,
followed by Enterobacter cloacae (19, 15%) and SA (15, 12%)
(23). In our study, in the
paediatric wards, the aetiology of blood cultures was as follows:
From the 10 isolates, 7 were represented by SA (70%), 2
non-fermenters bacilli (20%) and one CoNS (10%) (Table II).
The increase in bacteraemia with SA is significantly
associated with the increasing numbers of admissions to hospitals,
as observed in a study from Denmark over a 30-year period (24). Even if MRSA is responsible for the
majority of bacteraemia cases with SA, compared with
methicillin-sensitive SA, over the past decade, the incidence of
bacteraemia with SA is decreasing (12). Such a tendency was also observed in
our study, where the MRSA prevalence was 36.51% from the SA
strains, but only 2.36% from the total samples analysed (Table III). MRSA infection is associated
with poorer clinical outcomes (25).
In our study we also isolated strains of
Proteus spp., Bacillus subtilis and Clostridium
subterminale. These are considered contaminants of blood
cultures and therefore we will not discuss there resistance.
The significant effect of admission into the
cardiology unit (OR, 2.088; P=0.009) on the risk of acquisition of
invasive infection with any bacteria and with MRSA is easily
explained by the selection bias due to the fact that patients
suspected of infectious endocarditis or myocarditis are admitted
into the cardiology ward.
This study had certain limitations. The most
important limitation is the selection bias, as the study used as
negative controls people for which the physician ordered a blood
culture, which farther turned out to be negative. So the patients
were already at risk for invasive infections.
Although blood cultures are an important component
of diagnostic practice for antibiotic management in patients with
pneumonia, several studies have questioned whether they should be
performed (26,27). The objective of this study was to
evaluate the predictive factors of bacteraemia and the role of
blood cultures in patients with community-onset pneumonia
(community-acquired pneumonia and healthcare-associated pneumonia).
Sometimes, patients with septicaemia are treated with antibiotics
prior to the collection of the blood cultures and after the
isolation and identification of the pathogen they will receive the
proper treatment after the antibiogram results become
In conclusion, invasive infections are most commonly
caused by SA, a bacteria with resistance to multiple antibiotics.
This study identified key risk factors for invasive infections,
which may be addressed with therapy adjustments in high-risk
patients in order to reduce the incidence of invasive infections in
hospitals. In the light of these results, pharmacotherapeutic
management is difficult, and antibiotics administered require a
correct diagnosis and antibiogram.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analysed during the current
study are included in this published article or are available from
the corresponding author upon reasonable request.
Authors' contributions
All the authors were involved in conceiving and
designing the study. OZ, MB and OC contributed to sample collection
and intellectual input. OZ, MB, OC performed the experiments. OC,
ATB, GB and RM collected the data, OZ, MB, DC, performed the
statistical analysis. OZ, DC, AOD, ATB and RM drafted and wrote the
manuscript. DC, AOD, DAS and AMT gave advice on the experimental
design, interpreted the results and critically revised the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The access of the database for the purpose of this
study was approved by the Ethics Committee of Clinical County
Emergency Hospital of Craiova, Romania. Because we are a teaching
hospital, all patients admitted in our hospital signed a written
consent by which they agree that there medical data can be used in
scientific studies.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this
article.
References
|
1
|
Kaspersen ER, Ræder J and Dahl V:
Guidelines for treatment of sepsis. Tidsskr Nor Laegeforen.
138:2018.PubMed/NCBI
|
|
2
|
Wenzel RP and Edmond MB: The impact of
hospital-acquired bloodstream infections. Emerg Infect Dis.
7:174–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen SL, Lassen AT, Kolmos HJ, Jensen
TG, Gradel KO and Pedersen C: The daily risk of bacteremia during
hospitalization and associated 30-day mortality evaluated in
relation to the traditional classification of bacteremia. Am J
Infect Control. 44:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson DJ, Moehring RW, Sloane R,
Schmader KE, Weber DJ, Fowler VG Jr, Smathers E and Sexton DJ:
Bloodstream infections in community hospitals in the 21st century:
A multicenter cohort study. PLoS One. 9:e917132014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thaden JT, Park LP, Maskarinec SA, Ruffin
F, Fowler VG Jr and van Duin D: Results from a 13-year prospective
cohort study showincreased mortality associated with bloodstream
infections caused by Pseudomonas aeruginosa compared to other
bacteria. Antimicrob Agents Chemother. 61:e02671–e16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gradel KO, Nielsen SL, Pedersen C, Knudsen
JD, Østergaard C, Arpi M, Jensen TG, Kolmos HJ, Schønheyder HC,
Søgaard M, et al: Danish Collaborative Bacteraemia Network; Danish
Observational Registry of Infectious Syndromes: No specific time
window distinguishes between community-, healthcare-, and
hospital-acquired bacteremia, but they are prognostically robust.
Infect Control Hosp Epidemiol. 35:1474–1482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leibovici L, Schønheyder H, Pitlik SD,
Samra Z and Møller JK: Bacteraemia caused by hospital-type
micro-organisms during hospital stay. J Hosp Infect. 44:31–36.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buetti N, Marschall J, Atkinson A and
Kronenberg A; Swiss Centre for Antibiotic Resistance (ANRESIS), :
National Bloodstream Infection Surveillance in Switzerland
2008–2014: Different Patterns and Trends for University and
Community Hospitals. Infect Control Hosp Epidemiol. 37:1060–1067.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International forum of acute care trialists: Assessment of global
incidence and mortality of hospital-treated sepsis. Current
Estimates and Limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Călina D, Docea AO, Roşu L, Zlatian O,
Roşu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi
CM, et al: Antimicrobial resistance development following surgical
site infections. Mol Med Rep. 15:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Călina D, Roșu L, Roșu AF, Ianoşi G,
Ianoşi S, Zlatian O, Mitruț R, Docea AO, Rogoveanu O, Mitruț P, et
al: Etiological diagnosis and pharmacotherapeutic management of
parapneumonic pleurisy. Farmacia. 64:946–952. 2016.
|
|
12
|
Tong SY, Davis JS, Eichenberger E, Holland
TL and Fowler VG Jr: Staphylococcus aureus infections:
Epidemiology, pathophysiology, clinical manifestations, and
management. Clin Microbiol Rev. 28:603–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cahill TJ and Prendergast BD: Infective
endocarditis. Lancet. 387:882–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tănase A, Coliță A, Ianoşi G, Neagoe D,
Brănişteanu DE, Călina D, Docea AO, Tsatsakis A and Ianoşi SL: Rare
case of disseminated fusariosis in a young patient with graft vs.
host disease following an allogeneic transplant. Exp Ther Med.
12:2078–2082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamal AM, Mitrut P, Docea AO, Soşoi S,
Kamal KC, Mitrut R, Mărgăritescu D, Calina D, Banciu C, Tica OS, et
al: Double therapy with pegylated Interferon and Ribavirin for
chronic hepatitis C. A pharmacogenenetic guide for predicting
adverde events. Farmacia. 65:877–884. 2017.
|
|
16
|
Sader HS, Jones RN, Gales AC, Silva JB,
Pignatari AC and Group SP; SENTRY Participants Group (Latin
America), : SENTRY antimicrobial surveillance program report: Latin
American and Brazilian results for 1997 through 2001. Braz J Infect
Dis. 8:25–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahoo KC, Tamhankar AJ, Sahoo S, Sahu PS,
Klintz SR and Lundborg CS: Geographical variation in
antibiotic-resistant Escherichia coli isolates from stool, cow-dung
and drinking water. Int J Environ Res Public Health. 9:746–759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Central Laboratory Standards Institute, .
Performance standards for antimicrobial susceptibility testing;
eighteenth informational supplement. CLSI document M100-18. Wayne,
PA: 2008
|
|
19
|
Bonferroni CE: Teoria statistica delle
classi e calcolo delle probabilità. Pubblicazioni del R Istituto
Superiore di Scienze Economiche e Commerciali di Firenze. 8:3–62.
1936.
|
|
20
|
Ungureanu A, Zlatian O, Mitroi G, Drocaş
A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii
VN, et al: Staphylococcus aureus colonisation in patients from a
primary regional hospital. Mol Med Rep. 16:8771–8780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weisman LE: Coagulase-negative
staphylococcal disease: Emerging therapies for the neonatal and
pediatric patient. Curr Opin Infect Dis. 17:237–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Silva AR, Simões ML, Werneck LS and
Teixeira CH: Healthcare associated infections caused by
coagulase-negative Staphylococci in a neonatal intensive care unit.
Rev Bras Ter Intensiva. 25:239–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Macharashvili N, Kourbatova E, Butsashvili
M, Tsertsvadze T, McNutt LA and Leonard MK: Etiology of neonatal
blood stream infections in Tbilisi, Republic of Georgia. Int J
Infect Dis. 13:499–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frimodt-Møller N, Espersen F, Skinhøj P
and Rosdahl VT: Epidemiology of Staphylococcus aureus bacteremia in
Denmark from 1957 to 1990. Clin Microbiol Infect. 3:297–305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhlemann AC, Otto M, Lowy FD and DeLeo FR:
Evolution of community - and healthcare-associated
methicillin-resistant Staphylococcus aureus. Infect Genet Evol.
21:563–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JH and Kim YH: Predictive factors of
true bacteremia and the clinical utility of blood cultures as a
prognostic tool in patients with community-onset pneumonia.
Medicine (Baltimore). 95:e50582016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCulloh RJ, Koster MP, Yin DE, Milner TL,
Ralston SL, Hill VL, Alverson BK and Biondi EA: Evaluating the use
of blood cultures in the management of children hospitalized for
community-acquired pneumonia. PLoS One. 10:e01174622015. View Article : Google Scholar : PubMed/NCBI
|