Introduction
Lipopolysaccharide (LPS), a major component of the
outer membrane of Gram-negative bacteria, is involved in various
disorders including acute lung injury (ALI) that leads to the
accumulation of inflammation by inducing various inflammatory cells
activation (1). Toll-like receptor 4
(TLR4) is a member of the Toll-like receptor family, which mediates
regulation of endotoxin induced pro-inflammatory cellular responses
(2–4).
TLR4 contains an intracellular domain that is
involved in the activation of the cellular signaling pathways, a
transmembrane region and a leucine-rich extracellular domain
(5,6). Upon LPS binding, with the help of
myeloid differentiation protein 2 (MD-2), a multimer complex
composed of TLR4/MD-2-LPS is formed (7,8), which
initiates signaling cascades and results in the activation of
pro-inflammatory factors such as NF-κB and the IFN regulatory
factors.
A critical step in the response to infection is to
activate the innate immune response. LPS plays an importance role
in sepsis and septic shock pathogenesis, because more than half of
the patients have intermediate or higher levels of endotoxin in a
novel endotoxin activity assay (EAA) on the day of ICU admission
(9–11). Excessive activation of innate immune
responses leads to the pathogenesis of many inflammatory diseases
(12), so it is critical for innate
immunity to be strictly controlled and activated when necessary.
Inhibition of TLR4 signaling has become a hot topic in numerous
researches. Eritoran is a competitive inhibitor of LPS and has been
expected as a protective agent for endotoxin shock. Another method
of down-regulating TLR4 signaling is to produce an inhibitory
isoform by alternatively splicing specific genes encoding essential
signaling components such as IL-1R-associated kinase 2, TLR3, MD-2
and MyD88 (13–17). For instance, Gray P found the the
interaction of MD-2 with TLR4 could be competitively inhibited
in vitro by a novel isoform of human MD-2 (18). Kondo Y identified that Gb4 is an
endogenous ligand for TLR4/MD-2, which competes with LPS for
binding to TLR4/MD-2, suggesting that Gb4 can be a promising drug
for the treatment of LPS-induced endotoxin shock induced by
Gram-negative bacteria (19).
Mitsuzawa H testified soluble forms of extracellular TLR4 domain
(sTLR4) and MD-2 (sMD-2) inhibited LPS binding on cell surface and
attenuated LPS-induced lung injury in mice (20).
In the present study, we sought to make further
efforts to define the anti-inflammatory effects of sTLR4/sMD-2
complex in the LPS-induced inflammation in vitro and in
vivo. Our results demonstrated that sTLR4/sMD-2 complex
suppressed the expression of inflammatory cytokines and chemokine
CXCL1 in LPS-stimulated macrophages and reduced pulmonary
inflammation caused by LPS in mice. Taken together, these results
demonstrated that sTLR4/sMD-2 complex alleviates ALI through
inhibition of pro-inflammatory cytokines and chemokine CXCL1
expression and this mechanism indicated sTLR4/sMD-2 complex serves
as an anti-inflammatory agent for treating inflammatory
disease.
Materials and methods
Protein expression and
purification
The entire preparation process of extracellular TLR4
domain and MD-2, including construction of expression vectors of
sTLR4 and MD-2, removal of endotoxin, preparation and purification
of sTLR4/sMD2 complex according to the molar ratio of 1:1, was
carried out as described previously [21]. The molar ratio of the
sTLR4/sMD-2 complex (1:1) in treated mice was the same as that in
treated cells.
THP-1 cell culture and treatment
Macrophage-like cell line THP-1 was obtained from
China Cell Line Bank (Shanghai, China). The cells were cultured in
six-well plates (BD Biosciences, Franklin Lakes, NJ, USA) at a
density of 5×106 cells/ml. To induce THP-1 cells to the
mature macrophages-like state, cells were cultured in RPMI 1640
containing 10% FBS with 100 ng/ml PMA for 24 h and further
incubated in the culture medium without PMA for another 24 h. Then,
cells were incubated with medium alone (Con), LPS (1.0 µg/ml), LPS
+ sTLR4 (LPS 1.0 µg/ml +sTLR4 5.0 µg/ml), LPS + sMD-2 (LPS 1.0
µg/ml + sMD-2 1.25 µg/ml), LPS + sTLR4/sMD-2 (LPS 1.0 µg/ml
+sTLR4/sMD-2 6.25 µg/ml). After 24 h, cell supernatant was
collected and total RNA was extracted.
Reverse transcription
semi-quantitative polymerase chain reaction (PCR)
Total RNAs were extracted from cells using RNAiso
Plus reagent according to the manufacturer's instructions. Primers
used in our study were listed in Table
I. The 25 µl PCR reactions included Premix TaqTM (Ex Taq v.2.0
plus dye; Takara Biotechnology Co., Ltd., Dalian, China), 20 µM
primers, cDNA and dd H2O. The PCR reaction was carried
out by the manufacturer's instructions. The electrophoresis image
of PCR products were analyzed by the AlphaImager gel analysis
system (ProteinSimple, San Jose, CA, USA). Each analysis was
repeated three times. The semiquantitative value was expressed as
the ration of the integrated optical density, as previously
reported (21).
 | Table I.Primer sequences used for
RT-sqPCR. |
Table I.
Primer sequences used for
RT-sqPCR.
| Gene | Primers sequence |
|---|
| GAPDH |
|
|
Forward |
5′-TGATGACATCAAGAAGGTGGTGAAG-3′ |
|
Reverse |
5′-TCCTTGGAGGCCATGTAGGCCAT-3′ |
| IL-8 |
|
|
Forward |
5′-GACATACTCCAAACCTTTCCACC-3′ |
|
Reverse |
5′-CAACCCTACAACAGACCCACAC-3′ |
| TNF-α |
|
|
Forward |
5′-GCCCCAATCCCTTATTTACCC-3′ |
|
Reverse |
5′-GGCGATTACAGACACAACTCCC-3′ |
| CXCL1 |
|
|
Forward |
5′-ACGCATTTACTGTCACGGTTC-3′ |
|
Reverse |
5′-GTTGTATGGGGCATTGACTTTC-3′ |
Murine model of LPS-induced ALI
Health male BALB/c mice with the body weight of
22–28 g were obtained from the Experimental Animal Center of
Guangxi Medical University. All of the animals were kept in sterile
conditions. Mice were randomly divided into five groups
(n=5/group), i.e. phosphate buffered saline-treated group (PBS),
LPS-treated group (LPS), sTLR4+LPS-treated group (LPS+sTLR4),
sMD-2+LPS-treated group (LPS+sMD-2) and sTLR4/sMD-2+LPS-treated
group (LPS+sTLR4/sMD-2). Before surgery, all animals were
anaesthetized by i.p. with 60 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After successful
endotracheal intubation, LPS (400 µg/kg in 50 µl of PBS) was
instilled intratracheally with sTLR4 (650 µg/kg), sMD-2 (175 µg/kg)
or sTLR4/sMD-2 (825 µg/kg). Control mice received intratracheal
instillation of sterile PBS alone. The severity of lung injury was
evaluated by the wet lung-to-body weight ratio, histopathology
changes of the lung tissues and cellular profiles in the
bronchoalveolar lavage fluid (BALF) after LPS administration for 16
h. Experimental mice were used strictly in accordance with the
national animal experimental requirements, and the study was
approved by the Ethical Committee for Animal Experiments from the
Fourth Affiliated Hospital of Guangxi Medical University (Liuzhou,
China).
BALF collection
After LPS infection for 16 h, mice were euthanized
by anesthetic overdose with sodium pentobarbital (120 mg/kg, i.p.).
BALF was collected by washing the lungs four times with 0.5 ml of
ice-cold saline. Then the cells were centrifuged at 400 g for 10
min at 4°C. The supernatant was frozen at −80°C for further
analysis. Subsequently, the sedimentary cells were resuspended in
PBS and the total counts of cells were counted using a
hemocytometer, and the May-Giemsa staining (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) method was used for manual
classification count. We only counted the number of inflammatory
cells, including neutrophils, monocytes, macrophages and
lymphocytes for the total cells in BALF. A professional technician
performed cell staining and counting manually. At least 300
cells/slides were counted no less than twice and the mean was
calculated in each experiment.
Pulmonary index
The lung tissue samples were excised and extraneous
tissues were cleared away. Then the lung tissue were weighed and
the wet lung-to-body weight ratio was calculated to assess the
severity of lung inflammation.
Enzyme-linked immunosorbent assay
(ELISA)
To detect the TNF-α, IL-8 and IL-6 protein levels,
the cell supernatant and/or BALF of mice were determined by ELISA
kits from RayBiotech, Inc., (Norcross, GA, USA). Quantitation of
secreted CXCL1 protein levels was analyzed by ELISA kits (R&D
Systems, Inc., Minneapolis, MN, USA).
Histopathology
The right inferior lung lobe were fully fixed in 10%
neutral buffered formalin for one week, followed by
paraffin-embedding in line with the standard procedures. All
paraffin-embedded tissues were sectioned to 4 µm thick, and stained
with hematoxylin and eosin for examination under Nikon Eclipse E800
microscope (×200). The lung injury score was evaluated by one
pathologist in a blinded fashion as previously described (22). The extent of the pathological lesions
was scaled from 0 to 3 according to the criteria shown in Table II.
 | Table II.Extent of the pathological lesions
grade. |
Table II.
Extent of the pathological lesions
grade.
| Score | Alveolar
septae | Alveolar
hemorrhage | Intra-alveolar
fibrin | Intra-alveolar
infiltrations per field |
|---|
| 0 | All are thin and
delicate | No hemorrhage | No intra-alveolar
fibrin | <5
intra-alveolar cells |
| 1 | Congested alveolar
septae in less than 1/3 of the field | Erythrocyte per
alveolus in 1 to 5 alveoli | Fibrin strands in
less than 1/3 of the field | 5–10 intra-alveolar
cells |
| 2 | Congested alveolar
septae in 1/3 to 2/3 of the field | At least 5
erythrocyte per alveolus in 5 to 10 alveoli | Fibrin strands in
1/3 to 2/3 of the field | 10–20
intra-alveolar cells |
| 3 | Congested alveolar
septae in greater than 2/3 of the field | At least 5
erythrocytes per alveolus in more than 10 alveoli | Fibrin strands in
greater than 2/3 of the field | >20
intra-alveolar cells |
Immunohistochemical staining
All paraffin-embedded tissues were cut in 4 µm thick
for immunohistochemical analysis. 0.3% hydrogen peroxide in
methanol was used to block endogenous peroxidase activity. Then
sections were treated with 0.1 M citrate buffer (pH 6.0) as an
antigen retrieval system. Anti-MPO antibody was used for
immunostaining (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The avidin biotin peroxidase complex method was used to perform
immunostaining and the immunohistochemical analyses were performed
with the chromogen diaminobenzidine.
Statistical analysis
Data were presented as mean ± SD. The results were
statistically evaluated by one-way analysis of variance followed by
the LSD post hoc test. All data were analyzed using with SPSS
(v.17.0) software. P<0.05 was considered to indicate a
statistically significant difference.
Results
sTLR4/sMD-2 complex competes with cell
surface TLR4/MD-2 complex in binding LPS
Our laboratory has successfully constructed
extracellular TLR4 domain and sMD-2 [21] and we have reported the
binding assay of LPS to THP-1 cells in previous paper by Zou et
al (21). The fluorescence
intensity was significantly decreased in sTLR4/sMD-2 complex
treated group instead of sTLR4 or MD-2 alone. These results
suggested that recombinant sTLR4/sMD-2 complex competes with
TLR4/MD-2 complex on cell surface in binding LPS.
sTLR4/sMD-2 complex inhibies the
expression of pro-inflammatory cytokines and chemokines induced by
LPS in THP-1 cells
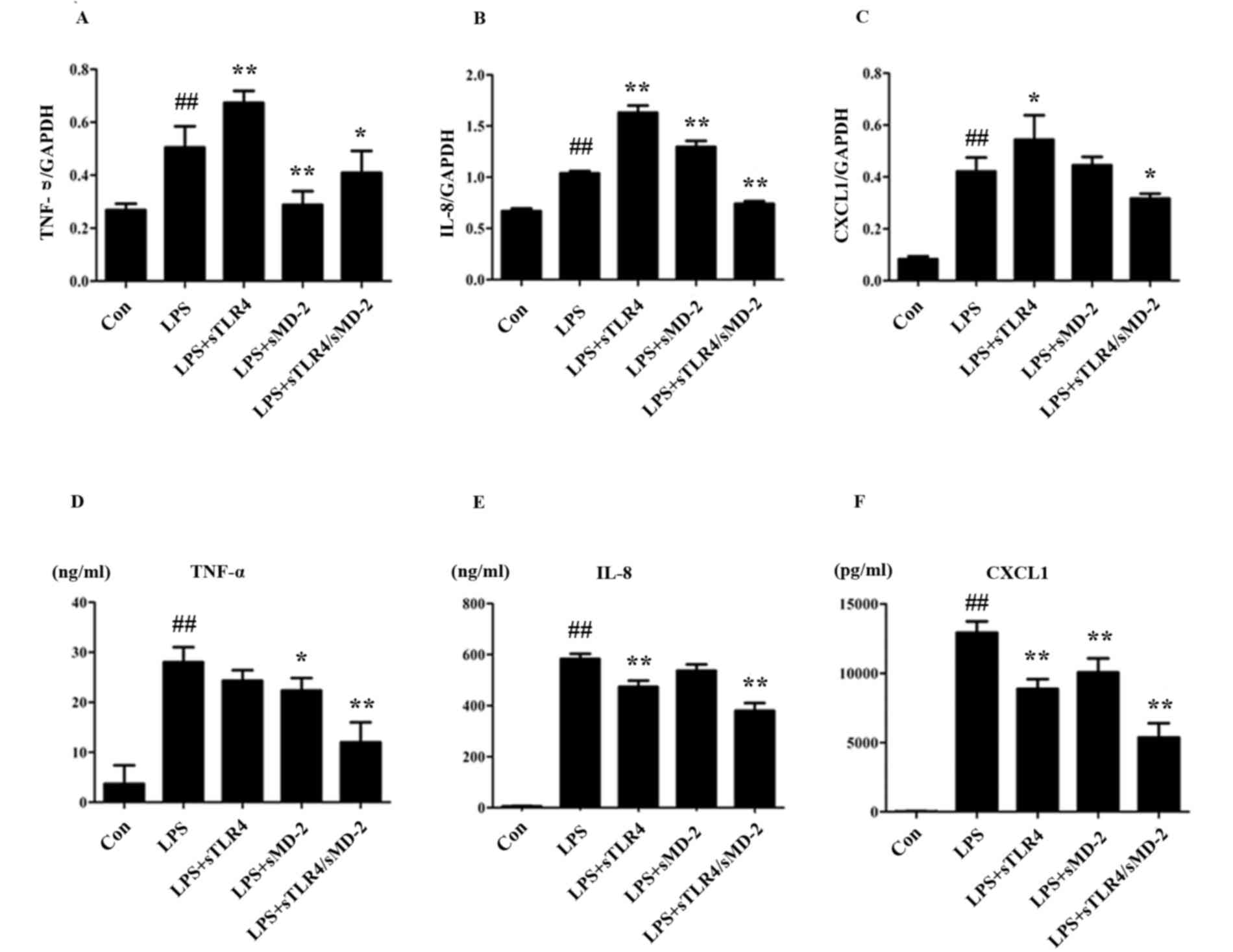
Cytokine microarray analysis of the changes in
transcription levels caused by LPS-treated THP-1 cells showed an
up-regulation of TNF-α, IL-8, MIP-1α and MIP-1β (23). We examined the expression of TNF-α,
IL-8 and CXCL1 in LPS stimulated THP-1 cells after being treated
with sTLR4, sMD-2 and sTLR4/sMD-2 complex. After 24 h, there was a
significant increase of TNF-α, IL-8 and CXCL1 on mRNA and protein
levels in LPS treated group, whereas the mRNA and protein levels of
TNF-α, IL-8 and CXCL1 were significantly inhibited in sTLR4/sMD-2
complex treated group (Fig. 1).
However, administration of sTLR4 or sMD-2 alone caused inconsistent
mRNA expression of TNF-α, IL-8, and CXCL1 (Fig. 1A-C). Furthermore, administration of
sTLR4 or sMD-2 alone gave a trend for decreasing the expression of
TNF-α, IL-8 and CXCL1 protein induced by LPS (Fig. 1D-F). Of note, the anti-inflammatory
effect of sTLR4/sMD-2 complex treatment group was better than that
of sTLR4 or sMD-2 alone group. Together, these findings suggested
that sTLR4/sMD-2 complex could suppress LPS-induced
pro-inflammatory mediators and chemokines production in THP-1
cells.
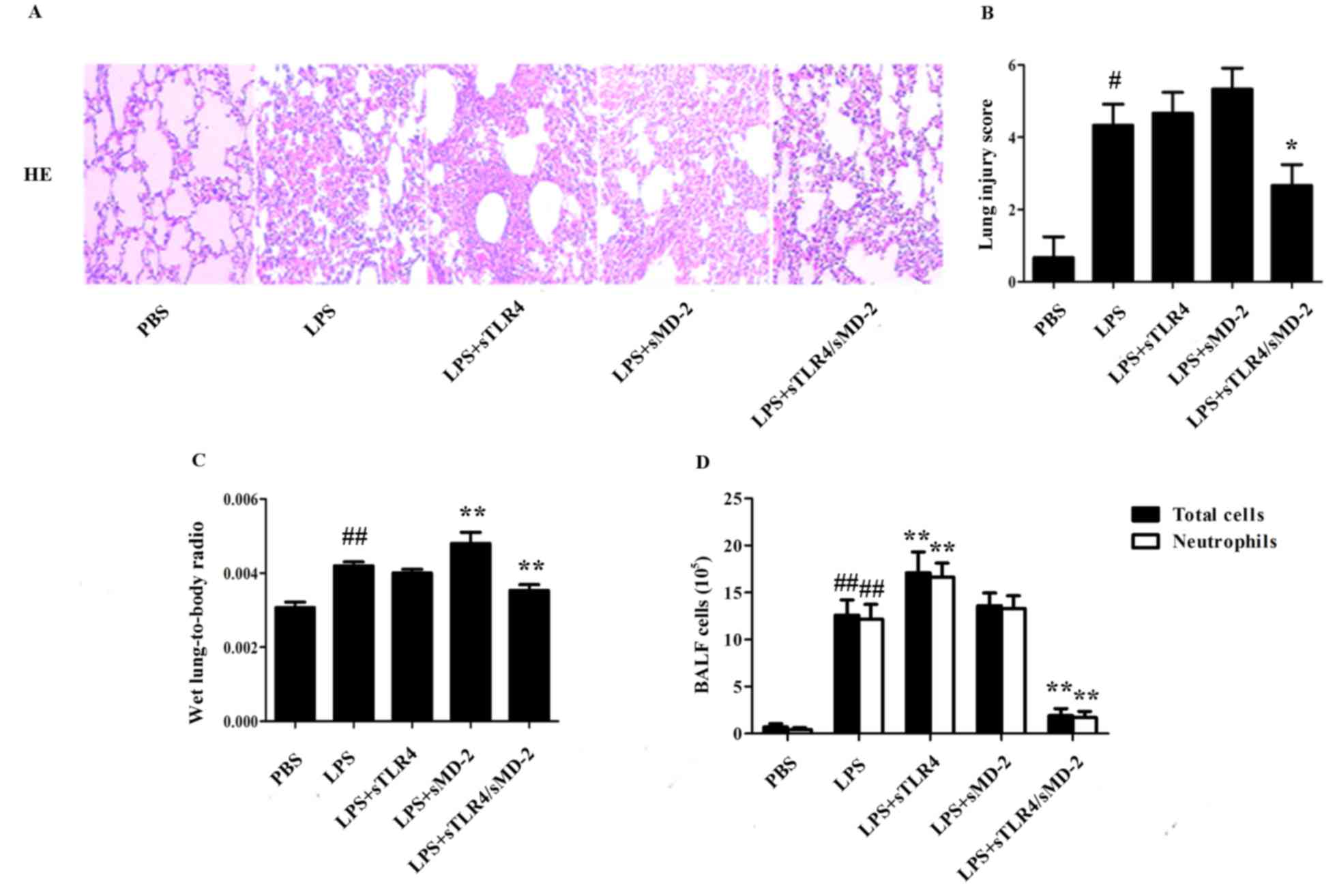
sTLR4/sMD-2 complex attenuates the
LPS-induced ALI
To evaluate the potential role of sTLR4/sMD-2
complex in the pathological changes of lung in LPS-treated ALI
mice, lung histological changes were evaluated after administration
of LPS with sTLR4, sMD-2 or sTLR4/sMD-2 complex. The results showed
marked inflammatory infiltrate, thick alveolar septum and severe
interstitial edema in LPS-induced ALI mice. When sTLR4 or sMD-2 was
separately treated, no significant decrease in LPS-induced
pulmonary inflammation was observed. In contrast, administration of
sTLR4/sMD-2 complex effectively reduced the airway inflammation
(Fig. 2A). Furthermore, the
semiquantitative histopathology score system was used to evaluate
the severity of lung injury as shown in Table II (22). We found that administration of
sTLR4/sMD-2 complex could significantly reduce lung injury score
(Fig. 2B). The pulmonary index was
also significantly decreased in sTLR4/sMD-2 complex treated mice.
In contrast, lung injury score and pulmonary index ratio in the
sTLR4 or sMD-2 groups did not decrease significantly compared to
that in the LPS group (Fig. 2B and
C).
Then we evaluated the potential role of sTLR4/sMD-2
complex in the changes of total cells and neutrophil count in BALF
of LPS-treated mice. LPS administration resulted in dramatically
increased total inflammatory cell count (P<0.05), most of which
were neutrophils (Fig. 2D). Notably,
we found that sTLR4/sMD-2 complex could significantly decrease the
total inflammatory cell and neutrophil count in BALF, whereas sTLR4
or sMD-2 alone did not have the same effect (Fig. 2D).
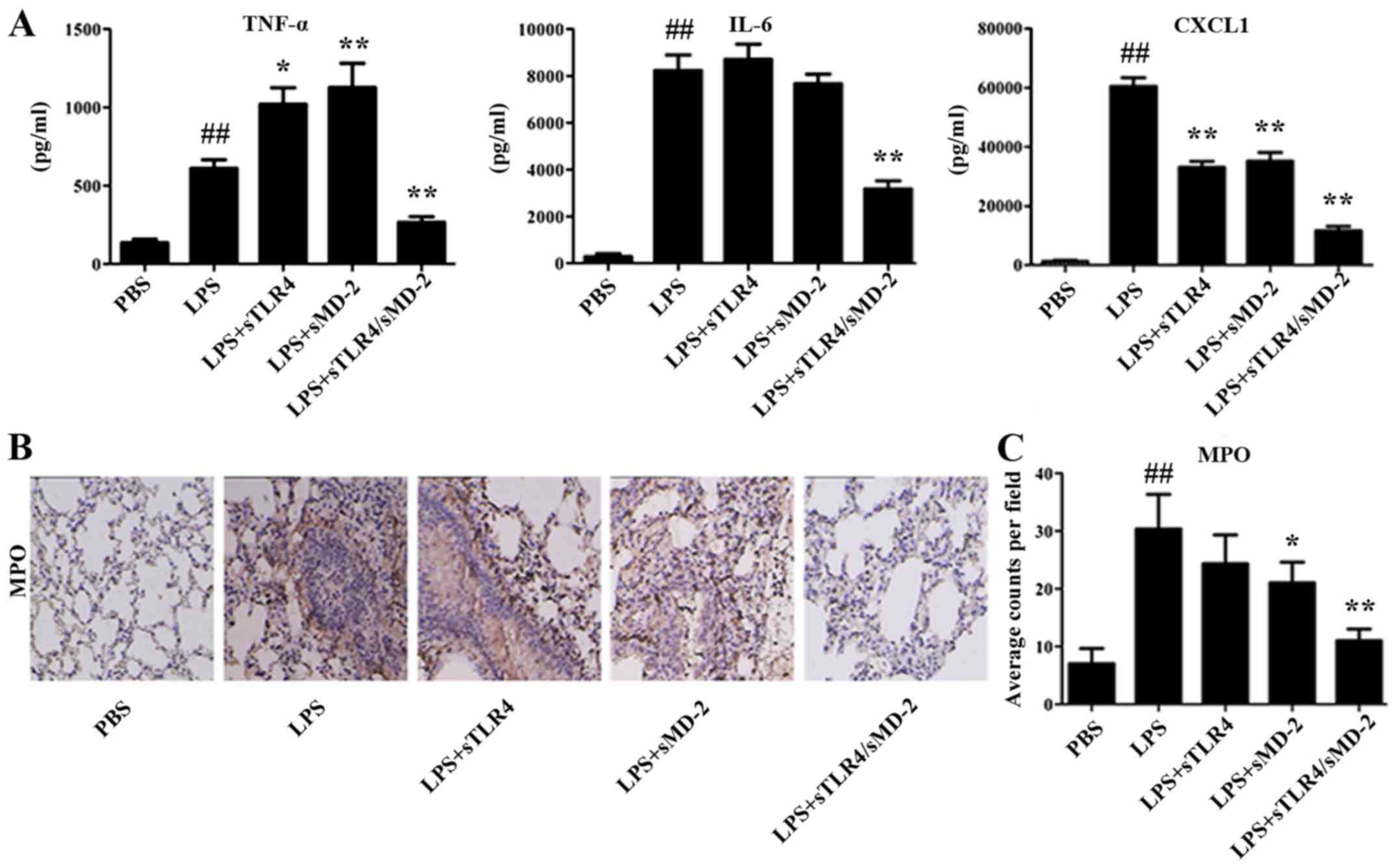
sTLR4/sMD-2 complex decreases
cytokines concentrations in BALF and MPO-positive cells in lung
tissues
The proinflammatory cytokines TNF-α, IL-6 and
chemokine CXCL1 in BALF were quantified using method of ELISA. We
found that the increased expression of TNF-α, IL-6 and CXCL1 in
BALF cells were significantly suppressed by sTLR4/sMD-2 complex
treatment (Fig. 3A). Administration
of sTLR4 or sMD-2 alone decreased the expression of CXCL1 in BALF
cells, but the effects were worse than that of sTLR4/sMD-2 complex.
However, administration of sMD-2 alone significantly increased the
expression of TNF-α in BALF cells, which was not consistent with
results in THP-1 cells. We believed that sMD-2 can combine with LPS
directly and induce homodimerization of the TLR4/MD-2 complex to
trigger a proinflammatory signaling cascade when treated with a
high concentration (400 µg/kg in 50 µl of PBS) of LPS in
vivo. And TNF-α is an early inflammatory factor, so the
expression of TNF-α increases first. However, when we used a low
concentration (1 µg/ml) of LPS in vitro, no extra LPS was
combined with sMD-2, so the addition of sMD-2 have no effects.
Immunohistochemical staining also revealed a large amount of
inflammatory cells infiltrated in lung tissue. The production of
MPO, which is an indicator of neutrophil infiltration, was also
detected in lung tissue. As shown in Fig. 3B and C, the number of MPO-positive
expression in the LPS group was greater than that in PBS group
(P<0.01). We found a significant reduction of MPO-positive cells
in the sMD-2 group compared with the LPS group (P<0.05), whereas
sTLR4/sMD-2 complex markedly decreased MPO-positive cells
(P<0.01). In summary, sTLR4/sMD-2 complex could effectively
protect against ALI induced by LPS.
Discussion
ALI is associated with an acute inflammatory process
and is characterized by increased vascular permeability, fibrin
deposition, and a large quantity of edema fluid accumulations in
alveoli (24). LPS inhalation
resulted in significant lung injury. To bind to LPS, the
extracellular domain of TLR4 interacts with secreted protein MD-2
(25,26). MD-2 combines with LPS directly at its
hydrophobic cavity and this recognition of LPS induces
homodimerization of the TLR4/MD-2 complex, which forms 1:1 of the
LPS-TLR4/MD-2 complex (27). The
formation of the LPS-TLR4/MD-2 complexes can recruit intracellular
adaptor protein MyD88 to activate downstream signaling pathways,
such as MAPKs and NF-κB (28).
Macrophages can release a large number of inflammatory mediators
after LPS stimulation (29).
Therefore, the inflammatory model of the macrophage induced by LPS
can be used to evaluate the anti-inflammatory effects of various
reagents. The effects of sTLR4/sMD-2 complex have not been
elucidated on THP-1 cells. So we tried to explore the underlying
mechanism of the therapeutic effects of sTLR4/sMD-2 complex in
ALI.
The main characteristics of acute pulmonary
inflammation are protein leakage, production of inflammatory
mediators and neutrophil influx (30). Inflammatory mediators TNF-α and IL-6
were reported to play a vital role in LPS-induced ALI (31). In this study, we investigated the
effects of sTLR4/sMD-2 complex on the inflammatory factors of
LPS-stimulated THP-1 cells. We found that administration of
sTLR4/sMD-2 complex significantly inhibited the mRNA and protein
level of TNF-α, IL-8 and CXCL1 induced by LPS. These results
indicated that sTLR4/sMD-2 complex may be useful for treating
inflammation diseases with an overproduction of pro-inflammatory
cytokines.
Studies have demonstrated that tissue macrophages
synthesize neutrophil chemoattractants CXCL1/CXCL2 in response to
LPS, which contributes to recruiting neutrophils into tissues
(32). When ALI occurs, the tissue
macrophages in the lungs produce CXCL1 to recruit neutrophil cells
in the infection site for killing pathogens. In this study, we
found that treatment with sTLR4/sMD-2 complex markedly attenuated
LPS-stimulated CXCL1 expression in THP-1 cells and BALF. So it is
important to inhibit CXCL1 production by sTLR4/sMD-2 complex for
treatment of ALI. The levels of MPO, a granule-derived mediator, is
increased in BALF samples obtained from mice with ALI (33). Furthermore, our results showed that
sTLR4/sMD-2 complex significantly decreased LPS-induced MPO
production. To analyze the severity of lung edema, the lung W/D
ratio was measured. We found that sTLR4/sMD-2 complex treatment
decreased the wet lung-to-body weight ratio, which suggested it
suppressed the excess accumulation of fluid in lung tissues. These
results showed that sTLR4/sMD-2 complex can act as an
anti-inflammatory reagent against LPS-induced ALI.
Early studies showed that sTLR4/sMD-2 complex
attenuates LPS induced-ALI (20). In
the present study, we found sTLR4/sMD-2 complex inhibited the
expression of TNF-α and CXCL1 on the cell surface and in the cell
supernatant of THP-1 cells treated with LPS at higher concentration
in vitro. Secondly, our results also showed that the
administration of sTLR4/sMD-2 complex decreased wet lung-to-body
weight ratio and lung injury score, pro-inflammatory cytokines
production of IL-6 and CXCL1 in BALF and MPO activity. Moreover,
sTLR4/sMD-2 complex significantly alleviated LPS-induced lung
histopathological changes, whereas sTLR4 and sMD-2 alone had no
effect. Furthermore, sTLR4/sMD-2 complex had the ability to protect
against ALI even when treated with high concentrations (400 µg/kg)
of LPS. These results are all the new findings of this study, which
provides powerful evidence to support sTLR4/sMD-2 complex's role as
a potential treatment for preventing ALI induced by LPS.
Therapeutic effects of the sTLR4/sMD-2 complex in other biological
fields still need to be investigated in the future studies.
Acknowledgements
The authors would like to thank Mr. Hao Wu, Mr. Qi
Sun, Mr. Dong Wei, Ms. Min Yi, Mr. Xi Qin, Mr. Siqiong Pan, Mr. Ni
Zheng, Mr. Ting Li and Mr. Meiying Lu (The Fourth Affiliated
Hospital of Guangxi Medical University, Liuzhou, Guangxi, China)
for their excellent technical assistance. We also thank Mr. Yujie
Huang (The Fourth Affiliated Hospital of Guangxi Medical
University, Liuzhou, Guangxi, China) for her work in revising this
manuscript.
Funding
This study was supported by grants from Key
Laboratory Construction of Tumor Diseases Prevention in Liuzhou,
Guangxi (grant no. 2014G020403) and National Natural Science
Foundation of China (grant no. 81160269).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JM and SD contributed to the conception and design
of the study. JM and YZ drafted the manuscript. JM, YZ, JC and FQ
performed the research. XC and XC performed the data analyses. SD
reviewed the manuscript and gave his approval to the submitted and
final versions. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were reviewed and approved by
the Ethical Committee for Animal Experiments from the Fourth
Affiliated Hospital of Guangxi Medical University (Liuzhou, China).
All efforts were made to minimize the suffering of the experimental
mice.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Ulich TR, Fann MJ, Patterson PH, Williams
JH, Samal B, Del Castillo J, Yin S, Guo K and Remick DG:
Intratracheal injection of LPS and cytokines. V. LPS induces
expression of LIF and LIF inhibits acute inflammation. Am J
Physiol. 267:L442–L446. 1994.PubMed/NCBI
|
|
2
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Means TK, Golenbock DT and Fenton MJ: The
biology of Toll-like receptors. Cytokine Growth Factor Rev.
11:219–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ulevitch RJ and Tobias PS: Recognition of
gram-negative bacteria and endotoxin by the innate immune system.
Curr Opin Immunol. 11:19–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beutler B and Poltorak A: Sepsis and
evolution of the innate immune response. Crit Care Med. 29 7
Suppl:S2–S7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medzhitov R and Janeway CA Jr: An ancient
system of host defense. Curr Opin Immunol. 10:12–15. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BS, Song DH, Kim HM, Choi BS, Lee H
and Lee JO: The structural basis of lipopolysaccharide recognition
by the TLR4-MD-2 complex. Nature. 458:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoshino K, Takeuchi O, Kawai T, Sanjo H,
Ogawa T, Takeda Y, Takeda K and Akira S: Cutting edge: Toll-like
receptor 4 (TLR4)-deficient mice are hyporesponsive to
lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J
Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
9
|
Marshall JC: Endotoxin in the pathogenesis
of sepsis. Contrib Nephrol. 167:1–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opal SM: Endotoxins and other sepsis
triggers. Contrib Nephrol. 167:14–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marshall JC, Foster D, Vincent JL, Cook
DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, et
al: Diagnostic and prognostic implications of endotoxemia in
critical illness: Results of the MEDIC study. J Infect Dis.
190:527–534. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwami KI, Matsuguchi T, Masuda A, Kikuchi
T, Musikacharoen T and Yoshikai Y: Cutting edge: Naturally
occurring soluble form of mouse Toll-like receptor 4 inhibits
lipopolysaccharide signaling. J Immunol. 165:6682–6686. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohta S, Bahrun U, Tanaka M and Kimoto M:
Identification of a novel isoform of MD-2 that downregulates
lipopolysaccharide signaling. Biochem Biophys Res Commun.
323:1103–1108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hardy MP and O'Neill LA: The murine IRAK2
gene encodes four alternatively spliced isoforms, two of which are
inhibitory. J Biol Chem. 279:27699–27708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leeman JR and Gilmore TD: Alternative
splicing in the NF-kappaB signaling pathway. Gene. 423:97–107.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lynch KW: Consequences of regulated
pre-mRNA splicing in the immune system. Nat Rev Immunol. 4:931–940.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gray P, Michelsen KS, Sirois CM, Lowe E,
Shimada K, Crother TR, Chen S, Brikos C, Bulut Y, Latz E, et al:
Identification of a novel human MD-2 splice variant that negatively
regulates Lipopolysaccharide-induced TLR4 signaling. J Immunol.
184:6359–6366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kondo Y, Ikeda K, Tokuda N, Nishitani C,
Ohto U, Akashi-Takamura S, Ito Y, Uchikawa M, Kuroki Y, Taguchi R,
et al: TLR4-MD-2 complex is negatively regulated by an endogenous
ligand, globotetraosylceramide. Proc Natl Acad Sci USA.
110:4714–4719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitsuzawa H, Nishitani C, Hyakushima N,
Shimizu T, Sano H, Matsushima N, Fukase K and Kuroki Y: Recombinant
soluble forms of extracellular TLR4 domain and MD-2 inhibit
lipopolysaccharide binding on cell surface and dampen
lipopolysaccharide-induced pulmonary inflammation in mice. J
Immunol. 177:8133–8139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou Y, Qin F, Chen J, Meng J, Wei L, Wu C,
Zhang Q, Wei D, Chen X, Wu H, et al: sTLR4/MD-2 complex inhibits
colorectal cancer in vitro and in vivo by targeting LPS.
Oncotarget. 7:52032–52044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Z, Li Q, Han Y, Liang Y, Xu Z and Ren
T: Prevention of LPS-induced acute lung injury in mice by
progranulin. Mediators Inflamm. 2012:5407942012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harrison LM, van den Hoogen C, van Haaften
WC and Tesh VL: Chemokine expression in the monocytic cell line
THP-1 in response to purified shiga toxin 1 and/or
lipopolysaccharides. Infect Immun. 73:403–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimazu R, Akashi S, Ogata H, Nagai Y,
Fukudome K, Miyake K and Kimoto M: MD-2, a molecule that confers
lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp
Med. 189:1777–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagai Y, Akashi S, Nagafuku M, Ogata M,
Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M and Miyake K:
Essential role of MD-2 in LPS responsiveness and TLR4 distribution.
Nat Immunol. 3:667–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akashi S, Saitoh S, Wakabayashi Y, Kikuchi
T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y,
et al: Lipopolysaccharide interaction with cell surface Toll-like
receptor 4-MD-2: Higher affinity than that with MD-2 or CD14. J Exp
Med. 198:1035–1042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JK, Sayers BC, Chun KS, Lao HC,
Shipley-Phillips JK, Bonner JC and Langenbach R: Multi-walled
carbon nanotubes induce COX-2 and iNOS expression via MAP
kinase-dependent and -independent mechanisms in mouse RAW264.7
macrophages. Part Fibre Toxicol. 9:142012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reutershan J, Morris MA, Burcin TL, Smith
DF, Chang D, Saprito MS and Ley K: Critical role of endothelial
CXCR2 in LPS-induced neutrophil migration into the lung. J Clin
Invest. 116:695–702. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Filippo K, Dudeck A, Hasenberg M, Nye
E, van Rooijen N, Hartmann K, Gunzer M, Roers A and Hogg N: Mast
cell and macrophage chemokines CXCL1/CXCL2 control the early stage
of neutrophil recruitment during tissue inflammation. Blood.
121:4930–4937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dooley JL, Abdel-Latif D, St Laurent CD,
Puttagunta L, Befus D and Lacy P: Regulation of inflammation by
Rac2 in immune complex-mediated acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 297:L1091–L1102. 2009. View Article : Google Scholar : PubMed/NCBI
|