Introduction
Budd-Chiari syndrome (BCS) is characterized by the
blockage of the hepatic veins (HVs) and/or the inferior vena cava
(IVC) (1). Myeloproliferative
disorders are the most common causes of BCS (1). Men and women are equally affected, with
patients mostly being diagnosed in the third or fourth decade of
life (2). Clinical manifestations
include: Abdominal pain, liver dysfunction and intractable ascites,
which is the most common clinical feature of BCS (3). Conventional management for BCS patients
includes the treatment of complications of portal hypertension,
surgery and endovascular intervention (4). The prevention of disease progression by
medical treatment alone is limited. However, endovascular
intervention has proven to be more effective than medical treatment
and exhibits a lower mortality rate than open surgery (5).
Doppler ultrasonography is the technique of choice
for initial investigation when BCS is suspected (1). Contrast-enhanced computed tomographic
(CT) scanning may be recommended to delineate venous anatomy and to
determine the configuration of the liver when a transjugular
intrahepatic portosystemic shunt is being considered (1). Digital subtraction angiography (DSA)
examination is considered as the ‘golden standard’ for the
diagnosis of BCS. However, it is an invasive examination, which
includes exposure to radiation and the injection of a contrast
agent (6). Magnetic resonance (MR)
angiography (MRA) has been suggested to be a suitable alternative
modality for diagnosing BCS (7).
However, to the best of our knowledge, no meta-analysis has been
performed for previous studies on MRA to fully elucidate the
qualities of this methodology for the diagnosis of BCS.
The aim of the present study was therefore to
perform a systematic review to obtain the best available estimates
of the diagnostic performance of MRA in patients with BCS.
Materials and methods
Publication search
The PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), MEDLINE
(https://www.nlm.nih.gov/bsd/pmresources.html), SCOPUS
(https://www.scopus.com/home.uri), EMBASE
(https://www.elsevier.com/solutions/embase-biomedical-research),
Cochrane Library (https://www.cochranelibrary.com/) and China National
Knowledge Infrastructure databases (http://oversea.cnki.net/) were all searched for
relevant studies published until November 2017. The following terms
were used for the search: (‘Budd-Chiari syndrome’ OR ‘hepatic
venous thrombosis’ OR ‘hepatic outflow obstruction’) and (‘magnetic
resonance angiography’ OR ‘MR angiography’ OR ‘MRA’). All of the
studies identified were retrieved, and their references were also
checked for other relevant publications.
Inclusion and exclusion criteria
Studies meeting the following selection criteria
were included in the present meta-analysis: i) Evaluation of the
diagnostic performance of MRA for detecting BCS, ii) true-positive
(TP), false-positive (FP), true-negative (TN) and false-negative
(FN) detection rate are contained in or may be calculated from the
data of the original published study, iii) articles were published
in English or Chinese and iv) DSA or surgery as the gold
standard.
The exclusion criteria were as follows: i) Review
articles, ii) animal studies, iii) studies with insufficient data,
and iv) case reports and small case series (number of patients,
<5). If the 2 reviewers disagreed on whether to include an
article, it was resolved by consensus with a third reviewer.
Data extraction and quality
assessment
Relevant studies were examined by two blinded
observers independently (PX and LL) with the Quality Assessment of
Diagnostic Studies (QUADAS) tool to assess the methodological
quality of each study included in the meta-analysis (8). A third reviewer (XL) served as a
blinded expert in cases of disagreement. The following data were
extracted: i) Characteristics of study participants (number of
patients, age, gender), ii) methodological details for MRA and iii)
relevant data (TP, FP, TN and FN).
Meta-analysis
The use of the random-effects or fixed-effects model
depends on the presence of statistical heterogeneity. Heterogeneity
was assessed by the χ2 test and the inconsistency index
(I2) (9). One of the
major causes of heterogeneity in test accuracy studies is the
threshold effect (10). A threshold
effect is indicated if assessment by computing the Spearman
correlation between the logarithm (logit) of sensitivity and logit
of (1-specificity) reveals a positive correlation between
sensitivity and 1-specificity. A positive correlation (P<0.05)
suggests a threshold effect. If heterogeneity is present due to a
threshold effect, accurate data should be pooled by fitting a
summary receiver operating characteristic curve and calculating the
area under the curve (AUC). Subgroup analysis and regression
meta-analysis were performed if there was no threshold effect but
significant heterogeneity. All statistical analyses were performed
using Meta-Disc software version 1.4 (11).
Results
Eligible studies
The initial search for studies that evaluated the
diagnostic accuracy of MRA for BCS yielded a total of 118
manuscripts. Manual searching of the reference lists of relevant
research studies did not yield any additional relevant studies. In
total, only 6 of these research studies contained the appropriate
data [sample size, sensitivity, specificity, positive predictive
value and negative predictive value] for inclusion in the
statistical calculations (12–17). The
major reasons for exclusion were as follows: i) No relevance
regarding the use of MRA for detecting or evaluating stenosis; ii)
insufficient data for creating a 2×2 table; iii) QUADAS score of
<9. The characteristics of each study included are presented in
Table I. The 6 research studies that
were finally included in the meta-analysis had been published
between 2007 and 2015, and comprised a total of 285 patients. The
mean number of patients per study was 47.5 (range, 35–108).
 | Table I.Baseline characteristics of included
studies on Budd-Chiari syndrome. |
Table I.
Baseline characteristics of included
studies on Budd-Chiari syndrome.
| Author, year | Patients (n) | Male (%) | Age (years) | Reference
standard | TP | FP | FN | TN | (Refs.) |
|---|
| Lu et al,
2011 | 44 | 26 | 46 (19–78) | DSA | 37 (84.1) | 3 (6.8) | 0 (0) | 4 (9.1) | (12) |
| Pu et al,
2015 | 51 | 28 | NA (19–61) | DSA | 41 (80.4) | 4 (7.8) | 1 (2.0) | 5 (9.8) | (13) |
| Qin et al,
2015 | 45 | 33 | 46.5 (27–71) | DSA/surgery | 40 (88.9) | 0 (0) | 1 (2.2) | 4 (8.9) | (14) |
| Ren et al,
2007 | 50 | 38 | 48.6 (NA) | DSA | 38 (76.0) | 1 (2.0) | 3 (6.0) | 8 (16.0) | (15) |
| Shen et al,
2013 | 108 | 57 | 46.5 (18–73) | DSA | 97 (89.8) | 3 (2.8) | 2 (1.9) | 6 (5.5) | (16) |
| Wu et al,
2014 | 35 | 23 | 43.9 (24–69) | DSA | 32 (91.4) | 1 (2.9) | 0 (0) | 2 (5.7) | (17) |
Threshold effect analysis
The Spearman correlation coefficient was determined
to be 0.600 (P=0.208), which suggested no threshold effect among
the individual studies that may have caused any variations in
accuracy estimates.
Data synthesis
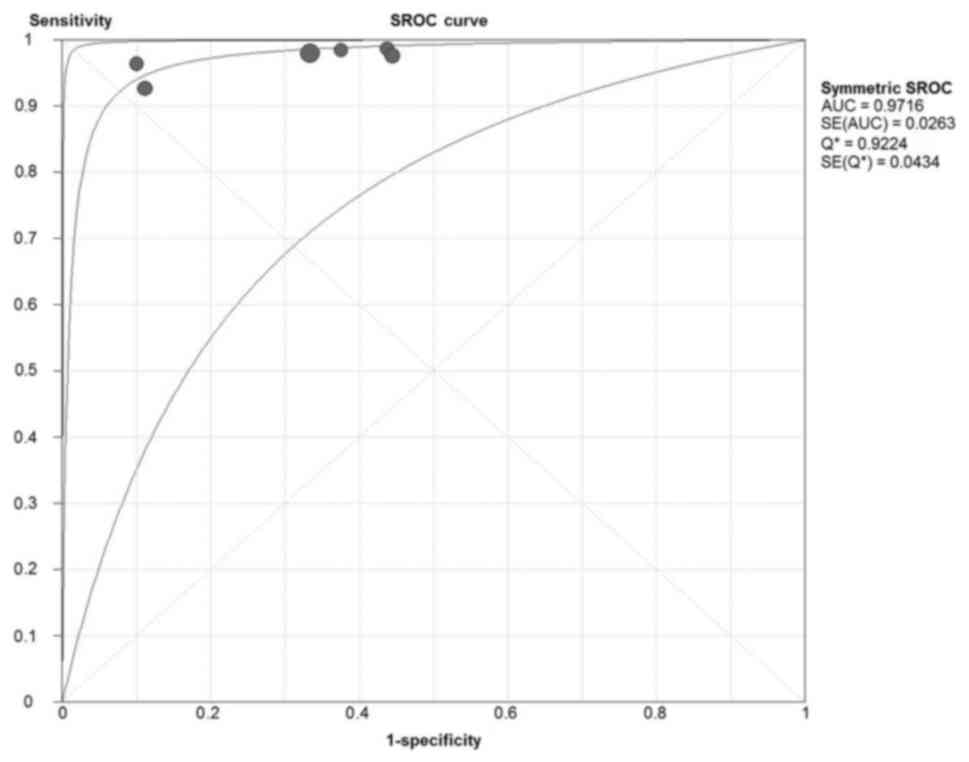
The overall AUC was 0.972, which suggested good
diagnostic accuracy (Fig. 1). Pooled
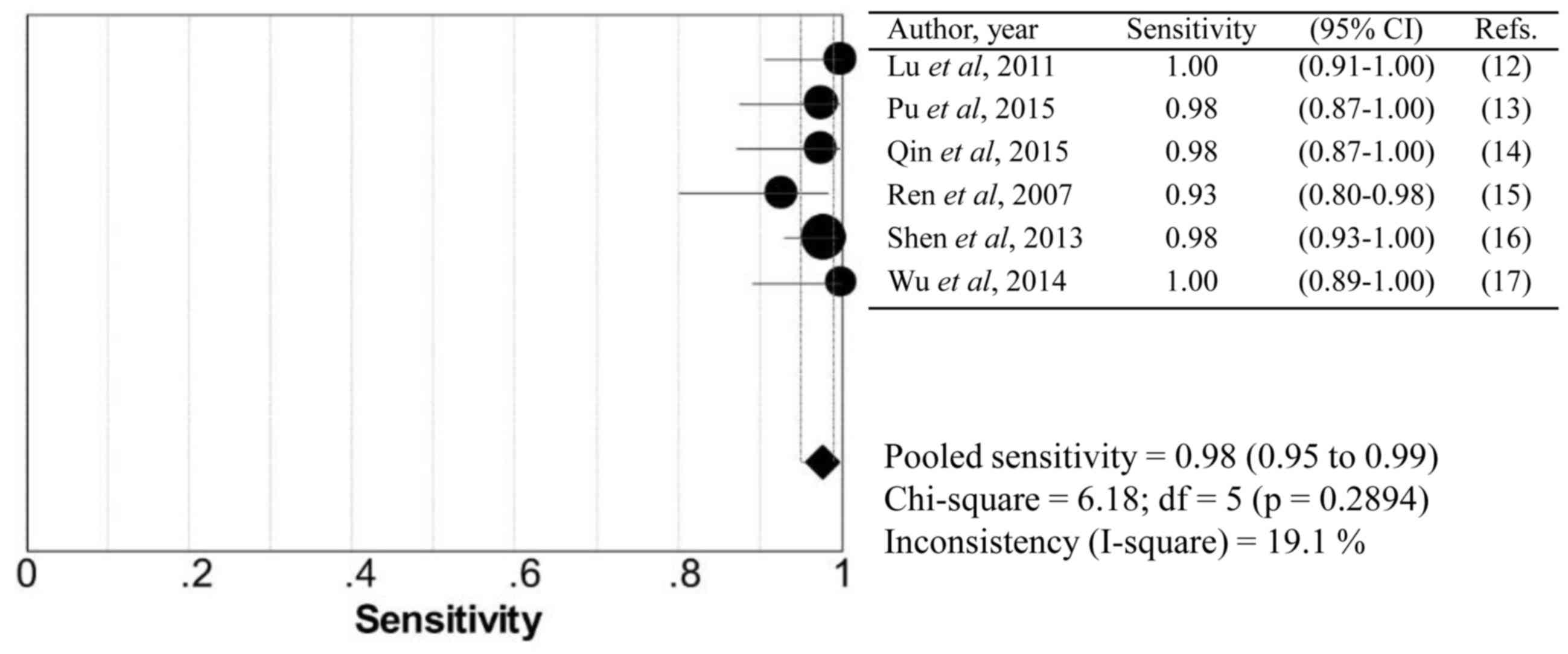
summary statistics for sensitivity (Fig.
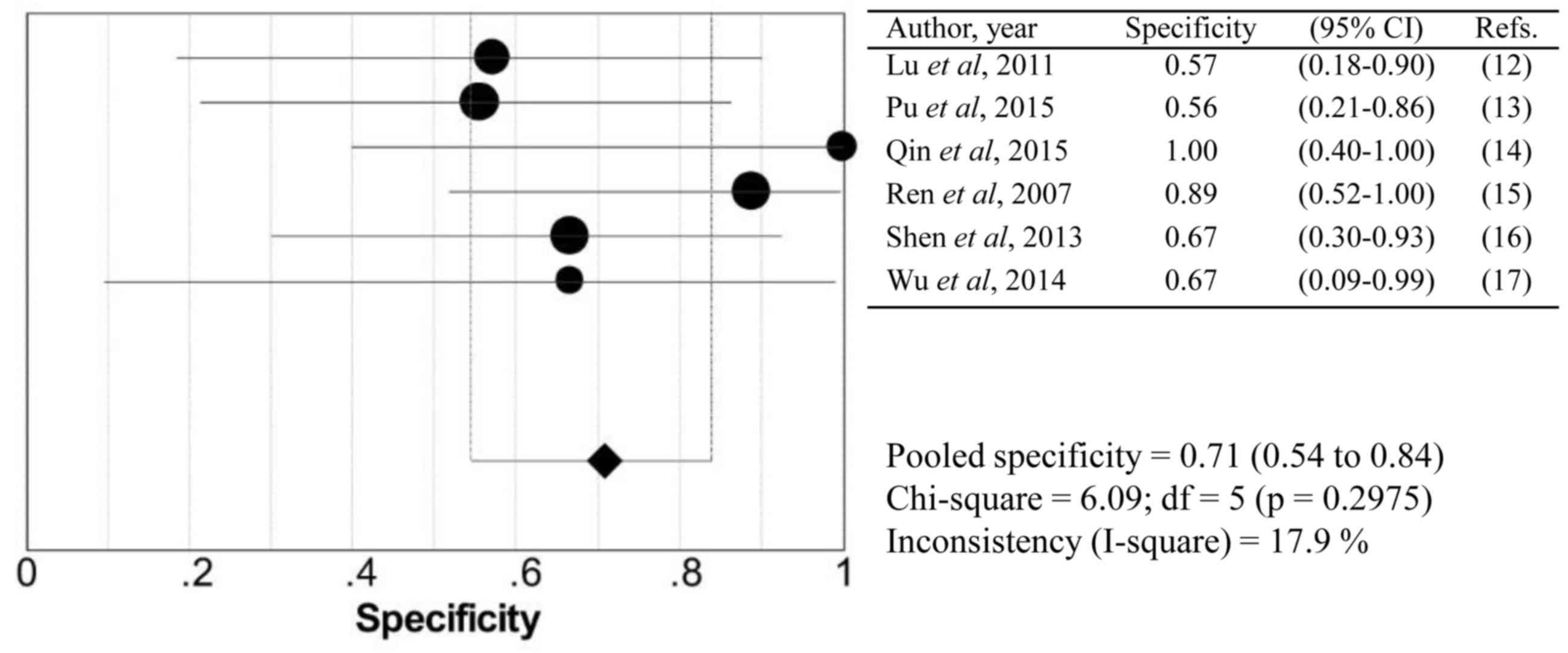
2), specificity (Fig. 3),
positive likelihood ratio, negative likelihood ratio, diagnostic
odds ratios (DOR), P-value for heterogeneity and
I2-value are summarized in Table II. No significant heterogeneity was
identified within these studies. None of the 95% confidence
intervals of the ORs included 1, which confirms a significant
diagnostic value of all modalities.
 | Table II.Weighted summary for each
modality. |
Table II.
Weighted summary for each
modality.
| Value | Sensitivity | Specificity | LR+ | LR- | DOR |
|---|
| Pooled | 0.976 | 0.707 | 3.163 | 0.045 | 94.053 |
| 95% CI | 0.95–0.99 | 0.55–0.84 | 2.03–4.94 | 0.02–0.09 | 32.71–270.41 |
| Chi-squared | 6.18 | 6.09 | 3.52 | 1.83 | 0.57 |
| P-value | 0.289 | 0.297 | 0.621 | 0.873 | 0.989 |
| I2 value
(%) | 19.1 | 17.9 | 0.0 | 0.0 | 0.0 |
Summary of scan modalities and
misdiagnosis analysis
Scan modalities (equipment, protocol, reconstruction
mode, contrast agent) and reasons for misdiagnosis were summarized
in Tables III and IV, respectively. In general, 54% of
patients used 1.5 T MRI equipment and 46% used 3.0 T. The maximum
intensity projection was the most common way to reconstruct images.
Patients with a heart rate of >100 bpm, uneven breathing,
massive ascites and membrane obstruction were more likely to be
misdiagnosed.
 | Table III.Summary of scan modalities for each
study. |
Table III.
Summary of scan modalities for each
study.
| Author, year | Equipment | Protocol | Reconstruction
mode | Contrast
medium | (Refs.) |
|---|
| Lu et al,
2011 | 3.0T GE Signa
EXCITE | FIESTA &
LAVA | MIP | Yes | (12) |
| Pu et al,
2015 | 1.5T GE Signa
HDxt | FIESTA &
LAVA | MIP & MPR &
VR | Yes | (13) |
| Qin et al,
2015 | 1.5T GE Signa
HDxt | IFIR | MIP | No | (14) |
| Ren et al,
2007 | 1.5T Toshiba
Visart | FBI | MIP | No | (15) |
| Shen et al,
2013 | 3.0T GE Signa
EXCITE | LAVA | NA | Yes | (16) |
| Wu et al,
2014 | 1.5T GE Signa
HDxt | IFIR &
FIESTA | MIP & MPR &
VR | No | (17) |
 | Table IV.Analysis of misdiagnoses in the
studies included. |
Table IV.
Analysis of misdiagnoses in the
studies included.
| Author, year | n (%) | Presentation | Misdiagnosis | (Refs.) |
|---|
| Lu et al,
2011 | 2 (4.5) | Segmental stenosis
of IVC | Segmental
obstruction | (12) |
|
| 1 (2.3) | Membranous
obstruction with hole and thrombus | Complete
obstruction | (12) |
| Pu et al,
2015 | 5 (9.8) | IVC compressed by
massive ascites | IVC stenosis | (13) |
| Qin et al,
2015 | 1 (2.2) | NA | NA | (14) |
| Ren et al,
2007 | 3 (6.0) | Heart rate >100
bpm and breathing uneven (disappearance of the signal from the
proximal part of the IVC) | IVC
obstruction | (15) |
|
| 3 (6.0) | Only one or two
hepatic vein stenoses (partial and gentle narrow) | No stenosis of the
hepatic vein | (15) |
| Shen et al,
2013 | 5 (4.6) | NA | NA | (16) |
| Wu et al,
2014 | 1 (2.9) | Membrane
obstruction | Membrane
stenosis | (17) |
Discussion
Budd-Chiari syndrome is an uncommon condition
induced by thrombotic or non-thrombotic obstruction of HV outflow
(18). DSA is considered as the
‘gold standard’ for this disease, but it is an invasive examination
involving exposure to radiation and injection of contrast agent.
Various of other imaging modalities are available for diagnosing
BCS in the clinic, including ultrasound (US), computed tomography
(CT) and MR imaging.
US should be performed as the first imaging method
of choice in patients with suspected BCS due to its high diagnostic
sensitivity (75–90%) and non-invasive nature (19), but this approach is
operator-dependent and maybe affected by excessive ascites and
intestinal gas (20). CT has also
been widely used for the diagnosis of BCS in the clinical setting
as a non-invasive diagnostic tool (21,22).
Certain studies have used CT to evaluate liver parenchyma, the
status of the HVs and IVC, as well as extrahepatic and intrahepatic
collaterals (23,24). With the development of CT technology,
the effectiveness of BCS diagnosis has markedly improved (25). Although CT is a good modality for
evaluating BCS, it has various disadvantages, including possible
allergic reactions and nephrotoxicity due to the use of contrast
agent (26).
MRA has been increasingly used as an alternative
technology for assessing BCS. However, the quality of this
methodology has not been assessed in previous studies. In the
present study, a systematic review was performed to obtain the best
available estimate of the diagnostic performance of MRA in patients
with BCS. The meta-analysis revealed that, compared with the
reference standard DSA, MRA is an accurate diagnostic tool for
diagnosing BCS with a pooled sensitivity of 97.6%, a specificity of
70.7% and a DOR of 94.1%. MRA is a useful method for the diagnosis
of BCS regardless of the use of contrast agent. Therefore, MRA
protocols without the use of contrast agent, e.g. fresh blood
imaging or in-flow inversion recovery, may become increasingly
important, particularly for patients who cannot receive any
contrast agent for various reasons. Time-spatial labeling inversion
pulse is another non-contrast-enhanced MRA technology with a high
success rate, high accuracy rate and fine image quality for the
diagnosis of BCS (27). This
technique based on true steady-state free-precession is a type of
spin labeling that yields the quantitative plus selective inflow of
information and suppressing the background (28–30).
In the present study, it was revealed that certain
factors may lead to misdiagnosis by MRA, including a heart rate of
>100 bpm, uneven breathing and massive ascites. All of these
factors may increase the misdiagnosis rate. At present, maximum
intensity projection is the most commonly used image reconstruction
method, which may facilitate an accurate diagnosis. Furthermore,
multiplanar reconstruction and volume rendering may be added as a
supplement. MRA may not only be used to diagnose BCS effectively,
but also provide more useful information on the classification of
BCS patients, the shape of the IVC obstruction, as well as the
position and direction of accessory HVs (AHVs), and this
information has a high degree of consistency with DSA. In 2016, Xu
et al (31) reported that
liver accelerated volume acquisition (LAVA) sequence had a high
sensitivity and specificity in detecting AHV in BCS patients as
indicated by a retrospective analysis. Lu et al (32) suggested that LAVA sequence is able to
detect more AHVs than DSA. In addition, MRA clearly reveals the
opening direction of AHV and the angle between AHV and distal vena
cava, which have practical value for interventional therapy
(33).
The present study has several limitations. One
limitation is that the number of studies included was low and the
sample size was small. The second limitation is that there were
differences in the equipment and protocols among the studies, among
which two studies used 3.0T magnetic resonance imaging equipment
and four studies used 1.5T equipment. In addition, BCS may be
categorized into three types depending on the type of venous
occlusion (2). The present study did
not analyze each type separately due to the small number of cases.
The third limitation is that only studies that were written in
English and Chinese were included for reasons of practicality.
Publication bias was not assessed due to the inclusion of a limited
number of studies (<10).
In conclusion, the high diagnostic accuracy of MRA
determined in the present meta-analysis suggests that this modality
has potential for improving the diagnosis and evaluation
Budd-Chiari syndrome. These results provide a larger-scale
reference for clinicians and it is recommended that MRA is
implemented in the clinic for diagnosing Budd-Chiari syndrome.
Acknowledgements
The authors would like to thank Mr Muhammad Bilal
(Department of Neurology, Affiliated Hospital of Xuzhou Medical
University, Xuzhou, China) for language editing of the
manuscript.
Funding
This study was supported by grants from the Clinical
Medical Science and Technology Project of Jiangsu Province (grant
no. BE2017637) and the Medical Innovation Team (leading talent) of
Jiangsu Province (grant no. CXTDA2017028).
Availability of data and materials
The datasets used and analyzed in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
PX and LL analyzed the patient data, and were the
major contributors in the preparation of the manuscript, HG and YR
analyzed part of the patient data, MUS and XL collected the
original data, and CH and KX made substantial contributions to the
conception of the study and drafted the manuscript. All authors
read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menon KN, Shah V and Kamath PS: The
Budd-Chiari syndrome. N Engl J Med. 350:578–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lupescu IG, Dobromir C, Popa GA, Gheorghe
L and Georgescu SA: Spiral computed tomography and magnetic
resonance angiography evaluation in Budd-Chiari syndrome. J
Gastrointestin Liver Dis. 17:223–226. 2008.PubMed/NCBI
|
|
3
|
Orloff MJ, Daily PO, Orloff SL, Girard B
and Orloff MS: A 27-year experience with surgical treatment of
Budd-Chiari syndrome. Ann Surg. 232:340–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Q, Shen B, Zhang Q, Xu H, Zu M, Gu
Y, Wei N, Cui Y and Huang R: Comparison of long-term outcomes of
endovascular management for membranous and segmental inferior vena
cava obstruction in patients with primary Budd-Chiari syndrome.
Circ Cardiovasc Interv. 9:e0031042016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng QY, Sun NF, Wang JX, Wang RH and Liu
ZX: Endovascular treatment of Budd-Chiari syndrome. Chin Med J
(Engl). 124:3289–3292. 2011.PubMed/NCBI
|
|
6
|
Kubo T, Shibata T, Itoh K, Maetani Y,
Isoda H, Hiraoka M, Egawa H, Tanaka K and Togashi K: Outcome of
percutaneous transhepatic venoplasty for hepatic venous outflow
obstruction after living donor liver transplantation. Radiology.
239:285–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Lu J, Wang F, Liu Q and Wang J:
Diagnosis of Budd-Chiari syndrome: Three-dimensional dynamic
contrast enhanced magnetic resonance angiography. Abdom Imaging.
36:399–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: A tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoh S, Kitazume Y, Ohdama S, Kimula Y,
Taura S and Endo Y: Can malignant and benign pulmonary nodules be
differentiated with diffusion-weighted MRI? AJR Am J Roentgenol.
191:464–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li B, Li Q, Chen C, Guan Y and Liu S:
Diagnostic accuracy of computer tomography angiography and magnetic
resonance angiography in the stenosis detection of autologuous
hemodialysis access: A meta-analysis. PLoS One. 8:e784092013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: A software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu X, Xu K, Zhang QQ, Yang C, Li SD, Li
JS, Rong YT and Zu MH: Study on between magnetic resonance
venography and digital subtraction angiography on the inferior vena
cava obstructive interface morphology of Budd-Chiari syndrome.
Zhonghua Gan Zang Bing Za Zhi. 19:923–926. 2011.(In Chinese).
PubMed/NCBI
|
|
13
|
Pu HB, You J, Chen HP, Zhang FZ and Xie Y:
The value of FIESTA sequence in the diagnostis of inferior vena
cava lesions with Budd-Chiari syndrome. Chin J Magn Reson Imaging.
6:422–466. 2015.(In Chinese).
|
|
14
|
Qin D, Shi D, Dou S, Lian JM and Yan FS:
Value of in-flow inversion recovery sequence in diagnosis of
Budd-Chiari syndrome. J Pract Radiol. 1:136–139. 2015.(In
Chinese).
|
|
15
|
Ren K, Xu K, Sun WG, Chen YS, Qi XX, Li RL
and Jin AY: Preliminary evaluation of magnetic resonance fresh
blood imaging for diagnosis of Budd-Chiari syndrome. Chin Med J
(Engl). 120:95–99. 2007.PubMed/NCBI
|
|
16
|
Shen PP and Wang XT: The comparison of the
imaging diagnosic value in Budd-Cmari syndrome. Acta Acad Med
Xuzhou. 33:111–113. 2013.(In Chinese).
|
|
17
|
Wu M, Xu J, Shi D, Shen H, Wang M, Li Y,
Han X and Zhai S: Evaluations of non-contrast enhanced MR
venography with inflow inversion recovery sequence in diagnosing
Budd-Chiari syndrome. Clin Imaging. 38:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferral H, Behrens G and Lopera J:
Budd-Chiari syndrome. AJR Am J Roentgenol. 199:737–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chawla Y, Kumar S, Dhiman RK, Suri S and
Dilawari JB: Duplex Doppler sonography in patients with Budd-Chiari
syndrome. J Gastroenterol Hepatol. 14:904–907. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Erden A: Budd-Chiari syndrome: A review of
imaging findings. Eur J Radiol. 61:44–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camera L, Mainenti PP, Di Giacomo A,
Romano M, Rispo A, Alfinito F, Imbriaco M, Soscia E and Salvatore
M: Triphasic helical CT in Budd-Chiari syndrome: Patterns of
enhancement in acute, subacute and chronic disease. Clin Radiol.
61:331–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Virmani V, Khandelwal N, Kang M, Gulati M
and Chawla Y: MDCT venography in the evaluation of inferior vena
cava in Budd-Chiari syndrome. Indian J Gastroenterol. 28:17–23.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho OK, Koo JH, Kim YS, Rhim HC, Koh BH
and Seo HS: Collateral pathways in Budd-Chiari syndrome: CT and
venographic correlation. AJR Am J Roentgenol. 167:1163–1167. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai SF, Gai YH and Liu QW: Computed
tomography angiography manifestations of collateral circulations in
Budd-Chiari syndrome. Exp Ther Med. 9:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LM, Zhang GY, Liu YL, Wu J, Cheng J
and Wang Y: Ultrasonography and computed tomography diagnostic
evaluation of Budd-Chiari syndrome based on radical resection
exploration results. Ultrasound Q. 31:124–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu SY, Xiao P, Cao HC, Jiang HS and Li
TX: Accuracy of computed tomographic angiography in the diagnosis
of patients with inferior vena cava partial obstruction in
Budd-Chiari syndrome. J Gastroenterol Hepatol. 31:1933–1939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang C, Li C, Zeng M, Lu X, Li JJ, Wang
JL, Sami MU and Xu K: Non-contrast-enhanced MR angiography in the
diagnosis of Budd-Chiari syndrome (BCS) compared with digital
subtraction angiography (DSA): Preliminary results. Magn Reson
Imaging. 36:7–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada K, Isoda H, Okada T, Kamae T,
Arizono S, Hirokawa Y, Shibata T and Togashi K:
Non-contrast-enhanced hepatic MR angiography: Do two-dimensional
parallel imaging and short tau inversion recovery methods shorten
acquisition time without image quality deterioration? Eur J Radiol.
77:137–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimada T, Amanuma M, Takahashi A and
Tsushima Y: Non-contrast renal MR angiography: Value of subtraction
of tagging and non-tagging technique. Ann Vasc Dis. 5:161–165.
2012.PubMed/NCBI
|
|
30
|
Shimada K, Isoda H, Okada T, Maetani Y,
Arizono S, Hirokawa Y, Kamae T and Togashi K: Non-contrast-enhanced
hepatic MR angiography with true steady-state free-precession and
time spatial labeling inversion pulse: Optimization of the
technique and preliminary results. Eur J Radiol. 70:111–117. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu HT, Xu K, He P, Zhang QQ, Dai Y, Lu L,
Li C and Sun JM: Value of three-dimensional liver acceleration
volume acquisition multiphase dynamic contrast-enhanced magnetic
resonance imaging in detection of accessory hepatic veins in
Budd-Chiari syndrome. Zhonghua Gan Zang Bing Za Zhi. 24:585–589.
2016.(In Chinese). PubMed/NCBI
|
|
32
|
Lu L, Xu K, Han C, Xu C, Xu H, Dai Y, Rong
Y, Li S and Xie L: Comparison of 3.0T MRI with 3D LAVA sequence and
digital subtraction angiography for the assessment of accessory
hepatic veins in Budd-Chiari syndrome. J Magn Reson Imaging.
45:401–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu H, Xu K, He P, Zhang QQ, Dai Y, Lu L,
Li C and Sun JM: CE-MRA in detecting the opening direction and
measuring the angle of accessory hepatic vein with the inferior
vena cava in Budd-Chiari syndrome. Chin J Hepatobiliary Surg.
21:596–599. 2015.(In Chinese).
|