Introduction
Diabetes mellitus (DM) has become a major public
health problem worldwide (1). It is
a major risk factor for ischemic heart disease and stroke, chronic
kidney disease and blindness, and is responsible for high rates of
morbidity and mortality among patients (2–4). Type 1
(T1) DM is characterized as a chronic autoimmune disease of the
pancreas, causing progressive destruction of β-cells (5). Patients with T1DM eventually require
exogenous insulin to control their blood glucose. Regarding the
treatment of DM patients, various methods have been evaluated,
including the use of cytostatic drugs (6,7),
monoclonal antibodies (e.g. rituximab, teplizumab) (8,9) and
vaccines for modifying the patient's abnormal immune system
response (10). Transplantation of
the entire human pancreas or isolated, cadaveric human islets,
which has provided proof of concept for β-cell replacement
therapies, has made T1DM patients insulin-independent (11,12).
However, the transplantation mentioned above has remained to be
fully developed and effectively implemented in clinical practice.
The major limitations include surgery-associated complications, the
requirement of donor cells (13) and
immunosuppressants (14), and the
risk of immune rejection (15) and
recurrence (16).
Stem cell (SC) transplantation has emerged as a
breakthrough treatment due to of the intrinsic regenerative
capacity and immunomodulatory properties of SCs (17), which may achieve arrest of autoimmune
β-cell destruction and generate functional β-cells (18). SC therapy may represent a novel
paradigm for optimizing glycemic control in T1DM. Meta-analyses of
studies on SC therapy for liver cirrhosis (19), chronic liver disease (20), inflammatory bowel disease (21) and ischemic heart disease (22) have indicated a certain potential for
cell therapy-based strategies. In animal studies, it was
demonstrated that SCs are able to relieve hyperglycemia by
differentiating into insulin-producing cells, thereby promoting the
transformation of α-cells to β-cells, improving pancreatic
regeneration and ameliorating insulin resistance. In the present
meta-analysis, the efficacy and safety of SC therapy for the
treatment of patients with T1DM was assessed based on published and
unpublished clinical trials. Representative outcome data were
analyzed, including the mean values of C-peptide levels, glycated
hemoglobin (HbA1c) levels, fasting plasma glucose (FPG) and daily
insulin requirement every three months over 12 months.
C-peptide, a connecting peptide, is a short
31-amino-acid polypeptide that connects the A-chain of insulin to
its B-chain in the pro-insulin molecule. In diabetes, measurement
of C-peptide blood serum levels may be performed to distinguish
between certain diseases with similar clinical features (23). HbA1c may be assessed to evaluate the
average blood sugar levels over a period of weeks/months (24). For patients with diabetes, this is
important, as higher HbA1c levels are linked with a greater risk of
developing diabetes-associated complications. A fasting plasma
glucose (FPG) test, a simple blood test taken after several h of
fasting, also known as the fasting glucose test, may be used to
diagnose diabetes or pre-diabetes (25). The insulin requirement reflects the
degree of insulin dependence in diabetic patients. In the present
study, two major subgroup analyses [data from randomized-controlled
trials (RCTs) and self-controlled trials (SCTs)] were performed.
Several subgroups were classified based on the origin and nature of
SCs within each major group, including hematopoietic stem cells
(HSC), mesenchymal stem cells (MSC) and umbilical cord blood (UCB).
The RCTs reflected the efficacy of a population-based study, while
that of individuals was demonstrated by the SCTs. In these two
major groups, the present study aimed to explore the therapeutic
efficacy of different SCs in patients with T1DM.
Materials and methods
Guidelines and data availability
This systematic review was registered at the
International prospective register of systematic reviews (no.
42018093930). Study inclusion and exclusion was performed in
accordance with the Preferred Reporting Items for Systematic
reviews and Meta Analyses (26) and
the Cochrane handbook for systematic reviews of interventions
(27).
Literature search
A systematic search of studies published from
inception until 13th January 2018 was performed in the databases
PubMed (https://www.ncbi.nlm.nih.gov/pubmed), MEDLINE
(https://www.medline.com/home.jsp),
WanFang (http://g.wanfangdata.com.cn/index.html) and the
Cochrane Library (including CENTRAL; http://www.cochranelibrary.com/). To accurately
identify clinical trials of SC transplantation in T1DM patients, a
search was performed using a combination of Me-SH terms and text
words: (‘type 1 diabetes’ OR ‘diabetes mellitus type 1’ OR
‘hyperglycemia’) AND (‘stem cells’ OR ‘progenitor cells’ OR
‘mesenchymal stem cell’ OR ‘mononuclear stem cell’ OR
‘hematopoietic stem cell’ OR ‘progenitor cell’ OR ‘beta cell’) AND
(‘cell therapy’ OR ‘treated’ OR ‘therapeutic’ OR ‘treatment’). No
language restrictions were applied. Furthermore, potentially
eligible studies were identified by screening the lists of
references of the retrieved articles as well as in review articles
and abstracts from relevant conferences. The most recent or
complete studies were selected for analysis when the same or
similar patient data were included. Furthermore, information of
prospective and ongoing trials was retrieved from http://www.ClinicalTrials.gov, a database created to
establish a registry of clinical trials involving investigational
drugs as a result of the Food and Drug Administration Modernization
Act of 1997 and a Final Rule issued by Department of Health and
Human Services (28). All of the
searches were performed by two investigators independently (GJD and
WYJ).
Study selection
The following inclusion criteria were applied to
select studies based on their titles and abstracts: i) The studies
were clinical trials, but not pre-clinical trials, reviews,
comments, case reports or basic scientific research; ii) the
subjects included had been diagnosed with T1DM; iii) the
participants received SC therapy (no restriction was applied
regarding the source and route of administration of the stem
cells). Studies were excluded if: i) They were animal studies; ii)
the included participants did not undergo SC therapy; iii) they
contained duplicate data; iv) the C-peptide levels, HbA1c levels
and FPG or insulin requirement assessed every 3 months over a 12
month period were not included and could not be calculated from the
data.
Furthermore, when the data overlapped or were
duplicated between two or more studies by the same group, the most
recent study, which contained more complete data were included. The
articles were independently evaluated by two reviewers (GJD and
WYJ) in duplicate and selected for inclusion if they met the
abovementioned criteria. Discrepancies were resolved by
consensus.
Data extraction and quality
assessment
Primary items were independently extracted by two
authors (GJD and WYJ), including the request for documentation and
recalculation of the following variables, through a standardized
data collection form. The form included: a) The first author's
name, b) year of publication, c) country, d) size of study
population, e) study design, f) mean age, g) gender of the
participants, h) history of DM, i) type of SCs, j) mean dose of
injected cells, k) path of injection, l) follow-up time, m)
C-peptide levels, n) HbA1c levels, o) FPG and p) insulin
requirement was assessed every 3 months over 12 months. The data
for C-peptide levels, HbA1c levels and FPG were extracted to
identify changes over time and evaluate the length of SC treatment.
For continuous outcomes, the mean, standard deviation and total
sample size were recorded. Furthermore, the corresponding authors
of studies with insufficient data or studies that could not be
located were contacted by email and asked whether they were willing
to share their unpublished data from these trials for inclusion in
the present analysis. If no reply was received, the study was
excluded from the meta-analysis. Those data that were the outcomes
of interest of the present study were pooled with the data from the
primary trials.
Data from SCTs were analyzed to evaluate whether the
SC therapy improved the symptoms of patients with T1DM. The data
from RCTs were pooled to evaluate whether the SC therapy was
superior to the standard or conventional therapy in improving the
symptoms of T1DM patients.
Two reviewers (GJD and WYJ) independently assessed
the methodological quality of the nine RCTs by rating them on the
Jadad scale (29). The quality scale
of Jadad ranges from 0 to 5 points, with a score of ≤2 indicating a
low-quality study and a score of ≥3 indicating a high-quality
study. Disagreements were resolved by discussion between the two
reviewers.
Statistical analysis
Meta-analysis was performed with a random-effects
model (DerSimonian-Laird method) by using Stata 14.0 (StataCorp LP,
College Station, TX, USA). The standardized mean difference (SMD)
was used to combine treatment effect data. A random-effects model
was used to compute the pooled standardized mean deviation of
C-peptide and HbA1c levels with 95% confidence intervals (CI), as
this model takes into account any differences between studies even
if there is no statistically significant heterogeneity (27). The heterogeneity among the studies
was assessed using the inconsistency index (I2)
statistics (30,31) [I2 describes the percentage
of total variation across studies that are due to heterogeneity
rather than to chance and I2>50% was considered to
indicate substantial heterogeneity based on the suggestion in the
Cochrane Handbook for Systemic Reviews of Interventions (27)] and the Chi-square test (the risk of
random error is the risk of drawing a false conclusion based on
sparse data). This risk is quantified as the P-value, and P<0.05
was considered to indicate significant statistical
heterogeneity.
To determine the primary outcomes, Egger's test was
employed to assess the publication bias (32). Clinical heterogeneity was assessed by
subgroup analysis with patients stratified according to age,
medical history and cell dose. Prespecified subgroup analyses were
performed to explore potential heterogeneity between subgroups
(P<0.05 was considered to indicate significant statistical
heterogeneity). To evaluate whether the association between
C-peptide levels or HbA1c levels and SC therapy was affected by
clinical characteristics, the subjects were stratified into
subgroups based on age (<18 or ≥18 years), medical history
(<3 or ≥3 months) and cell dose (<107 or
≥107 IU/kg/day). Data were analyzed using Stata
statistical software version 14.0 (StataCorp LP).
Results
Literature search and study
selection
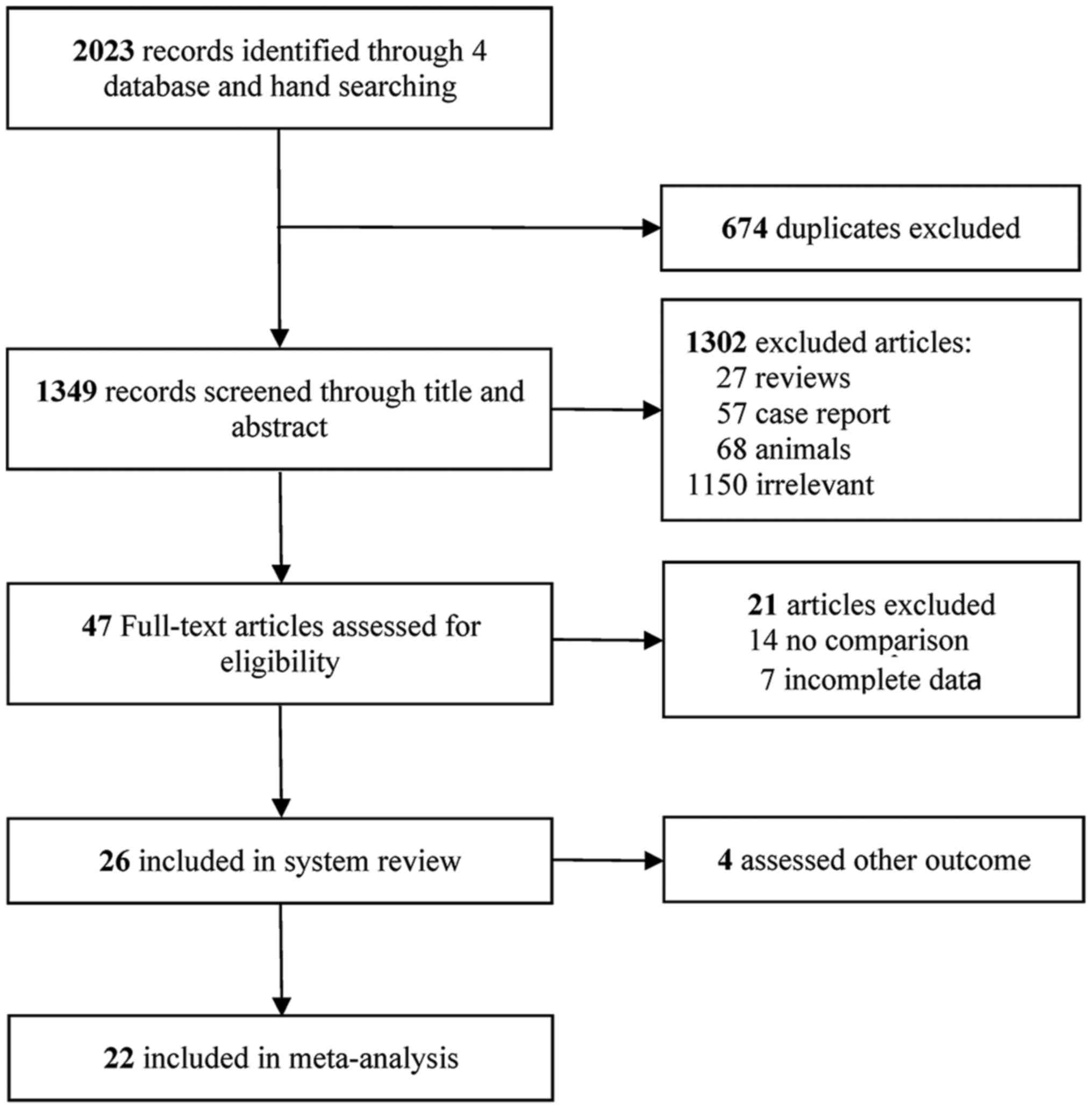
The literature search performed in the present study
yielded 2,023 records of potentially eligible studies, of which 674
were excluded due to being duplicates. Following screening of
titles and abstracts of the remaining 1,349 studies, a total of
1,302 studies were excluded based on the inclusion and exclusion
criteria, among which 27 were reviews, 57 were case reports, 68
included animal studies and 1,150 were irrelevant. Finally, 47
full-text articles were considered for inclusion, among which 22
included original research papers (33–54). A
flowchart illustrating the selection process is presented in
Fig. 1.
Study and patient characteristics
A total of 9 RCTs and 14 SCTs were included in the
final meta-analysis. Their details are summarized in Table I. Of the studies, 2 were published
prior to 2009 and 19 were published from 2010 onwards. The eligible
studies represented an international population, as they were
performed in a large range of countries, including China, India,
Poland, Spain, USA, Sweden, Argentina, Germany and Brazil, with the
age of participants ranging from 3 to 40 years. Individual patient
data were obtained for 455 participants, including 189 from the
RCTs and 266 from SCTs. The length of follow-up period ranged
between 1 and 12 months. The most common injection route of the SCs
in the studies included was intravenous. Thakkar et al
(34) and Vanikar et al
(50) used intra-pancreatic
injection. Furthermore, Cai et al (37) used a pancreatic artery cannulation
method.
 | Table I.Characteristics of the trials
included. |
Table I.
Characteristics of the trials
included.
| First author
(year) | Country | Cases/Total | Design | Mean age
(years) | Gender (F/M) | BMI
(kg/m2) | History of DM | Intervention | Dose | Injection path | Time to follow-up
(months) | (Refs.) |
|---|
| Ye (2017) | China | 10/18 | RCT | 18.9±1.5 | 6/4 | 19.2 ± 1.1 | <6 months | HSC |
2.00×105 | IV | 12 | (33) |
|
|
| 8/18 |
| 20.2±4.0 | 5/3 | 18.2±1.3 | <6 months | Insulin |
|
|
|
|
| Thakkar (2015) | India | 10/20 | RCT | 20.2 | 1/9 | NA | 8.1 years | MSC+HSC |
2.07×104 | IP | 3,6,9,12,24 | (34) |
|
|
| 10/20 |
| 19.7 | 4/6 | NA | 8.1 years | Insulin |
|
|
|
|
| Snarski (2016) | Poland | 20 | SCT | 24.3±4.5 | 5/15 | 22.2±2.3 | NA | HSC |
3.0×106 | IV | 3,6,12,24 | (35) |
| Delgado (2015) | Spain | 6/15 | SCT |
33.8±10.9 | 2/4 |
| 6.5 years | UCB |
1.0×106 | IV | 12 | (36) |
| Cai (2016) | USA | 21/42 | RCT | 18.3 | 12/9 | 22.0 | 9.2 years | MSC+HSC | 106.8×106 | IP | 3,6,9,12 | (37) |
|
|
| 21/42 |
| 20.4 | 10/11 | 22.1 | 7 years | Insulin |
|
|
|
|
| Giannopoulou | Germany | 7/17 | RCT | 3.0 | 2/5 | NA | <3 months | UCB |
1.27×106 | IV | 3,6,9,12 | (38) |
| (2014) |
| 10/17 |
| 6.6 | 6/4 | NA | <4 months | Insulin |
|
|
|
|
| D'Addio (2014) | USA | 65 | SCT | 20.4±5.5 | 24/41 | 18.1±3.1 | <3 months | HSC |
5.8×106 | IV | 12,24 | (39) |
| Carlsson
(2015) | Sweden | 9/18 | RCT | 24±2 | 1/8 | 23.3±1.1 | <3 weeks | MSC |
2.75×106 | IV | 12 | (40) |
|
|
| 9/18 |
| 27±2 | 4/5 | 22.5±0.9 | <3 weeks | Insulin |
|
|
|
|
| Hu (2013) | China | 15/29 | RCT | 17.6±8.7 | 6/9 | 20.9±3.7 | New onset | MSC |
2.6×107 | IV | 3,6,9,12,24 | (41) |
|
|
| 14/29 |
| 18.2±7.9 | 6/8 | 21.3±4.2 | New onset | Insulin |
|
|
|
|
| Haller (2013) | USA | 10/15 | RCT | 7.2 | NA | NA | 3.9 months | UCB |
1.1×107 | IV | 6,12 | (42) |
|
|
| 5/15 |
| 6.6 | NA | NA | 3.9 months | Insulin |
|
|
|
|
| Zhao (2012) | USA | 6/9 | RCT | 30±9 | 4/2 | NA | 6 years | UCB-SC |
1.0×106 | IV | 12 | (43) |
|
|
| 3/9 |
| 33±9 | 0/3 | NA | 6 years | Insulin |
|
|
|
|
| Zhang (2012) | China | 9 | SCT | 17.5±3.5 | 4/5 | 18.5±1.5 | 2 years | HSC |
10.49×106 | IV | 6,12 | (44) |
| Li (2012) | China | 13 | SCT | 14.1±4.0 | 3/10 | 18.1±2.3 | New onset | HSC |
4×106 | IV | 12 | (45) |
| Gu (2012) | China | 28 | SCT | 18.4±4.1 | 9/8 | 19.2±1.6 | 3 months | HSC |
1.0×106 | IV | 12 | (46) |
| Yu (2011) | China | 6/12 | RCT | 19.6±2.5 | 3/3 | 21.5±1.6 | <3 months | MSC |
1.0×107 | IV | 12 | (47) |
|
|
| 6/12 |
| 14.8±8.1 | 2/4 | 20.0±3.4 | <3 months | Insulin |
|
|
|
|
| Haller (2011) | USA | 24 | SCT | 5.1 | NA | NA | 4 months | UCB |
1.88×107 | IV | 3,6,9,12,24 | (48) |
| Feng (2011) | China | 17 | SCT | 13.0±3.8 | 9/8 | NA | 4 months | HSC |
3×106 | IV | 12 | (49) |
| Vanikar (2010) | India | 11 | SCT | 21.1 | 4/7 | NA | 8.2 years | MSC+HSC |
3.15×106 | IP | 12 | (50) |
| Snarski (2011) | Poland | 8 | SCT | 25.8 | 4/4 | NA | 2 years | HSC |
4.14×106 | IV | 3,6 | (51) |
| Gu (2010) | China | 18 | SCT | 18.8±4.4 | 12/6 | NA | <6 months | HSC | NA | IV | 3,6,12 | (52) |
| Haller (2009) | USA | 15 | SCT | 5.5 | 7/8 | NA | 4.1 months | UCB |
1.5×107 | IV | 12 | (53) |
| Couri (2009) | Brazil | 23 | SCT | 18.4±4.6 | 6/17 | 19.7±2.2 | <3 months | HSC | 10.52×106 | IV | 12 | (54) |
Quality assessment
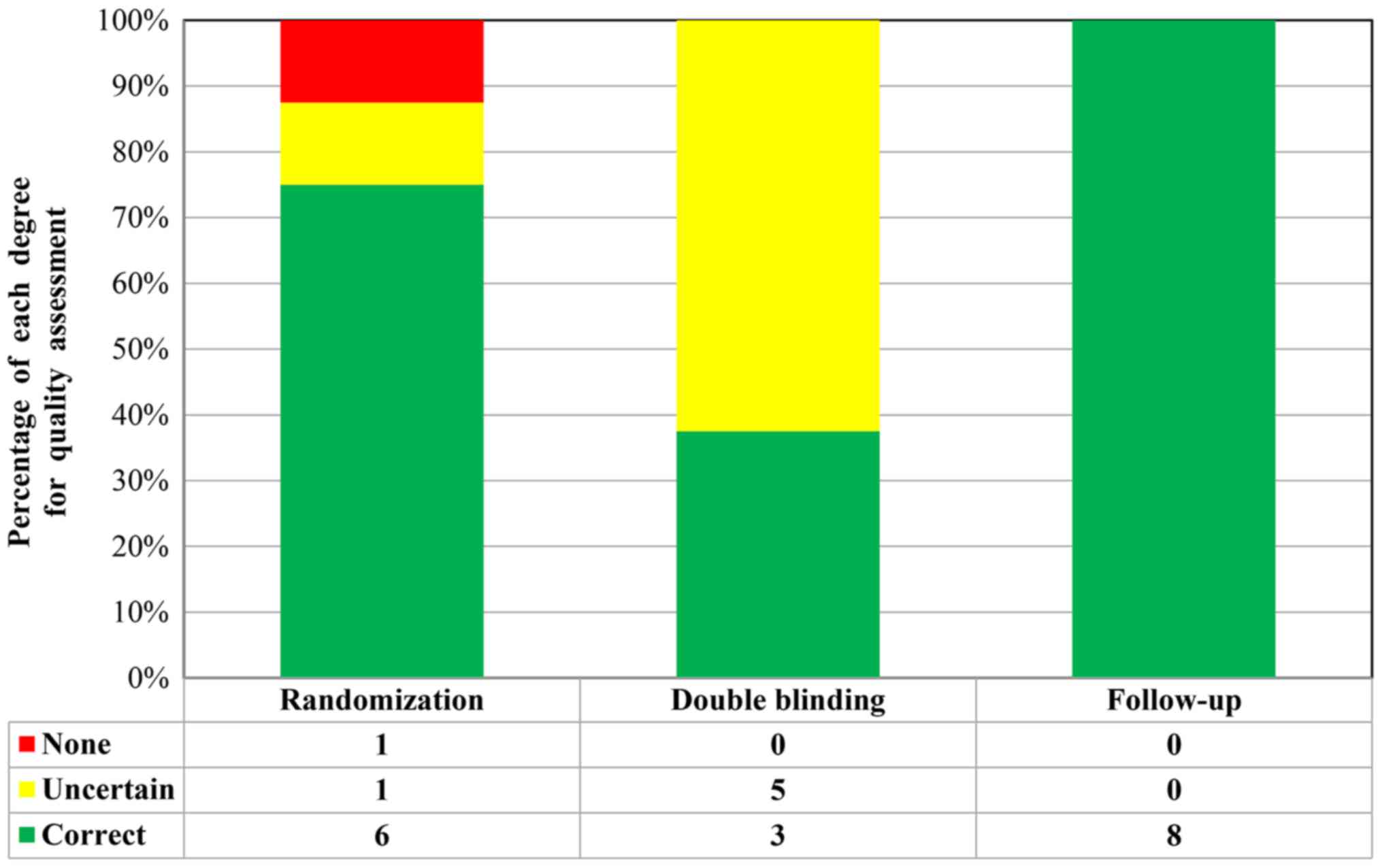
A total of 9 RCTs included a control group (placebo
or hypoglycemic agent) in a double-blinded design. Through Jadad
score tools, 5 studies [Cai et al (37), Carlsson et al (40), Hu et al (41), Haller et al (42) and Yu et al (46)] received 5 points, one study [Zhao
et al (43)] received 4
points and one study [Ye et al (33)] received 3 points. These eight studies
were considered to be of high quality, whereas another study
[Giannopoulou et al (38)]
received 2 points and was considered a low-quality study (Fig. 2). In addition, 13 studies were
designed as SCTs, for which no approved assessment tools were
available to evaluate the SCTs. However, these studies were also
repeatedly reviewed and quality assessments were performed. Each of
these SCTs included detailed selection procedures, strict
descriptions of the procedures and an adequate follow-up
period.
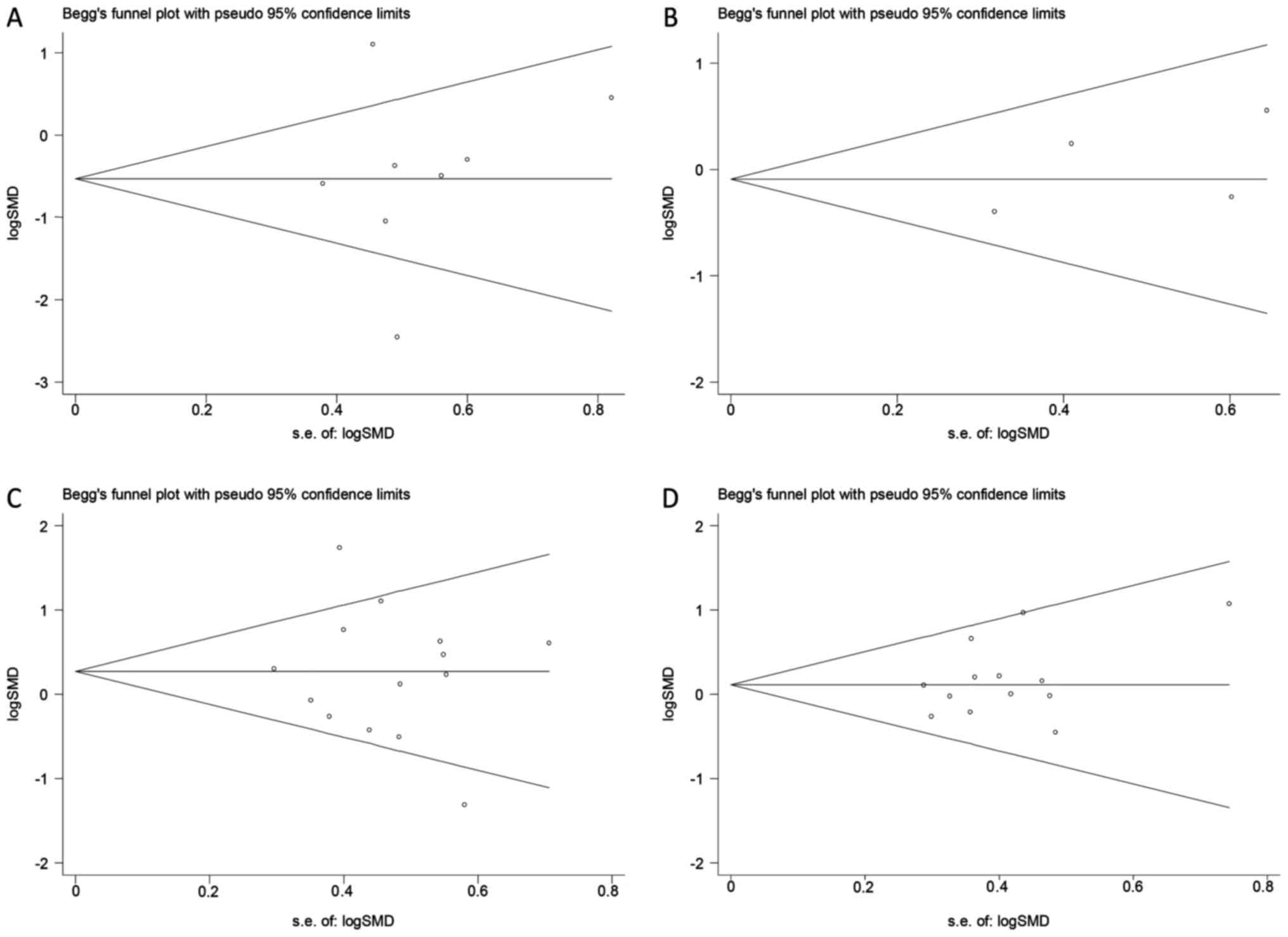
Publication bias
Begg's funnel plot revealed no evidence of
publication bias for the outcomes examined (P>0.05; Fig. 3). This indicated that the present
results may slightly overestimate the true effect size if
non-significant studies are not identified. The potential
publication bias of the effects of SC therapy on C-peptide and
HbA1c estimated in RCTs (Fig. 3A and
B). The potential publication bias of the effects of SC therapy
on C-peptide and HbA1c estimated in SCTs (Fig. 3C and D).
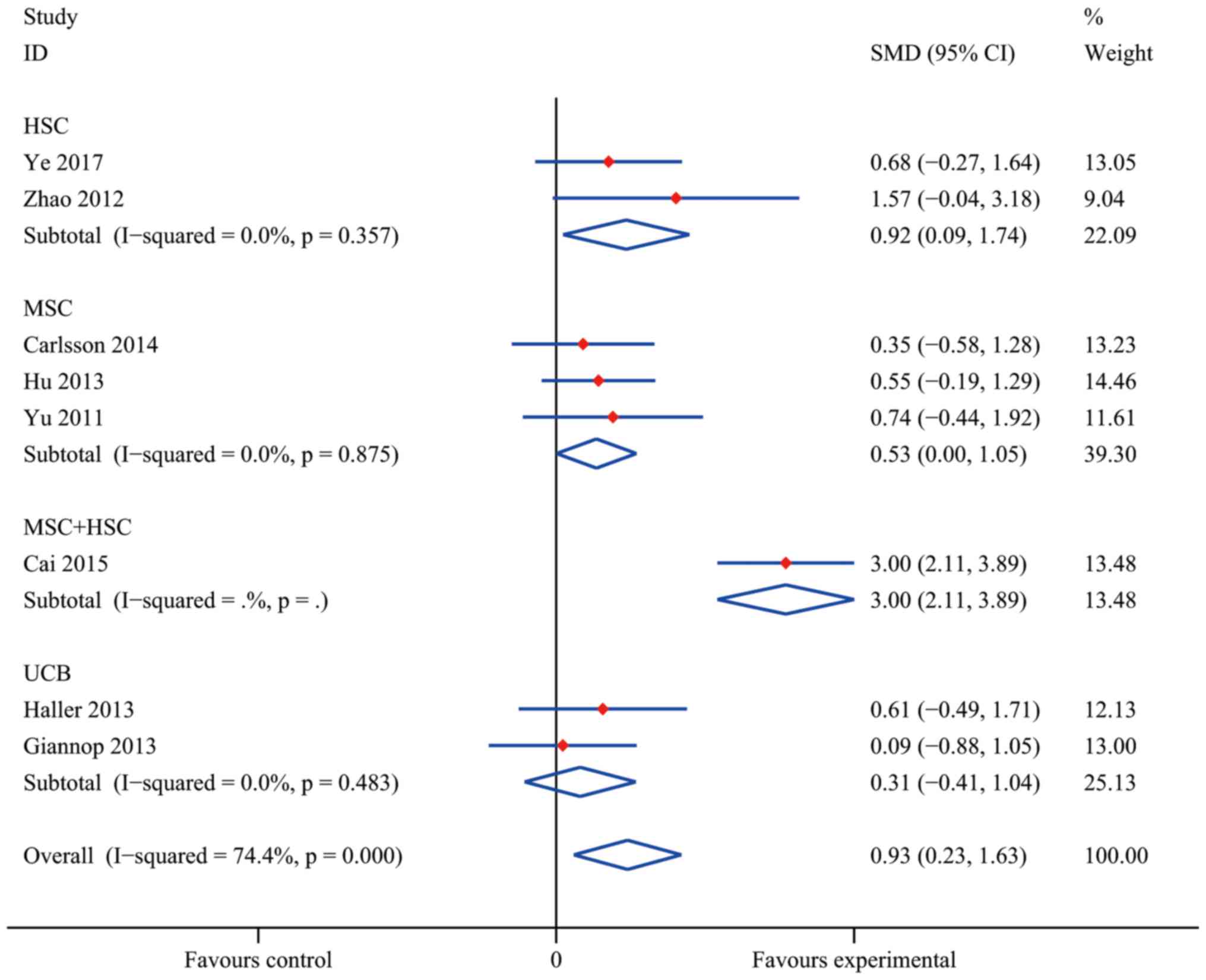
Efficacy of SC treatment in RCTs
Compared with conventional insulin therapy, SC
treatment resulted in a statistically significant difference in the
increase of C-peptide levels in T1DM patients with an overall SMD
of 0.93 (95% CI, 0.23–1.63; I2=74.4%; P=0.009). As
presented in Fig. 4, a statistically
significant pooled effect was determined in the HSC group (SMD,
0.92; 95% CI, 0.09–1.74; I2=0.0%; P=0.029) and in the
MSC group (SMD, 0.53; 95% CI, 0.00–1.05; I2=0.0%;
P=0.048), whereas in the UCB group, no statistically significant
effect was observed (SMD, 0.31; 95% CI, −0.41 to 1.04;
I2=0.0%; P=0.397). The data obtained by Cai et al
(37) indicated a significant
efficacy of MSC+HSC (SMD, 3.00; 95% CI, 2.11–3.89). At the same
time, the pooled effect of HbA1c reduction was significantly
different from that in the insulin treatment group (the control
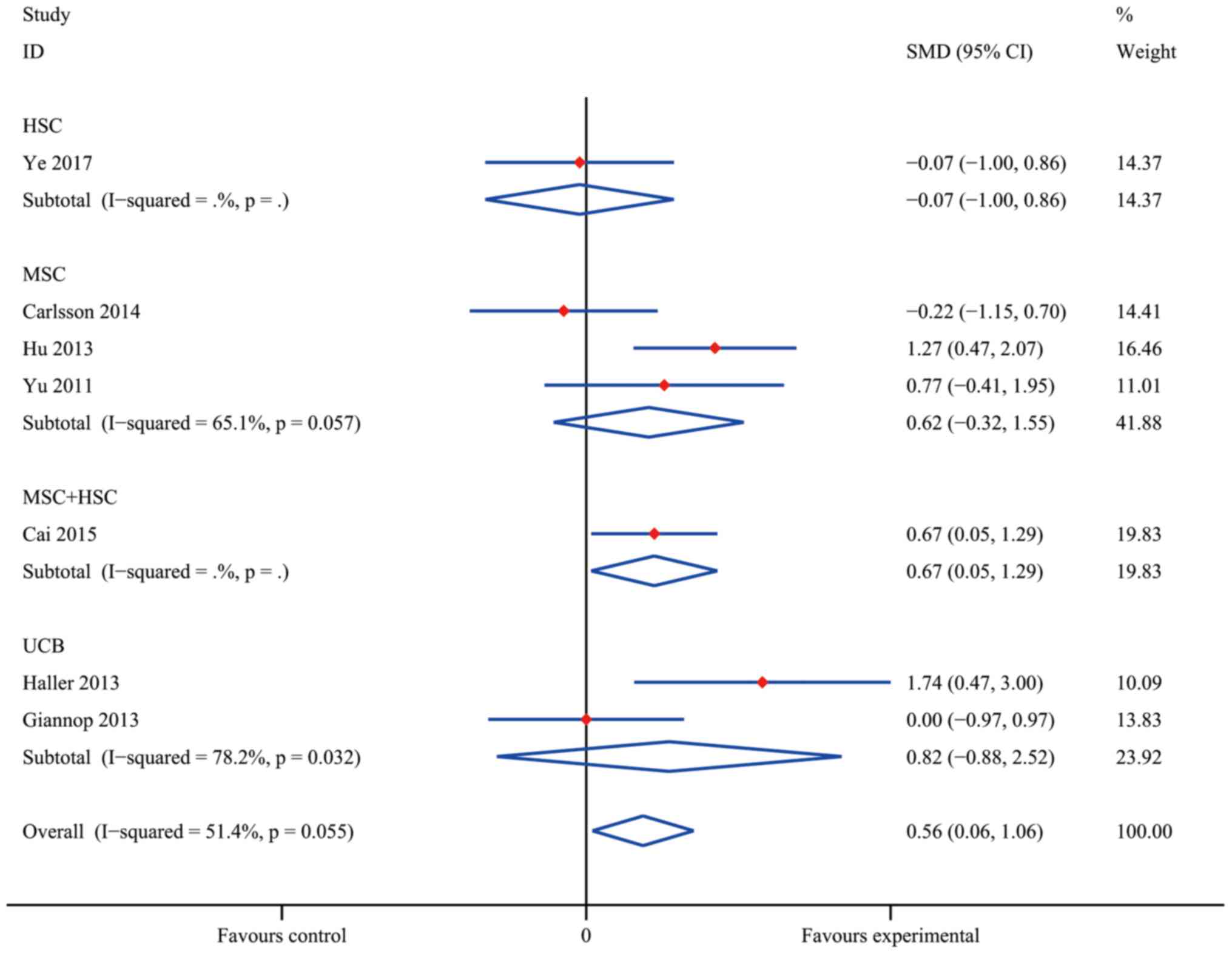
group) with an overall SMD of 0.56 (95% CI, 0.06–1.06;
I2=51.4%; P=0.028; Fig.
5). Furthermore, MSC+HSC exerted a decent therapeutic effect on
T1DM patients in terms of HbA1c with an SMD of 0.67 (95% CI,
0.05–1.29; P=0.034). However, the effect in the groups treated with
HSC (SMD, −0.07; 95% CI, −1.00 to 0.86; P=0.089), UCB (SMD, 0.82;
95% CI, −0.88 to 2.52; I2=78.2%; P=0.345) and MSC (0.62;
95% CI, −0.32 to 1.55; I2=65.1%; P=0.197) overlapped
with the no effect lines, indicating that the differences in these
groups were not statistically significant.
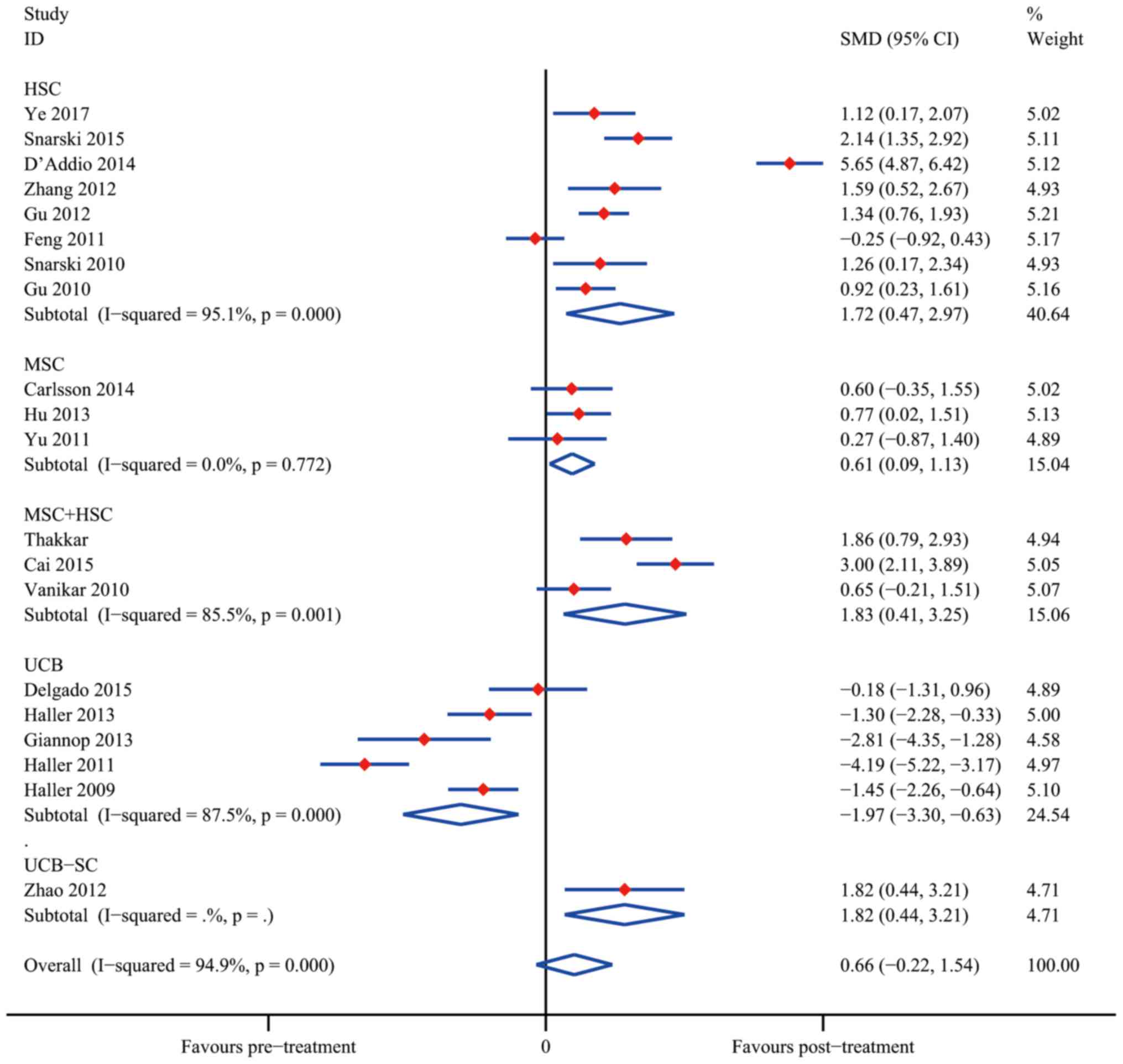
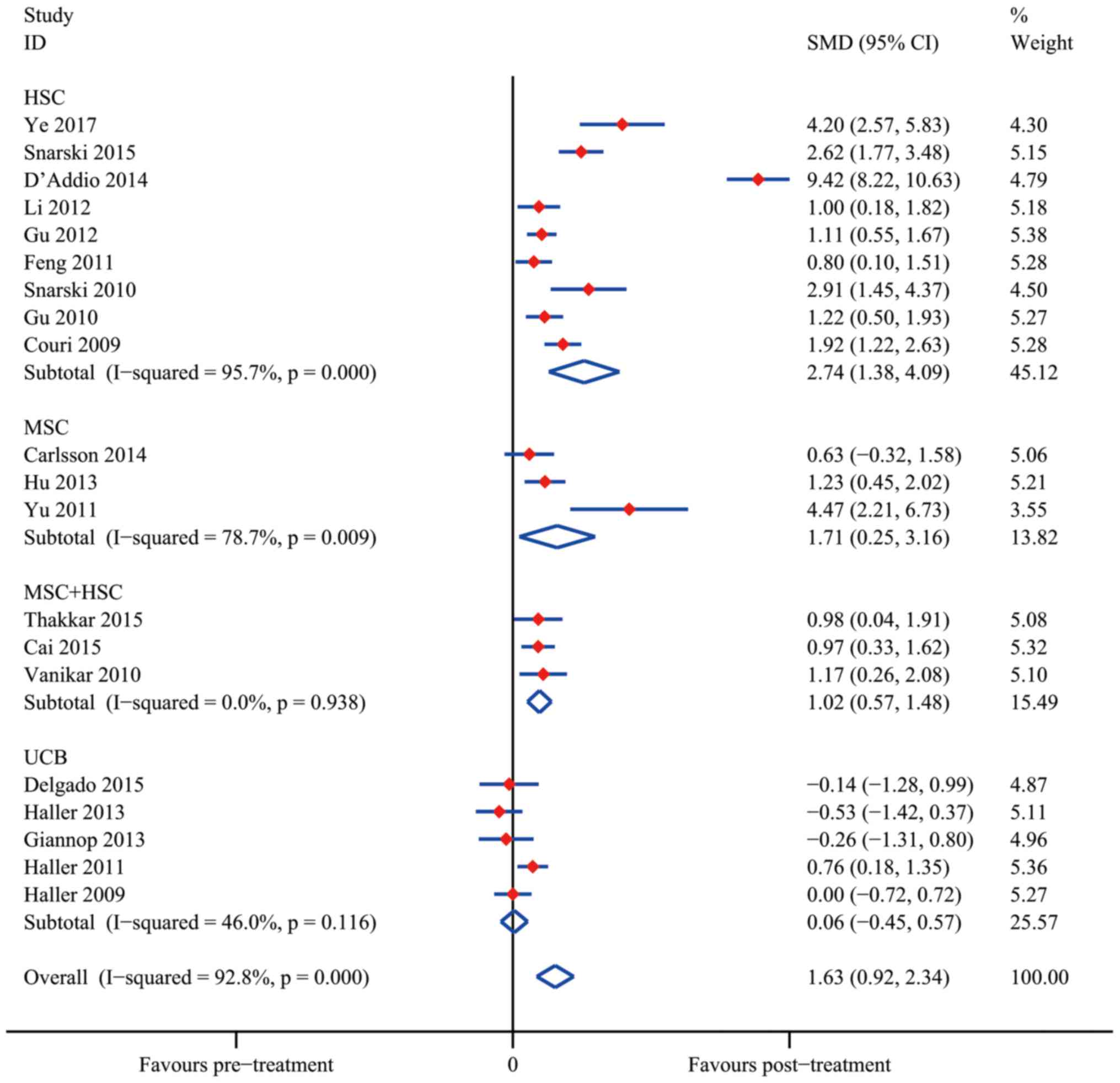
Efficacy of SC therapy in SCTs
The overall meta-analyses of data from SCTs are
presented in Figs. 6 and 7. The results indicated that SC treatment
significantly increased C-peptide levels after 12 months in the HSC
group (SMD, 1.72; 95% CI, 0.47–2.97; I2=95.1%; P=0.007),
the MSC group (SMD, 0.61; 95% CI, 0.09–1.13; I2=0.0%;
P=0.021) and the MSC+HSC group (SMD, 1.83; 95% CI, 0.41–3.25;
I2=85.5%; P=0.011). On the contrary, the results
obtained using UCB suggested that SC therapy reduced C-peptide
levels (SMD, −1.97; 95% CI, −3.30 to −0.63; I2=87.5%;
P=0.004); however, Zhao et al (43) reported a decent curative effect with
UCB-SC therapy (SMD, 1.82; 95% CI, 0.44–3.21; P=0.010). It was
therefore apparent that UCB-SC therapy had a different effect when
compared with that of treatment with UCB.
The analysis of changes in HAb1c levels demonstrated
that SC therapy resulted in a statistically significant difference
from the control group after 12 months in the groups treated with
HSC (SMD, 2.74; 95% CI, 1.38–4.09; I2=95.7%;
P<0.001), MSC (SMD, 1.71; 95% CI, 0.25–3.16;
I2=78.7%; P=0.022) and MSC+HSC (SMD, 1.02; 95% CI,
0.57–1.48; I2=0.0%; P<0.001). Similar to the result
regarding the C-peptide levels, UCB treatment did not significantly
reduce the HbA1c levels (SMD, 0.06; 95% CI, −0.45 to 0.57;
I2=46.0%; P=0,831).
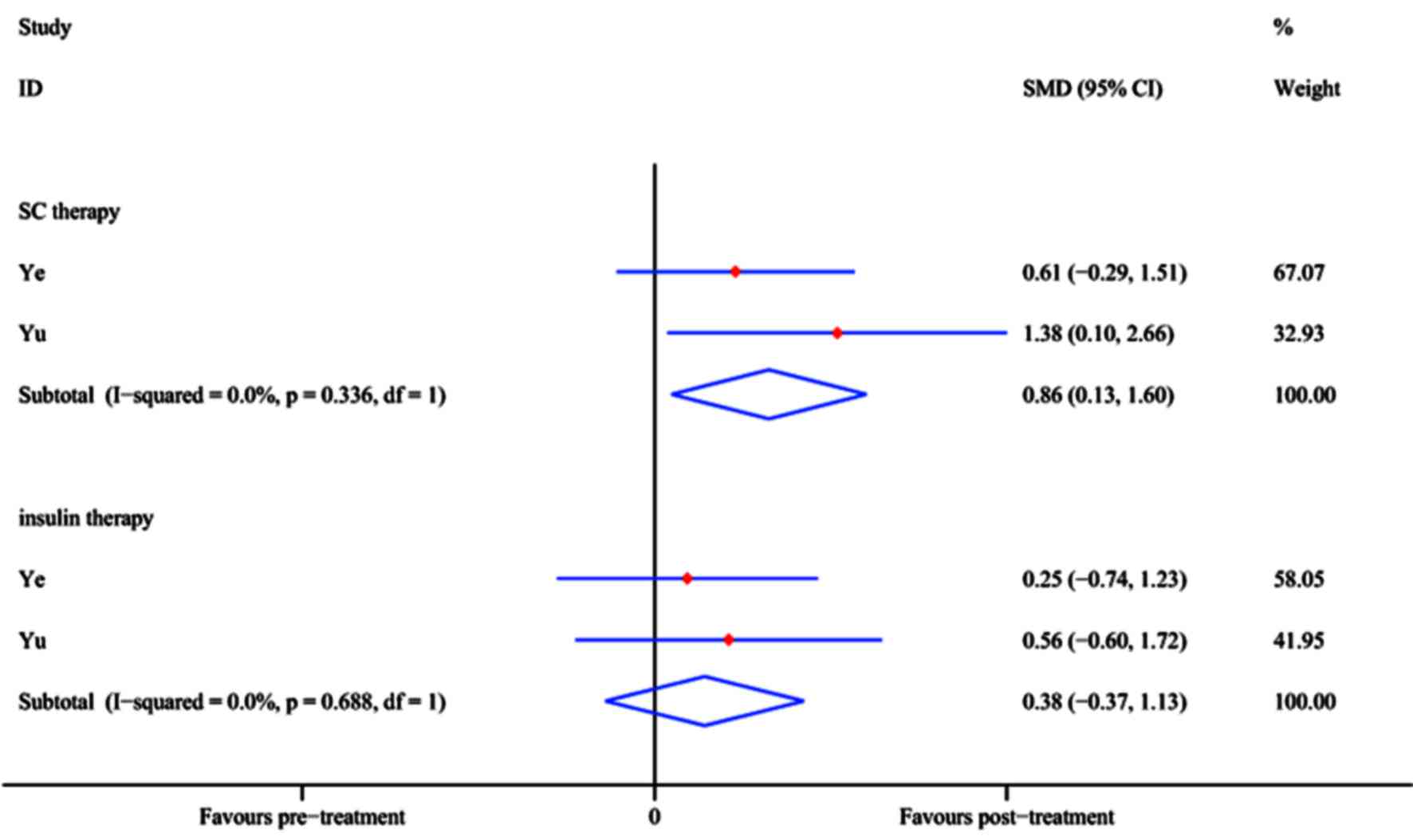
FPG and insulin requirement
In two RCTs [Ye et al (33) and Yu et al (47)], the FPG level (mmol/l) was reported
at the 12-month follow-up time-point. The pooled effect of FPG
reduction at 12 months after SC treatment achieved an SMD of 0.86
(95% CI, 0.13–1.60; P=0.021), whereas in the control group, no
significant difference was identified with insulin treatment (SMD,
0.38; 95% CI, −0.37 to 1.13; P=0.323; Fig. 8) compared with baseline. The results
demonstrated that SC therapy is an efficient option while insulin
may not.
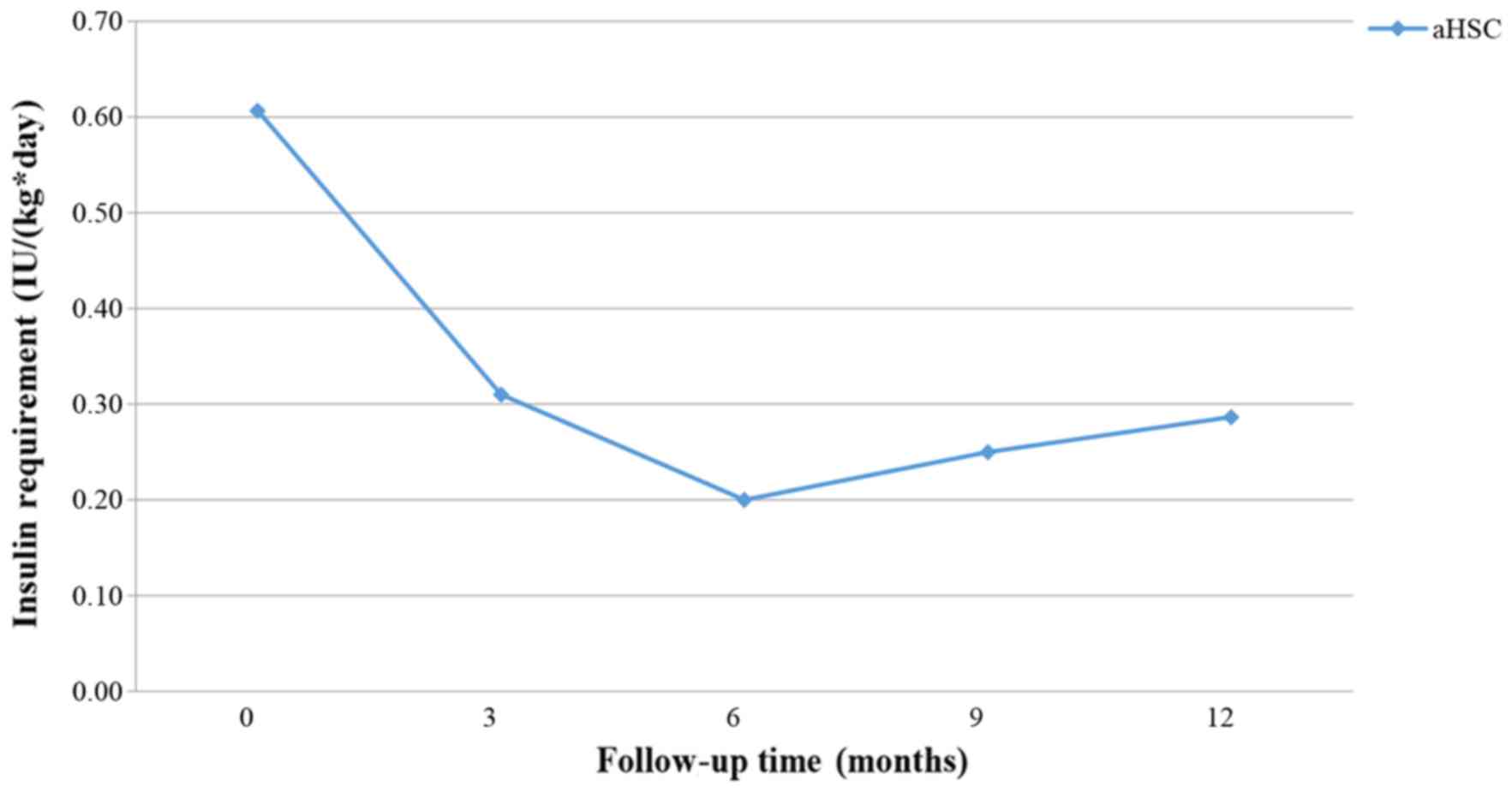
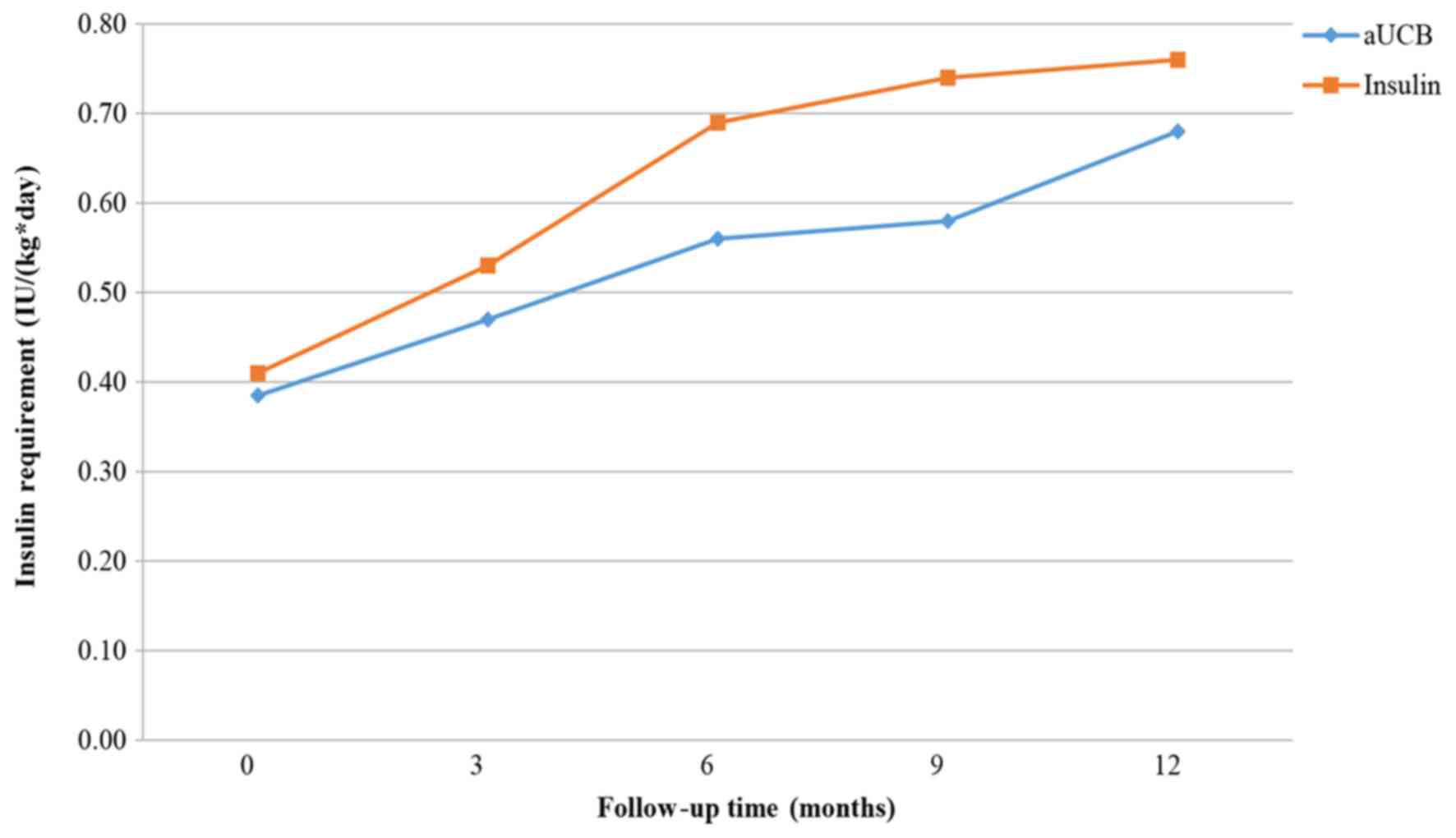
Figs. 9–11 present the trend of insulin requirement
after treatment. The insulin requirement vs. time curves exhibited
a downward trend, demonstrating that SC transplantation resulted in
a reduction in exogenous insulin requirement in patients with
T1DM.
Subgroup analysis
A subgroup analysis was performed to assess the
influence of clinical characteristics and the type of SCs on the
therapeutic effect compared with the insulin-treated control group.
As presented in Table IIA, an age
of ≥18 years and a disease history of <3 months were associated
with a greater increase in C-peptide levels in patients
transplanted with HSC (P<0.001), whereas in patients treated
with MSC+HSC, an age of <18 years and a cell dose of
≥107 IU/kg/day was associated with higher C-peptide
levels. The subgroup analysis also indicated that patient age,
disease history and the dose of SCs used led to different results
in patients treated with UCB. However, UCB therapy was not
recommended since it had a worse outcome compared with the other
treatments. No significant differences were identified in patients
transplanted with MSC.
 | Table II.Subgroup analysis of C-peptide and
glycated hemoglobin changes at 12-month follow-up. |
Table II.
Subgroup analysis of C-peptide and
glycated hemoglobin changes at 12-month follow-up.
| A, C-peptide |
|---|
|
|---|
| Variable | N | SMD | 95% CI | P-value |
|---|
| HSC | 211 |
|
|
|
|
| Age
(years) |
|
|
|
|
|
|
<18 | 118 | 0.87 | 0.54, 1.20 | <0.001 | <0.001 |
|
≥18 | 93 | 3.37 | 2.88, 3.87 | <0.001 |
|
| Disease
history (months) |
|
<3 | 159 | 1.74 | 1.43, 2.04 | <0.001 | <0.001 |
|
≥3 | 52 | 1.31 | 0.71, 1.90 | <0.001 |
|
| MSC | 30 |
|
|
|
|
| Age
(years) |
|
<18 | 15 | 0.77 | 0.02, 1.51 | 0.043 | 0.254 |
|
≥18 | 15 | 0.46 | −0.26, 1.19 | 0.212 |
|
| Dose
(IU/kg/day) |
|
<107 | 9 | 0.60 | −0.35, 1.55 | 0.214 | 0.946 |
|
≥107 | 21 | 0.62 | −0.01, 1.24 | 0.052 |
|
| MSC+HSC | 42 |
|
|
|
|
| Age
(years) |
|
<18 | 21 | 3.00 | 2.11, 3.89 | <0.001 | <0.001 |
|
≥18 | 21 | 1.13 | 0.06, 1.80 | 0.001 |
|
| Dose
(IU/kg/day) |
|
<107 | 21 | 1.13 | 0.06, 1.80 | 0.001 | <0.001 |
|
≥107 | 21 | 3.00 | 2.11, 3.89 | <0.001 |
|
| UCB | 62 |
|
|
|
|
| Age
(years) |
|
<18 | 56 | −2.21 | −2.72, −1.71 | <0.001 | <0.001 |
|
≥18 | 6 | −0.18 | −1.31, 0.96 | 0.757 |
|
| Dose
(IU/kg/day) |
|
<107 | 13 | −1.11 | −2.02, −0.19 | 0.017 | <0.001 |
|
≥107 | 49 | −2.14 | −2.67, −1.61 | <0.001 |
|
| Disease
history (months) |
|
<3 | 6 | −2.81 | −4.35, −1.28 | <0.001 | <0.001 |
|
≥3 | 56 | −1.79 | −2.27, −1.31 | <0.001 |
|
|
| B, Glycated
hemoglobin |
|
|
Variable | N | SMD | 95% CI | P-value |
|
| HSC | 211 |
|
|
|
|
| Age
(years) |
|
<18 | 118 | 1.31 | 1.01, 1.61 | <0.001 | <0.001 |
|
≥18 | 93 | 4.53 | 3.90, 5.16 | <0.001 |
|
| Dose
(IU/kg/day) |
|
<107 | 188 | 1.91 | 1.62, 2.20 | <0.001 | 0.899 |
|
≥107 | 23 | 1.92 | 1.22, 2.63 | <0.001 |
|
| Disease
history (months) |
|
<3 | 159 | 2.26 | 1.93, 2.59 | <0.001 | <0.001 |
|
≥3 | 52 | 1.21 | 0.73, 1.68 | <0.001 |
|
| MSC | 30 |
|
|
|
|
| Age
(years) |
|
|
|
|
|
|
<18 | 15 | 1.23 | 0.45, 2.02 | 0.007 | 0.947 |
|
≥18 | 15 | 1.21 | 0.33, 2.08 | 0.002 |
|
| Dose
(IU/kg/day) |
|
<107 | 9 | 0.63 | −0.32, 1.58 | 0.192 | 0.006 |
|
≥107 | 21 | 1.58 | 0.84, 2.32 | <0.001 |
|
| MSC+HSC | 42 |
|
|
|
|
| Age
(years) |
|
<18 | 21 | 0.97 | 0.33, 1.62 | 0.001 | 0.616 |
|
≥18 | 21 | 1.07 | 0.42, 1.73 | 0.003 |
|
| Dose
(IU/kg/day) |
|
<107 | 21 | 1.07 | 0.42, 1.73 | 0.003 | 0.616 |
|
≥107 | 21 | 0.97 | 0.33–1.62 | 0.001 |
|
| UCB | 62 |
|
|
|
|
| Age
(years) |
|
<18 | 56 | −0.14 | −1.28, 0.99 | 0.327 | 0.485 |
|
≥18 | 6 | 0.19 | −0.19, 0.57 | 0.804 |
|
| Dose
(IU/kg/day) |
|
<107 | 13 | −0.20 | −0.98, 0.57 | 0.603 | 0.005 |
|
≥107 | 49 | 0.25 | −0.15, 0.66 | 0.217 |
|
| Disease
history (months) |
|
<3 | 6 | 0.19 | −0.19, 0.57 | 0.804 | 0.483 |
|
≥3 | 56 | −0.14 | −1.28, 0.99 | 0.327 |
|
As presented in Table
IIB, regarding the change in HbA1c levels at the 12-month
follow-up, statistically significant differences in the effect of
HSC treatment were identified in patients of different age and
disease history subgroups (an age of ≥18 years and a disease
history of <3 months were associated with lower HbA1c levels).
Furthermore, an SC dose of ≥107 IU/kg/day was associated
with lower HbA1c levels in patients with MSC therapy, which
demonstrated SC therapy at the aforementioned dose was more
efficient. Regarding UCB therapy, a significant difference between
dose groups was identified. Regarding UCB therapy, a significant
difference between the two dose groups was identified, but the
treatment did not significantly improve the overall outcome. No
statistically significant differences were identified for any of
the other comparisons.
Discussion
In recent years, significant progress has been made
regarding the treatment of diabetes. With the development of
genomics, genetics, cell biology, and other major advances in
science and technology, a major breakthrough has emerged in the
treatment of diabetes (55,56). The present systematic review and
meta-analysis suggested that SC therapy has a potential therapeutic
effect in T1DM patients.
As presented by the pooled effect of RCTs, HSC, MSC
and MSC+HSC therapy resulted in a higher C-peptide level increase
when compared with that achieved by conventional insulin treatment,
whereas UCB did not. The higher C-peptide levels significantly
proved the regeneration of β-cell function after SC therapy. As for
the reduction of HbA1c levels, MSC+HSC had a significantly better
efficacy when compared to insulin and MSC treatment resulted in a
similar trend. However, the results did not support that HSC and
UCB had a better effect on the regulation of HbA1c levels when
compared to insulin, with the reduction in HbA1c indirectly
representing the degree of glycemic control.
As indicated by the pooled effect of SCTs, HSC, MSC
and MSC+HSC all had a significant effect on the C-peptide increase
after 12 months, compared to the baseline level. These results
indicated that these three methods all improved β-cell function. In
addition, UCB treatment had an opposite effect to that of and
UCB-SC: UCB-SC was effective, whereas UCB was not or even resulted
in a worse outcome. This result demonstrated that the dose of SCs
may affect the effect of SC therapy, which may result from the
variation of SCs volume between UCB and UCB-SC. Similar results
were obtained regarding the reduction of HbA1c levels. HSC, MSC and
MSC+HSC all reduced the HbA1c levels over a period of 12 months
after SC transplantation, whereas the UCB was not effective.
Given that the present analysis was obtained from
measurements every three months the results may have some
deviation, since the measurement of HbA1c is slightly delayed in
the diagnosis of hyperglycemia (57). In addition, C-peptide levels have a
moderate capacity to reflect the regeneration of β-cell
function.
Based on the results of the subgroup analysis, it
may be concluded that the group aged ≥18 years and patients with a
medical history of <3 months benefited significantly more from
HSC transplantation. An SC injection dose of ≥107
IU/kg/day may be more suitable when MSC+HSC therapy is employed in
patients aged ≥18 years with a recent (<3 months) diagnosis of
DM.
At present, methods for SC therapy are limited by
certain bottlenecks. To implement novel cell therapy-based
strategies in the clinic, numerous issues require addressing. For
instance, the mechanism for the differentiation of SCs into islet
β-cells remains to be fully elucidated, reproducible induction
methods require to be established and there is no consensus
regarding the definitive functional criteria for induced cells.
Therefore, it is challenging to determine whether the obtained
cells are suitable for replacing the islet β-cells. Furthermore,
the number of implanted SCs, the site of transplantation, the
host's immune response after transplantation and carcinogenicity
are all issues that require addressing by future studies. In a
previous study, only few of the pediatric T1DM patients treated by
autologous umbilical cord blood transfusion no longer required
insulin (58). Thus, the major
function of β-cells, namely robust glucose-induced insulin
secretion, may not be easily realized by SCs. The combination of
multiple approaches and methods may be the safest and most
effective type of immunomodulatory therapy.
One issue that must be considered is the safety of
SC therapy. Besides the immune reaction mentioned above, Gu et
al (46) demonstrated that
patients experienced different degrees of gastrointestinal
reactions, hair loss, fever, bone marrow suppression and other
adverse reactions during the process of transplantation.
Fortunately, no significant organ, heart, liver or kidney
dysfunction was observed and adverse reactions gradually
disappeared at 2–4 weeks after SC reinfusion.
Furthermore, a major safety issue exists regarding
the numerous rounds of replication that these SCs undergo prior to
transplantation into the patient, which may result in the
accumulation of chromosomal abnormalities and the cells may
eventually become malignant.
The analysis of the present study has several
strengths. Firstly, the robust methodology is a major strength. It
was attempted to consider all available published studies via
searching four major databases, and the data quality was assessed
and to obtain suitable data. Furthermore, no language restrictions
were imposed on the articles included. In addition, the present
study compared data between SC therapy and conventional insulin
treatment groups, and also included data from prior to and after SC
therapy, which not only indicated the individuality of the
treatments, but also reflected their applicability. In the present
analysis, a random-effects model was employed, which was able to
correlate individual observations to fit non-independent
observation data. In addition, subgroup analyses were performed to
confirm the consistency of conclusions among the different subgroup
populations.
Several limitations of the present analysis reduce
the value of the information obtained. First, in several trials
included, the baseline C-peptide levels, HbA1c levels and FPG
concentrations of participants were not assessed. Therefore,
results of the subgroup analyses may have been different if all of
the indexes had been assessed. Furthermore, several of the studies
included [Ye et al (33),
Giannopoulou et al (38) and
Zhao (43)] were of poor quality,
e.g. due to using unclear allocation concealment. In addition,
there was a lack of original trials and the sample size of the
studies included was limited. Finally, one inevitable limitation of
the present study was the heterogeneity between the studies, which
was based on differences between the institutions from various
parts of the world.
In conclusion, according to the present
meta-analysis, the use of SCs, including HSC, MSC or MSC+HSC, may
be considered an effective approach for the therapy of T1DM. These
results supported the routine application of these therapies in
patients with T1DM. The use of USB is not recommended; however, a
USB-SC component may be considered. Furthermore, patients aged ≥18
years or with a duration of T1DM of <3 months demonstrated a
greater benefit from HSC transplantation. A SC injection dose of
≥107 IU/kg/day is more suitable in patients undergoing
MSC+HS transplantation. Taken together, the present results may
guide the further development of SC therapy and its implementation
in the clinic, and indicate that SC therapy holds great promise for
treating T1DM. In the future, additional trials are required to
further elucidate the subject.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of China (grant nos. 81160307 and 81560395), the Jiangxi
Science & Technology Pillar Program and the Science Foundation
for Young Scholars of Jiangxi Province (grant no. 2007GQY1167) and
the Voyage Project of Jiangxi Province Science and Technology
Association.
Availability of data and materials
The analyzed data sets generated during the study
are available in the published article.
Authors' contributions
JG performed the meta-analysis, YW was responsible
for the statistical analysis and XZ prepared the manuscript. All
authors have read and approved the final version of the manuscript
prior to submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garofalo C, Iazzetta N, Camocardi A,
Pacilio M, Iodice C, Minutolo R, De Nicola L and Conte G:
Anti-diabetics and chronic kidney disease. G Ital Nefrol.
32:pii2015.
|
|
4
|
Gholamhossein Y, Behrouz H and Asghar Z:
Diabetic retinopathy risk factors: Plasma erythropoietin as a risk
factor for proliferative diabetic retinopathy. Korean J Ophthalmol.
28:373–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bluestone JA, Herold K and Eisenbarth G:
Genetics, pathogenesis and clinical interventions in type 1
diabetes. Nature. 464:1293–1300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cook JJ, Hudson I, Harrison LC, Dean B,
Colman PG, Werther GA, Warne GL and Court JM: Double-blind
controlled trial of azathioprine in children with newly diagnosed
type I diabetes. Diabetes. 38:779–783. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludvigsson J, Carlsson A, Deli A,
Forsander G, Ivarsson SA, Kockum I, Lindblad B, Marcus C, Lernmark
Å and Samuelsson U: Decline of C-peptide during the first year
after diagnosis of Type 1 diabetes in children and adolescents.
Diabetes Res Clin Pract. 100:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pescovitz MD, Greenbaum CJ,
Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA,
Marks JB, McGee PF, Moran AM, et al: Rituximab, B-lymphocyte
depletion and preservation of beta-cell function. N Engl J Med.
361:2143–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hagopian W, Ferry RJ Jr, Sherry N, Carlin
D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold
KC, et al: Teplizumab preserves C-peptide in recent-onset type 1
diabetes: Two-year results from the randomized, placebo-controlled
Protégé trial. Diabetes. 62:3901–3908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Herold KC and Miller SD:
Immunotherapy of type 1 diabetes: Where are we at and where should
we be going. Immunity. 32:488–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Posselt AM, Bellin MD, Tavakol M, Szot GL,
Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering
BJ, et al: Islet transplantation in type 1 diabetics using an
immunosuppressive protocol based on the anti-LFA-1 antibody
efalizumab. Am J Transplant. 10:1870–1880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCall M and Shapiro AM: Update on islet
transplantation. Cold Spring Harb Perspect Med. 2:a0078232012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong JH, Yook S, Hwang JW, Jung MJ, Moon
HT, Lee DY and Byun Y: Synergistic effect of surface modification
with Poly(ethylene glycol) and immunosuppressants on repetitive
pancreatic islet transplantation into antecedently sensitized rat.
Transplant Proc. 45:585–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evgenov NV, Medarova Z, Pratt J,
Pantazopoulos P, Leyting S, Bonner-Weir S and Moore A: In vivo
imaging of immune rejection in transplanted pancreatic islets.
Diabetes. 55:2419–2428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stegall MD, Lafferty KJ, Kam I and Gill
RG: Evidence of recurrent autoimmunity in human allogeneic islet
transplantation. Transplantation. 61:1272–1274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newman RG, Ross DB, Barreras H, Herretes
S, Podack ER, Komanduri KV, Perez VL and Levy RB: The allure and
peril of hematopoietic stem cell transplantation: Overcoming immune
challenges to improve success. Immunol Res. 57:125–139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chhabra P and Brayman KL: Stem cell
therapy to cure type 1 diabetes: From hype to hope. Stem Cells
Transl Med. 2:328–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi X, Guo X and Su C: Clinical outcomes of
the transplantation of stem cells from various human tissue sources
in the management of liver cirrhosis: A systematic review and
meta-analysis. Curr Stem Cell Res Ther. 10:166–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim G, Eom YW, Baik SK, Shin Y, Lim YL,
Kim MY, Kwon SO and Chang SJ: Therapeutic effects of mesenchymal
stem cells for patients with chronic liver diseases: Systematic
review and Meta-analysis. J Korean Med Sci. 30:1405–1415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dave M, Mehta K, Luther J, Baruah A, Dietz
AB and Faubion WA Jr: Mesenchymal stem cell therapy for
inflammatory bowel disease: A systematic review and Meta-analysis.
Inflamm Bowel Dis. 21:2696–2707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong H, Yim HW, Park HJ, Cho Y, Hong H,
Kim NJ and Oh IH: Mesenchymal stem cell therapy for ischemic heart
disease: Systematic review and Meta-analysis. Int J Stem Cells.
11:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoekstra JB, van Rijn HJ, Erkelens DW and
Thijssen JH: C-peptide. Diabetes Care. 5:438–446. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gomez-Perez FJ, Aguilar-Salinas CA,
Almeda-Valdes P, Cuevas-Ramos D, Lerman Garber I and Rull JA: HbA1c
for the diagnosis of diabetes mellitus in a developing country. A
position article. Arch Med Res. 41:302–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Preedy VR and Watson RR: Fasting Plasma
Glucose. Springer; New York: 25. 2010
|
|
26
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, . Preferred reporting items for systematic
reviews and Meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269, W64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.0 [updated
March 2011]. naunyn-schmiedebergs archiv für experimentelle
pathologie und pharmakologie. 5 Suppl:S382014.
|
|
28
|
Anderson ML and Peterson ED: Compliance
with results reporting at ClinicalTrials.gov. N Engl J Med.
372:2370–2371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deeks JJ, Altman DG and Bradburn MJ:
Statistical methods for examining heterogeneity and combining
results from several studies in Meta-analysis. Systematic Reviews
in Health Care Systematic Reviews in Health Care: Meta-Analysis in
Context. 2nd. Wiley-Blackwell; Chichester: 2008
|
|
32
|
Sterne JA, Gavaghan D and Egger M:
Publication and related bias in meta-analysis: Power of statistical
tests and prevalence in the literature. J Clin Epidemiol.
53:1119–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye L, Li L, Wan B, Yang M, Hong J, Gu W,
Wang W and Ning G: Immune response after autologous hematopoietic
stem cell transplantation in type 1 diabetes mellitus. Stem Cell
Res Ther. 8:902017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thakkar UG, Trivedi HL, Vanikar AV and
Dave SD: Insulin-secreting adipose-derived mesenchymal stromal
cells with bone marrow-derived hematopoietic stem cells from
autologous and allogenic sources for type 1 diabetes mellitus.
Cytotherapy. 17:940–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Snarski E, Milczarczyk A, Hałaburda K,
Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P,
Jedynasty K, Franek E and Wiktor-Jedrzejczak W: Immunoablation and
autologous hematopoietic stem cell transplantation in the treatment
of new-onset type 1 diabetes mellitus: Long-term observations. Bone
Marrow Transplant. 51:398–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delgado E, Perez-Basterrechea M,
Suarez-Alvarez B, Zhou H, Revuelta EM, Garcia-Gala JM, Perez S,
Alvarez-Viejo M, Menendez E, Lopez-Larrea C, et al: Modulation of
autoimmune T-cell memory by stem cell educator therapy: Phase 1/2
clinical trial. EBiomedicine. 2:2024–2036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai J, Wu Z, Xu X, Liao L, Chen J, Huang
L, Wu W, Luo F, Wu C, Pugliese A, et al: Umbilical cord mesenchymal
stromal cell with autologous bone marrow cell transplantation in
established type 1 diabetes: A pilot randomized controlled
open-label clinical study to assess safety and impact on insulin
secretion. Diabetes Care. 39:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giannopoulou EZ, Puff R, Beyerlein A, von
Luettichau I, Boerschmann H, Schatz D, Atkinson M, Haller MJ, Egger
D, Burdach S and Ziegler AG: Effect of a single autologous cord
blood infusion on beta-cell and immune function in children with
new onset type 1 diabetes: A non-randomized, controlled trial.
Pediatr Diabetes. 15:100–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Addio F, Valderrama Vasquez A, Ben Nasr
M, Franek E, Zhu D, Li L, Ning G, Snarski E and Fiorina P:
Autologous nonmyeloablative hematopoietic stem cell transplantation
in new-onset type 1 diabetes: A multicenter analysis. Diabetes.
63:3041–3046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carlsson PO, Schwarcz E, Korsgren O and Le
Blanc K: Preserved β-cell function in type 1 diabetes by
mesenchymal stromal cells. Diabetes. 64:587–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H,
Chen Y, Zhao W, Jia Z, Yan S and Wang Y: Long term effects of the
implantation of Wharton's jelly-derived mesenchymal stem cells from
the umbilical cord for newly-onset type 1 diabetes mellitus. Endoc
J. 60:347–357. 2013. View Article : Google Scholar
|
|
42
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster
JJ, Ferguson RJ, et al: Autologous umbilical cord blood infusion
followed by oral docosahexaenoic acid and vitamin D supplementation
for C-peptide preservation in children with type 1 diabetes. Biol
Blood Marrow Transplant. 19:1126–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin
Z, Li H, Zhang Y, Diao Y, Li Y, et al: Reversal of type 1 diabetes
via islet β cell regeneration following immune modulation by cord
blood-derived multipotent stem cells. BMC Med. 10:32012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Ye L, Hu J, Tang W, Liu R, Yang
M, Hong J, Wang W, Ning G and Gu W: Acute response of peripheral
blood cell to autologous hematopoietic stem cell transplantation in
type 1 diabetic patient. PLoS One. 7:e318872012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W,
Zhang N, Zhuge YZ, Chen B, Xu J and Zhu D: Autologous hematopoietic
stem cell transplantation modulates immunocompetent cells and
improves β-cell function in Chinese patients with new onset of type
1 diabetes. J Clin Endocrinol Metab. 97:1729–1736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu W, Hu J, Wang W, Li L, Tang W, Sun S,
Cui W, Ye L, Zhang Y, Hong J, et al: Diabetic ketoacidosis at
diagnosis influences complete remission after treatment with
hematopoietic stem cell transplantation in adolescents with type 1
diabetes. Diabetes Care. 35:1413–1419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu W, Gao H, Yu X, Wang L, Yan S and Wang
Y: Umbilical cord mesenchymal stem cells transplantation for
newly-onset type 1 diabetes. J Clin Rehabil Tissue Engineering Res.
15:4363–4366. 2011.
|
|
48
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Sumrall TM, Wingard JR, Theriaque
DW, Shuster JJ, et al: Autologous umbilical cord blood transfusion
in young children with type 1 diabetes fails to preserve C-peptide.
Diabetes Care. 34:2567–2569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng K, Xu YW, Ye FG, Xiao L, Ma XH, Gao
Y, Zhang X, Yao SZ and Shi BY: Autologous peripheral blood
hematopoietic stem cell transplantation in the treatment of type 1
diabetic mellitus: A report of 16 cases. Zhonghua Yi Xue Za Zhi.
91:1966–1969. 2011.(In Chinese). PubMed/NCBI
|
|
50
|
Vanikar AV, Dave SD, Thakkar UG and
Trivedi HL: Cotransplantation of adipose tissue-derived
insulin-secreting mesenchymal stem cells and hematopoietic stem
cells: A novel therapy for insulin-dependent diabetes mellitus.
Stem Cells Int. 2010:5823822010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Snarski E, Milczarczyk A, Torosian T,
Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K,
Franek E and Wiktor-Jedrzejczak W: Independence of exogenous
insulin following immunoablation and stem cell reconstitution in
newly diagnosed diabetes type I. Bone Marrow Transplant.
46:562–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu WQ, Sun SY, Hu J, Tang W, Wei JS, Zhu
LP, Hong J, Tang ZY, Liu JM, Li XY, Wang WQ, et al: Efficacy and
safety of autologous hematopoietic stem cell transplantation in
treating type 1 diabetes mellitus. Chin J Endocrinol Metabol.
53:1023–1026. 2010.(In Chinese).
|
|
53
|
Haller MJ, Wasserfall CH, Mcgrail KM,
Cintron M, Brusko TM, Wingard JR, Kelly SS, Shuster JJ, Atkinson MA
and Schatz DA: Autologous umbilical cord blood transfusion in very
young children with type 1 diabetes. Diabetes Care. 32:2041–2046.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Couri CE, Oliveira MC, Stracieri AB,
Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC,
Foss-Freitas MC, Simões BP, et al: C-peptide levels and insulin
independence following autologous nonmyeloablative hematopoietic
stem cell transplantation in newly diagnosed type 1 diabetes
mellitus. JAMA. 301:1573–1579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Li Y, Zhao J, Zhang J and Huang Y:
Mesenchymal stem cells ameliorate podocyte injury and proteinuria
in a type 1 diabetic nephropathy rat model. Biol Blood Marrow
Transplant. 19:538–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yanai G, Hayashi T, Zhi Q, Yang KC,
Shirouzu Y, Shimabukuro T, Hiura A, Inoue K and Sumi S:
Electrofusion of mesenchymal stem cells and islet cells for
diabetes therapy: A rat model. PLoS One. 8:e644992013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Glycosylated hemoglobin assays in the
management and diagnosis of diabetes mellitus. Health and Public
Policy Committee, American College of Physicians. Ann Intern Med.
101:710–713. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Haller MJ, Viener H, Brusko T, Wasserfall
C, Mcgrail K, Staba S, Cogle C, Atkinson M and Schatz DA: Insulin
requirements, HbA1c and stimulated C-peptide following autologous
umbilical cord blood transfusion in children with T1D. Diabetes.
56:A822007.
|