|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garofalo C, Iazzetta N, Camocardi A,
Pacilio M, Iodice C, Minutolo R, De Nicola L and Conte G:
Anti-diabetics and chronic kidney disease. G Ital Nefrol.
32:pii2015.
|
|
4
|
Gholamhossein Y, Behrouz H and Asghar Z:
Diabetic retinopathy risk factors: Plasma erythropoietin as a risk
factor for proliferative diabetic retinopathy. Korean J Ophthalmol.
28:373–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bluestone JA, Herold K and Eisenbarth G:
Genetics, pathogenesis and clinical interventions in type 1
diabetes. Nature. 464:1293–1300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cook JJ, Hudson I, Harrison LC, Dean B,
Colman PG, Werther GA, Warne GL and Court JM: Double-blind
controlled trial of azathioprine in children with newly diagnosed
type I diabetes. Diabetes. 38:779–783. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludvigsson J, Carlsson A, Deli A,
Forsander G, Ivarsson SA, Kockum I, Lindblad B, Marcus C, Lernmark
Å and Samuelsson U: Decline of C-peptide during the first year
after diagnosis of Type 1 diabetes in children and adolescents.
Diabetes Res Clin Pract. 100:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pescovitz MD, Greenbaum CJ,
Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA,
Marks JB, McGee PF, Moran AM, et al: Rituximab, B-lymphocyte
depletion and preservation of beta-cell function. N Engl J Med.
361:2143–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hagopian W, Ferry RJ Jr, Sherry N, Carlin
D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold
KC, et al: Teplizumab preserves C-peptide in recent-onset type 1
diabetes: Two-year results from the randomized, placebo-controlled
Protégé trial. Diabetes. 62:3901–3908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Herold KC and Miller SD:
Immunotherapy of type 1 diabetes: Where are we at and where should
we be going. Immunity. 32:488–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Posselt AM, Bellin MD, Tavakol M, Szot GL,
Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering
BJ, et al: Islet transplantation in type 1 diabetics using an
immunosuppressive protocol based on the anti-LFA-1 antibody
efalizumab. Am J Transplant. 10:1870–1880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCall M and Shapiro AM: Update on islet
transplantation. Cold Spring Harb Perspect Med. 2:a0078232012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong JH, Yook S, Hwang JW, Jung MJ, Moon
HT, Lee DY and Byun Y: Synergistic effect of surface modification
with Poly(ethylene glycol) and immunosuppressants on repetitive
pancreatic islet transplantation into antecedently sensitized rat.
Transplant Proc. 45:585–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evgenov NV, Medarova Z, Pratt J,
Pantazopoulos P, Leyting S, Bonner-Weir S and Moore A: In vivo
imaging of immune rejection in transplanted pancreatic islets.
Diabetes. 55:2419–2428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stegall MD, Lafferty KJ, Kam I and Gill
RG: Evidence of recurrent autoimmunity in human allogeneic islet
transplantation. Transplantation. 61:1272–1274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newman RG, Ross DB, Barreras H, Herretes
S, Podack ER, Komanduri KV, Perez VL and Levy RB: The allure and
peril of hematopoietic stem cell transplantation: Overcoming immune
challenges to improve success. Immunol Res. 57:125–139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chhabra P and Brayman KL: Stem cell
therapy to cure type 1 diabetes: From hype to hope. Stem Cells
Transl Med. 2:328–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi X, Guo X and Su C: Clinical outcomes of
the transplantation of stem cells from various human tissue sources
in the management of liver cirrhosis: A systematic review and
meta-analysis. Curr Stem Cell Res Ther. 10:166–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim G, Eom YW, Baik SK, Shin Y, Lim YL,
Kim MY, Kwon SO and Chang SJ: Therapeutic effects of mesenchymal
stem cells for patients with chronic liver diseases: Systematic
review and Meta-analysis. J Korean Med Sci. 30:1405–1415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dave M, Mehta K, Luther J, Baruah A, Dietz
AB and Faubion WA Jr: Mesenchymal stem cell therapy for
inflammatory bowel disease: A systematic review and Meta-analysis.
Inflamm Bowel Dis. 21:2696–2707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong H, Yim HW, Park HJ, Cho Y, Hong H,
Kim NJ and Oh IH: Mesenchymal stem cell therapy for ischemic heart
disease: Systematic review and Meta-analysis. Int J Stem Cells.
11:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoekstra JB, van Rijn HJ, Erkelens DW and
Thijssen JH: C-peptide. Diabetes Care. 5:438–446. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gomez-Perez FJ, Aguilar-Salinas CA,
Almeda-Valdes P, Cuevas-Ramos D, Lerman Garber I and Rull JA: HbA1c
for the diagnosis of diabetes mellitus in a developing country. A
position article. Arch Med Res. 41:302–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Preedy VR and Watson RR: Fasting Plasma
Glucose. Springer; New York: 25. 2010
|
|
26
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, . Preferred reporting items for systematic
reviews and Meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269, W64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.0 [updated
March 2011]. naunyn-schmiedebergs archiv für experimentelle
pathologie und pharmakologie. 5 Suppl:S382014.
|
|
28
|
Anderson ML and Peterson ED: Compliance
with results reporting at ClinicalTrials.gov. N Engl J Med.
372:2370–2371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deeks JJ, Altman DG and Bradburn MJ:
Statistical methods for examining heterogeneity and combining
results from several studies in Meta-analysis. Systematic Reviews
in Health Care Systematic Reviews in Health Care: Meta-Analysis in
Context. 2nd. Wiley-Blackwell; Chichester: 2008
|
|
32
|
Sterne JA, Gavaghan D and Egger M:
Publication and related bias in meta-analysis: Power of statistical
tests and prevalence in the literature. J Clin Epidemiol.
53:1119–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
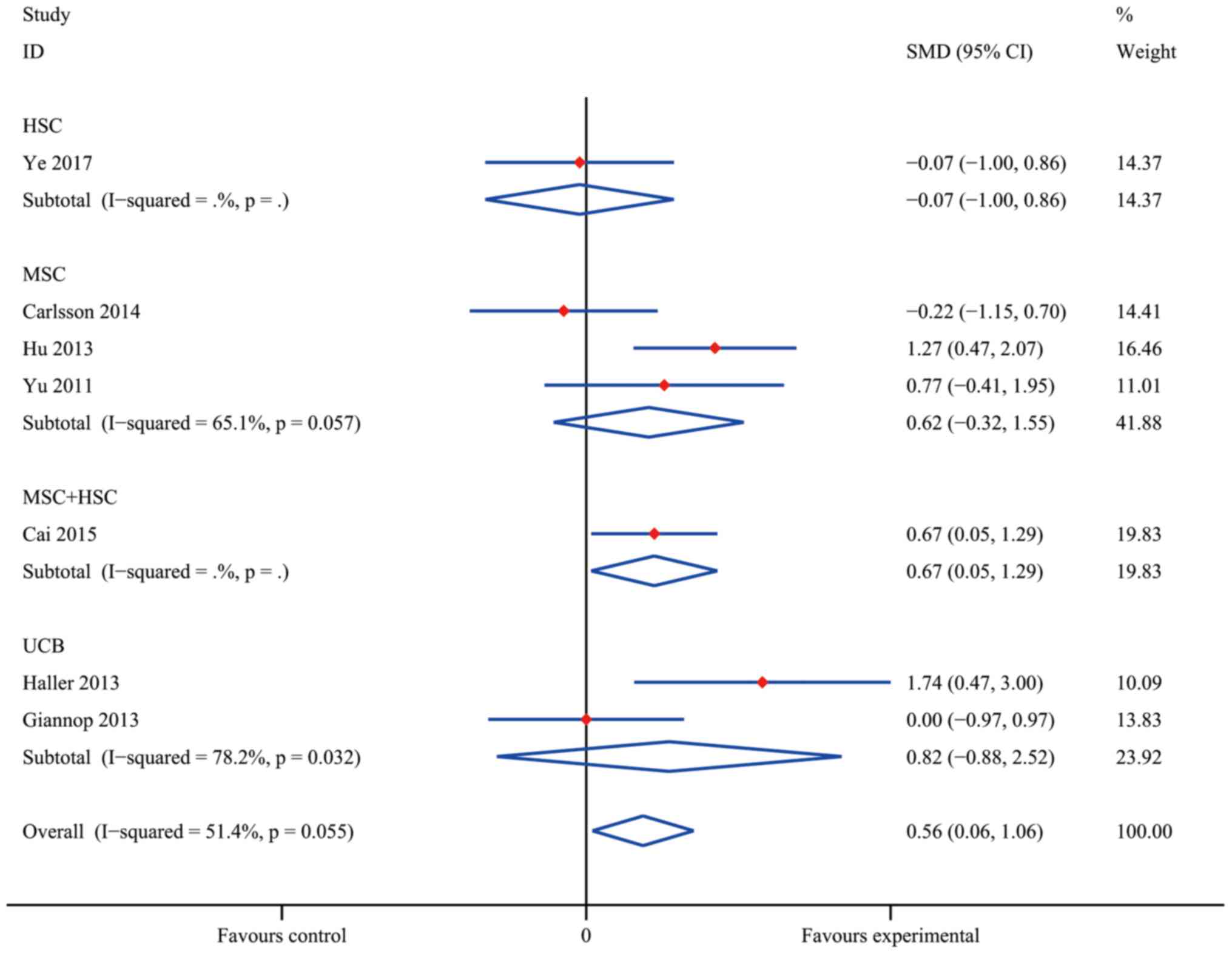
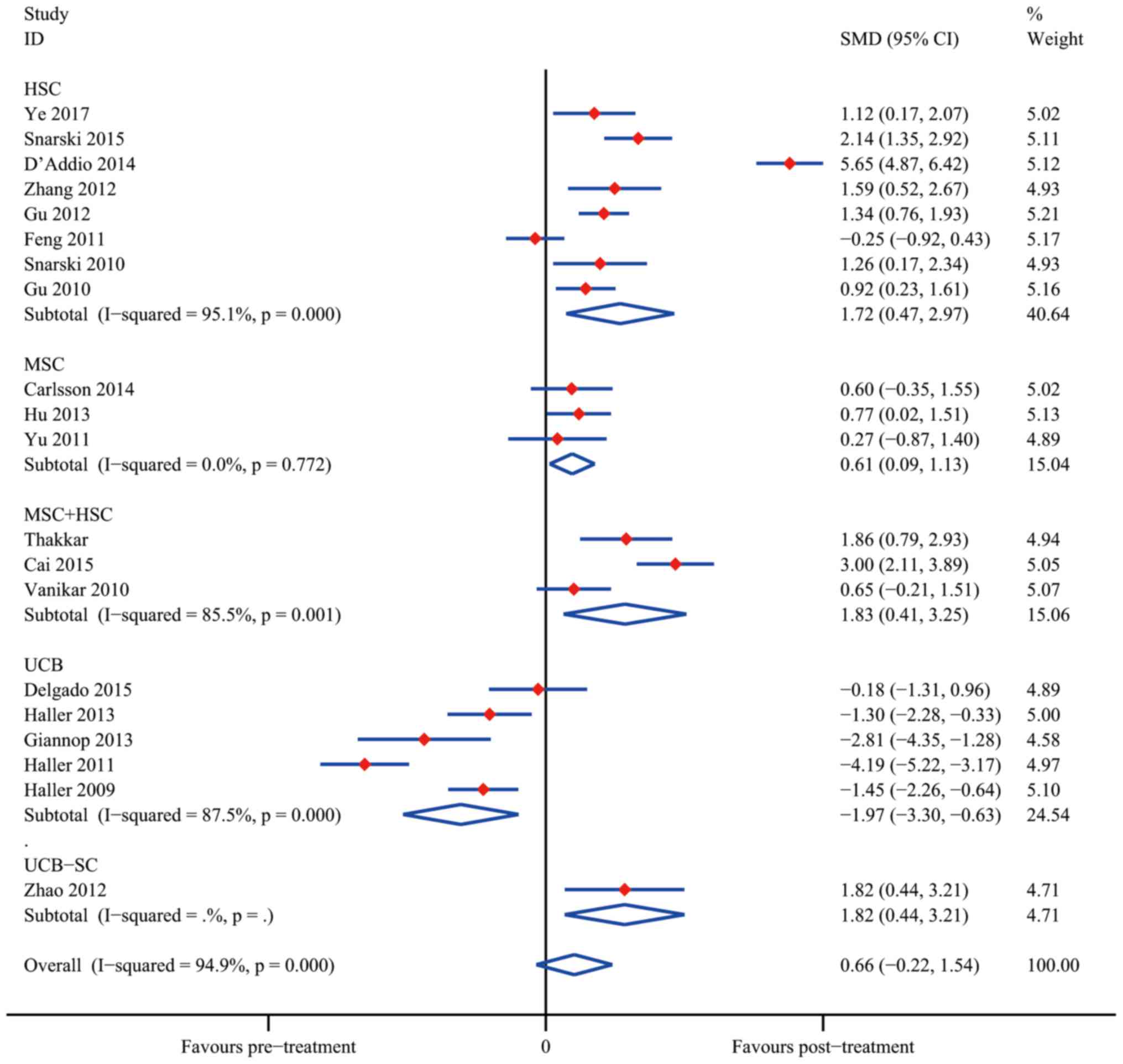
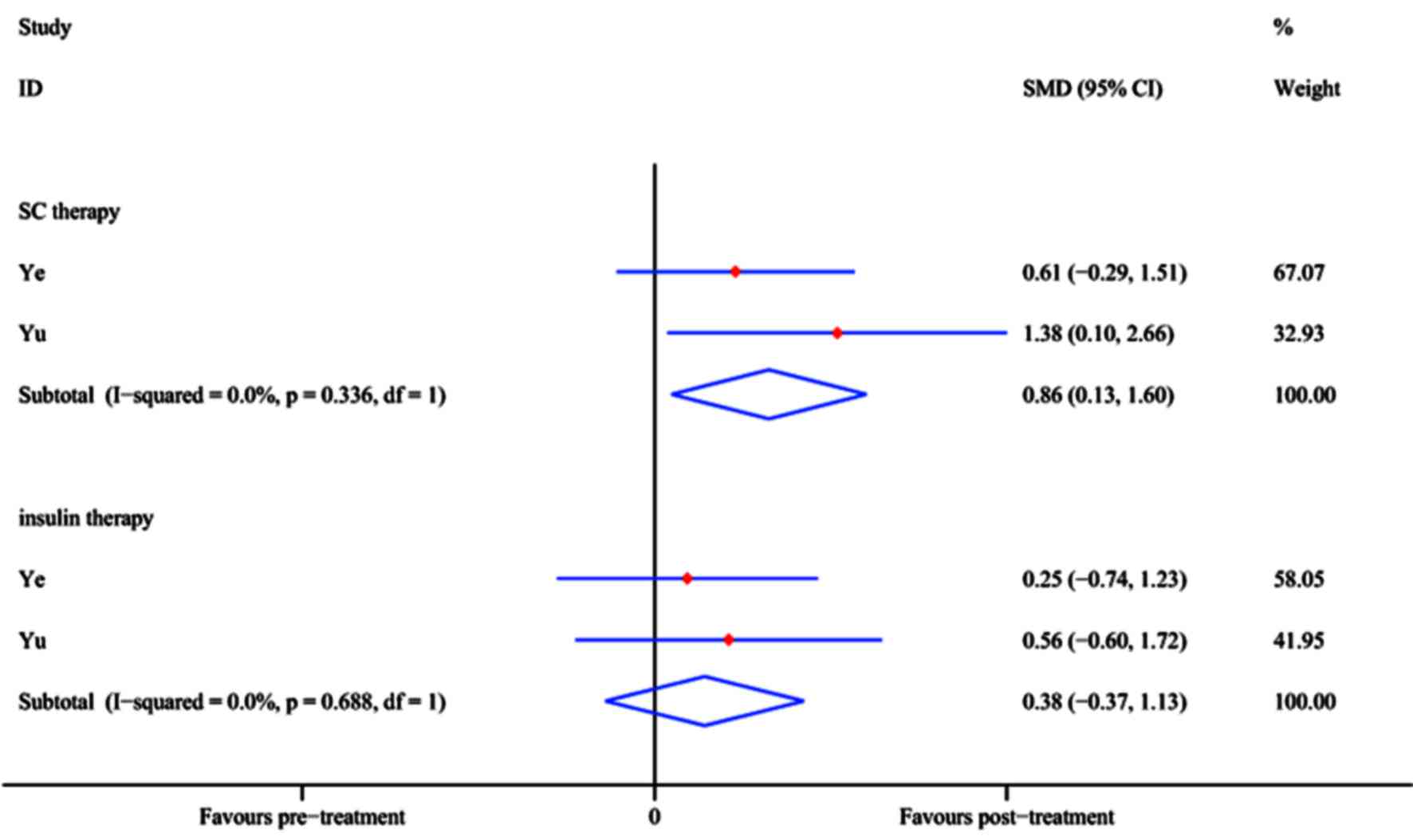
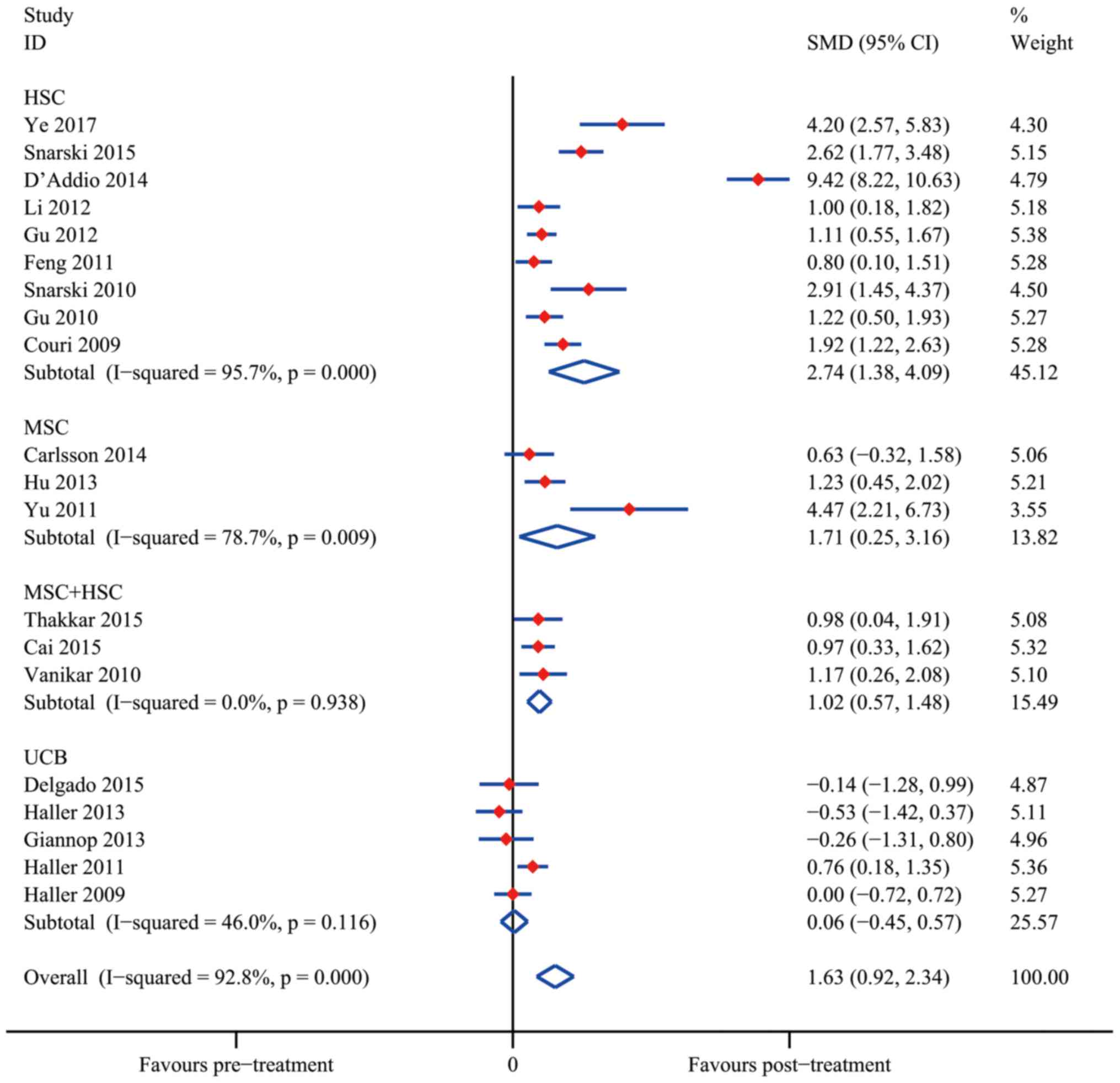
Ye L, Li L, Wan B, Yang M, Hong J, Gu W,
Wang W and Ning G: Immune response after autologous hematopoietic
stem cell transplantation in type 1 diabetes mellitus. Stem Cell
Res Ther. 8:902017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thakkar UG, Trivedi HL, Vanikar AV and
Dave SD: Insulin-secreting adipose-derived mesenchymal stromal
cells with bone marrow-derived hematopoietic stem cells from
autologous and allogenic sources for type 1 diabetes mellitus.
Cytotherapy. 17:940–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Snarski E, Milczarczyk A, Hałaburda K,
Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P,
Jedynasty K, Franek E and Wiktor-Jedrzejczak W: Immunoablation and
autologous hematopoietic stem cell transplantation in the treatment
of new-onset type 1 diabetes mellitus: Long-term observations. Bone
Marrow Transplant. 51:398–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delgado E, Perez-Basterrechea M,
Suarez-Alvarez B, Zhou H, Revuelta EM, Garcia-Gala JM, Perez S,
Alvarez-Viejo M, Menendez E, Lopez-Larrea C, et al: Modulation of
autoimmune T-cell memory by stem cell educator therapy: Phase 1/2
clinical trial. EBiomedicine. 2:2024–2036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai J, Wu Z, Xu X, Liao L, Chen J, Huang
L, Wu W, Luo F, Wu C, Pugliese A, et al: Umbilical cord mesenchymal
stromal cell with autologous bone marrow cell transplantation in
established type 1 diabetes: A pilot randomized controlled
open-label clinical study to assess safety and impact on insulin
secretion. Diabetes Care. 39:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giannopoulou EZ, Puff R, Beyerlein A, von
Luettichau I, Boerschmann H, Schatz D, Atkinson M, Haller MJ, Egger
D, Burdach S and Ziegler AG: Effect of a single autologous cord
blood infusion on beta-cell and immune function in children with
new onset type 1 diabetes: A non-randomized, controlled trial.
Pediatr Diabetes. 15:100–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Addio F, Valderrama Vasquez A, Ben Nasr
M, Franek E, Zhu D, Li L, Ning G, Snarski E and Fiorina P:
Autologous nonmyeloablative hematopoietic stem cell transplantation
in new-onset type 1 diabetes: A multicenter analysis. Diabetes.
63:3041–3046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carlsson PO, Schwarcz E, Korsgren O and Le
Blanc K: Preserved β-cell function in type 1 diabetes by
mesenchymal stromal cells. Diabetes. 64:587–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H,
Chen Y, Zhao W, Jia Z, Yan S and Wang Y: Long term effects of the
implantation of Wharton's jelly-derived mesenchymal stem cells from
the umbilical cord for newly-onset type 1 diabetes mellitus. Endoc
J. 60:347–357. 2013. View Article : Google Scholar
|
|
42
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster
JJ, Ferguson RJ, et al: Autologous umbilical cord blood infusion
followed by oral docosahexaenoic acid and vitamin D supplementation
for C-peptide preservation in children with type 1 diabetes. Biol
Blood Marrow Transplant. 19:1126–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin
Z, Li H, Zhang Y, Diao Y, Li Y, et al: Reversal of type 1 diabetes
via islet β cell regeneration following immune modulation by cord
blood-derived multipotent stem cells. BMC Med. 10:32012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Ye L, Hu J, Tang W, Liu R, Yang
M, Hong J, Wang W, Ning G and Gu W: Acute response of peripheral
blood cell to autologous hematopoietic stem cell transplantation in
type 1 diabetic patient. PLoS One. 7:e318872012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W,
Zhang N, Zhuge YZ, Chen B, Xu J and Zhu D: Autologous hematopoietic
stem cell transplantation modulates immunocompetent cells and
improves β-cell function in Chinese patients with new onset of type
1 diabetes. J Clin Endocrinol Metab. 97:1729–1736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu W, Hu J, Wang W, Li L, Tang W, Sun S,
Cui W, Ye L, Zhang Y, Hong J, et al: Diabetic ketoacidosis at
diagnosis influences complete remission after treatment with
hematopoietic stem cell transplantation in adolescents with type 1
diabetes. Diabetes Care. 35:1413–1419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu W, Gao H, Yu X, Wang L, Yan S and Wang
Y: Umbilical cord mesenchymal stem cells transplantation for
newly-onset type 1 diabetes. J Clin Rehabil Tissue Engineering Res.
15:4363–4366. 2011.
|
|
48
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Sumrall TM, Wingard JR, Theriaque
DW, Shuster JJ, et al: Autologous umbilical cord blood transfusion
in young children with type 1 diabetes fails to preserve C-peptide.
Diabetes Care. 34:2567–2569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng K, Xu YW, Ye FG, Xiao L, Ma XH, Gao
Y, Zhang X, Yao SZ and Shi BY: Autologous peripheral blood
hematopoietic stem cell transplantation in the treatment of type 1
diabetic mellitus: A report of 16 cases. Zhonghua Yi Xue Za Zhi.
91:1966–1969. 2011.(In Chinese). PubMed/NCBI
|
|
50
|
Vanikar AV, Dave SD, Thakkar UG and
Trivedi HL: Cotransplantation of adipose tissue-derived
insulin-secreting mesenchymal stem cells and hematopoietic stem
cells: A novel therapy for insulin-dependent diabetes mellitus.
Stem Cells Int. 2010:5823822010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Snarski E, Milczarczyk A, Torosian T,
Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K,
Franek E and Wiktor-Jedrzejczak W: Independence of exogenous
insulin following immunoablation and stem cell reconstitution in
newly diagnosed diabetes type I. Bone Marrow Transplant.
46:562–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu WQ, Sun SY, Hu J, Tang W, Wei JS, Zhu
LP, Hong J, Tang ZY, Liu JM, Li XY, Wang WQ, et al: Efficacy and
safety of autologous hematopoietic stem cell transplantation in
treating type 1 diabetes mellitus. Chin J Endocrinol Metabol.
53:1023–1026. 2010.(In Chinese).
|
|
53
|
Haller MJ, Wasserfall CH, Mcgrail KM,
Cintron M, Brusko TM, Wingard JR, Kelly SS, Shuster JJ, Atkinson MA
and Schatz DA: Autologous umbilical cord blood transfusion in very
young children with type 1 diabetes. Diabetes Care. 32:2041–2046.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Couri CE, Oliveira MC, Stracieri AB,
Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC,
Foss-Freitas MC, Simões BP, et al: C-peptide levels and insulin
independence following autologous nonmyeloablative hematopoietic
stem cell transplantation in newly diagnosed type 1 diabetes
mellitus. JAMA. 301:1573–1579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Li Y, Zhao J, Zhang J and Huang Y:
Mesenchymal stem cells ameliorate podocyte injury and proteinuria
in a type 1 diabetic nephropathy rat model. Biol Blood Marrow
Transplant. 19:538–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yanai G, Hayashi T, Zhi Q, Yang KC,
Shirouzu Y, Shimabukuro T, Hiura A, Inoue K and Sumi S:
Electrofusion of mesenchymal stem cells and islet cells for
diabetes therapy: A rat model. PLoS One. 8:e644992013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Glycosylated hemoglobin assays in the
management and diagnosis of diabetes mellitus. Health and Public
Policy Committee, American College of Physicians. Ann Intern Med.
101:710–713. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Haller MJ, Viener H, Brusko T, Wasserfall
C, Mcgrail K, Staba S, Cogle C, Atkinson M and Schatz DA: Insulin
requirements, HbA1c and stimulated C-peptide following autologous
umbilical cord blood transfusion in children with T1D. Diabetes.
56:A822007.
|