Introduction
Colorectal cancer (CRC) is one of the most common
and aggressive cancer types in the digestive tract and poses a
serious threat to human life and health (1). Among all cancer types, the incidence
and mortality of CRC are the third and fourth highest, respectively
(2). The development and progression
of CRC are induced by a cascade of multiple events, including
genetic and epigenetic deregulation, with changes at the genetic
and protein expression level (3). To
develop novel diagnostic biomarkers and effective therapeutic
targets for CRC patients, it is urgently required to better
understand the mechanisms involved in CRC progression.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNAs with a length of >200 nucleotides and no protein
coding ability (4,5). Accumulating studies have illustrated
that certain lncRNAs are involved in tumorigenesis (6). For instance, Wei and Li (7) reported that lncRNA sex-determining
region Y 21 antisense diverging transcript 1 (AS1) sponges microRNA
(miRNA/miR)-145 to promote the tumorigenesis of CRC by targeting
myosis, class VI. Wang et al (8) reported that lncRNA nuclear paraspeckle
assembly transcript 1 regulates the proliferation, migration and
invasion of gastric cancer cells via inhibiting the binding of
miR-335-5p to Rho associated coiled-coil containing protein kinase
1. Fu et al (9) indicated
that long intergenic non-protein coding RNA (LINC)00210 drives
Wnt/β-catenin signaling activation and liver tumor progression in a
catenin β interacting protein 1-dependent manner. In addition, Xie
et al (10) indicated that
lncRNA zinc finger NFX1-type containing 1 antisense RNA 1 sponges
miR-484 to promote cell proliferation and invasion in CRC.
Therefore, it has been widely acknowledged that lncRNAs have
essential roles in human cancer, including CRC.
A recent study implied that LINC01503 contributes to
squamous cell carcinoma progression (11). However, the function of LINC01503 in
CRC has remained to be elucidated. In the present study, it was
identified that LINC01503 was highly expressed in CRC tissues.
Furthermore, knockdown of LINC01503 significantly inhibited the
proliferation and invasion of CRC cells, while ectopic expression
of LINC01503 had the opposite effect. Regarding the mechanism of
action, LINC01503 was indicated to promote the expression of
forkhead box K1 (FOXK1) by sponging miR-4492. Through regulating
the miR-4492/FOXK1 axis, LINC01503 contributes to CRC progression.
Taken together, the present results demonstrated the pivotal role
of LINC01503 in CRC progression and elucidated the underlying
mechanisms, which suggested that LINC01503/miR-4492/FOXK1 axis may
be a potential therapeutic target.
Materials and methods
Clinical specimens
All tissue samples (47 cases) and pair-matched
non-cancerous tissues (28 cases; 1.5 cm from the lesions) from
patients with CRC were collected at the Shanghai East Hospital,
Shanghai Tongji University (Shanghai, China) between January 2014
and December 2016. None of the patients included in the present
study had received any therapy prior to surgery. Informed consent
was obtained from all patients prior to sample collection. All
tissues obtained during surgery were confirmed by histopathological
evaluation and then immediately frozen until use. The protocol of
the present study was approved by the Ethics Committee of Shanghai
Tongji University.
Cell culture and transfection
The CRC cell lines (HT29, HCT8, LS513, SW620 and
HCT116) and a non-cancerous colon epithelial FHC cell line were
purchased from the American Type Culture Collection (Manassas, VA,
USA). CRC cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (FBS; HyClone; GE Healthcare, Logan,
UT, USA), 100 units/ml penicillin and 100 mg/ml streptomycin at
37°C in a humidified atmosphere containing 5% CO2. The
small interfering (si)RNA against LINC01503
(5′-TCTGACAAGTGTGTACCTA-3′), siRNA negative control (siLINC;
5′-AATTCTCCGAACGTGTCACGT-3′), miR-4492 mimics
(5′-GGGGCUGGGCGCGCGCC-3′), miR negative controls (NC mimics;
5′-UCACAACCUCCUAGAAAGAGUAGA-3′) and pCDNA3-LINC01503 were
synthesized and purchased from RiboBio Co. (Guangzhou, China).
Transfection of SW620 and HCT8 cells was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Cell proliferation assay
For the CRC cell proliferation assay, SW620 and HCT8
cells were transfected with siLINC01503 or pCDNA3-LINC01503 for 0.5
h according to manufacturer's protocol. Subsequently, the cells
were re-seeded in 96-well plates at 3×103 cells/well.
Following culture for 24, 48 or 72 h, the proliferation of CRC
cells was measured using a Cell Counting kit-8 (Dojindo, Kumamoto,
Japan) according to the manufacturer's protocol. The absorbance of
CRC cells was measured at 450 nm to draw cell proliferation curves.
The CCK-8 assay was performed independently three times.
Transwell assay
Cell invasion was determined by Transwell assays.
The membrane of the Transwell chambers (pore size, 8 µm; cat. no.
PSET010R5; EMD Millipore, Billerica, MA, USA) was coated with 30
mg/cm2 Matrigel® (BD Biosciences, San Jose,
CA, USA) for 1 h at 37°C to form a matrix barrier. The lower
chambers were filled with 600 µl DMEM supplemented with 10% FBS.
The SW620 or HCT8 cells were suspended in DMEM and 1×105
cells were loaded into each upper well in a volume of 200 µl. After
the chambers cultured at 37°C with 5% CO2 for 24 h, the
cells at the upper side of the membrane were wiped off. The cells
in the lower side of the membrane were fixed with methanol for 10
min at 25°C and stained with 0.1% crystal violet for 10 min at
25°C. Subsequently, the cells were washed with PBS. Counts were
obtained from five random fields at a magnification of ×200. Three
replicates per condition were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 1×107
cultured SW620 and HCT8 cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol and complementary (c)DNA was synthesized from total RNA
with a PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu, Japan).
miRNA from total RNA was reverse transcribed using the PrimeScript
miRNA cDNA Synthesis kit (Takara Bio, Inc.). Real-time PCR was
performed with the SYBR-Green Premix Ex Taq II (Takara Bio, Inc.)
on an Applied Biosystems Step One Plus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Denaturation at 95°C for
10 min, followed by 40 cycles of denaturation at 95°C for 15 sec,
60°C for 20 sec and 72°C for 30 sec. GAPDH was used as the
endogenous control for detection of mRNA and lncRNA expression
levels, while U6 was used as an endogenous control for miRNA
expression analysis. Relative gene expression levels were
calculated using the 2−ΔΔCq method (12). Primer sequences were as follows:
LINC01503 forward, 5′-GGGACGGAGACAAATGACGG-3′ and reverse,
5′-GCAGGCTCCCTGACACGTA-3′; miR-4492 forward,
5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-GGGGCTGGGCGCGCGCC-3′);
FOXK1 forward, 5′-ACGTTTGGTGACCAGAGGGA-3′ and reverse,
5′-CGACAGAATTCAAGCCGCAC-3′; U6 forward, 5′-AACGAGACGACGACAGAC-3′
and reverse, 5′-GCAAATTCGTGAAGCGTTCCATA-3′ and GAPDH forward,
5′-ATGTTGCAACCGGGAAGGAA-3′ and reverse,
5′-AGGAAAAGCATCACCCGGAG-3′.
Luciferase reporter assay
The putative interacting sites between LINC01503 and
miR-4492, and between miR-4492 and FOXK1 were predicted using miRDB
(http://mirdb.org/miRDB/index.html)
and TargetScan7 (http://www.targetscan.org/vert_71/) tools. The
targeting association between miR-4492 and LINC01503 or the
3′-untranslated region (3′-UTR) of FOXK1 was validated by
luciferase reporter assays. The wild-type (WT) LINC01503 sequence
or the WT 3′-UTR fragment from FOXK1 mRNA containing the predicted
miR-4492 binding site was amplified and inserted into a pmirGLO
dual-luciferase miRNA target expression vector (Promega Corp.,
Madison, WI, USA) to construct the reporter vector
pmirGLO-LINC01503-WT or pmirGLO-FOXK1-WT as previously described
(13). The putative binding site #2
of miR-4492 in the LINC01503 or FOXK1 3′-UTR was mutated using a
GeneArt™ Site-Directed Mutagenesis PLUS System (cat. no. A14604;
Thermo Fisher Scientific, Inc.). The mutant (mut) LINC01503 or
FOXK1 3′-UTR was inserted into a pmirGLO vector to form the
reporter vector pmirGLO-LINC01503-Mut or pmirGLO-FOXK1-Mut. The
respective reporter vector and miR-4492 mimics or NC mimics were
co-transfected into 2×104 SW620 and HCT8 cells, followed
by incubation for 48 h. Subsequently, luciferase activities were
measured using a Dual-Luciferase Reporter Assay System (Promega
Corp.). The values were normalized to those of the Renilla
luciferase transfection control using the Dual-Luciferase Reporter
Gene Assay kit (Promega Corp.) according to the manufacturer's
protocol.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM, Corp., Armonk, NY, USA) and GraphPad Prism (version 6;
GraphPad Inc., La Jolla, CA, USA). Student's t-test and one-way
analysis of variance followed by Tukey's post-hoc test was used to
assess differences between 2 or multiple groups, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LINC01503 is overexpressed in CRC
tissues
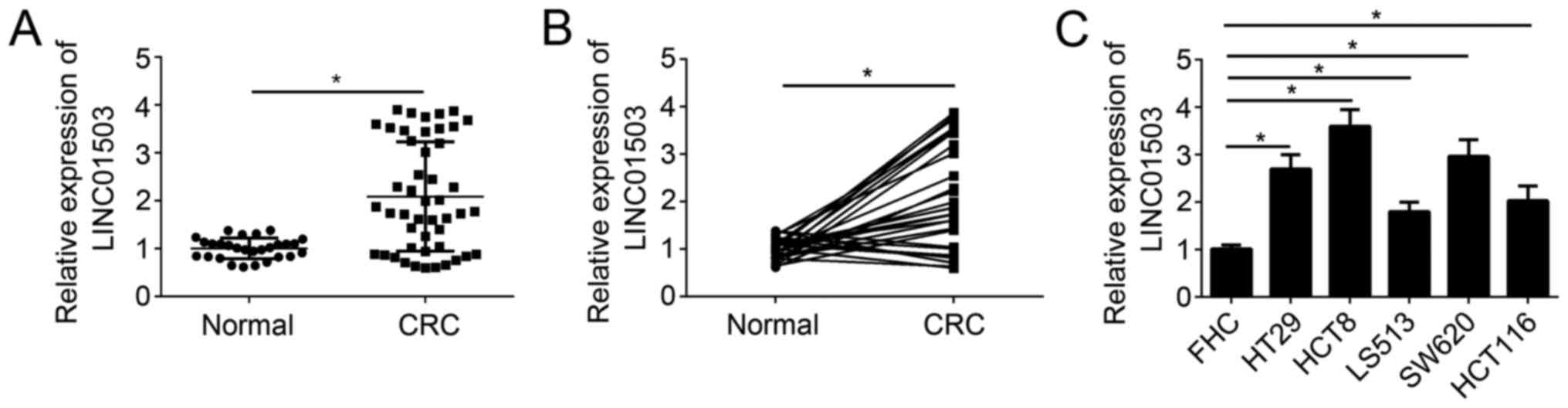
A total of 47 CRC samples and 28 adjacent normal
tissues were collected and the expression levels of LINC01503 were
determined in these tissue samples by RT-qPCR. The results
indicated that LINC01503 expression was significantly upregulated
in CRC tissues compared with that in adjacent normal tissues
(Fig. 1A). Furthermore, the
expression of LINC01503 was higher in most CRC tissues compared
with that in paired adjacent normal tissues (Fig. 1B). Similarly, the expression pattern
of LINC01503 was measured in CRC cell lines and the normal colon
epithelial FHC cell line. The results indicated that LINC01503
expression was significantly upregulated in CRC cell lines compared
with that in the non-cancerous reference cell line (Fig. 1C). These results implied that
LINC01503 may be involved in the genesis of CRC.
Silencing of LINC01503 inhibits CRC
cell proliferation and invasion
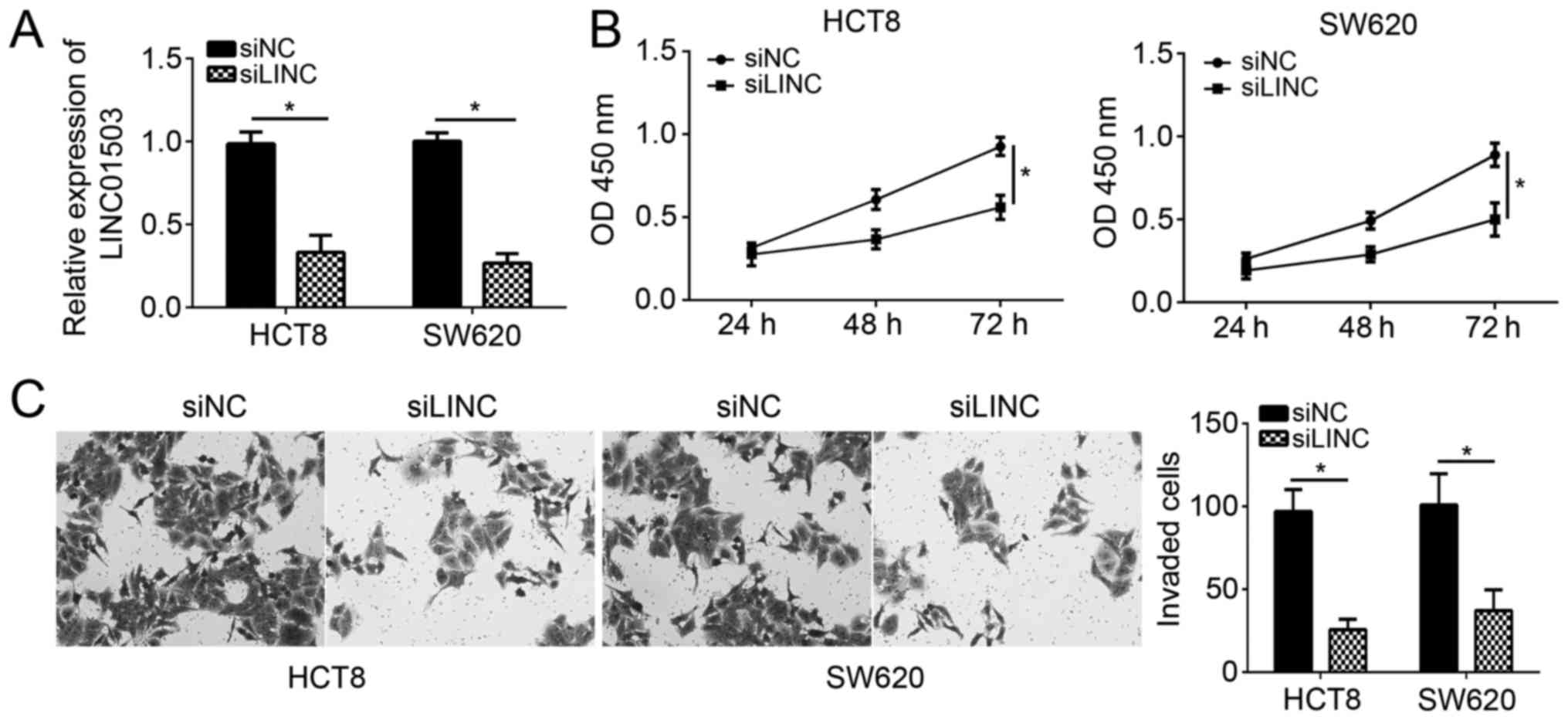
To determine the function of LINC01503 in CRC, HCT8
and SW620 cells were selected for the subsequent experiments. After
transfection with siLINC01503, the expression of LINC01503 was
significantly downregulated in HCT8 and SW620 cells (Fig. 2A). A CCK-8 and Transwell assays were
then performed to detect the effects of LINC01503 on CRC cell
proliferation and invasion. The results suggested that LINC01503
knockdown significantly impaired the proliferation of HCT8 and
SW480 cells (Fig. 2B). Consistently,
HCT8 and SW480 cells transfected with siLINC01503 displayed an
attenuated potential of invasion (Fig.
2C). These results indicated that LINC01503 contributes to
malignant behavior of CRC cells that is associated with cancer
progression.
LINC01503 overexpression promotes CRC
cell proliferation and invasion
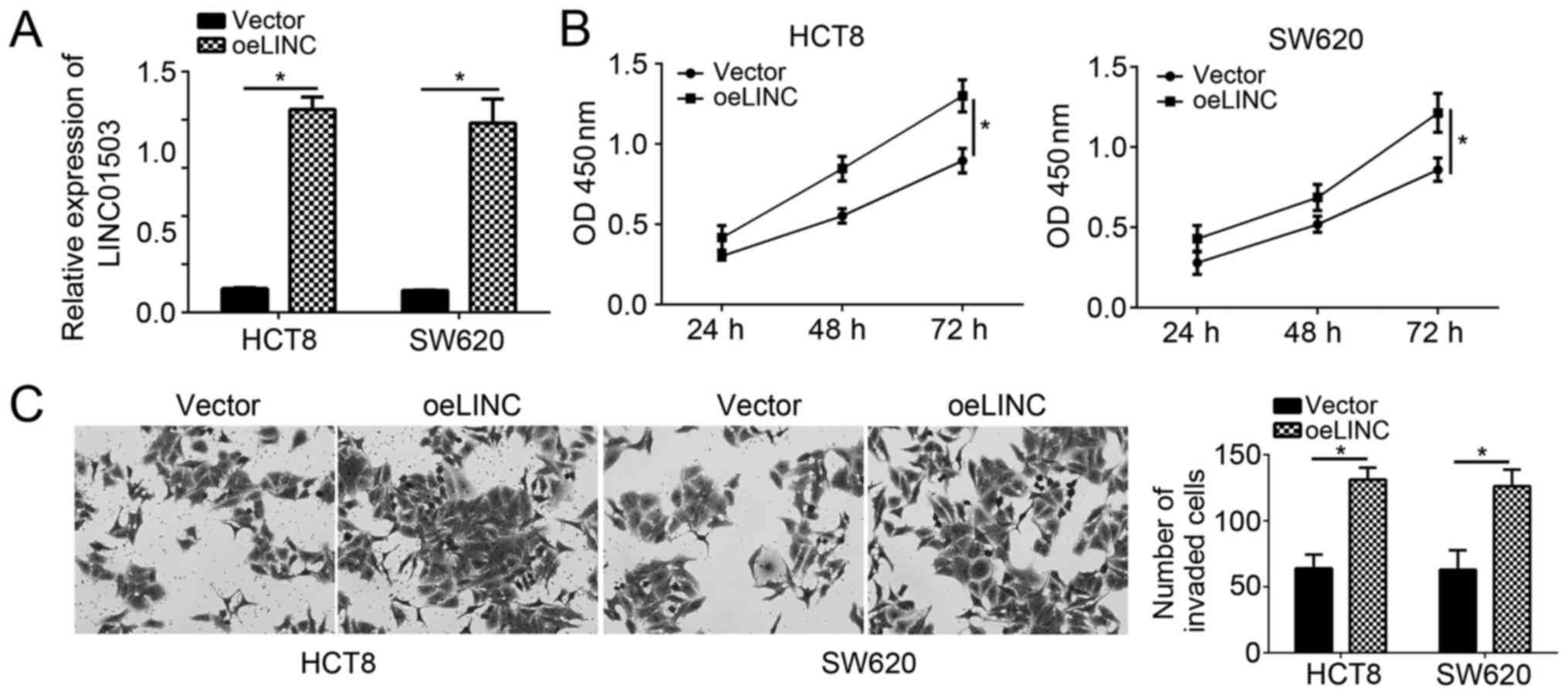
To further confirm the effects of LINC01503 on CRC
cells, LINC01503 was overexpressed in HCT8 and SW620 cells by
transfection with pCDNA3-LINC01503. RT-qPCR analysis indicated that
LINC01503 expression was significantly upregulated in HCT8 and
SW620 cells after transfection (Fig.
3A). CCK8 and Transwell assays were then performed. Conversely
to the observations made following silencing of LINC01503,
overexpression of LINC01503 markedly promoted the proliferation of
HCT8 and SW480 cells (Fig. 3B).
Furthermore, the invasion ability of HCT8 and SW480 cells was also
enhanced following transfection with LINC01503 ectopic expression
plasmid (Fig. 3C). These results
demonstrated that LINC01503 has an oncogenic role in CRC cells.
LINC01503 facilitates FOXK1 expression
via sponging of miR-4492
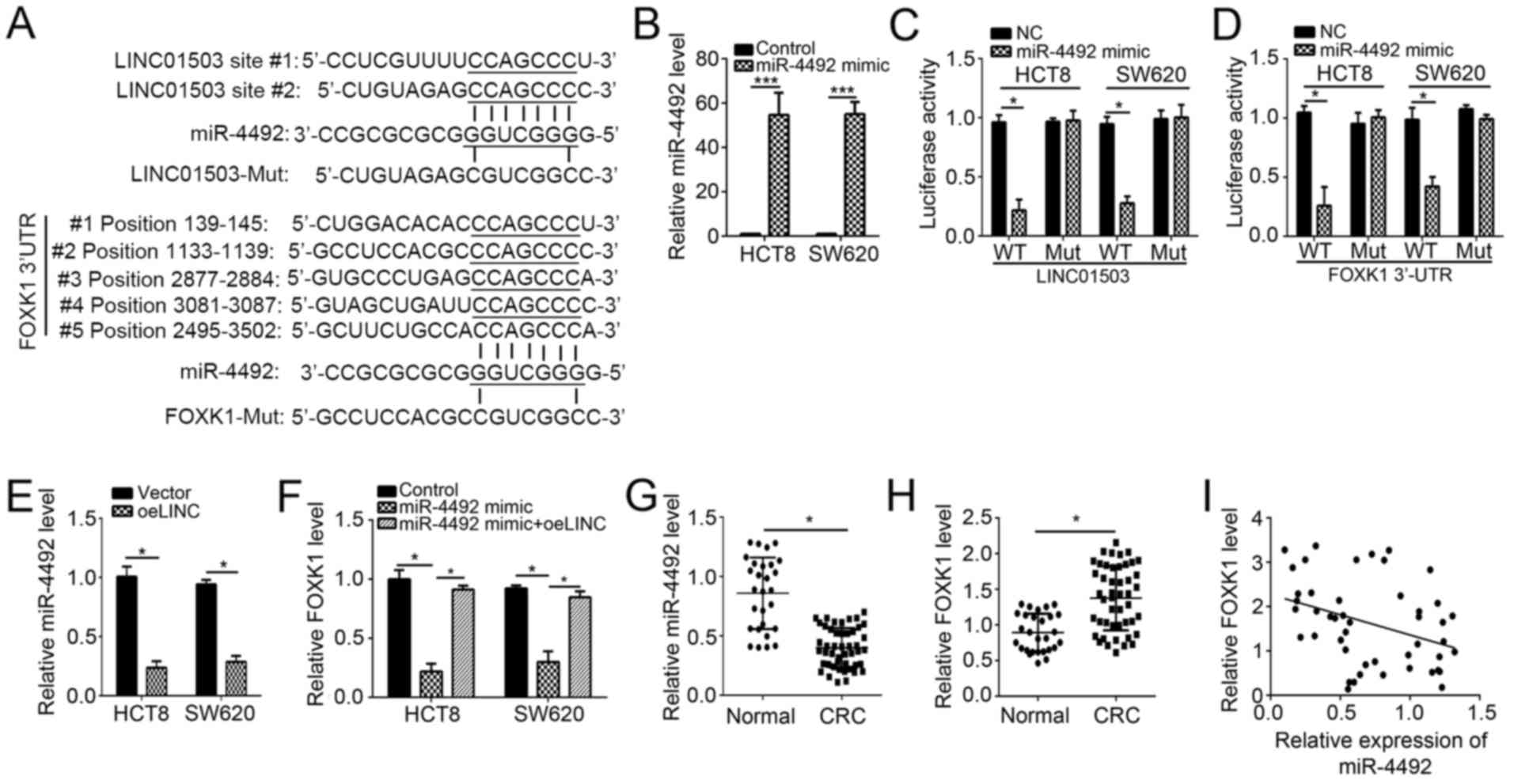
To further explore the downstream mechanisms of the
effects of LINC01503 in CRC cells, the target miRNAs were predicted
by a bioinformatics analysis. It was identified that miR-4492 was
the most probable target miRNA of LINC01503, as two potential
binding sites for miR-4492 were identified in LINC01503 (Fig. 4A). Furthermore, bioinformatics
analysis also suggested FOXK1 as a potential downstream target of
miR-4492, as five potential binding sites of miR-4492 were
identified in the 3′-UTR of FOXK1 mRNA (Fig. 4A). To verify the predicted binding
interactions between miR-4492 and LINC01503 or FOXK1, luciferase
reporter assays were performed. First, the efficiency of the
transfection of miR-4492 mimics into HCT8 and SW620 cells was
confirmed by RT-qPCR (Fig. 4B).
Subsequently, HCT8 and SW620 cells were co-transfected with the
constructed WT or mut LINC01503 reporter plasmid as well as
miR-4492 mimics or controls, and the luciferase activity was then
measured. The results indicated that overexpression of miR-4492
significantly repressed the luciferase activity of
pmirGLO-LINC01503-WT but not pmirGLO-LINC01503-Mut in HCT8 and
SW620 cells (Fig. 4C). Similarly,
miR-4492 mimics also inhibited the luciferase activity of
pmirGLO-FOXK1-WT but not pmirGLO-FOXK1-Mut in HCT8 and SW620 cells
(Fig. 4D). These results
demonstrated that miR-4492 interacted with LINC01503 and FOXK1 mRNA
in CRC cells. To further prove this, RT-qPCR analysis was
performed, which demonstrated that overexpression of LINC01503
significantly reduced the levels of miR-4492 in HCT8 and SW620
cells (Fig. 4E). Furthermore,
miR-4492 mimics caused a decrease in the mRNA levels of FOXK1 in
HCT8 and SW620 cells, while simultaneous overexpression of
LINC01503 abrogated this effect (Fig.
4F). In addition, miR-4492 expression was significantly
downregulated, while FOXK1 expression was significantly increased
in CRC tissues compared with that in adjacent normal tissues
(Fig. 4G and H). Of note, the
expression levels of miR-4492 and FOXK1 were negatively correlated
in CRC tissues (Fig. 4I). Taken
together, these results indicated that LINC01503 promotes FOXK1
expression via inhibition of miR-4492 in CRC cells.
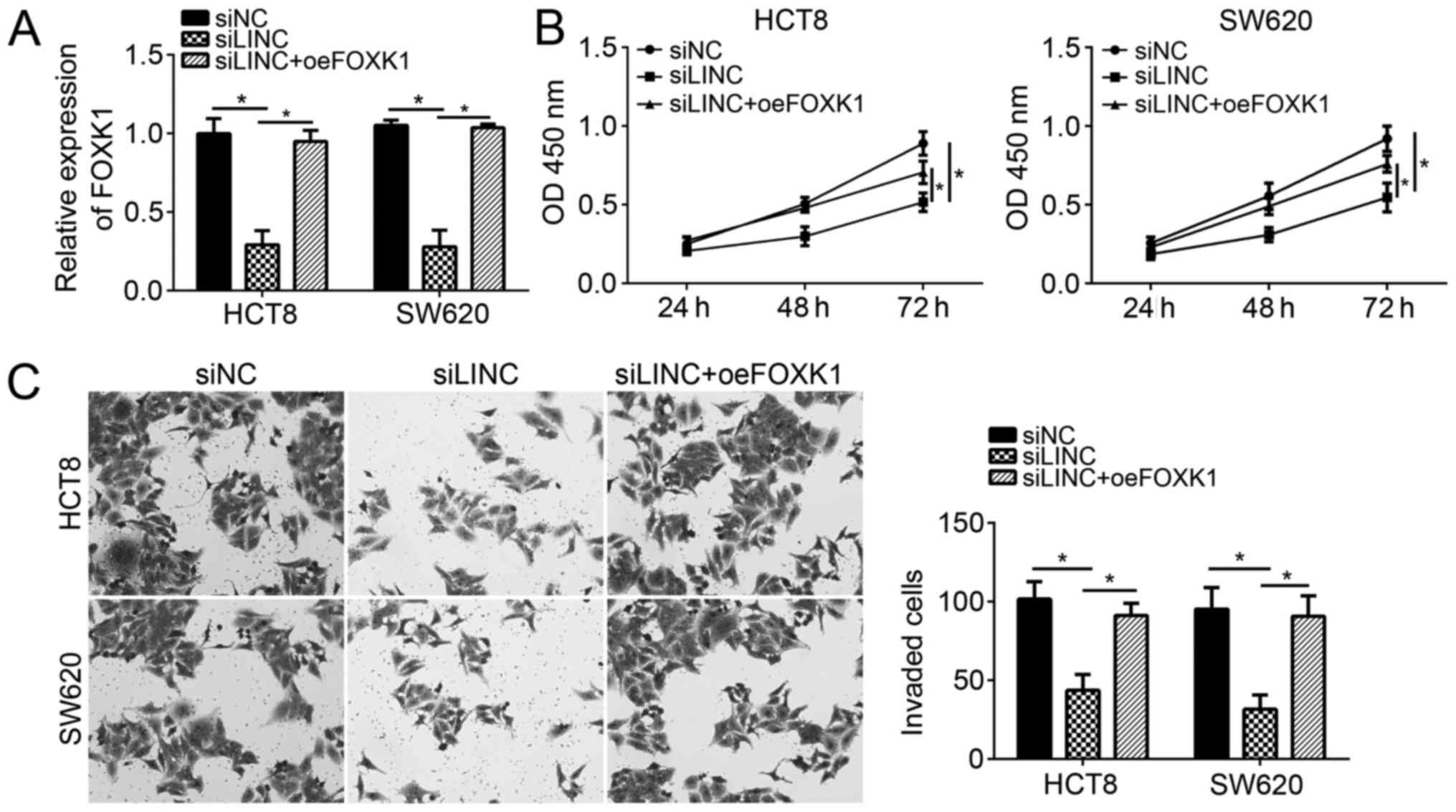
Restoration of FOXK1 expression
abrogates the effects of LINC01503 knockdown
The present study then sought to determine whether
FOXK1 expression is responsible for the effects of LINC01503 on CRC
cells. LINC01503-silenced CRC cells were subjected to ectopic
overexpression of FOXK1. RT-qPCR analysis indicated that FOXK1
expression, which was markedly declined after silencing of
LINC01503, was significantly rescued after simultaneous
transfection of FOXK1 expression plasmid in CRC cells (Fig. 5A). CCK8 and Transwell assays were
then performed. The results indicated that LINC01503 knockdown
suppressed the proliferation and invasion of CRC cells, whereas
restoration of FOXK1 significantly reversed the effects of
LINC01503 on CRC cells (Fig. 5B and
C). Taken together, the present results demonstrated that
LINC01503 promoted the proliferation and invasion of CRC cells via
enhancing FOXK1 expression.
Discussion
CRC is one of the most prevalent cancer types
worldwide and the fourth leading cause of cancer-associated death
(2). Most patients are diagnosed
with CRC at the advanced stage, which is usually accompanied with
metastasis, leading to poor outcomes for CRC patients. Therefore,
the discovery of novel diagnostic biomarkers and development of
therapeutic targets for CRC is an urgent priority. In the present
study, it was identified that LINC01503 is overexpressed in CRC
tissues and cell lines compared with normal adjacent tissues and a
reference cell line, respectively. Furthermore, it was demonstrated
that overexpression of LINC01503 enhanced the proliferation and
invasion of CRC cells, while knockdown of LINC01503 had the
opposite effect. It was also demonstrated that LINC01503 exerts its
effects by promoting FOXK1 expression through acting as a sponge
for miR-4492 in CRC cells.
Besides miRNAs, lncRNAs have also been reported to
be essential post-transcriptional regulators of gene expression
that regulate various biological processes, including cell
proliferation, survival and mobility (14). For instance, Fu et al
(15) indicated that lncRNA
LINC00978 promotes cancer growth and acts as a diagnostic biomarker
in gastric cancer. Furthermore, Chen et al (16) reported that lncRNA FOXD2-AS1 promotes
the genesis of nasopharyngeal carcinoma by modulating the
miR-363-5p/S100 calcium binding protein A1 pathway. In addition,
Xie et al (17) indicated
that hepatocellular carcinoma (HCC)-associated lncRNA facilitates
the growth and metastasis of HCC by acting as a competing
endogenous (ce)RNA of lysosomal-associated transmembrane protein
4B. The abovementioned studies evidenced that dysregulation of
lncRNA expression is associated with various types of human cancer.
Another study suggested that LINC01503 was upregulated in squamous
cell carcinoma and contributed to its progression (11). However, the function of LINC01503 in
CRC has remained largely elusive. In the present study, LINC01503
was identified to have an oncogenic role in CRC and to be
associated with its progression.
An increasing number of studies demonstrate that
lncRNAs serve as ceRNAs to act as a sponge on miRNAs to
consequently regulate gene expression (18). For instance, Chang et al
(19) reported that lncRNA for Pvt1
oncogene promotes epithelial to mesenchymal transition via
mediating the targeting of twist family basic helix-loop-helix
transcription factor 1 by miR-186 in prostate cancer. The present
study assessed whether LINC01503 may also serve as a ceRNA in CRC.
To investigate this, the target miRNAs of LINC01503 were predicted
by a bioinformatics analysis. miR-4492 was suggested as a direct
target of LINC01503 in CRC cells, which was then experimentally
verified. It was demonstrated that miR-4492 mimics repressed the
luciferase activity of a LINC01503 reporter vector in CRC cells.
Furthermore, ectopic overexpression of LINC01503 suppressed the
levels of miR-4492 in HCT8 and SW620 cells. These results
demonstrated that LINC01503 acted as a sponge for miR-4492 and
reduced its availability. To the best of our knowledge, the
function of miR-4492 has not been explored in cancer cells
previously. The present study was the first to suggest that
miR-4492 acts as a tumor suppressor in CRC. However, the expression
patterns and functions of miR-4492 require further exploration.
In addition, the present study further identified
FOXK1 as a direct target gene of miR-4492. A luciferase reporter
assay confirmed the interaction between miR-4492 and FOXK1 mRNA.
Furthermore, it was indicated that miR-4492 mimics significantly
repressed the mRNA expression of FOXK1 in CRC cells. However,
simultaneous overexpression of LINC01503 rescued the expression of
FOXK1 in CRC cells transfected with miR-4492 mimics. These results
demonstrated that LINC01503 promotes the expression of FOXK1 by
acting as a sponge for miR-4492. Previous studies have indicated
that FOXK1 has an oncogenic role in various cancer types, including
esophageal cancer (20), ovarian
cancer (21), gastric cancer
(22), CRC (23) and prostate cancer (24). To determine whether LINC01503 exerts
its oncogenic effects by regulating FOXK1 expression, loss- and
gain-of-function assays were performed. CCK-8 and Transwell assays,
demonstrated that restoration of FOXK1 significantly abrogated the
effects of LINC01503 knockdown on CRC cell proliferation and
invasion, which suggested that FOXK1 has an oncogenic role in CRC.
This result was consistent with that of a previous study on FOXK1
(25). Therefore, these results
demonstrated that LINC01503 promotes CRC progression via enhancing
the expression of FOXK1.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that LINC01503 promotes
CRC cell proliferation and invasion by reducing the formation of
the miR-4492/FOXK1 complex through acting as a ceRNA. These results
suggested that the LINC01503/miR-4492/FOXK1 interaction may be a
promising therapeutic target for CRC.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
J-ZL, S-RL and QL conceived of and designed the
current study, and performed some of the experiments. J-LL, CL and
XX collected the samples and performed some of the experiments. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Regarding the use of human samples, the protocol of
the present study was approved by the Institutional Ethics
Committee of Shanghai East Hospital, Shanghai Tongji University,
and all enrolled patients signed a written informed consent
document.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Almasi Z, Rafiemanesh H and Salehiniya H:
Epidemiology characteristics and trends of incidence and morphology
of stomach cancer in Iran. Asian Pac J Cancer Prev. 16:2757–2761.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krah NM and Murtaugh LC: Differentiation
and inflammation: ‘Best enemies’ in gastrointestinal
carcinogenesis. Trends Cancer. 2:723–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu
P, Du Y, Wu J, Qin X, Chen R, et al: Long noncoding RNA lncKdm2b is
required for ILC3 maintenance by initiation of Zfp292 expression.
Nat Immunol. 18:499–508. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y, Ouyang J, Wei J, Maarouf M and Chen
JL: Involvement of host non-coding RNAs in the pathogenesis of the
influenza virus. Int J Mol Sci. 18:E392016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei AW and Li LF: Long non-coding RNA
SOX21-AS1 sponges miR-145 to promote the tumorigenesis of
colorectal cancer by targeting MYO6. Biomed Pharmacother.
96:953–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Zhang M and Sun G: Long non-coding
RNA NEAT1 regulates the proliferation, migration and invasion of
gastric cancer cells via targeting miR-335-5p/ROCK1 axis.
Pharmazie. 73:150–155. 2018.PubMed/NCBI
|
|
9
|
Fu X, Zhu X, Qin F, Zhang Y, Lin J, Ding
Y, Yang Z, Shang Y, Wang L, Zhang Q and Gao Q: Linc00210 drives
Wnt/β-catenin signaling activation and liver tumor progression
through CTNNBIP1-dependent manner. Mol Cancer. 17:732018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie S, Ge Q, Wang X, Sun X and Kang Y:
Long non-coding RNA ZFAS1 sponges miR-484 to promote cell
proliferation and invasion in colorectal cancer. Cell Cycle.
17:154–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie JJ, Jiang YY, Jiang Y, et al:
Increased expression of the long non-coding RNA LINC01503,
regulated by TP63, in squamous cell carcinoma and effects on
oncogenic activities of cancer cell lines. Gastroenterology.
154:2137–2151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu
XY, Yu ZW, Jia YH, Bai XF, Li L, et al: The lncRNA CRNDE promotes
colorectal cancer cell proliferation and chemoresistance via
miR-181a-5p-mediated regulation of Wnt/beta-catenin signaling. Mol.
Cancer. 16:92017.
|
|
14
|
Wang L, Wang F, Na L, Yu J, Huang L, Meng
ZQ, Chen Z, Chen H, Ming LL and Hua YQ: LncRNA AB209630 inhibits
gemcitabine resistance cell proliferation by regulating PI3K/AKT
signaling in pancreatic ductal adenocarcinoma. Cancer Biomark.
22:169–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu M, Huang Z, Zang X, Pan L, Liang W,
Chen J, Qian H, Xu W, Jiang P and Zhang X: Long noncoding RNA
LINC00978 promotes cancer growth and acts as a diagnostic biomarker
in gastric cancer. Cell Prolif. 51:Feb;2018. View Article : Google Scholar
|
|
16
|
Chen G, Sun W, Hua X, Zeng W and Yang L:
Long non-coding RNA FOXD2-AS1 aggravates nasopharyngeal carcinoma
carcinogenesis by modulating miR-363-5p/S100A1 pathway. Gene.
645:76–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie CR, Wang F, Zhang S, Wang FQ, Zheng S,
Li Z, Lv J, Qi HQ, Fang QL, Wang XM and Yin ZY: Long noncoding RNA
HCAL facilitates the growth and metastasis of hepatocellular
carcinoma by acting as a ceRNA of LAPTM4B. Mol Ther Nucleic Acids.
9:440–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan Z, An N, Qin J, Yang J, Sun H and
Yang W: Long non-coding RNA Linc00675 suppresses cell proliferation
and metastasis in colorectal cancer via acting on miR-942 and
Wnt/β-catenin signaling. Biomed Pharmacother. 101:769–776. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang Z, Cui J and Song Y: Long noncoding
RNA PVT1 promotes EMT via mediating microRNA-186 targeting of
Twist1 in prostate cancer. Gene. 654:36–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen D, Wang K, Li X, Jiang M, Ni L, Xu B,
Chu Y, Wang W, Wang H, Kang H, et al: FOXK1 plays an oncogenic role
in the development of esophageal cancer. Biochem Biophys Res
Commun. 494:88–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Gong M, Zhao Y, Zhao X and Li Q:
FOXK1 facilitates cell proliferation through regulating the
expression of p21, and promotes metastasis in ovarian cancer.
Oncotarget. 8:70441–70451. 2017.PubMed/NCBI
|
|
22
|
Zhang P, Tang WM, Zhang H, Li YQ, Peng Y,
Wang J, Liu GN, Huang XT, Zhao JJ, Li G, et al: MiR-646 inhibited
cell proliferation and EMT-induced metastasis by targeting FOXK1 in
gastric cancer. Br J Cancer. 117:525–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie R, Wang J, Liu X, Wu L, Zhang H, Tang
W, Li Y, Xiang L, Peng Y, Huang X, et al: RUFY3 interaction with
FOXK1 promotes invasion and metastasis in colorectal cancer. Sci
Rep. 7:37092017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Xiong W, Dou K and Ran Q:
Knockdown of FOXK1 suppresses proliferation, migration, and
invasion in prostate cancer cells. Oncol Res. 25:1261–1267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu M, Wang J, Tang W, Zhan X, Li Y, Peng
Y, Huang X, Bai Y, Zhao J, Li A, et al: FOXK1 interaction with FHL2
promotes proliferation, invasion and metastasis in colorectal
cancer. Oncogenesis. 5:e2712016. View Article : Google Scholar : PubMed/NCBI
|