Introduction
Propofol is widely used for general anesthesia or
sedation in critically ill patients (1). Intensive insulin therapy can reduce
morbidity and mortality in patients in surgical intensive care
units, and, therefore, insulin resistance is an important factor
affecting the prognosis of critically ill patients (2,3). It has
been reported that anesthesia with propofol could induce systemic
insulin resistance and decrease insulin-stimulated glucose uptake
in skeletal and heart muscles and attenuate the insulin-mediated
suppression of hepatic glucose output in rats, however the specific
molecular mechanisms underlying this phenomenon remain unknown
(4). Previous studies have revealed
that propofol can inhibit the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt)/glycogen synthase kinase (GSK)-3b
signaling pathway and glycogen synthesis in mouse primary
hepatocytes, and the target of propofol-induced insulin resistance
in primary mouse hepatocytes was suggested to be upstream of GSK-3β
(5). In this study cell viability
was assessed by MTT reduction assay, as previously described
(5). Propofol was added at a final
concentration of 10 µg/ml, based on the results of previous studies
(5–8). PTEN is an important regulatory gene of
the PI3K/Akt/GSK-3β signaling pathway (9–11), and
inhibition of PTEN activity can activate the Akt signal
transduction pathway (12). RNA
interference is the process of sequence-specific,
post-transcriptional gene silencing in animals and plants,
initiated by double-stranded RNA (dsRNA) homologous in sequence to
the silenced gene (13). The
mediators of sequence-specific messenger RNA degradation are 21-
and 22-nucleotide long small interfering RNAs (siRNAs) generated by
ribonuclease III cleavage from longer dsRNAs (13). The current study used siRNA-996 to
silence the endogenous PTEN gene expression, observed the
alterations of the signaling pathway and glycogen synthesis in
primary mouse hepatocytes, and investigated the role of PTEN in
propofol-induced insulin resistance. The present study demonstrated
that propofol enhanced PTEN expression in mouse primary
hepatocytes. In addition, PTEN knockdown reversed propofol-induced
inhibition of the PI3K/Akt/GSK-3β signaling pathway and glycogen
synthesis in mouse primary hepatocytes. These results indicated
that PTEN may be the target of propofol-induced insulin resistance
in mouse primary hepatocytes, and, therefore, PTEN could be a
potential target for therapeutic intervention against
propofol-induced adverse effects.
Materials and methods
Animals
Male C57BL/6J mice (n=10; age, 8 weeks; weight,
24–28 g) were provided by Peking University Health Science Center
(Beijing, China). Mice were housed at a constant temperature
(22±2°C) and 55±10% relative humidity with a 12 h light/dark cycle
and free access to food and water. The mice were fasted for 12 h
prior to all experiments. Animal procedures were performed in
accordance with the National Institutes of Health Animal Care and
Use Guidelines (14), and animal
experimental protocols were approved by the Ethics Committee of
Shenzhen Maternity and Child Healthcare Hospital (Shenzhen,
China).
Isolation of mouse primary
hepatocytes
Primary hepatocytes were isolated using a two-step
collagenase perfusion method, as previously described (15,16). The
hepatocytes were plated in collagen-coated 25-cm2 flasks
at a density of 1×106 cells/flask and were used as the
control group in the following experiment. Dimethylsulfoxide (DMSO)
at a final concentration of 0.1% was added to the DMSO group
cells.
Western blot analysis
Protein was extracted with radioimmunoprecipitation
assay lysis buffer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The protein content of cells was assessed using Pierce BCA
protein assay kit (Thermo Fisher Scientific Inc.). The proteins
(15–30 µg/lane) were separated on SDS-PAGE 10% gels (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and transferred onto a
polyvinylidene difluoride membranes (EMD Millipore; Billerica, MA,
USA). The membranes were blocked with 5% nonfat dry milk at 4°C
overnight, and incubated with primary antibodies against PTEN (cat.
no. 9188), Akt (cat. no. 9272), phosphorylated Akt (cat. no. 9271),
GSK (cat. no. 9315), phosphorylated GSK (cat. no. 9323) and β-actin
(cat. no. 4970; all 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) overnight at 4°C. Following primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:5,000; cat. no. ab6721;
Abcam) at room temperature for 2 h. The blots were visualized using
an enhanced chemiluminescence detection system (EMD Millipore) and
quantified by densitometry using Image-Pro Plus software 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). β-actin was used as the
internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the M-MLV reverse transcriptase
kit (Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocol. qPCR was subsequently performed using the
SYBR®-Green PCR mastermix (Takara Biotechnology Co.,
Ltd., Dalian, China), according to manufacturer's protocol The
following primer pairs were used for the qPCR: hypoxanthine
phosphoribosyltransferase 1 (HPRT1) forward,
5′-AATTATGGACAGGACTGAACGTCTTGCT-3′ and reverse,
5′-TCCAGCAGGTCAGCAAAGAATTTATAGC-3′; and mouse PTEN forward,
5′-AATTCCCAGTCAGAGGCGCTATGT-3′ and reverse,
5′-GATTGCAAGTTCCGCCACTGAACA-3′. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
10 min; 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
relative mRNA levels were quantified using the 2−ΔΔCq
method (17) and normalized to the
reference gene HPRT1-R.
Cell culture and transfections
Mouse primary hepatocytes were plated on 24-well
plates at a density of 6×104 cells/well and grown in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin, at 37°C in a 5% CO2-humidified incubator.
The siRNA (GenePharma Co., Ltd., Shanghai, China) sequences used in
this study are as follows. si-PTEN (Cy3-labeled siR-996) sense,
5′-GGUGUAUACAGGAACAAUATT-3′ and anti-sense,
5′-UAUUGUUCCUGUAUACACCTT-3′; negative control siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. A total of 100 µl siR-996 at a final
concentration of 50 nmol/l was mixed with 3 µl HiPerFect
Transfection reagent (Qiagen China Co., Ltd., Shanghai, China) in
serum-free DMEM at room temperature for 10 min, then the cells were
transfected for 24 h at 37°C. Negative control siRNA was added at a
final concentration of 50 nmol/l in the negative control (NC)
group. Cells were incubated with 2 µg/ml Hoechst 33342 solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 10 min at room
temperature and washed twice with PBS. The transfection efficiency
determined by counting the red fluorescent Cy3-labeled siR-996 in
transfected cells under an inverted fluorescent microscope (Nikon
Corporation, Tokyo, Japan).
Analysis of glycogen content
Glycogen levels were measured following incubation
of cells with 10 nmol/l insulin (United States Biological, Salem,
MA, USA) at room temperature for 3 h using a glycogen assay kit
(BioVision, Inc., Milpitas, CA, USA).
Statistical analysis
The results are reported as the mean ± standard
deviation of at least three independent experiments with a minimum
sample size of three. Statistical analysis was performed with SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). All
experimental data were analyzed using one-way analysis of variance
followed by Tukey's test to confirm statistical differences among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
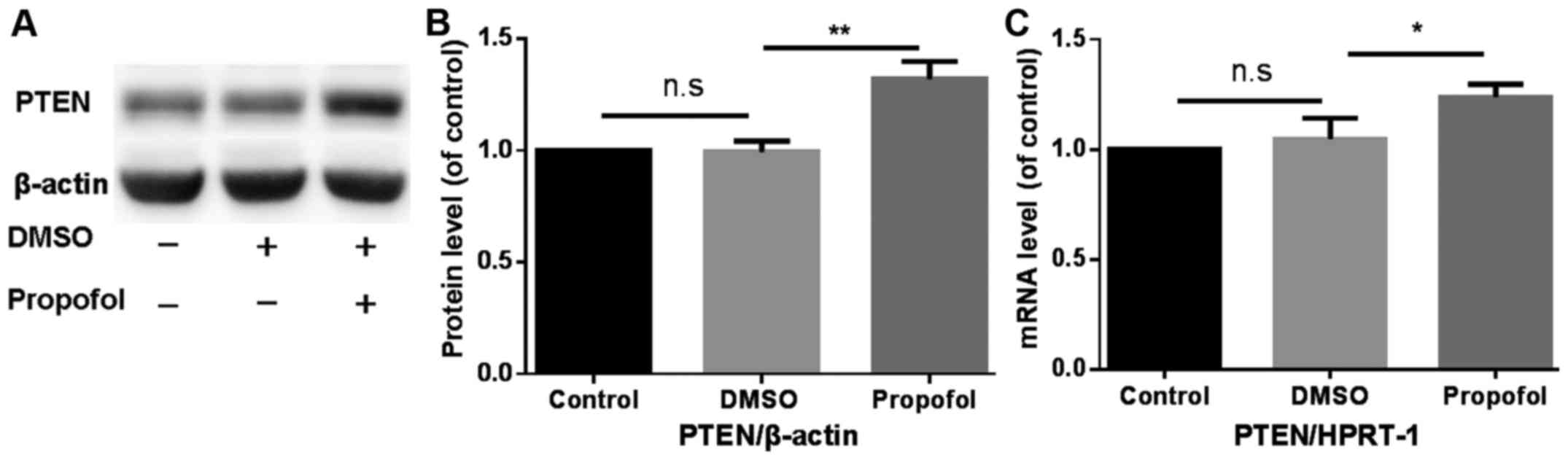
Propofol enhances PTEN expression in
mouse primary hepatocytes
The effect of treatment with propofol on PTEN
protein and mRNA expression in mouse primary hepatocytes was
analyzed using western blotting and RT-qPCR, respectively.
Treatment with propofol significantly increased PTEN protein and
mRNA expression compared with the control group (Fig. 1).
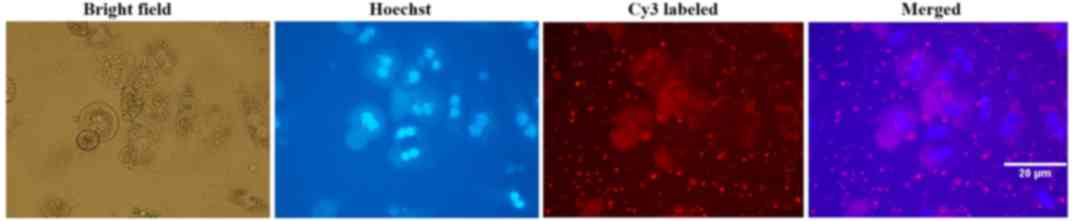
Transfection efficiency of
Cy3-siRNA
Mouse primary hepatocytes were transfected with
Cy3-labelled siR-996. Cells were initially observed under an
inverted fluorescent microscope. The subcellular localization and
distribution of cells transfected with Cy3-labelled siRNA were
observed using the fluorescent microscope. Transfection efficiency
of Cy3-labelled siRNA was >95% in mouse primary hepatocytes, 24
h after transfection (Fig. 2).
PTEN knockdown reverses
propofol-induced inhibition of the PI3K/Akt/GSK-3β signaling
pathway and glycogen synthesis in mouse primary hepatocytes
To further confirm the biological functions of PTEN
in propofol-induced insulin resistance in mouse hepatocytes,
endogenous PTEN expression was silenced by transfecting siR-996
into mouse primary hepatocytes, with simultaneous treatment with
propofol (final concentration, 10 µg/ml) for 24 h. Following PTEN
knockdown and treatment with propofol, western blot analysis was
used to detect the protein expression levels of PTEN and components
of the PI3K/Akt/GSK-3β signaling pathway (Fig. 3A). Compared with the NC+DMSO group,
the protein expression level of PTEN in the NC+propofol group
significantly increased (P<0.01), while the protein expression
level of PTEN in the DMSO+siR-996 group significantly decreased
(P<0.001) (Fig. 3B). There was no
significant difference in the protein expression level of PTEN
between the siR-996+Propofol group and the control group (Fig. 3B). Compared with the NC+DMSO group,
the phosphorylation levels of Akt (Ser473) in the NC+propofol group
decreased (P<0.05), while the phosphorylation levels of Akt
(Ser473) in the DMSO+siR-996 group increased (P<0.05) (Fig. 3C). Compared with the NC+DMSO group,
the phosphorylation levels of GSK-3β (Ser9) in the NC+propofol
group significantly decreased (P<0.01), while the
phosphorylation levels of GSK-3β (Ser9) in the DMSO+siR-996 group
increased (P<0.05) (Fig. 3D).
There was no significant difference in the phosphorylation levels
of Akt (Ser473) and GSK-3β (Ser9) between the siR-996+Propofol
group and the control group (Fig. 3C and
D). In addition, the glycogen assay kit was used to detect the
level of glycogen synthesis. Compared with the NC+DMSO group, the
glycogen level in the NC+propofol group decreased (P<0.05),
while the glycogen level in the DMSO+siR-996 group was increased
(P<0.05) (Fig. 3D). However,
there was no significant difference in the rate of glycogen
synthesis between the siR-996+Propofol group and the control group
(Fig. 3E).
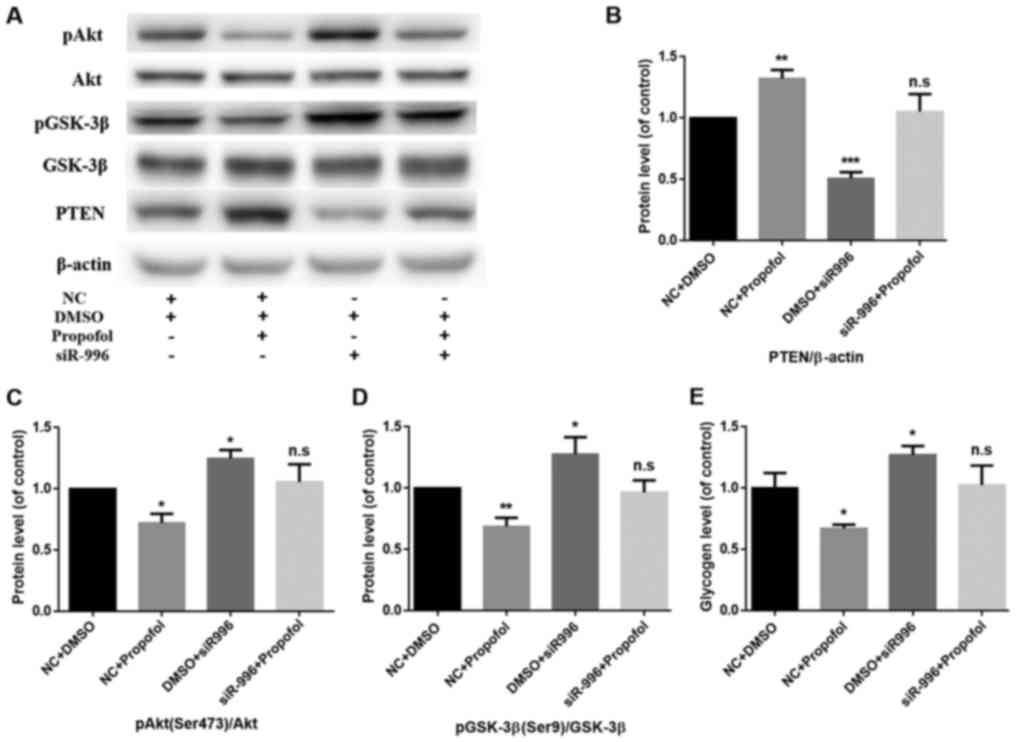 | Figure 3.PTEN knockdown reverses
propofol-induced insulin resistance in mouse primary hepatocytes.
(A) The protein expression levels of pAkt (Ser473), Akt, pGSK-3β
(Ser9), GSK-3β and PTEN were determined by western blot analysis.
β-actin was used as the loading control. (B) PTEN knockdown
reversed the propofol-induced enhancement of PTEN protein
expression. Data are presented as the mean ± standard deviation.
**P<0.01 and ***P<0.001 vs. NC+DMSO group. (C) PTEN knockdown
reversed the propofol-induced inhibition of pAkt (Ser473)/Akt. Data
are presented as the mean ± standard deviation. *P<0.05 vs.
NC+DMSO group. (D) PTEN knockdown reversed the propofol-induced
inhibition of pGSK-3β (Ser9)/GSK-3β. Data are presented as the mean
± standard deviation. *P<0.05 and **P<0.01 vs. NC+DMSO group.
(E) Glycogen levels were measured in mouse primary hepatocytes
using a glycogen assay kit. PTEN knockdown reversed the
propofol-induced inhibition of glycogen synthesis. Data are
presented as the mean ± standard deviation. *P<0.05 vs. NC+DMSO
group. PTEN, phosphatase and tensin homolog; Akt, protein kinase B;
GSK, glycogen synthase kinase; DMSO, dimethylsulfoxide; NC,
negative control; n.s., not significant; p, phosphorylated. |
Discussion
Insulin resistance is a physiological condition in
which normal or elevated insulin levels produce an attenuated
biological effect (18). In 1988,
Reaven (19) first suggested the
idea of insulin resistance being a common phenomenon, which can
occur in a number of pathological and physiological conditions,
other than diabetes. Insulin resistance severely affects the
prognosis of critically ill patients (2,3,20). A clinical study revealed that
intensive insulin therapy could reduce mortality by 42.5% as well
as significantly reducing other complications in surgical intensive
care unit patients (21). Propofol
is the most common intravenous anesthetic agent used in clinical
practice (1). Propofol has been
demonstrated to cause systemic insulin resistance in rats (4). In addition, previous studies have
indicated that propofol can induce insulin resistance in mouse
primary hepatocytes (5). The
molecular mechanism through which propofol influences insulin
resistance in mouse primary hepatocytes remains unknown, however
the present study focused on PTEN as a potential target for
therapeutic intervention to treat the adverse reaction of
propofol.
PTEN was first identified, cloned and named in 1997
by three independent research groups (22–24). The
protein encoded by PTEN is a dual-specificity phosphatase,
with both lipid and protein phosphatase activity. The
phosphorylation of proteins can affect a number of signal
transduction pathways and regulate gene transcription in the
nucleus. PTEN is the first tumor-suppressor gene identified with
phosphatase activity (23). It
serves a role in cell apoptosis, cell cycle arrest and cell
migration (25). PTEN is an
important regulator of the PI3K/Akt signaling pathway (9–11,26).
PTEN negatively regulates PI3K/Akt signal transduction by
catalyzing the dephosphorylation of the lipid signaling
intermediate phosphatidylinositol-3,4,5-trisphosphate (27). Inhibition of PTEN activity can
activate the Akt signal transduction pathway (12). The present study indicated that
protein and mRNA expression levels of PTEN increased in mouse
primary hepatocytes following treatment with propofol for 24 h. In
addition, PTEN knockdown reversed propofol-induced inhibition of
the PI3K/Akt/GSK-3β signaling pathway and glycogen synthesis in
mouse primary hepatocytes. In conclusion, the present study
suggested that PTEN may be the target of propofol-induced insulin
resistance in mouse primary hepatocytes, and, therefore, could be a
potential target for therapeutic prevention of propofol-induced
adverse effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Shenzhen Science and Technology Plan Project (grant no.
JCYJ20160427145626702) and Shenzhen Health Commission Project
(grant no. 201605019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and LW designed and performed the experiments,
and wrote the manuscript. XH has participated in data analysis. YL
has participated in the design of this experiment.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shenzhen Maternity and Child Healthcare Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller RD: Miller's anesthesia. Eighth.
Philadelphia: Elsevier Saunders; pp. 822–831. 2015
|
|
2
|
Vanhorebeek I, Gunst J and Van den Berghe
G: Critical care management of stress-induced hyperglycemia. Curr
Diab Rep. 18:172018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van den Berghe G, Wilmer A, Hermans G,
Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H
and Bouillon R: Intensive insulin therapy in the medical ICU. N
Engl J Med. 354:449–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasuda Y, Fukushima Y, Kaneki M and Martyn
JA: Anesthesia with propofol induces insulin resistance
systemically in skeletal and cardiac muscles and liver of rats.
Biochem Biophys Res Commun. 431:81–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou L, Wang L, Yang B, Zeng J, Zhang Q,
Lei H and Xu S: Protective effect of pretreatment with propofol
against tumor necrosis factor-α-induced hepatic insulin resistance.
Exp Ther Med. 10:289–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milne SE, Troy A, Irwin MG and Kenny GN:
Relationship between bispectral index, auditory evoked potential
index and effect-site EC50 for propofol at two clinical end-points.
Br J Anaesth. 90:127–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith C, McEwan AI, Jhaveri R, Wilkinson
M, Goodman D, Smith LR, Canada AT and Glass PS: The interaction of
fentanyl on the Cp50 of propofol for loss of consciousness and skin
incision. Anesthesiology. 81:820–828, Discussion 26A. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsing CH, Chen YH, Chen CL, Huang WC, Lin
MC, Tseng PC, Wang CY, Tsai CC, Choi PC and Lin CF: Anesthetic
propofol causes glycogen synthase kinase-3β-regulated
lysosomal/mitochondrial apoptosis in macrophages. Anesthesiology.
116:868–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Driessen GJ, IJspeert H, Wentink M, Yntema
HG, van Hagen PM, van Strien A, Bucciol G, Cogulu O, Trip M,
Nillesen W, et al: Increased PI3K/Akt activity and deregulated
humoral immune response in human PTEN deficiency. J Allergy Clin
Immunol. 138:1744–1747.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wishart MJ and Dixon JE: PTEN and
myotubularin phosphatases: From 3-phosphoinositide
dephosphorylation to disease. Trends Cell Biol. 12:579–585. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi XP, Zong AN, Tao J and Wang LZ: Study
of instructive notions with respect to caring for laboratory
animals. J China Med Univ. 36:4932007.
|
|
15
|
Seglen PO: Preparation of isolated rat
liver cells. Methods Cell Biol. 13:29–83. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casciano DA: Development and utilization
of primary hepatocyte culture systems to evaluate metabolism, DNA
binding, and DNA repair of xenobiotics. Drug Metab Rev. 32:1–13.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lebovitz HE: Insulin resistance:
Definition and consequences. Exp Clin Endocrinol Diabetes. 109
Suppl 2:S135–S148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reaven GM: Banting lecture 1988. Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fahy BG, Sheehy AM and Coursin DB: Glucose
control in the intensive care unit. Crit Care Med. 37:1769–1776.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van den Berghe G, Wouters P, Weekers F,
Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P,
Lauwers P and Bouillon R: Intensive insulin therapy in critically
ill patients. N Engl J Med. 345:1359–1367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li DM and Sun H: TEP1, encoded by a
candidate tumor suppressor locus, is a novel protein tyrosine
phosphatase regulated by transforming growth factor beta. Cancer
Res. 57:2124–2129. 1997.PubMed/NCBI
|
|
25
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
26
|
Sha SH, Chen FQ and Schacht J: PTEN
attenuates PIP3/Akt signaling in the cochlea of the aging CBA/J
mouse. Hear Res. 264:86–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leslie NR, Bennett D, Lindsay YE, Stewart
H, Gray A and Downes CP: Redox regulation of PI 3-kinase signalling
via inactivation of PTEN. EMBO J. 22:5501–5510. 2003. View Article : Google Scholar : PubMed/NCBI
|