Introduction
Chronic urticaria (CU) is a common distressing skin
disorder characterized by recurrent wheals and/or angioedema
lasting for >6 weeks. CU can be divided into chronic spontaneous
urticaria (CSU), inducible urticaria and other types of urticaria
according to different etiology. CSU is a mast cell-driven disease
that is defined as the recurrence of wheals, angioedema or both for
>6 weeks due to known or unknown causes, excluding chronic
inducible urticaria and urticaria vasculitis (1). The incidence of CSU and the etiology is
unclear, the prevalence in the general population is ~0.5–1%, and
the annual incidence is ~1.4% (1,2). Lapi
et al (3) found that the
annual prevalence of CSU in Italy increased from 0.02 to 0.38% from
2002 to 2013. CSU is the most common type of CU, accounting for
~2/3 of all cases (4), which is the
same as that found in China (5). Due
to the lack of specific treatment, the symptoms easily recur and
are difficult to cure. CSU seriously affects the patients' quality
of life and the stability of the immune environment. Antihistamine
is the most commonly used drug for CSU treatment. However, this is
far from satisfactory. Due to its complicated disease mechanism,
most clinicians diagnose and treat CSU according to their symptoms.
The objective and measurable indicators of disease activity are
absent, so it is hard to evaluate the degree of disease, treatment
efficiency and prognosis correctly. Thus, further investigation is
required.
Although a large number of studies have been
conducted on the pathogenesis of CSU, many hypotheses on CSU still
have no conclusive evidence to confirm its mechanism. The specific
allergen can not be found in most CSU patients. So the mechanism
can not be well elaborated through IgE-mediated classic type I
hypersensitivity. It is also difficult to explain with autoimmune
theory, or genetic factors. Several researchers have suggested that
blood parameters may indicate disease activity and duration. This
would assist monitor treatment and could provide potential
prognostic biomarkers of CSU (6–9). Some
strong evidence has shown significant differences between patients
with CSU and healthy controls in blood levels or values of D-dimer,
C-reactive protein (CRP), matrix metalloproteinase-9 (MMP-9), mean
platelet volume (MPV), factor VIIa, prothrombin 1+2 (F1+2), tumur
necrosis factor, dehydroepiandrosterone sulphate and vitamin D
(10). Data have shown that plasma
FDP, D-dimer and serum CRP may be well associated with each other
and significantly associated with the disease severity of CU, but
not with the skin reactions of the autologous serum (11). However, Korean scholars Kim et
al (12) have found that the
expression level of serum clusterin increases in patients with a
positive autologous serum skin test (ASST) than a negative one.
Fujii et al (13) further
found that, after the urticaria subsided the elevated circulating
thrombin-antithrombin (TAT) III complex level recovered to within
the normal range, and so did the elevated D-dimer level in all
cases except two. This may explain the clinical symptoms of wheals
in urticaria patients that vanished gradually by themselves.
Researches (11,14) have shown that C3, C4 and CRP are
closely related with the severity of CSU in the diagnosis of CSU
and/or prognosis. Some authors have studied the inflammatory and
cytokines in serum and lesions of CSU patients. This can not
clearly explain the exact relationship with the pathogenesis of the
disease. Conflicting evidence might also be explained by different
patient populations and differences in the analysis of the results
(e.g., difference in standardization between the various producers
of the assay kits, the existence of various cut-offs and distinct
methodological measurements of blood parameters).
With the further development of genomics and
proteomics research, as well as the continuous accumulation of
protein research data, proteomics research will lead to
breakthroughs in the pathogenesis, diagnosis, treatment and new
drug development of various skin diseases. Increasing number of
proteomic techniques have been applied to skin diseases (15–17),
including some allergic skin diseases (18–22). So
far, proteomics technology has not been applied in the field of
urticaria and is rarely reported. Isobaric tags for relative and
absolute quantitation (iTRAQ) technology is a new relative and
absolute quantification technique of in vitro isotope
labeling of peptides developed by ABI Scientific, Inc. (Sterling,
VA, USA) in 2004. Proteomics research based on the iTRAQ labeling
method is a powerful tool for discovering protein markers. This
technique can quantitatively compare proteins in 8 different
samples at the same time. Therefore, it can distinguish the protein
changes in quality and quantity between normal and disease samples,
pre- and post-treatment, disease progression and cell culture under
different conditions.
In this study, iTRAQ labeling combined with
two-dimensional liquid chromatography/tandem mass spectrometry
(2D-LC-MS/MS) technique was used to study the protein profiling of
sera from CSU patients with different durations of wheals and
normal subjects. A total of 370 serum proteins associated with CSU
were initially identified. Among those identified proteins, ~30
were found to have significant differences between the groups.
According to the classification of biological functions and
upregulated/downregulated values, SAA, CFL1, TPM4 and monocyte
differentiation antigen (CD14) were chosen and validated by
enzyme-linked immunosorbent assay (ELISA).
The results showed that in CSU patients with wheal
duration of 12–24 h the expression level of SAA increased
(P=0.047767), while CFL1 and TPM4 levels decreased (P=0.049229 and
P=0.0049, respectively) in comparison with the healthy control
group. Compared with the group of patients with wheal duration of
<2 h, the expression level of SAA in CSU patients with 12–24 h
wheal duration had no significant difference, while the expression
levels of CFL1 and TPM4 decreased (P=0.01684 and P=0.0186,
respectively). However, there was no significant difference in
serum levels of SAA, CFL1 and TPM4 between CSU patients with wheal
duration of <2 h and the healthy control group. The expression
level of CD14 in serum was no significantly different among the
three groups. SAA, CFL1 and TPM4 proteins were associated with
wheal duration in CSU patients and might be considered as new
potential inflammatory biomarkers associated with CSU.
Materials and methods
Specimens and sample collection
A total of 20 CSU patients were selected and divided
into group A and B according to the duration of the wheals. Group
A: wheal duration <2 h, 10 cases; group B: wheal duration 12–24
h, 10 cases. All the serum samples were collected before the wheals
subsided. All patients conformed to the diagnostic criteria of CSU
in EAACI/GA (2) LEN/EDF/WAO
guidelines (1).
At the same time, group D, whose outpatient physical
examination was positive, served as a healthy control group. The 10
cases comprising the control group underwent blood tests. Liver and
kidney function, blood glucose and blood lipids were normal, and
there were no previous allergic and systemic diseases.
The study was approved by the Ethics Committee of
Pudong New Area People's Hospital (Shanghai, China). Signed
informed consents were obtained from the patients or the
guardians.
After the differentially expressed CSU
serum-associated proteins were identified among the groups, 42 CSU
patients were re-selected: 21 patients with wheal duration <2 h
(group Y) and 21 patients with wheal duration of 12–24 h (group G),
and 21 healthy controls (group J) were selected. The level of
differentially expressed serum protein was examined by ELISA
test.
The proteins that flowed through the depletion
column were precipitated using ReadyPrep 2-D Cleanup kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions. After precipitation, protein pellets
were re-suspended in dissolution buffer using the iTRAQ experiment
kit (AB Sciex, Framingham, MA, USA). The total protein
concentration of each sample was determined by Bradford protein
assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA), as
previously described (7). A total of
100 µg aliquots of each of the 3 samples were then reduced,
alkylated, digested with trypsin, and labeled individually with one
iTRAQ tag (Applied Biosystems: Thermo Fisher Scientific, Inc.,
Foster City, CA, USA) according to the manufacturer's instructions.
Group A, B and D samples were labeled with 113, 114 and 115 tags,
respectively. The labeled samples were then pooled and dried by
centrifugal evaporation (Christ Alpha 1–2 and Christ RVC 2–25;
Martin Christ Gefriertrocknungsanlagen GmbH, Osterode,
Germany).
2D-LC conditions
Chromatographic separation of the pooled samples was
performed on an Acquity Ultra Performance LC system (Waters Corp.,
Milford, MA, USA). Tryptic digested and labeled peptides were first
fractionated by a strong cation exchange (SCX) liquid chromatograph
using a 0.5×23 mm, 5 µm, 300 Å column (Waters Corp.). Samples were
loaded onto the column and eluted stepwise by injecting salt plugs
of 10 different molar concentrations of 25, 50, 75, 100, 150, 200,
300, 400, 500, 1,000 mM of NH4Ac. Ten fractions were
collected from the SCX column. Each of the fractions was then
loaded onto a reverse phase (RP) column, ZORBAX 300SB-C18 column (5
µm, 300 Å, 4.6×50 mm; Agilent Technologies, Inc., Santa Clara, CA,
USA). The flow rate used for separation on RP column was 0.4
µl/min. Buffer A was 5% acetonitrile, 95% water, 0.1% formic acid
and buffer B was 95% acetonitrile, 5% water, 0.1% formic acid.
Elution was performed using a gradient ranging from 5 to 45% buffer
B for >90 min.
MS/MS conditions
The LC eluent was subjected to positive ion nanoflow
electrospray analysis using a Qstar XL MS/MS system (Applied
Biosystems: Thermo Fisher Scientific, Inc.) in an
information-dependent acquisition (IDA) mode. In IDA mode, a TOFMS
survey scan was acquired (m/z 400–1,800), with up to six most
intense multiply charged ions in the survey scan sequentially
subjected to product ion analysis. Product ion spectra were
accumulated for 2 sec in the mass range m/z 100–2,000 with a
modified Enhance All mode Q2 transition setting favoring low mass
ions, so that the reporting iTRAQ ion (113, 114, and 115 m/z)
intensities were enhanced for quantitation.
Data analysis
All LC MS/MS data were acquired by Analyst QS 3.1
(Applied Biosystems: Thermo Fisher Scientific, Inc.). MS/MS data
were analyzed using ProteinPilot v3.0 (Applied Biosystems: Thermo
Fisher Scientific, Inc.) which uses the Paragon Algorithm to
perform database searching. The search results were further
processed by the Pro Group Algorithm to remove redundant hits and
comparative quantitation so that the minimal set of justifiable
identified proteins could be found. The protein database used for
all searches was Swiss-Prot human (downloaded on Sept. 15, 2016).
Loading error was normalized by bias correction calculated using
ProteinPilot software. All reported data were based on 95%
confidence for protein identification as determined by ProteinPilot
(Prot score ≥1.3). The relative protein quantitation was calculated
as an average ratio. The confidence level of the altered expression
of proteins was calculated by ProteinPilot as P-value, which allows
the results to be evaluated based on the confidence level of
expression change, not just by the magnitude of the change.
ELISA
Blood samples taken from the patients and normal
control, were grouped and numbered (5 ml elbow vein blood, low
temperature centrifugation, at the speed of 1,500 × g for 15 min at
4°C, supernatant sub-packaging, −80°C in storage) and then
recorded. Specific detection was performed according to iTRAQ
technology and ELISA kit.
Preparation of the reagents, samples and standards:
100 µl of sample were added, standard or blank to each well, and
incubated for 90 min at 37°C. A total of 100 µl of 1X biotinylated
detection anti-human SAA (EA8001-1; AssayPro, St. Charles, MO,
USA), CD14 (227920; Biomol GmbH, Hamburg, Germany), CFL1
(LS-F20976; LifeSpan BioSciences, Inc., Seattle, WA, USA) and TPM4
(EKC35896; Biomatik Corp., Cambridge, ON, Canada) polyclonal
antibodies (1:300) were aspirated and added, and then incubated for
1 h at 37°C. Then aspirated and washed 3 times. 1X HRP conjugate
(100 µl) was added and incubated for 30 min at 37°C. Aspirated and
washed 5 times. TMB substrate solution (90 µl) was added and
incubated for 15 min at 37°C. Finally, 50 µl of stop solution were
added and reading was performed immediately at 450 nm.
Serum measurement: ELISA assays for IGFBP2 and LCAT
(Cloud-Clone Corp., Wuhan, China), SHBG (R&D Systems Europe,
Ltd., Abingdon, UK), GRP78 (Enzo Life Sciences, Inc., Exeter, UK)
and calprotectin (BioLegend, Inc., San Diego, CA, USA) were
performed in duplicate using commercial kits following the
manufacturer's instructions. The mean coefficients of variance for
duplicate analysis for each assay were as follows: IGFBP2, 8.1%;
LCAT, 8.4%; SHBG, 7.4%; GRP78, 3.1%; and calprotectin, 4.5%.
Statistical analysis
All reported data were based on 95% confidence for
protein identification as determined by ProteinPilot (Prot score
≥1.3). The relative protein quantitation was calculated as an
average ratio. The confidence level of the altered expression of
proteins was calculated by ProteinPilot as P-value, which allows
the results to be evaluated based on the confidence level of
expression change, not just by the magnitude of the change.
P<0.05 was considered as a statistically significant
difference.
Results
Proteomic and biological functional
analysis in serum
There were in total 370 proteins identified in sera
of groups A, B and D using iTRAQ labeling combined with
2D-LC-MS/MS. There were 18 significant differentially expressed
proteins between group B and A. Among them, 4 proteins were
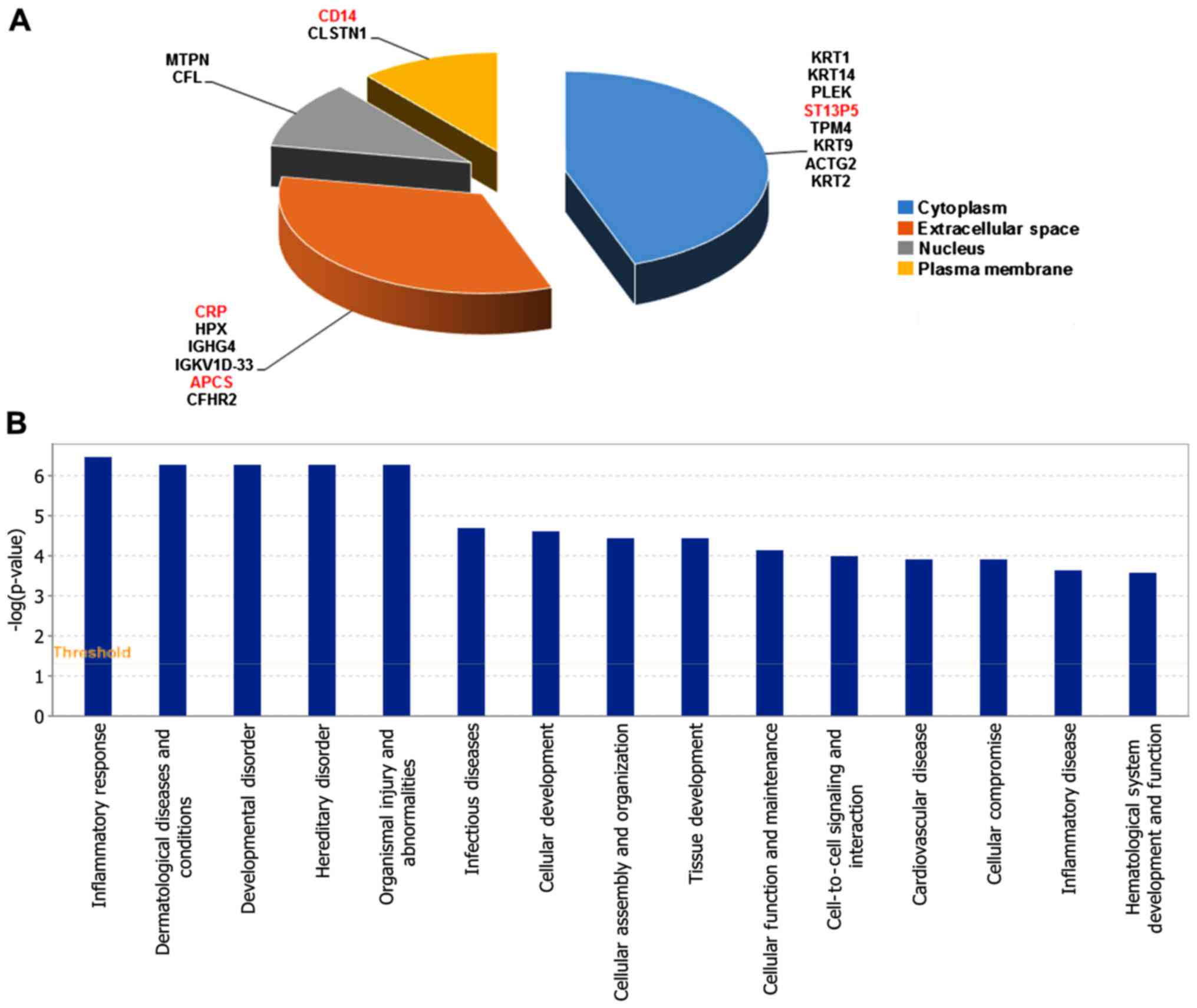
upregulated and 14 were downregulated (Table I). The cell location and functional
distribution of the 18 differentially expressed proteins are
displayed in Fig. 1A and B,
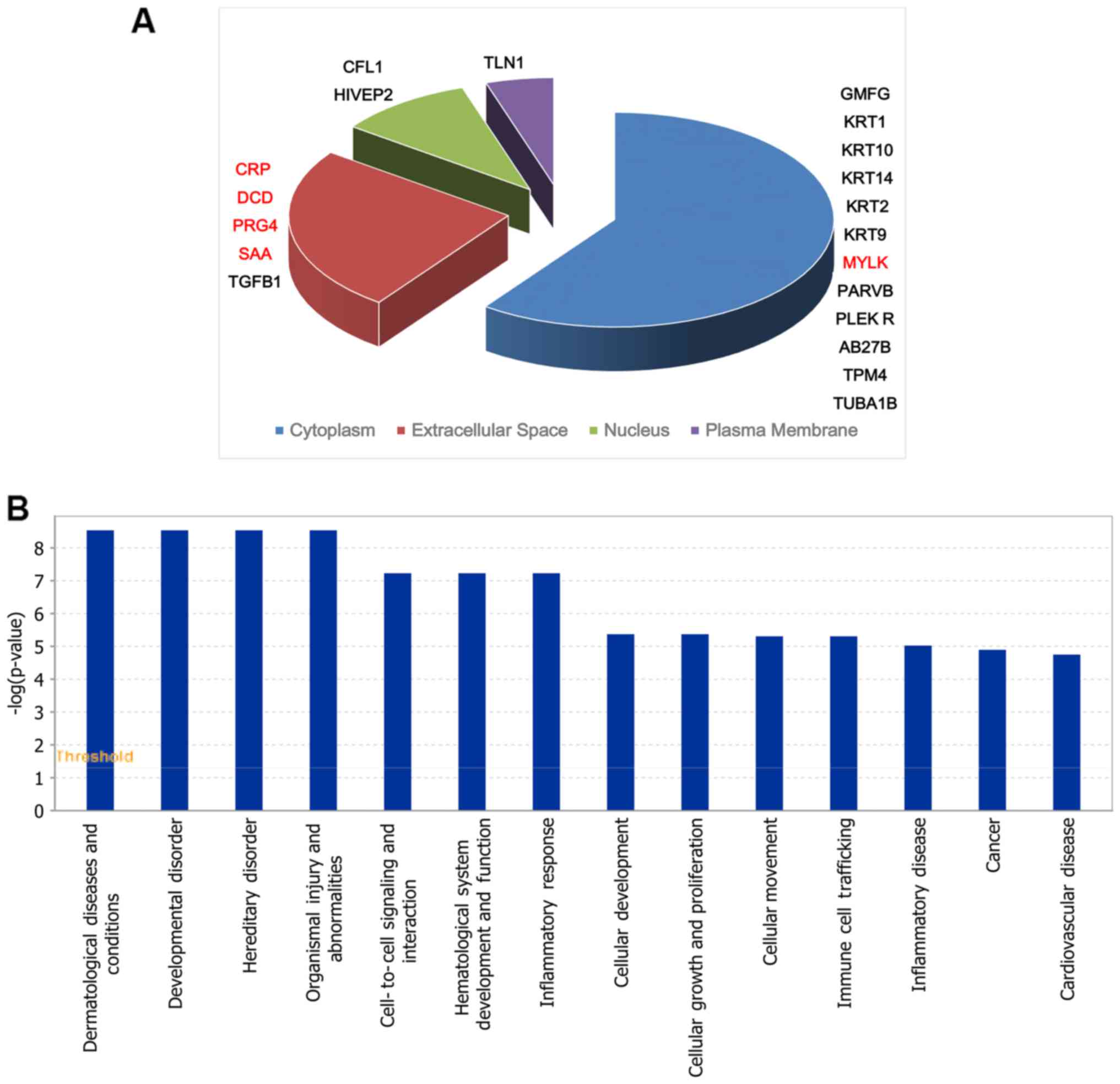
respectively. There were 20 significantly different proteins in
group D compared with group B, 5 proteins were upregulated and 15
were downregulated (Table II).
Fig. 2A and B, shows the 20
differentially expressed proteins in cell location and functional
distribution. These differentially expressed proteins were
classified in cell components by GO. They were both mainly
distributed in cytoplasm, outer matrix components, plasma membrane
and the nucleus.
 | Table I.Differentially expressed proteins of
group A and B. |
Table I.
Differentially expressed proteins of
group A and B.
| Accession no. | Entry name | Protein names | Ratio B/A |
|---|
| P02743 | SAMP_HUMAN | SAP-component | 1.719164386 |
| P67936 | TPM4_HUMAN | Tropomyosin α-4
chain (TM30p1) | 0.55495 |
| P02741 | CRP_HUMAN | CRP | 2.707280979 |
| P35527 | K1C9_HUMAN | CK-9 | 0.378568685 |
| P04264 | K2C1_HUMAN | CK-1 | 0.458821195 |
| P01861 | IGHG4_HUMAN | Ig γ-4 chain C
region | 0.654559002 |
| P08567 | PLEK_HUMAN | Pleckstrin | 0.605110697 |
| P35908 | K22E_HUMAN | CK-2e | 0.490515564 |
| P23528 | COF1_HUMAN | Cofilin-1 | 0.283736309 |
| P36980 | FHR2_HUMAN | Complement factor
H-related protein 2 | 0.56605 |
| P02790 | HEMO_HUMAN | Hemopexin | 0.6199 |
| P02533 | K1C14_HUMAN | K14 | 0.664 |
| P08571 | CD14_HUMAN | Monocyte
differentiation antigen CD14 | 1.66208565 |
| Q8NFI4 | F10A5_HUMAN | Putative protein
FAM10A5 | 1.5919 |
| P58546 | MTPN_HUMAN | Myotrophin (protein
V-1) | 0.5265 |
| O94985 | CSTN1_HUMAN | Calsyntenin-1 | 0.5115 |
| P01608 | KVD33_HUMAN | Immunoglobulin κ
variable 1D-33 | 0.6313 |
| P63267 | ACTH_HUMAN | Actin | 0.66 |
 | Table II.Differentially expressed proteins of
group B and D. |
Table II.
Differentially expressed proteins of
group B and D.
| Accession no. | Entry name | Protein names | Ratio B/D |
|---|
| P0DJI8 | SAA1_HUMAN | SAA-1 protein | 3.118620690 |
| P67936 | TPM4_HUMAN | Tropomyosin α-4
chain | 0.225594016 |
| Q9Y490 | TLN1_HUMAN | Talin-1 | 0.425138687 |
| P02741 | CRP_HUMAN | CRP | 4.628658177 |
| P35527 | K1C9_HUMAN | CK-9 | 0.377987984 |
| P13645 | K1C10_HUMAN | CK-10 | 0.424446977 |
| P04264 | K2C1_HUMAN | CK-1 | 0.393632417 |
| Q92954 | PRG4_HUMAN | Proteoglycan 4 | 1.678792288 |
| P08567 | PLEK_HUMAN | Pleckstrin | 0.180288202 |
| Q15746 | MYLK_HUMAN | Myosin light chain
kinase | 1.783790265 |
| P81605 | DCD_HUMAN | Dermcidin | 2.342048293 |
| P35908 | K22E_HUMAN | CK-2e | 0.226904558 |
| P31629 | ZEP2_HUMAN | Transcription
factor HIVEP2 | 0.666550825 |
| P01137 | TGFB1_HUMAN | Transforming growth
factor β-1 | 0.629120347 |
| Q9HBI1 | PARVB_HUMAN | β-parvin
(affixin) | 0.182152123 |
| P23528 | COF1_HUMAN | Cofilin-1 | 0.226166454 |
| P02533 | K1C14_HUMAN | CK-14 | 0.431996357 |
| O00194 | RB27B_HUMAN | Ras-related protein
Rab-27B | 0.405605322 |
| P68363 | TBA1B_HUMAN | Tubulin α-1B
chain | 0.609584125 |
| O60234 | GMFG_HUMAN | Glia maturation
factor γ | 0.447163743 |
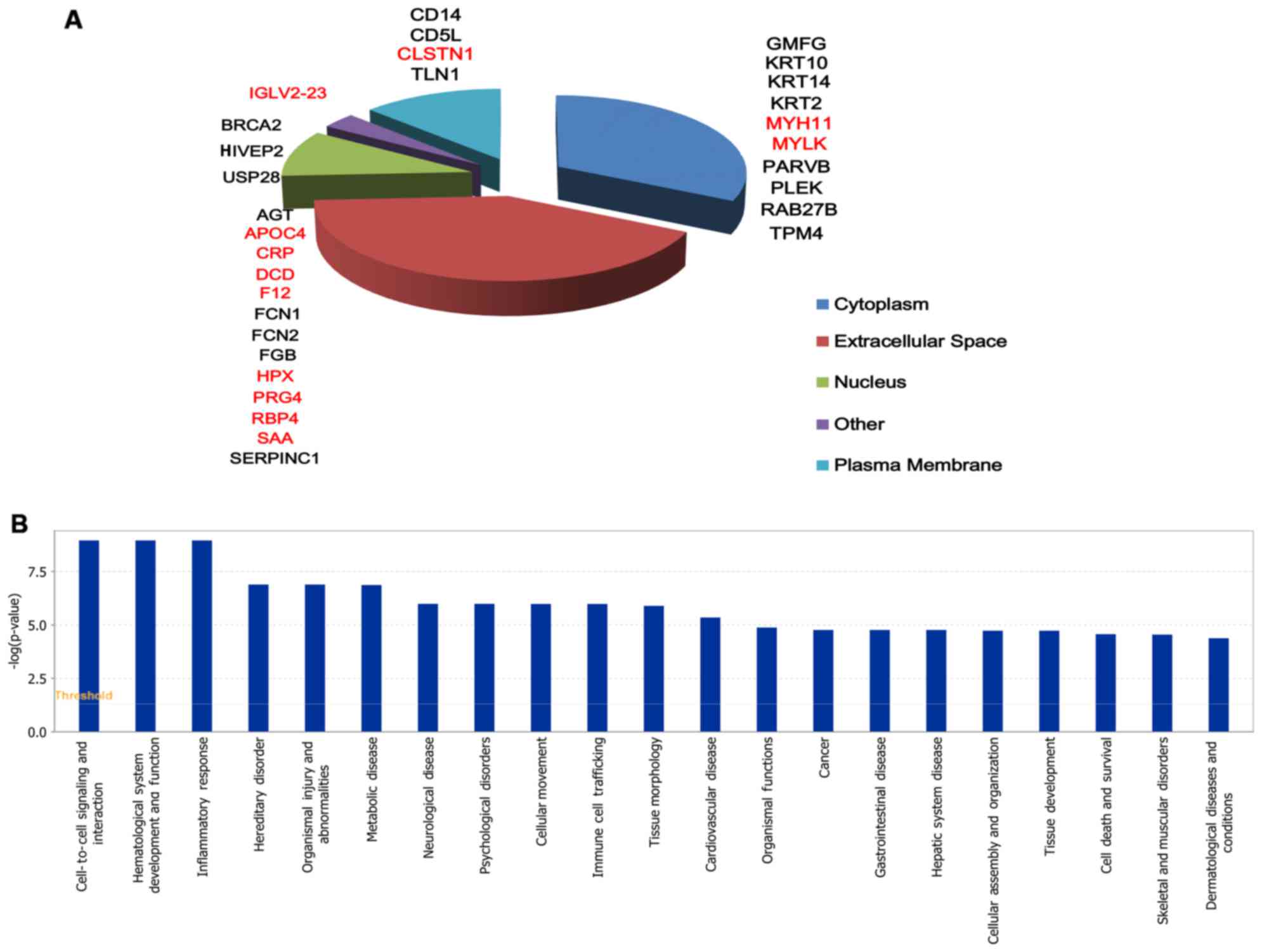
There were 31 significantly different proteins
between group A and D, 12 proteins were upregulated and 19 were
downregulated (Table III). These
31 differentially expressed proteins were classified in cell
components by GO, and were mainly distributed in extracellular
matrix components, cytoplasm, plasma membrane and nucleus (Fig. 3A). For IPA bio function, the first
three ranks are cell-to-cell signaling and interaction,
hematological system development and function and inflammatory
response (Fig. 3B).
 | Table III.Differentially expressed proteins of
group A and D. |
Table III.
Differentially expressed proteins of
group A and D.
| Accession no. | Entry name | Protein names | Ratio A/D |
|---|
| P01008 | ANT3_HUMAN | ATIII | 0.522774597 |
| P0DJI8 | SAA1_HUMAN | SAA-1 protein | 2.838068966 |
| Q96RU2 | UBP28_HUMAN | Ubiquitin
carboxyl-terminal hydrolase 28 | 0.662010526 |
| P67936 | TPM4_HUMAN | Tropomyosin-4 | 0.406512327 |
| Q9Y490 | TLN1_HUMAN | Talin-1 | 0.595211961 |
| P02741 | CRP_HUMAN | CRP | 1.709707346 |
| Q15485 | FCN2_HUMAN | Ficolin-2 | 0.553688195 |
| P13645 | K1C10_HUMAN | CK-10 | 0.554630198 |
| O43866 | CD5L_HUMAN | CD5
antigen-like | 0.662852444 |
| P02753 | RET4_HUMAN | Retinol-binding
protein 4 | 1.523612657 |
| Q92954 | PRG4_HUMAN | Proteoglycan 4 | 1.818843216 |
| P08567 | PLEK_HUMAN | Pleckstrin | 0.297942514 |
| Q15746 | MYLK_HUMAN | MLCK, smooth
muscle | 1.598593238 |
| P02675 | FIBB_HUMAN | Fibrinogen β
chain | 0.648407879 |
| P51587 | BRCA2_HUMAN | Breast cancer type
2 susceptibility protein | 0.413992962 |
| P55056 | APOC4_HUMAN | Apolipoprotein
C-IV | 2.046185998 |
| P81605 | DCD_HUMAN | Dermcidin | 1.578517902 |
| P35749 | MYH11_HUMAN | Myosin-11 | 1.744439599 |
| P35908 | K22E_HUMAN | CK-2e | 0.46258381 |
| P31629 | ZEP2_HUMAN | Transcription
factor HIVEP2 | 0.579206487 |
| P01705 | LV223_HUMAN | Immunoglobulin λ
variable 2–23 | 1.68477803 |
| Q9HBI1 | PARVB_HUMAN | β-parvin
(affixin) | 0.273112113 |
| P01019 | ANGT_HUMAN | Angiotensinogen
(serpin A8) | 0.644745326 |
| P00748 | FA12_HUMAN | Coagulation factor
XII | 1.885902876 |
| P02790 | HEMO_HUMAN | Hemopexin
(β-1B-glycoprotein) | 1.76584849 |
| P02533 | K1C14_HUMAN | CK-14 | 0.650596923 |
| P08571 | CD14_HUMAN | Monocyte
differentiation antigen CD14 | 0.537576093 |
| O94985 | CSTN1_HUMAN | Calsyntenin-1 | 2.147996993 |
| O00194 | RB27B_HUMAN | Ras-related protein
Rab-27B (C25KG) | 0.480318932 |
| O00602 | FCN1_HUMAN | Ficolin-1 | 0.644163875 |
| O60234 | GMFG_HUMAN | Glia maturation
factor γ (GMF-γ) | 0.584795322 |
The differentially expressed proteins identified
among these three groups were also compared. SAA, CD14, CFL1 and
TPM4 proteins had significant change. CRP, as a non-specific
inflammatory marker, has been studied and reported in literature.
Further 4 proteins, SAA, TPM4, CFL1 and CD14, based on the
biological and functional information have close relationship with
dermatological diseases and therefore were chosen to be validated
as potential disease biomarkers by ELISA.
ELISA
The expression level of SAA in the sera of group G
(wheal duration of 12–24 h) was higher than that of healthy
control; while there was no significant difference in the
expression of SAA between group Y (wheal duration <2 h) and G,
and between group Y and J (healthy group) (Fig. 4A) indicating that serum SAA levels
are significantly elevated in CSU patients with longer duration of
wheals. In other words, the severity of symptoms in CSU patients is
directly proportional to the serum level of SAA.
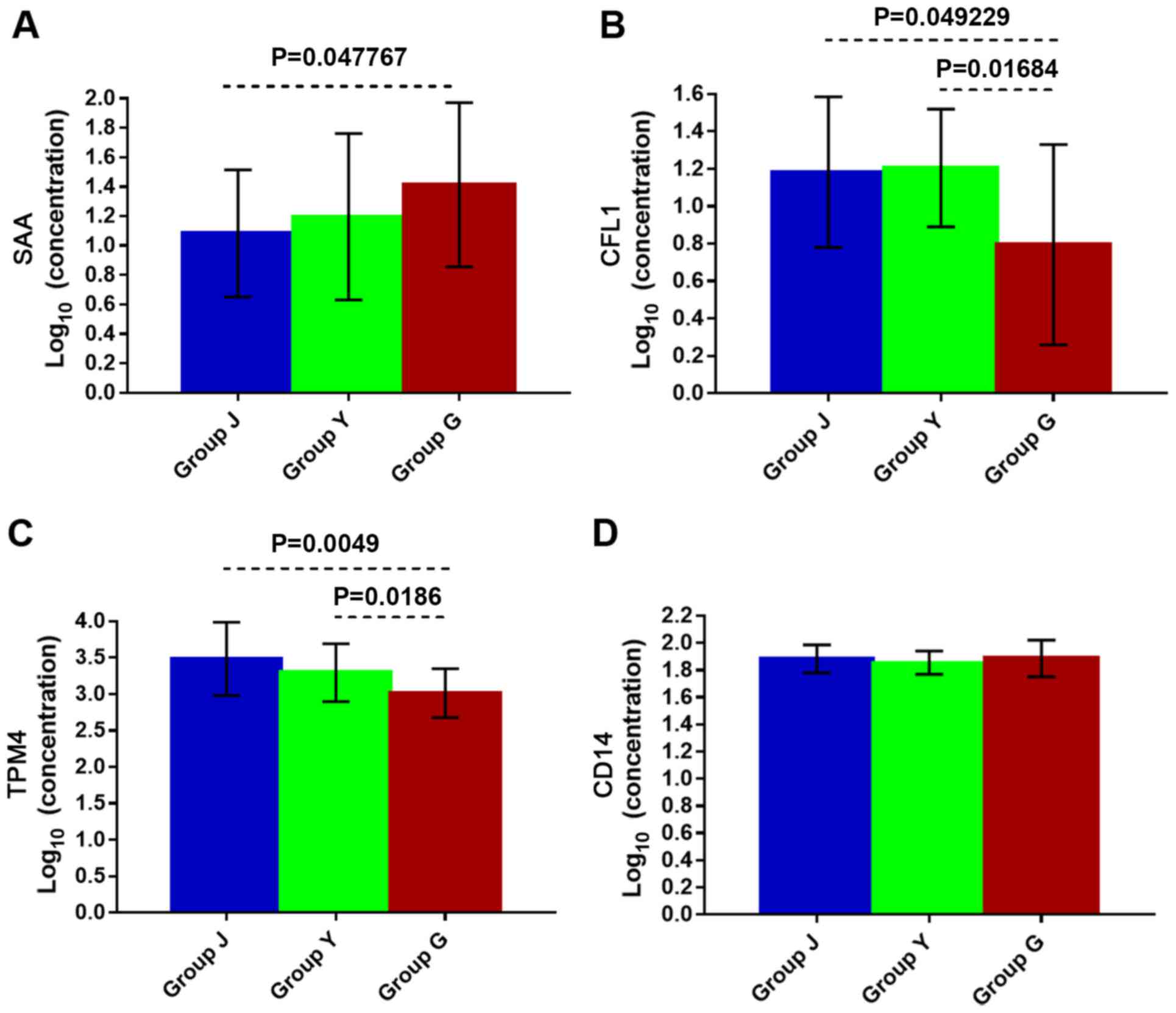 | Figure 4.(A) The SAA, (B) the CFL1, (C) the
TPM4, and (D) the CD14 levels in serum of CSU patients of different
groups by ELISA. Group Y: wheal duration <2 h, 21 cases; group
G: wheal duration 12–24 h, 21 cases; group J: healthy control
group, 21 cases. Statistical significance, P<0.05. SAA, serum
amyloid A; CSU, chronic spontaneous urticaria; ELISA, enzyme-linked
immunosorbent assay. |
The expression levels of CFL1 and TPM4 were
decreased in group G compared to group Y and J (Fig. 4B and C). The expression levels of
CFL1 and TPM4 were significantly decreased in the serum of CSU
patients with longer wheal duration compared to shorter wheal
duration and healthy control as shown by ELISA. The expression
levels of CFL1 and TPM4 had no significant change in the serum of
CSU patients with shorter wheal duration and healthy control.
The results showed that in CSU patients with wheal
duration of 12–24 h the expression level of SAA increased
(P=0.047767), but CFL1 and TPM4 levels decreased (P=0.049229 and
P=0.0049, respectively) in comparison with healthy controls.
Compared with the group of wheal duration of <2 h, the
expression level of SAA in CSU patients with 12–24 h wheal duration
had no significantly difference, while the expression level of CFL1
and TPM4 decreased (P=0.01684 and P=0.0186, respectively). However,
there was no significant difference in sera levels of SAA, CFL1 and
TPM4 between CSU patients with wheal duration of <2 h group and
healthy control. There was no statistically significant change in
the expression level of CD14 of the three groups (Fig. 4D).
Discussion
CSU is an inflammatory disease, characterized by
acute phase response (APR) (23,24). The
impact of APR on the body is a change in plasma protein
concentration accompanied by a series of physiological and
biochemical changes. Among them, the plasma protein concentration
increased by >25%, is known as positive acute phase protein
(APP), such as CRP, SAA and fibrinogen (FIB). CSU might be caused
by an interactive combination of immune, genetic, and environmental
factors, including infections (25–27). In
addition, the altered function of the neuroendocrine-immune system
has been recognized in CSU pathogenesis (28,29).
Relevant research between coagulation and inflammation markers and
the severity of acute exacerbation of the level of indicators and
CU severity is also one of the hot spots in recent years; D-dimer
and CRP levels are closely related to the activation of CU
(11,30,31).
In the present study, the expression level of SAA in
serum of CSU patients with wheal duration of 12–24 h increased
significantly compared with the healthy control group as resulted
by ELISA test. While there was no difference between healthy group
and wheal duration of <2 h group. Serum amyloid A (SAA) is one
of the most dominent positive acute-phase proteins, which increases
sharply in serum due to inflammation, infection, neoplasia and
tissue injury (32–34). The high expression level of SAA in
the serum of CSU patients plays an important role in the long
duration of the wheals. SAA is highly induced during the
inflammatory response and is involved in the systemic regulation of
the innate and adaptive immune responses. SAA possesses a number of
cytokine-like properties reported in recent research. It can also
stimulate the release of mature IL-1β from neutrophils, mast cells,
macrophages and fibroblasts (33,35,36).
IL-1β is a potent pro-inflammatory cytokine involved in many
inflammatory diseases, including autoimmune and allergic diseases
(37). The expression level of SAA
was also significantly increased in allergic rhinitis and asthma
patients (38).
Interestingly, in our experiment the expression
levels of CFL1 and TPM4 were significantly downregulated in sera of
CSU patients with longer duration of wheals compared to healthy and
CSU patients with shorter duration of wheals. There was no
difference between the serum in CSU patients with shorter duration
of wheals and healthy groups. The role of CFL1 and TPM4 in the
disease mechanism of CSU is still unclear. CFL1 is a key member of
the actin-binding protein family that cleaves microfilaments and
binds to actin monomers, to accelerate actin conversion rate in
vivo.
Eosinophils are rich in actin, and the cytoskeleton
is dynamic and very sensitive to changes in the cell's environment.
This desirable property may benefit a motile cell such as the
eosinophil to migrate into an inflammatory site. The dual function
of cofilin, namely depolymerization and severing, serve as a key
moducule controlling the actin dynamics. Thus, cofilin might be a
possible candidate which connects early signal transduction events
with the functional role of the cytoskeleton upon eosinophil
activation (39). The number of
eosinophils is increased in patients with CSU peripheral blood
(40) and skin tissue (39). It has also been reported that the
immunopathology of CSU is based on eosinophils and
basophil-mediated hypersensitivity reactions characterized by
secretion of Th0 cytokines (41).
Therefore, CFL1 is abnormally expressed in CSU patients with longer
periods of wheals and is probably due to eosinophil changes of
function, which needs to be further confirmed.
Tropomyosins are coiled-coil dimmers, one of the
major allergenic proteins of crustacean aquatic animals, that is
located from head-to-tail polymers along actin filaments and
regulate interactions of other proteins, including actin
depolymerizing factor (ADF)/cofilins and myosins, with actin
(42–45).
There are at least 40 tropomyosin subtypes in
eukaryotes, and not all tropomyosins can compete with cofilin for
binding to F-actin. The results of this experiment showed that TPM4
expression was consistent with that of CFL1 in the sera of CSU
patients with longer duration of wheals, which seemed to be not
competitive with F-actin binding. The role of TPM4 and CFL1 in the
pathogenesis of CSU and the relationship between them need also to
be further studied.
The pathogenesis of CSU remains unclear. As one of
the major allergens of crustacean aquatic animals, TPM4 is
abnormally expressed in the sera of CSU patients with longer period
of wheals. The possible explanation is that the crustacean aquatic
animal is one of the main causes in CSU patients with longer
duration of the wheals and this result would be further observed in
combination with clinical cases.
CD14, one of glycosylphosphatidylinositol-anchored
proteins, plays multiple roles in microbial recognition and
signaling. It assists to recognize the ligands of TLR1, 2, 3, 4, 6,
7, and 9, and it contributes in many ways to the trigger of the
signaling pathways activated in response to LPS (46). Although CD14 protein was expressed in
all serum of CSU patients, the expression of CD14 in serum of
patients with wheal duration of <2 h was lower than that in
healthy controls (ratio of A/D was ~0.54), and higher in sera of
patients with long duration of wheals (ratio of B/A was ~1.66).
However, the results of further validation did not confirm
statistically significant differences between groups of patients
with different duration of the wheals and with healthy individuals.
CSU is a chronic inflammatory disease caused by the interaction of
immune, genetic, environmental and infection factors. The results
of ELISA validated that CD14 has no practical significance in the
pathogenesis of CSU.
In conclusion, the results of serum identification
of CSU patients with different durations of wheals using iTRAQ
technique showed that there were different proteins in the serum of
CSU patients with different duration. The level of SAA was
positively associated with the duration of the wheals in sera of
CSU patients, as shown by ELISA. The expression levels of CFL1 and
TPM4 were different in sera of CSU patients with different duration
of wheals; the expression levels were decreased in the serum of CSU
patients with wheal duration of 12–24 h. The results suggest that
there is an association between SAA, CFL1 and TPM4 and the duration
of wheals in CSU patient, and therefore may be considered as new
potential inflammatory biomarkers associated with CSU.
Acknowledgements
We would like to thank Mr. Lei Zhang, who works at
IBS Core Facility Platform in Fudan University (Shanghai, China),
for his assistance with the iTRAQ labeling.
Funding
This study was supported by the research project of
Shanghai Pudong New Area Science and Technology Bureau (no.
PKJ2015-Y28) and the key research program of Shanghai Pudong New
Area Health and Family Planning Commission (no. PWZzk2017-28).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and SX conceived and designed this study. WK and
HC analyzed the data and interpreted the results. YX acquired the
data, performed the ELISA assays and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Pudong New Area People's Hospital (Shanghai, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zuberbier T, Aberer W, Asero R,
Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF,
Giménez-Arnau A, Godse K, et al: European Academy of Allergy and
Clinical Immunology; Global Allergy and Asthma European Network;
European Dermatology Forum; World Allergy Organization: The
EAACI/GA(2) LEN/EDF/WAO Guideline for the definition,
classification, diagnosis, and management of urticaria: The 2013
revision and update. Allergy. 69:868–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernstein JA, Lang DM, Khan DA, Craig T,
Dreyfus D, Hsieh F, Sheikh J, Weldon D, Zuraw B, Bernstein DI, et
al: The diagnosis and management of acute and chronic urticaria:
2014 update. J Allergy Clin Immunol. 133:1270–1277. 2014.
|
|
3
|
Lapi F, Cassano N, Pegoraro V, Cataldo N,
Heiman F, Cricelli I, Levi M, Colombo D, Zagni E, Cricelli C, et
al: Epidemiology of chronic spontaneous urticaria: Results from a
nationwide, population-based study in Italy. Br J Dermatol.
174:996–1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maurer M, Weller K, Bindslev-Jensen C,
Giménez-Arnau A, Bousquet PJ, Bousquet J, Canonica GW, Church MK,
Godse KV, Grattan CE, et al: Unmet clinical needs in chronic
spontaneous urticaria. A GA2LEN task force report.
Allergy. 66:317–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong H, Song Z, Chen W, Li H, He L, Gao
T, Fang H, Guo Z, Xv J, Yu B, et al: Chronic urticaria in Chinese
population: A hospital-based multicenter epidemiological study.
Allergy. 69:359–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabelo-Filardi R, Daltro-Oliveira R and
Campos RA: Parameters associated with chronic spontaneous urticaria
duration and severity: A systematic review. Int Arch Allergy
Immunol. 161:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asero R: D-dimer: A biomarker for
antihistamine-resistant chronic urticaria. J Allergy Clin Immunol.
132:983–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Altrichter S, Boodstein N and Maurer M:
Matrix metalloproteinase-9: A novel biomarker for monitoring
disease activity in patients with chronic urticaria patients?
Allergy. 64:652–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grzanka A, Machura E, Misiolek M, Polaniak
R, Kasperski J and Kasperska-Zajac A: Systemic inflammatory
response and calcification markers in patients with long lasting
moderate-severe chronic spontaneous urticaria. Eur J Dermatol.
25:26–28. 2015.PubMed/NCBI
|
|
10
|
Kolkhir P, André F, Church MK, Maurer M
and Metz M: Potential blood biomarkers in chronic spontaneous
urticaria. Clin Exp Allergy. 47:19–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahagi S, Mihara S, Iwamoto K, Morioke
S, Okabe T, Kameyoshi Y and Hide M: Coagulation/fibrinolysis and
inflammation markers are associated with disease activity in
patients with chronic urticaria. Allergy. 65:649–656. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JH, Lee HY, Ban GY, Shin YS, Park HS
and Ye YM: Serum clusterin as a prognostic marker of chronic
spontaneous urticaria. Medicine (Baltimore). 95:e36882016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujii K, Usuki A, Kan-No Y and Ohgou N:
Elevation of circulating thrombin-antithrombin III complex and
fibrin degradation products in urticaria: A laboratory finding
unrelated to intravascular coagulopathy. J Dermatol. 35:308–310.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasperska-Zajac A, Grzanka A, Machura E,
Misiolek M, Mazur B and Jochem J: Increased serum complement C3 and
C4 concentrations and their relation to severity of chronic
spontaneous urticaria and CRP concentration. J Inflamm (Lond).
10:222013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lundberg KC, Fritz Y, Johnston A, Foster
AM, Baliwag J, Gudjonsson JE, Schlatzer D, Gokulrangan G, McCormick
TS, Chance MR, et al: Proteomics of skin proteins in psoriasis:
From discovery and verification in a mouse model to confirmation in
humans. Mol Cell Proteomics. 14:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao Q, Byrum SD, Moreland LE, Mackintosh
SG, Kannan A, Lin Z, Morgan M, Stack BC Jr, Cornelius LA, Tackett
AJ, et al: A proteomic study of human merkel cell carcinoma. J
Proteomics Bioinform. 6:275–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meral O and Uysal H: Comparative proteomic
analysis of fibrosarcoma and skin fibroblast cell lines. Tumour
Biol. 36:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erler A, Hawranek T, Krückemeier L, Asam
C, Egger M, Ferreira F and Briza P: Proteomic profiling of birch
(Betula verrucosa) pollen extracts from different origins.
Proteomics. 11:1486–1498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Broccardo CJ, Mahaffey S, Schwarz J, Wruck
L, David G, Schlievert PM, Reisdorph NA and Leung DY: Comparative
proteomic profiling of patients with atopic dermatitis based on
history of eczema herpeticum infection and Staphylococcus aureus
colonization. J Allergy Clin Immunol. 127:186–193, e1-193.e11.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holm T, Rutishauser D, Kai-Larsen Y,
Lyutvinskiy Y, Stenius F, Zubarev RA, Agerberth B, Alm J and
Scheynius A: Protein biomarkers in vernix with potential to predict
the development of atopic eczema in early childhood. Allergy.
69:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Neil SE, Sitkauskiene B, Babusyte A,
Krisiukeniene A, Stravinskaite-Bieksiene K, Sakalauskas R, Sihlbom
C, Ekerljung L, Carlsohn E and Lötvall J: Network analysis of
quantitative proteomics on asthmatic bronchi: Effects of inhaled
glucocorticoid treatment. Respir Res. 12:1242011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okamoto T, Miyazaki Y, Shirahama R,
Tamaoka M and Inase N: Proteome analysis of bronchoalveolar lavage
fluid in chronic hypersensitivity pneumonitis. Allergol Int.
61:83–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasperska-Zajac A: Acute-phase response in
chronic urticaria. J Eur Acad Dermatol Venereol. 26:665–672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasperska-Zając A, Grzanka A, Czecior E,
Misiolek M, Rogala B and Machura E: Acute phase inflammatory
markers in patients with non-steroidal anti-inflammatory drugs
(NSAIDs)-induced acute urticaria/angioedema and after aspirin
challenge. J Eur Acad Dermatol Venereol. 27:1048–1052. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wedi B, Raap U, Wieczorek D and Kapp A:
Urticaria and infections. Allergy Asthma Clin Immunol. 5:102009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabroe RA, Grattan CE, Francis DM, Barr
RM, Black Kobza A and Greaves MW: The autologous serum skin test: A
screening test for autoantibodies in chronic idiopathic urticaria.
Br J Dermatol. 140:446–452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dreyfus DH: Autoimmune disease: A role for
new anti-viral therapies? Autoimmun Rev. 11:88–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kasperska-Zajac A, Brzoza Z and Rogala B:
Sex hormones and urticaria. J Dermatol Sci. 52:79–86. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kasperska-Zajac A, Brzoza Z and Rogala B:
Serum concentration of dehydroepiandrosterone sulphate in female
patients with chronic idiopathic urticaria. J Dermatol Sci.
41:80–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeda T, Sakurai Y, Takahagi S, Kato J,
Yoshida K, Yoshioka A, Hide M and Shima M: Increase of coagulation
potential in chronic spontaneous urticaria. Allergy. 66:428–433.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jansen PM, Boermeester MA, Fischer E, de
Jong IW, van der Poll T, Moldawer LL, Hack CE and Lowry SF:
Contribution of interleukin-1 to activation of coagulation and
fibrinolysis, neutrophil degranulation, and the release of
secretory-type phospholipase A2 in sepsis: Studies in nonhuman
primates after interleukin-1 α administration and during lethal
bacteremia. Blood. 86:1027–1034. 1995.PubMed/NCBI
|
|
32
|
Sodin-Šemrl S, Žigon P, Čučnik S, Kveder
T, Blinc A, Tomšič M and Rozman B: Serum amyloid A in autoimmune
thrombosis. Autoimmun Rev. 6:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Migita K, Izumi Y, Jiuchi Y, Kozuru H,
Kawahara C, Izumi M, Sakai T, Nakamura M, Motokawa S, Nakamura T,
et al: Effects of Janus kinase inhibitor tofacitinib on circulating
serum amyloid A and interleukin-6 during treatment for rheumatoid
arthritis. Clin Exp Immunol. 175:208–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dogan S and Atakan N: Is serum amyloid A
protein a better indicator of inflammation in severe psoriasis? Br
J Dermatol. 163:895–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niemi K, Baumann MH, Kovanen PT and Eklund
KK: Serum amyloid A (SAA) activates human mast cells which leads
into degradation of SAA and generation of an amyloidogenic SAA
fragment. Biochim Biophys Acta. 1762:424–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niemi K, Teirilä L, Lappalainen J,
Rajamäki K, Baumann MH, Öörni K, Wolff H, Kovanen PT, Matikainen S
and Eklund KK: Serum amyloid A activates the NLRP3 inflammasome via
P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol.
186:6119–6128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krause K, Metz M, Makris M, Zuberbier T
and Maurer M: The role of interleukin-1 in allergy-related
disorders. Curr Opin Allergy Clin Immunol. 12:477–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Büyüköztürk S, Gelincik AA, Genç S, Koçak
H, Oneriyidogan Y, Erden S, Dal M and Colakoglu B: Acute phase
reactants in allergic airway disease. Tohoku J Exp Med.
204:209–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lorenzo GD, Mansueto P, Melluso M, Candore
G, Cigna D, Pellitteri ME, Salvo AD and Caruso C: Blood eosinophils
and serum eosinophil cationic protein in patients with acute and
chronic urticaria. Mediators Inflamm. 5:113–115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pazdrak K, Young TW, Straub C, Stafford S
and Kurosky A: Priming of eosinophils by GM-CSF is mediated by
protein kinase C betaII-phosphorylated L-plastin. J Immunol.
186:6485–6496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng W, Wang J, Zhu W, Xu C and He S:
Upregulated expression of substance P in basophils of the patients
with chronic spontaneous urticaria: Induction of histamine release
and basophil accumulation by substance P. Cell Biol Toxicol.
32:217–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gunning PW, Hardeman EC, Lappalainen P and
Mulvihill DP: Tropomyosin - master regulator of actin filament
function in the cytoskeleton. J Cell Sci. 128:2965–2974. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
von der Ecken J, Müller M, Lehman W,
Manstein DJ, Penczek PA and Raunser S: Structure of the
F-actin-tropomyosin complex. Nature. 519:114–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Manstein DJ and Mulvihill DP:
Tropomyosin-mediated regulation of cytoplasmic myosins. Traffic.
17:872–877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ono S and Ono K: Tropomyosin inhibits
ADF/cofilin-dependent actin filament dynamics. J Cell Biol.
156:1065–1076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zanoni I and Granucci F: Role of CD14 in
host protection against infections and in metabolism regulation.
Front Cell Infect Microbiol. 3:322013. View Article : Google Scholar : PubMed/NCBI
|