Introduction
Ovarian cancer is the fifth leading cause of
cancer-associated mortality in women (1). In the United States of America, ovarian
cancer is the second most common malignancy of the female genital
tract, with an estimated 22,440 cases diagnosed and 14,080
mortalities in 2017 (1). It was
demonstrated that in 2012 all over Asia, there were 110,526
diagnosed cases of ovarian cancer and China was the country with
the highest number of cases (34,575 patients) (2). Ovarian cancer is classified based on
the tissue of origin, for example epithelial, stromal endocrine or
germ cells. Although certain types of cancer arise from cells that
exist in histologically normal ovaries, the majority of ovarian
cancer cases are derived from cells that typically reside in
extra-ovarian tissue (3), including
endometrioid carcinoma. Epithelial ovarian cancer (EOC) is the most
prevalent type of ovarian cancer, accounting for 90% of all cases
of ovarian cancer (4). The median
age at diagnosis is 63 years. Although a clear etiological factor
responsible for the development of ovarian cancer has not been
identified at present, an increasing amount of evidence has been
obtained on the initiation, progress, and treatment of ovarian
cancer (5,6). Therefore, the development of novel
diagnostic and therapeutic targets is necessary for EOC.
The Hippo pathway represents a novel tumor
suppressor pathway, and dysregulation of Hippo signaling has been
demonstrated to be a key regulator of tumor cell proliferation and
survival (7). In mammalian systems,
the Hippo pathway is composed of four core kinase complexes:
Mammalian Ste2-like kinases 1/2 and large tumor suppressor kinases
1/2 (7,8). Activation of the Hippo tumor suppressor
pathway increases the phosphorylation level of the transcription
co-activator yes-associated protein 1 (YAP)/transcriptional
co-activator with PDZ binding motif (TAZ), which results in the
cytoplasmic retention of YAP/TAZ and protein degradation (9,10). High
nuclear YAP expression was observed in ovarian cancer and
associated with poor prognosis of patients with ovarian cancer
(11,12). YAP expression was demonstrated to
regulate ovarian cancer cell proliferation, chemoresistance,
migration and pluripotency (13–17).
Tumor necrosis factor-α-induced protein 8 (TNFAIP8)
belongs to the TNFAIP8 gene family, which consists of TNFAIP8,
TNFAIP8 like 1, TNFAIP8 like 2 and TNFAIP8 like 3. The TNFAIP8 gene
was subsequently demonstrated to be involved in regulating human
cancer cell proliferation, inflammation, metastasis and
chemoresistance (18–21). TNFAIP8 messenger RNA and protein
expression were upregulated in invasive ductal carcinoma tissues,
and TNFAIP8 overexpression was markedly associated with axillary
lymph node metastasis and a shorter survival time (22). Furthermore, the status of TNFAIP8
expression was an independent prognostic factor for cancer-specific
and disease-free survival of patients with EOC (23). TNFAIP8 overexpression was associated
with platinum resistance in EOCs with optimal cytoreduction, and
was closely associated with residual tumor size (24). These data suggest that TNFAIP8 is an
oncogene in human cancer development. However, the exact biological
role of TNFAIP8 in ovarian cancer remains unclear.
The present study aimed to identify the functional
role of TNFAIP8 in EOC through in vitro and in vivo
experimental models. Additionally, the potential downstream targets
of TNFAIP8 during regulating EOC growth were also investigated. The
results of the current study suggested that TNFAIP8 was an oncogene
in EOC, as supported by the decreased number of proliferative cells
identified in TNFAIP8-knockdown EOC cells. Further, it demonstrated
that the knockdown of TNFAIP8 inhibited EOC growth through
regulation of Hippo signaling in vitro and in vivo.
The results provided evidence to suggest the functional role and
underlying mechanism of TNFAIP8 in regulating EOC.
Materials and methods
Cell culture and transfection
The ovarian cancer SKOV3, A2780s, A2780cp, PA-1 and
CAOV3 cell lines were obtained from American Type Culture
Collection (Manassas, VA, USA) and normal human ovarian epithelial
cells (HOEC) were obtained from The Cell Bank of Type Culture
Collection of Shanghai Chinese Academy of Sciences (Shanghai,
China). All cells were cultured in Dulbecco's modified Eagle's
medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (EMD Millipore, Billerica,
MA, USA) at 37°C with 5% CO2.
Small interfering RNA (siRNA) targeting TNFAIP8
(siTNFAIP8, CTGCGTGCGTTTCAGTGTTTAA) and negative control siRNA
(siNC, CGAGGGCGACTTAACCTTAGG) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). A total of 5 nM siTNFAIP8 and siNC
were transfected into A2780s and A2780cp cells using the
transfection agent riboFECT CP (cat. no. C10511; Guangzhou RiboBio
Co., Ltd) following the manufacturer's protocol. A pVax-based YAP
overexpression system and blank vector pVax vectors were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China) and 2 µg/well
were used to transfect the A2780s and A2780cp cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. At 48 h post transfection, transfected
A2780s and A2790cp were collected for further experiments.
Cell viability assay
siRNA-transfected A2780s and A2780cp cells were
seeded in a 96-well plate (1,500 cells/well), and the cell
viability was measured daily using a Cell Counting kit-8 assay
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) for 4 days,
according to the manufacturer's protocol.
Colony formation assay
siRNA-transfected A2780s and A2780cp cells were
seeded in a 6-well plate (1,000 cells/well), and the cells were
cultured for 14 days. The cells fixed with 4% paraformaldehyde for
15 min at room temperature, followed by staining with crystal
violet (cat. no. C0121; Beyotime Institute of Biotechnology,
Haimen, China) at room temperature for 15 min. The number of
colonies (>50 cells) in each well was counted and analyzed. A
total of three independent experiments were performed.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. A total of 1 µg RNA
samples were subjected to RT-PCR (Takara Bio, Inc., Otsu, Japan),
and resulting cDNA was analyzed in triplicate using SYBR-Green
(Takara Bio, Inc.). qPCR conditions were as follows: Denaturation
at 94°C for 2 min, amplification for 35 cycles at 94°C for 0.5 min,
annealing at 60°C for 0.5 min and extension at 72°C for 1 min,
followed by a terminal elongation step at 72°C for 10 min. The qPCR
was performed with the CX-96 system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Relative expression of TNFAIP8 were determined
by 2−ΔΔCq, where Cq is the mean threshold cycle
difference calculated following normalization to U6 values
(25). The primer sequences were as
follows: TINFAIP8 forward, 5′-GCCGTTCAGGCACAAAAGA-3′ and reverse,
5′-GCACCTCACTACTTGTGTCGTCTATT-3′; and U6 forward,
5′-TGCGTGACATTAAGGAGAA-3′ and reverse, 5′AAGGAAGGCTGGAAGAGT-3′.
Apoptosis detection
Flow cytometric analysis of apoptosis was performed
using the Fluorescein isothiocyanate-Annexin V Apoptosis Detection
kit I (BD Biosciences, San Jose, CA, USA), according to the
manufacturer's protocol. The flow cytometry was performed on a
Cytomics FC500 flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA). Flow cytometry data were analyzed using FlowJo 7.6 software
(Tree Star Inc., Ashland, OR, USA).
Cell cycle analysis
For cell cycle analysis, cells were collected at the
logarithmic stage of growth, centrifuged (4°C; 3 min; 1,200 × g)
and then resuspended at 2×106 cells/ml. Cells were fixed in 70%
ethanol at 4°C for 15 min, washed in PBS, incubated in 50 µg/ml
propidium iodide (PI; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) containing 0.1 mg/ml RNase A (Sigma-Aldrich; Merck KGaA)
and 0.1% Triton X (Sigma-Aldrich; Merck KGaA) for 30 min and were
analyzed on a Cytomics FC500 flow cytometer (Beckman Coulter,
Inc.). Flow cytometry data were analyzed using FlowJo 7.6
software.
Western blot analysis
Cells were harvested in cell lysis buffer (Beyotime
Institute of Biotechnology) for total protein extraction as
described previously (26). The
nuclear and cytoplasmic proteins were extracted with a subcellular
protein extraction kit (EMD Millipore), according to the
manufacturer's protocol. The concentration was determined using a
BCA kit (Beyotime Institute of Biotechnology). Equal quantities (10
µg) of denatured protein samples were resolved by 10% SDS-PAGE, and
then transferred onto polyvinylidene fluoride membranes (EMD
Millipore). Following blocking with 5% non-fat dry milk in
TBS/0.05% Tween-20 at room temperature for 2 h, membranes were
incubated with specific primary antibodies against TNFAIP8 (1:800;
cat. no. 119-17060; RayBiotech Life, Norcross, GA, USA), cellular
tumor protein p53 (p53; 1:1,000; cat. no. 2542; Cell Signaling
Technology, Inc., Danvers, MA, USA), caspase-3 (1:600; cat. no.
9662; Cell Signaling Technology, Inc.), cyclin B1 (1:1,000; cat.
no. 4138; Cell Signaling Technology, Inc.), cyclin D1 (1:1,000;
cat. no. 2922; Cell Signaling Technology, Inc.), YAP (1:800; cat.
no. 4932; Cell Signaling Technology, Inc.) and phosphorylated
(p)-YAP (1:600; cat. no. 13008; Cell Signaling Technology, Inc.),
followed by a horseradish peroxidase-conjugated secondary antibody
(ZSGB-BIO; OriGene Technologies, Inc., Beijing, China). Proteins
were visualized using enhanced chemiluminescent reagents (Pierce;
Thermo Fisher Scientific, Inc.). GAPDH (1:5,000; cat. no. ABS16;
EMD Millipore) was used as a loading control. Image-Pro Plus
(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used
to perform densitometry.
Animal model
The present study was approved by the Ethics
Committee of Sichuan University (Chengdu, China) and complied with
the animal guidelines of Sichuan University. A total of 10
6-week-old female BALB/c nude mice (weight, ~20 g) were purchased
from Beijing HFK Bioscience Co. Ltd. (Beijing, China) and housed at
specific-pathogen-free room (temperature, 22–25°C) with a 12 h
dark/light cycle and ad libitum access to food and water. Short
hairpin (sh)RNA targeting TNFAIP8 (CTGCGTGCGTTTCAGTGTTTAA) and
negative control (shNC, CGAGGGCGACTTAACCTTAGG) were purchased from
Shanghai GenePharma Co., Ltd. and used to transfect the A2780s and
A2780cp cells (2 µg/well) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. At 48 h post transfection, puromycin (2
µg/ml) was added into the medium to select the transfected cells.
Stably transfected cells were collected for TNFAIP8 detection by
western blotting and further in vivo study. To establish the tumor
model, 5×106 A2780s cells that exhibited stable knockdown of
TNFAIP8 (A2780s-shTNFAIP8) and negative control cells (A2780s-shNC)
were subcutaneously injected into the right flank of (n=5 per
experimental group). Tumor length and width were measured every
five days from the tenth day post-cell injection. Tumor volumes
were calculated as ellipsoids (length × width2/2). At 25 days
post-cell injection, the mice were sacrificed, and the tumors were
removed and measured.
Statistical analysis
All values are presented as the means ± standard
deviation, representative of 3–5 repeats. Significant differences
were determined using GraphPad 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). The two-tailed Student's t-test was used
to evaluate the significance of differences between two groups of
data, and one-way analysis of variance with Tukey's post-hoc test
was used to determine statistical differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockdown of TNFAIP8 inhibits EOC cell
growth
To investigate the biological function of TNFAIP8 in
EOC, EOC cell lines (SKOV3, A2780s, A2780cp, PA-1 and CAOV3) and
normal HOEC were cultured and collected for qPCR and western blot
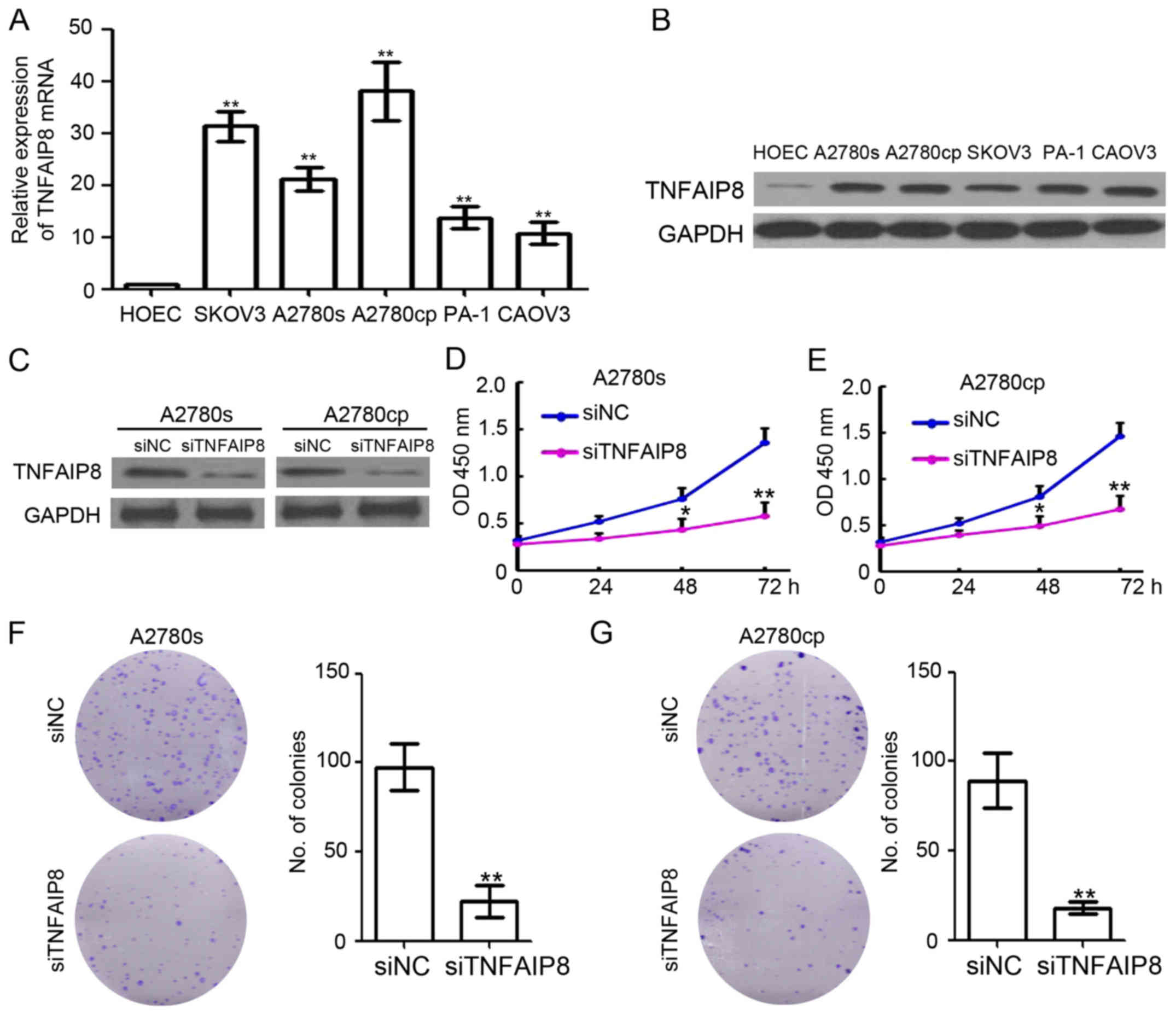
analysis. As demonstrated in Fig.
1A, TNFAIP8 mRNA was significantly upregulated in all EOC cell
lines. The western blot analysis results confirmed the upregulation
of TNFAIP8 in EOC cells (Fig. 1B).
siRNA specifically targeting TNFAIP8 (siTNFAIP8) were used to
transfect A2780s and A2780cp cells and were demonstrated to
efficiently inhibit TNFAIP8 expression (Fig. 1C). Notably, knockdown of TNFAIP8
markedly inhibited the proliferation of A2780s and A2780cp cells by
58.4 and 61.3%, respectively (Fig. 1D
and E). Furthermore, the colony formation ability of A2780s and
A2780cp cells were also significantly downregulated in
TNFAIP8-silenced cells (Fig. 1F and
G). Collectively, TNFAIP8 was demonstrated to be an oncogene in
EOC.
Knockdown of TNFAIP8 promotes EOC
apoptosis
To additionally investigate the potential mechanism
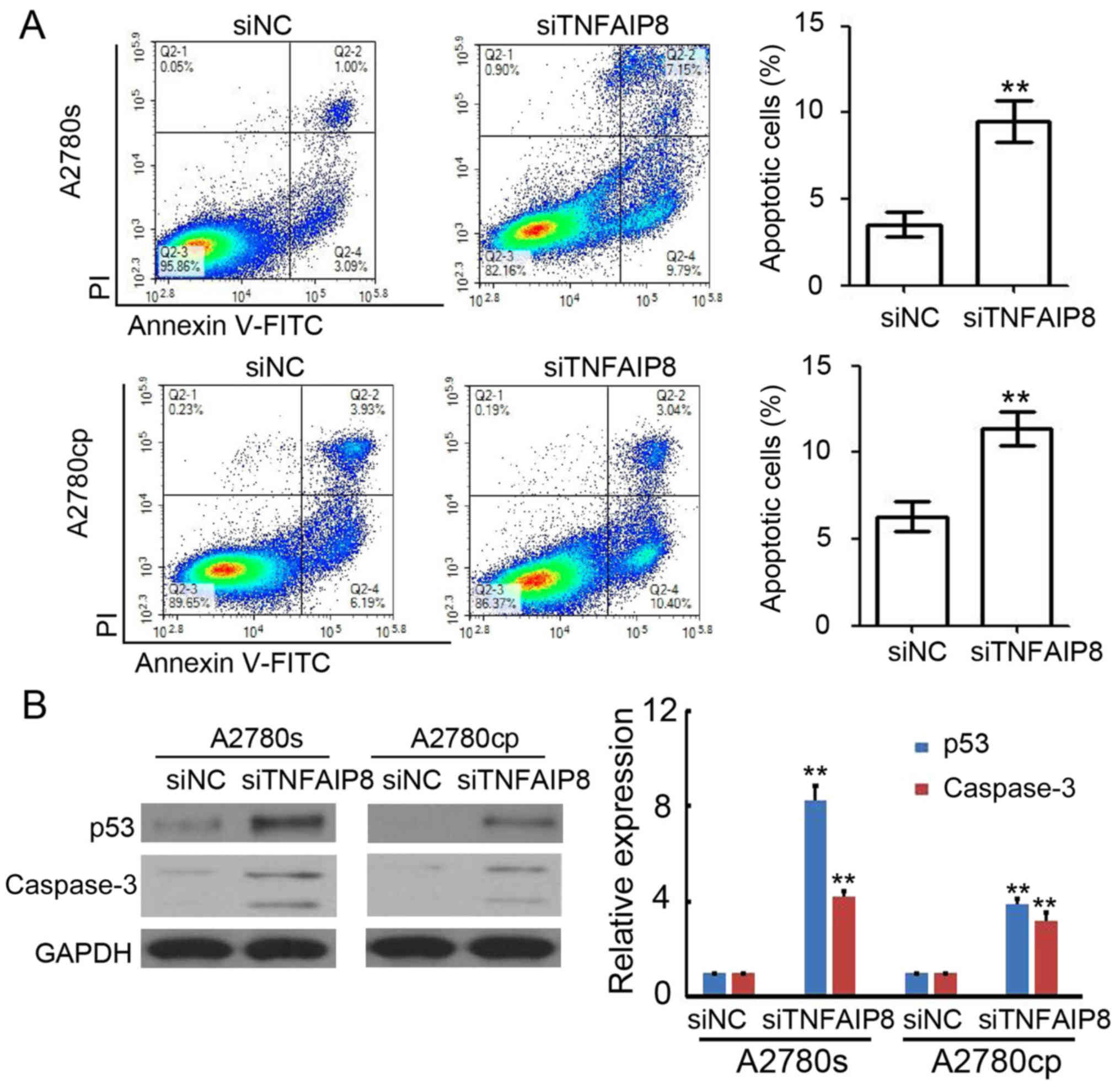
of TNFAIP8-based regulation of EOC growth, the levels of apoptosis
were analyzed by flow cytometry. The results suggested that the
knockdown of TNFAIP8 efficiently promoted apoptosis of A2780s
[(siNC) 4.2±0.6% vs. (siTNFAIP8) 9.8±1.6%] and A2780cp cells
[(siNC) 5.9±1.2% vs. (siTNFAIP8) 11.7±1.3%)] (Fig. 2A). Furthermore, the transfected cells
were collected for western blot analysis and a significant
upregulation of p53 and caspase-3 expression levels was observed in
TNFAIP8-silenced A2780s and A2780cp cells (Fig. 2B). These results demonstrated that
siTNFAIP8 promoted apoptosis in EOC cells.
Knockdown of TNFAIP8 induces EOC cell
cycle arrest
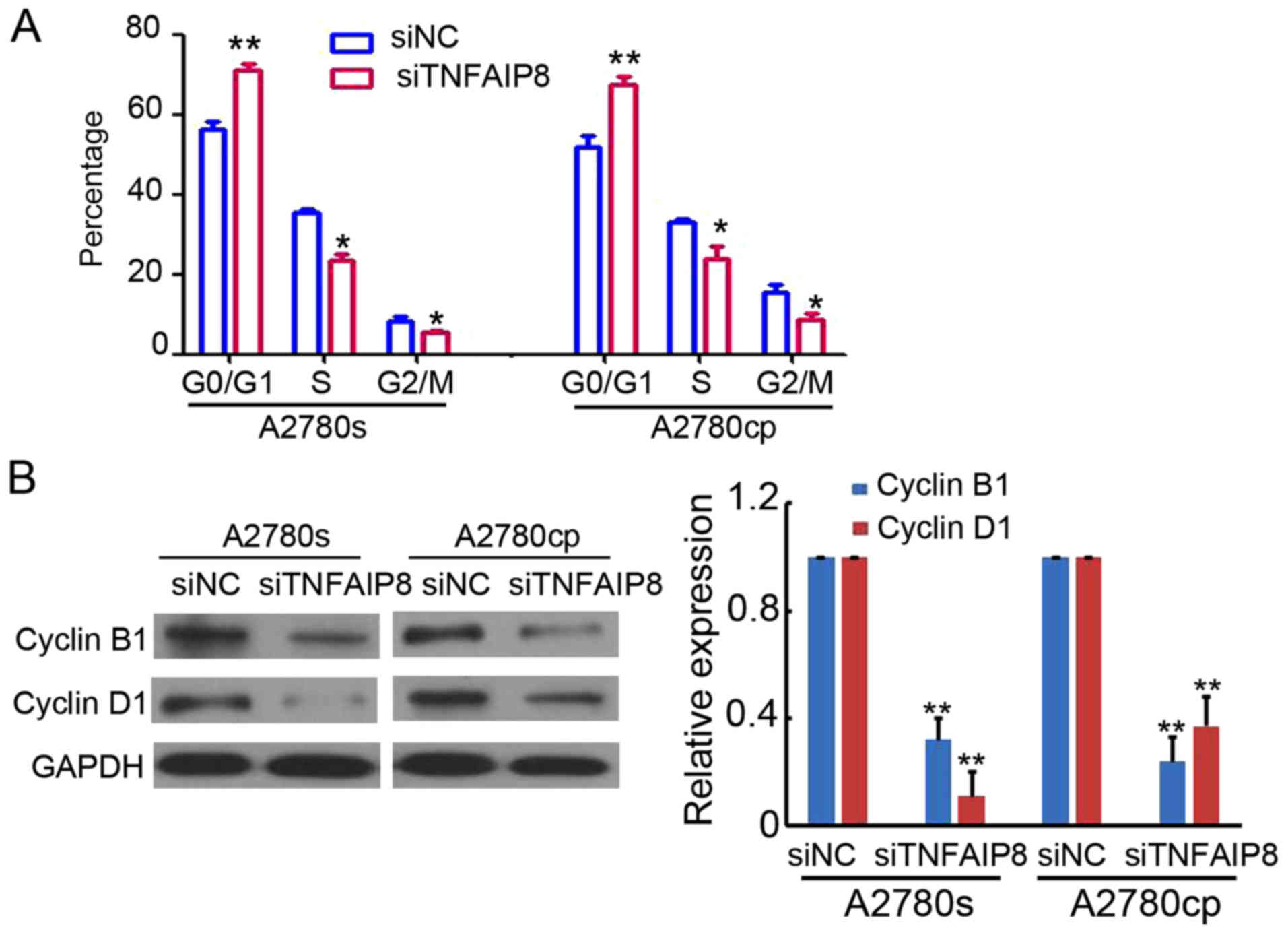
Next, the cell cycle in A2780s and A2780cp cells
transfected with siNC or siTNFAIP8 were analyzed by PI staining and
flow cytometry. As demonstrated in Fig.
3A, TNFAIP8 knockdown in A2780s and A2780cp cells increased the
proportion of cells in the G0/G1 phase and decreased the proportion
of cells in the S phase (Fig. 3A).
In addition, the cell cycle checkpoint proteins cyclin B1 and
cyclin D1 were markedly downregulated in TNFAIP8-knockdown A2780s
and A2780cp cells (Fig. 3B). These
results demonstrated the regulatory role of TNFAIP8 in the cell
cycle.
TNFAIP8 promotes EOC growth through
regulating Hippo signaling
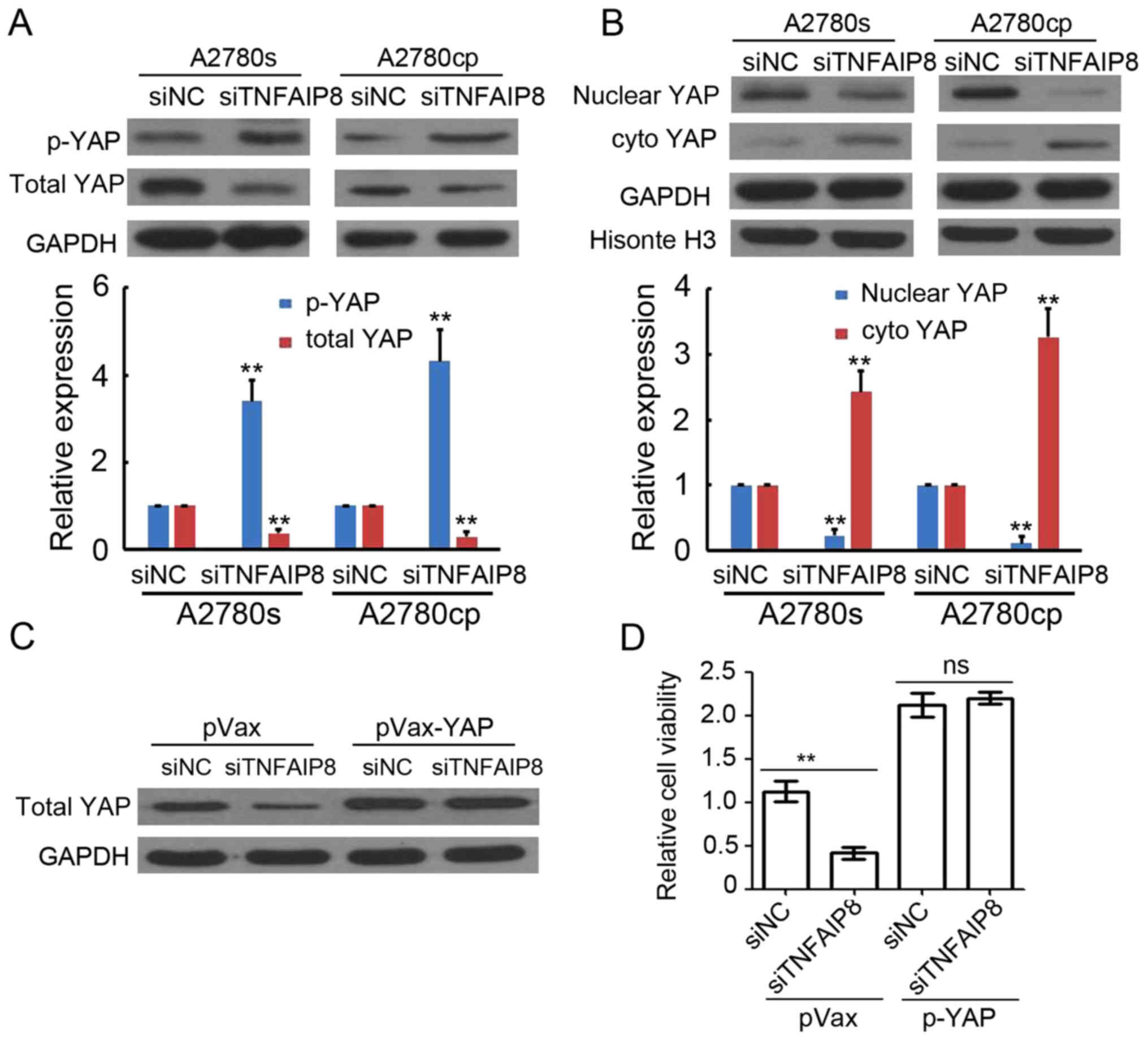
To investigate the underlying mechanism of
TNFAIP8-based regulation of EOC growth, the YAP expression was
determined by western blot analysis. The whole proteins of A2780s
and A2780cp cells transfected with siNC or siTNFAIP8 were
collected. The results suggested that TNFAIP8 knockdown promoted
p-YAP expression, but inhibited total YAP expression (Fig. 4A). Furthermore, TNFAIP8-knockdown
decreased nuclear YAP expression levels, but increased cytoplasmic
YAP expression levels (Fig. 4B). To
additionally determine whether Hippo signaling served a crucial
role in the TNFAIP8-based regulation of EOC growth, a plasmid-based
YAP expression system was used to transfect A2780s cells. As
indicated in Fig. 4C,
siTNFAIP8-mediated total YAP expression downregulation was
attenuated by pVax-YAP transfection. Notably, the cell growth
inhibition in TNFAIP8-knockdown EOC cells was also abrogated by
pVax-YAP transfection (Fig. 4D).
Collectively, these results suggest that TNFAIP8 promoted EOC
growth through regulating Hippo signaling.
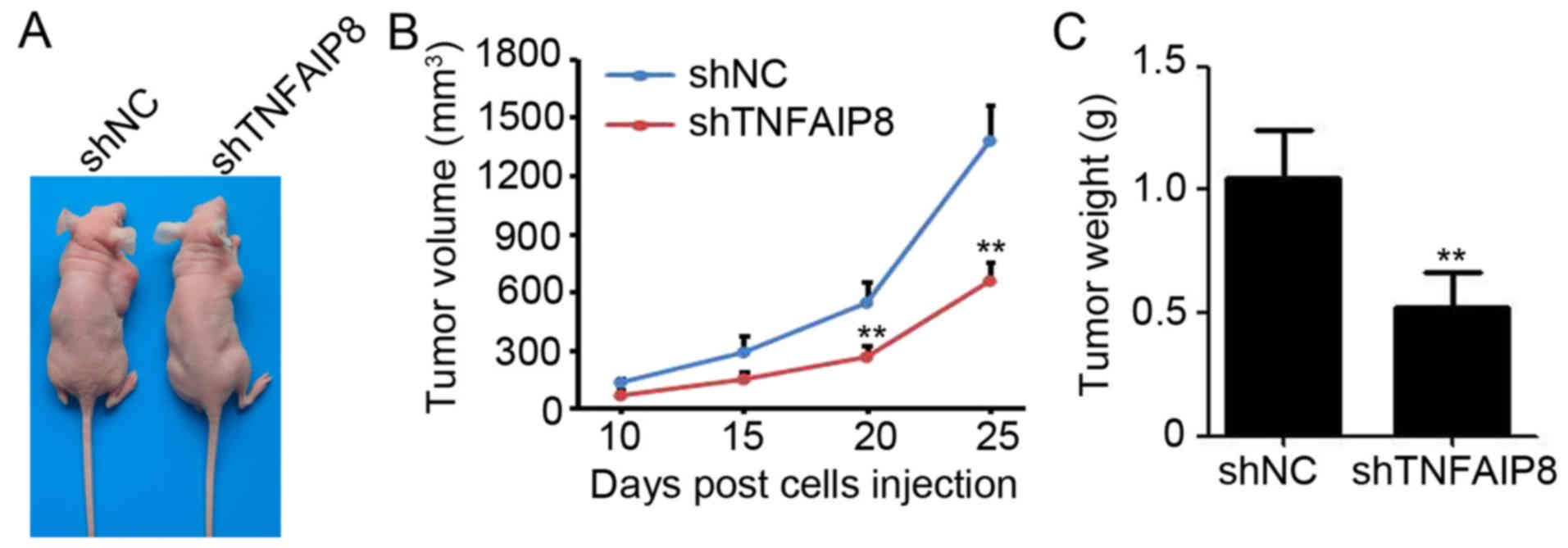
Knockdown of TNFAIP8 impairs EOC tumor
growth in vivo
To additionally investigate the functional role of
TNFAIP8 on EOC growth in vivo, A2780s-shTNFAIP8 and A2780s-shNC
cells were subcutaneously injected into the right flank of
6-week-old female BALB/c nude mice. At 30 days post-cell injection,
the mice were sacrificed and the tumors were measured (Fig. 5A). The results revealed that the
knockdown of TNFAIP8 significantly inhibited the tumor volume by
52.5% (final tumor volume of shNC vs. shTNFAIP8: 1376.2±187.2 mm3
vs. 654.3±97.2 mm3) (Fig. 5B) and
tumor weight by 50.5% (tumor weight of shNC vs. shTNFAIP8:
1.05±0.09 g vs. 0.52±0.07 g) (Fig.
5C). These results suggested that the knockdown of TNFAIP8
inhibited the EOC tumor growth generated from A2780s cells.
Discussion
In the present study, TNFAIP8 was identified as an
oncogene in EOC, as evidenced by the decreased number of
proliferative cells identified in TNFAIP8-knockdown EOC cells. It
was also demonstrated that the knockdown of TNFAIP8 inhibited EOC
growth through Hippo signaling regulation in vitro and in vivo. A
clearer understanding of the role and mechanisms of TNFAIP8 in EOC
may provide novel therapeutic targets.
TNFAIP8 was demonstrated to be a prognostic factor
for cancer-specific and disease-free survival of patients with EOC
(23). In the present study, it was
demonstrated that the knockdown of TNFAIP8 inhibited EOC cell
growth and colony formation, accompanied by increased levels of
apoptosis and cell cycle arrest. Mechanistic studies indicated that
the downregulation of TNFAIP8 promoted p-YAP expression while
inhibiting nuclear and total YAP expression. To the best of our
knowledge, the present study provided, for the first time,
biological evidence for the oncogenic role of TNFAIP8 in EOC,
suggesting that TNFAIP8 may serve as a potential therapeutic target
for EOC.
Various studies have demonstrated the upregulation
of TNFAIP8 in a diverse range of cancer types: In esophageal
squamous cell carcinoma, TNFAIP8 overexpression was identified in
73 (59.8%) tumor specimens and was significantly increased in
patients with lymphatic recurrence (27). TNFAIP8 was also overexpressed in
primary hepatocellular carcinoma (HCC) samples and was associated
with Tumor Node Metastasis staging, recurrence and poor prognosis,
whilst also serving as an independent favorable prognostic factor
(28). In accordance with the
upregulation of TNFAIP8 in EOC tissues from patients (23), the mRNA and protein expression levels
of TNFAIP8 were upregulated in all EOC cell lines assessed in the
present study. This result indicated that TNFAIP8 may function as
an oncogene in EOC development.
TNFAIP8 was demonstrated to regulate the progression
of various types of cancer. Dai et al (26) demonstrated that TNFAIP8 upregulated
cell proliferation, migration, invasion, and xenograft tumor growth
in HCC cells. TNFAIP8 also promoted cell proliferation and invasion
in lung cancer (29). Concurrently,
downregulated expression of TNFAIP8 via the overexpression of
microRNA (miR)-9 markedly inhibited gastric cancer cell
proliferation in vitro and tumor growth in vivo (20). miR-99a may induce osteosarcoma cell
cycle progression and cell apoptosis by directly targeting TNFAIP8
(30). The results of the present
study suggested that TNFAIP8 promoted cell growth and colony
formation by inhibiting apoptosis and cell cycle arrest in EOC
cells. Although additional in vivo studies are required, these data
provide evidence to elucidate the functional role of TNFAIP8 in EOC
development.
The Hippo pathway effector YAP increased cell
proliferation, resistance to cisplatin-induced apoptosis, cell
migration and anchorage-independent growth, and was associated with
poor survival in ovarian cancer (31). Xia et al (14) demonstrated that the YAP/TEA domain
transcription factors co-activator promoted ovarian
cancer-initiated cell pluripotency and chemoresistance. YAP
phosphorylation is regulated by its interactions with other
proteins including serine/threonine-protein kinase LATS1,
serine/threonine protein kinase 4/3 and angiomotin (32). Activation of the Hippo tumor
suppressor pathway increases the phosphorylation level of the
transcription co-activator YAP/TAZ, which results in the
cytoplasmic retention of YAP/TAZ and protein degradation (9,10). The
present study indicated that TNFAIP8 inhibited the expression of
p-YAP while promoting total and nuclear YAP expression.
Overexpression of YAP in EOC cells efficiently attenuated cell
growth inhibition in TNFAIP8-deficient EOC cells. These data are
consistent with those of previous studies that demonstrated the
regulatory role of TNFAIP8 in Hippo signaling (28,29).
Additional experimental studies also suggest the involvement of
TNFAIP8 in apoptosis and cell cycle checkpoint protein expression
in EOC cells, which were identified as downstream targets of YAP
(7,8).
Collectively, the data from the present study
provide experimental evidence that TNFAIP8 functions as an oncogene
in EOC development and may be used as a therapeutic target for EOC.
In future, additional studies are required to determine the direct
targets of TNFAIP8 during the regulation of EOC growth.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included within this published article.
Authors' contributions
YX and FZ were involved in acquisition of the data.
YX was involved in analysis and interpretation of the data. XZ was
involved in developing the study concept and design.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sichuan University and complied with the animal
guidelines of Sichuan University.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Razi S, Ghoncheh M, Mohammadian-Hafshejani
A, Aziznejhad H, Mohammadian M and Salehiniya H: The incidence and
mortality of ovarian cancer and their relationship with the Human
Development Index in Asia. Ecancermedicalscience. 10:6282016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karnezis AN, Cho KR, Gilks CB, Pearce CL
and Huntsman DG: The disparate origins of ovarian cancers:
Pathogenesis and prevention strategies. Nat Rev Cancer. 17:65–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voutsadakis IA: On adjuvant hormone
therapy in epithelial ovarian cancer. J Clin Oncol. 34:2070–2071.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung C and Lee R: An update on current
and emerging therapies for epithelial ovarian cancer: Focus on poly
(adenosine diphosphate-ribose) polymerase inhibition and
antiangiogenesis. J Oncol Pharm Pract. 23:454–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rojas V, Hirshfield KM, Ganesan S and
Rodriguez-Rodriguez L: Molecular characterization of epithelial
ovarian cancer: Implications for diagnosis and treatment. Int J Mol
Sci. 17(pii): E21132016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wierzbicki PM and Rybarczyk A: The Hippo
pathway in colorectal cancer. Folia Histochem Cytobiol. 53:105–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Y, Yang Y, Wang F, Wei Q and Qin H:
Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int
J Cancer. 137:2275–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M
and Fan HY: YAP promotes ovarian cancer cell tumorigenesis and is
indicative of a poor prognosis for ovarian cancer patients. PloS
One. 9:e917702014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho SY, Kim K, Park MS, Jang MY, Choi YH,
Han S, Shin HM, Chung C, Han HY, Yang JB, et al: Expression of
yes-associated protein 1 and its clinical significance in ovarian
serous cystadenocarcinoma. Oncol Rep. 37:2620–2632. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong GO, Shin SH, Seo EJ, Kwon YW, Heo
SC, Kim KH, Yoon MS, Suh DS and Kim JH: TAZ mediates
lysophosphatidic acid-induced migration and proliferation of
epithelial ovarian cancer cells. Cell Physiol Biochem. 32:253–263.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia Y, Zhang YL, Yu C, Chang T and Fan HY:
YAP/TEAD co-activator regulated pluripotency and chemoresistance in
ovarian cancer initiated cells. PloS One. 9:e1095752014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu M, Xiao J, Chen M, Yuan L, Li J, Shen H
and Yao S: miR1495p promotes chemotherapeutic resistance in ovarian
cancer via the inactivation of the Hippo signaling pathway. Int J
Oncol. 52:815–827. 2018.PubMed/NCBI
|
|
16
|
Yagi H, Asanoma K, Ohgami T, Ichinoe A,
Sonoda K and Kato K: GEP oncogene promotes cell proliferation
through YAP activation in ovarian cancer. Oncogene. 35:4471–4480.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB, Bowtell DD and Harvey KF: The Hippo pathway
transcriptional co-activator, YAP, is an ovarian cancer oncogene.
Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun H, Lou Y, Porturas T, Morrissey S, Luo
G, Qi J, Ruan Q, Shi S and Chen YH: Exacerbated experimental
colitis in TNFAIP8-deficient mice. J Immunol. 194:5736–5742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao HY, Huo FC, Wang HY and Pei DS:
MicroRNA-9 inhibits the gastric cancer cell proliferation by
targeting TNFAIP8. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
21
|
Lowe JM, Nguyen TA, Grimm SA, Gabor KA,
Peddada SD, Li L, Anderson CW, Resnick MA, Menendez D and Fessler
MB: The novel p53 target TNFAIP8 variant 2 is increased in cancer
and offsets p53-dependent tumor suppression. Cell Death Differ.
24:181–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao M, Xu Q, Lou C, Qin Y, Ning X, Liu T,
Zhao X, Jia S and Huang Y: Overexpression of TNFAIP8 is associated
with tumor aggressiveness and poor prognosis in patients with
invasive ductal breast carcinoma. Hum Pathol. 62:40–49. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu T, Gao H, Chen X, Lou G, Gu L, Yang M,
Xia B and Yin H: TNFAIP8 as a predictor of metastasis and a novel
prognostic biomarker in patients with epithelial ovarian cancer. Br
J Cancer. 109:1685–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Xia B, Lu Y, Xu Y and Lou G:
TNFAIP8 overexpression is associated with platinum resistance in
epithelial ovarian cancers with optimal cytoreduction. Hum Pathol.
45:1251–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Z, Liu X, Song JH, Cheng Y, Liu Y, Jia
Y, Meltzer SJ and Wang Z: TNFAIP8 overexpression: a potential
predictor of lymphatic metastatic recurrence in pN0 esophageal
squamous cell carcinoma after Ivor Lewis esophagectomy. Tumour
Biol. 37:10923–10934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong Q, Fu L, Zhao Y, Xie C, Li Q and Wang
E: TNFAIP8 interacts with LATS1 and promotes aggressiveness through
regulation of Hippo pathway in hepatocellular carcinoma.
Oncotarget. 28:15689–15703. 2017.
|
|
29
|
Han Y, Tang Z, Zhao Y Li Q and Wang E:
TNFAIP8 regulates Hippo pathway through interacting with LATS1 to
promote cell proliferation and invasion in lung cancer. Mol
Carcinog. 57:159–166. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing B and Ren C: Tumor-suppressive
miR-99a inhibits cell proliferation via targeting of TNFAIP8 in
osteosarcoma cells. Am J Transl Res. 8:1082–1090. 2016.PubMed/NCBI
|
|
31
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim M and Jho EH: Cross-talk between
Wnt/β-catenin and Hippo signaling pathways: A brief review. BMB
reports. 47:540–545. 2014. View Article : Google Scholar : PubMed/NCBI
|