Introduction
Acute lymphoblastic leukemia (ALL) affects both
children and adults, with peak prevalence between the ages of 2–5
years and again after the age of 50 years (1). Overall age-adjusted incidence is 1.7
per 100,000 persons; roughly 60% of cases are diagnosed in patients
younger than 20 years (2). ALL
development includes genetic instability such as translocation and
fusion genes. The relevance of the prognosis and cytogenetic
abnormalities between adult ALL and pediatric ALL has been studied
for decades (3–5). Retrospective studies (6,7) by
diverse departments and various institutions worldwide have
revealed that 20% of adult patients with ALL have the BCR-ABL
fusion protein, while only ~5% of pediatric patients with ALL
harbor the Philadelphia (Ph) chromosome at present. Due to the high
frequency of unfavorable cytogenetic features such as
t(9;22)(q34;q11) and t(1;19)(q23;p13), the outcome of treatment in
ALL worsens with age (8).
During the last decade, the advances in our
understanding of the clinical, immunobiological and genetic
characteristics of ALL have led to improved risk stratification and
to risk-adapted treatment strategies. In addition to conventional
chemotherapy, stem cell (or bone marrow) transplant and chimeric
antigen receptor T-cell therapy are used for certain unusual
subtypes of ALL or refractory B-cell ALL. Monoclonal antibodies,
new immunotherapy treatments and other targeted approaches are
promising novel therapeutic approaches to ALL. With the current
therapies, the vast majority of pediatric patients with ALL are now
long-term survivors. In China, the 5-year and 10-year overall
survival rates are 80.0±1.2 and 76.3±1.6%, respectively (9). In developed countries, the 5-year
overall survival rate for ALL has increased to 90% in the last 30
years (10). Unfortunately, the same
positive results have not been obtained for adult patients with ALL
(11). In adult ALL, the cure rate
is estimated to be 20–40% (12).
Adults present with higher risk features at diagnosis, which
predispose them to chemotherapy resistance and disease relapse
after an initial achievement of complete remission (CR). The
incorporation of targeted agents into adult ALL therapy has
improved survival in several subsets (13,14). In
the present study, the cryptic chromosomal alterations and genomic
changes in a cohort of 75 adult ALL samples and 207 pediatric ALL
samples were investigated. The cytogenetic abnormalities and
prognosis of the two groups were analyzed using fluorescence in
situ hybridization (FISH) in order to determine why survival is
poorer in adult ALL than in pediatric ALL. The results indicate
that adult ALL has a poorer prognosis compared with pediatric ALL
based on Ph+ status and presence of trisomy 4 or 10. Ph+ is an
independent prognosis factor of ALL.
Patients and methods
Patient characteristics
All ALL cases enrolled in the present study were
diagnosed and treated at the Department of Hematology, Xiangya
Hospital (Changsha, China) from January 2008-December 2012.
Consecutive patients were enrolled, giving a total of 207 pediatric
patients and 75 adult patients. Written informed consent was
obtained from all patients. In order to maintain consistent
treatment, patients who received HSCT or had never received
chemotherapy were excluded from the study. All patients underwent
blood routine examination, bone marrow (BM) aspiration, Wright's
and peroxidase (POX) staining, cerebrospinal fluid,
immunophenotyping and FISH examination. In addition, the
pretreatment workup included a complete medical history, physical
examination, chest X-ray, B-ultrasound scan of the abdomen and neck
and routine laboratory analysis. Table
I presents the characteristics of the whole cohort. The Xiangya
Hospital Ethics Committee approved the present study (approval no.
201212478).
 | Table I.Characteristics of adult and
pediatric patients with ALL. |
Table I.
Characteristics of adult and
pediatric patients with ALL.
| Characteristic | Adult ALL (n=75)
(%) | Pediatric ALL
(n=207) (%) | P-value |
|---|
| Sex |
|
| 0.865 |
|
Male | 39 (52.0) | 110 (53.1) |
|
|
Female | 36 (48.0) | 97 (46.9) |
|
| WBC
×109/l |
|
| 0.462 |
|
<5 | 20 (26.7) | 52 (25.1) |
|
|
5–50 | 30 (40.0) | 99 (47.8) |
|
|
>50 | 25 (33.3) | 56 (27.1) |
|
| Median
(range) | 16.9
(1.2–462.0) | 11.9
(1.5–910.0) |
|
| Hemoglobin level,
g/l |
|
| 0.061 |
|
≥80 | 40 (53.3) | 60 (29.0) |
|
|
<80 | 35 (46.7) | 147 (71.0) |
|
| LDH |
|
| 0.551 |
|
High | 49 (65.3) | 143 (69.1) |
|
|
Normal/low | 26 (34.7) | 64 (30.9) |
|
| CNS
involvement |
|
| 0.187 |
|
Yes | 11 (14.7) | 19 (9.2) |
|
| No | 64 (85.3) | 188 (90.8) |
|
| Minimal residual
disease |
|
| 0.009 |
|
Yes | 16 (25.3) | 21 (10.1) |
|
| No | 56 (74.7) | 186 (89.9) |
|
Immunophenotyping and FISH
Immunophenotype analysis was performed on BM
specimens of 269 of 282 cases using a BD FACSCanto II flow
cytometry instrument (BD Biosciences, Franklin Lakes, NJ, USA). The
other 13 patients had no available immunophenotype analysis and
were excluded from this assay. BM samples were stored at 4°C prior
to the assay and all samples were assessed within 24 h of
collection. All antibody reagents were purchased from BD
Biosciences. The ALL marker panel used was as follows: (CD)
45-PerCP (cat no. 347464), CD34-APC (cat no. 743533), CD19-PE Cy7
(cat no. 745907), CD10-FITC (cat no. 745553), CD20-APC (cat no.
743611), cytoplasmic CD22-PE (cat no. 562859), CD2-FITC (cat no.
745734), CD3-APC (cat no. 746776), cytoplasmic CD3-APC (cat no.
340440), CD5-APC (cat no. 742554), CD7-FITC (cat no. 562635),
CD13-PE (cat no. 555394), CD33-APC (cat no. 551378), surface
immunoglobulin M (IgM)-APC (cat no. 560575) and cytoplasmic IgM-APC
(cat no. 560914). All the antibodies were diluted 1:100 and were
detected by direct immunofluorescence using a flow cytometer. Data
were analyzed by FACSDiva 6.0 (BD Biosciences).
The multiprobe ALL panel was designed to detect
eight FISH probes: BCR/ABL translocation, mixed
lineage leukemia (MLL) rearrangement, translocation ETS
leukemia-acute myeloid leukemia 1 (TEL-AML1) fusion gene and
trisomy 4/10. The probes are located at 22q11.2 9q34, 11q23, 12p13
21q22, and 4p11.1-q11.1/10p11.1-q11.1, which represent the
chromosome abnormalities t(9;22)(q34;qll), 11q23, t(12;21)(p13;q22)
and trisomy 4/10, respectively. A total of 2 ml fresh BM was taken
from each patient prior to treatment. The BM was centrifuged at
12,00 rpm for 10 min at room temperature to extract nucleated
cells. Cells were subjected to hypotonic shock, fixed with methanol
and acetic acid, then a cell suspension was prepared for FISH
detection. FISH probes were purchased from Beijing GP Medical
Technologies, Ltd., Beijing, China) and marked by nick translation.
FISH was performed according to the manufacturer's instructions
FISH probes are reversibly bound to the surface of a glass device,
and dissolve upon contact with hybridization buffer, which is
composed of formamide, SSC and glucan sulfate; probe and target DNA
denaturation occurs simultaneously upon heating at 78°C for 5 min.
Hybridization conditions were 42°C for 14–16 h. Probe cut-off
values were established by testing 20 BM samples from healthy
individuals (median age, 31 years; range, 18–50 years old; male to
female ratio, 1.5:1; enrolled at Xiangya Hospital from May to
December 2011, with informed consent). Data from 500 interphase
cells were gathered from each individual in the control group to
establish a normal database for the probes. The cut-off values for
the Ph chromosome, trisomy 4, trisomy 10, MLL gene, and
TEL-AML1 gene were 4.08, 2.32, 2.88, 3.24 and 3.76%,
respectively.
Diagnosis and subclassification
ALL diagnosis was mainly based on morphology of BM
and immunotyping of flow cytometry (15,16): The
proportion of primitive and juvenile lymphocytes in BM was >20%,
which was the basic diagnosis requirement. Morphology diagnosis
performed using routine procedures: Methanol fixing, Wright
staining and POX staining, then cell morphology was examined with
an optical microscope (Olympus CX21; Olympus, Japan, Tokyo). Flow
cytometry served a crucial role in differential diagnosis and
subclassification: Strong CD19 expression associated with weak
expression of CD10, CD22, or CD79a or weak CD19 expression plus
strong expression of two of the same markers were considered as B
lineage; strong cytoplasmic or surface CD3 expression was
considered as T lineage; CD5 or CD7 indicated pre-T-ALL and early
pre-T-ALL; and ambiguous B-lineage or T-lineage markers, or
‘bilineal’ cases were considered as uncertain-lineage subtypes. All
patients received the standard treatment strategies according to
the National Comprehensive Cancer Network guideline (17). Remission-induction therapy,
consolidation phase and maintenance chemotherapy comprise an
integrity program for patients who achieved continued CR until the
analysis of the present study. Adult patients received CVMD
(cyclophosphamide, vincristine, mitoxantrone and dexamethasone),
R-CVMDL (rituximab in combination with cyclophosphamide,
vincristine, mitoxantrone, dexamethasone and L-asparaginase), or
R-CHOP (rituximab in combination with cyclophosphamide,
doxorubicin, vincristine and prednisone) treatment regimens,
whereas pediatric patients were treated with VDLD (vincristine,
daunorubicin, L-asparaginase and dexamethasone) or VDLP
(vincristine, daunorubicin, L-asparaginase and prednisone)
therapeutic regimens.
Follow-up
Follow-up was measured from the initial day of
treatment to the final follow-up date (January 2015), or the day
the patient succumbed. Following treatment, follow-up examinations
were conducted every 3–6 months in the first 2 years, every month
in the following 3 years and annually thereafter. The duration of
overall survival (OS) was calculated from the day of treatment
completion to the day of mortality or the final follow-up; the
event-free survival (EFS) rate was calculated from the day of
treatment completion to the day of tumor progression, the
occurrence of fatal or intolerable side effects or mortality.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). The χ2 test was used for
comparing clinical and biological features between the 282 adults
and pediatric ALL cases. Prognostic factors, including leukocyte
count, hemoglobin level, BCR-ABL translocation, MLL
rearrangement, and immunophenotype were analyzed. Multivariate
analysis was performed to identify the independent prognostic
factors. The OS and EFS rates were calculated using the
Kaplan-Meier method and were compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of
patients
The median follow-up time was 46 months (range, 3–68
months), with 96.8% of patients finishing a complete 3-year
follow-up. The median age of the 207 pediatric ALL cases was 5.4
years (range, 0.5–14 years); that of the 75 adult ALL cases was
32.9 years (range, 15–68 years). Table
I presents the other patient clinical characteristics. Only
minimal residual disease was significantly different in the
pediatric and adult ALL groups. Sex, leukocyte count, hemoglobin
level and lactate dehydrogenase level were not significantly
different between the two groups.
Immunophenotyping and FISH
results
The expression of lineage markers in the two groups
did not differ significantly. Table
II shows that 82.6 and 14.7% of the adult ALL cases had
B-lineage markers and T-lineage markers, respectively. In pediatric
ALL cases, 81.2% had B-lineage markers and 12.1% had T-lineage
markers.
 | Table II.Immunophenotype and cytogenetic
features in adult and pediatric ALL. |
Table II.
Immunophenotype and cytogenetic
features in adult and pediatric ALL.
| Feature | Adult ALL (n=75)
(%) | Pediatric ALL
(n=207) (%) | χ2 | P-value |
|---|
|
Immuno-phenotype |
|
| 1.933 | 0.380 |
|
B-lineage | 62 (82.6) | 168 (81.2) |
|
|
|
T-lineage | 11 (14.7) | 25 (12.1) |
|
|
|
Uncertain-lineage | 2 (2.7) | 14 (6.7) |
|
|
| Ph |
|
| 33.893 | <0.001 |
|
Positive | 29 (38.6) | 19 (9.2) |
|
|
|
Negative | 46 (61.4) | 188 (90.8) |
|
|
| +4/+10 |
|
| 29.553 | <0.001 |
|
Positive | 1 (1.3) | 71 (34.3) |
|
|
|
Negative | 74 (98.7) | 146 (65.7) |
|
|
|
TEL-AML1 |
|
| 6.146 | 0.013 |
|
Positive | 0 (0) | 16 (7.7) |
|
|
|
Negative | 75 (100) | 191 (92.3) |
|
|
| MLL |
|
| 2.134 | 0.144 |
|
Positive | 12 (16.0) | 50 (24.2) |
|
|
|
Negative | 63 (84.0) | 157 (75.8) |
|
|
Cytogenetically, genetic rearrangement, including
the BCR-ABL fusion gene and MLL rearrangement, were
detected in all adult patients. In adult patients, the rate of Ph
chromosome positivity (Ph+) and MLL rearrangement was 38.6%
(29/75) and 16.0% (12/75), respectively, and 1.3% (1/75) and 0% of
patients were trisomy 4/10 and had the TEL-AML1 fusion gene,
respectively. In pediatric ALL, 24.2% of patients (50/207) had
MLL rearrangement and only 9.2% of patients (19/207) were
Ph+, while 34.3% (71/207) and 7.7% (16/207) of patients were
trisomy 4/10 or had the TEL-AML1 fusion gene, respectively.
Fig. 1 depicts the FISH results. In
Ph-negative and trisomy 4/10 negative patients, two red and two
green particles were observed in the nucleus (Fig. 1A). In Ph-positive patients, there was
one red, one green and one yellow particle in the nucleus (Fig. 1B). In MLL-negative patients, two
yellow particles were observed in the nucleus (Fig. 1C). One red, one green and one yellow
particle were observed in MLL-positive patients (Fig. 1D). Chromosome 4 amplification was
observed as three red and two green particles (Fig. 1E); Chromosome 10 amplification
resulted in two red and three green particles (Fig. 1F). In the TEL-AML1-negative sample,
there are two red and two green particles (Fig. 1G); The TEL-AML1-positive sample
usually presented with one red, one green and one yellow particle
(Fig. 1H).
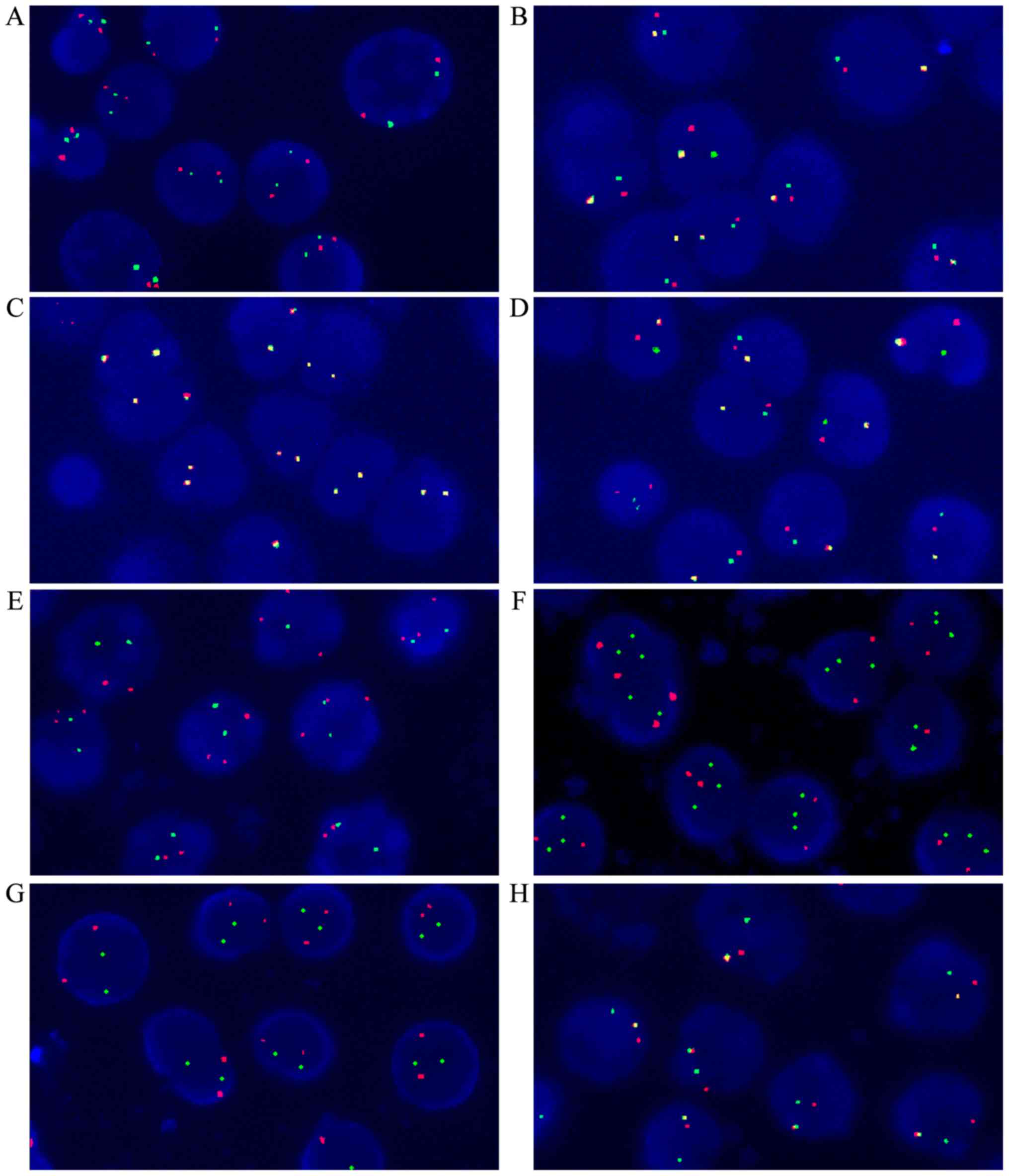 | Figure 1.Fluorescence in situ
hybridization results (original magnification, ×1,000). (A) Normal
signal of Ph-negative and chromosomes 4/10 (two red, two green
particles). (B) Ph-positive sample (one red, one green, one yellow
particle). (C) MLL-negative sample (two yellow particles).
(D) MLL-positive sample (one red, one green, one yellow
particle). (E) Chromosome 4 amplification (three red, two green
particles). (F) Chromosome 10 amplification (two red, three green
particles). (G) TEL-AML1-negative sample (two red, two green
particles). (H) TEL-AML1-positive sample (one red, one
green, one yellow particle). Ph, Philadelphia; MLL, mixed lineage
leukemia; TEL-AML1, translocation ETS leukemia-acute myeloid
leukemia 1. |
Prognosis analysis of cases with
different chromosomes or immunophenotypes
Survival rates were calculated using the
Kaplan-Meier method and compared with the log-rank test. The mean
3-year OS rate for the entire cohort was 59.5% (range, 56.3–62.7%),
74% (range, 70.7–77.3%) in the pediatric ALL group, and 18.8%
(range, 13.6–24%) in the adult ALL group [hazard ratio (HR) =5.28;
95% confidence interval (CI), 3.59–7.78; P<0.001]. The 3-year
EFS rate for the entire cohort was 50.6% (range, 47.4–53.8%), 62.8%
(range, 59.1–66.5%) in the pediatric ALL group, and 16.5% (range,
12.0–21.0%) in the adult ALL group (HR=4.58; 95% CI, 3.22–6.52;
P<0.001).
The multivariate analysis suggested that age, i.e.,
pediatric vs. adult patients, was most associated with OS (HR=4.44;
P<0.001); Ph chromosome status (HR=2.71; P<0.001), MLL
status (HR=1.65; P=0.020), TEL-AML1 fusion gene (HR=0.76;
P=0.067) and white blood cell (WBC) level (HR=1.001; P=0.045) were
independent prognostic factors of OS (Table III).
 | Table III.Multivariate analysis of prognostic
factors of overall survival in acute lymphoblastic leukemia. |
Table III.
Multivariate analysis of prognostic
factors of overall survival in acute lymphoblastic leukemia.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variable | Regression
coefficient | Standard error | P-value | HR | Lower | Upper |
|---|
| Sex | −0.131 | 0.206 | 0.524 | 0.877 | 0.586 | 1.313 |
| Age (adult or
pediatric) | 1.491 | 0.221 | <0.001 | 4.441 | 2.878 | 6.852 |
| MLL | 0.503 | 0.217 | 0.020 | 1.653 | 1.081 | 2.529 |
| TEL-AML/4–10 | −0.275 | 0.150 | 0.067 | 0.759 | 0.566 | 1.019 |
| Ph | 0.997 | 0.218 | <0.001 | 2.711 | 1.767 | 4.159 |
|
B/T/uncertain-lineage | 0.246 | 0.186 | 0.187 | 1.278 | 0.888 | 1.841 |
| WBC | 0.001 | 0.001 | 0.045 | 1.001 | 1.000 | 1.003 |
| Hb | 0.002 | 0.004 | 0.599 | 1.002 | 0.994 | 1.010 |
| Pt | 0.000 | 0.001 | 0.847 | 1.000 | 0.997 | 1.002 |
In pediatric ALL cases, Ph chromosome (HR=3.56;
P<0.001) and MLL status (HR=2.27; P=0.007) were
independent prognostic factors of OS (Table IV). In adult ALL cases, Ph
chromosome status (HR=2.21; P=0.016) and WBC level (HR=1.003;
P=0.012) were independent prognostic factors of OS (Table V).
 | Table IV.Multivariate analysis of prognostic
factors of overall survival in pediatric acute lymphoblastic
leukemia. |
Table IV.
Multivariate analysis of prognostic
factors of overall survival in pediatric acute lymphoblastic
leukemia.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variable | Regression
coefficient | Standard error | P-value | HR | Lower | Upper |
|---|
| Sex | −.0117 | 0.301 | 0.698 | 0.890 | 0.493 | 1.605 |
| Age | 0.034 | 0.039 | 0.386 | 1.035 | 0.958 | 1.118 |
| MLL | 0.818 | 0.305 | 0.007 | 2.266 | 1.247 | 4.118 |
| TEL-AML/4–10 | −0.317 | 0.184 | 0.085 | 0.729 | 0.508 | 1.045 |
| Ph | 1.269 | 0.350 | 0.000 | 3.558 | 1.790 | 7.070 |
|
B/T/uncertain-lineage | 0.349 | 0.263 | 0.185 | 1.417 | 0.846 | 2.372 |
| WBC | 0.001 | 0.001 | 0.497 | 1.001 | 0.999 | 1.002 |
| Hb | 0.004 | 0.007 | 0.530 | 1.004 | 0.991 | 1.018 |
| Pt | −0.002 | 0.002 | 0.355 | 0.998 | 0.995 | 1.002 |
 | Table V.Multivariate analysis of prognostic
factors of overall survival in adult acute lymphoblastic
leukemia. |
Table V.
Multivariate analysis of prognostic
factors of overall survival in adult acute lymphoblastic
leukemia.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variable | Regression
coefficient | Standard error | P-value | HR | Lower | Upper |
|---|
| Sex | −0.003 | 0.302 | 0.992 | 0.997 | 0.551 | 1.803 |
| Age | 0.001 | 0.010 | 0.934 | 1.001 | 0.982 | 1.020 |
| MLL | −0.221 | 0.411 | 0.591 | 0.802 | 0.358 | 1.795 |
| TEL-AML/4–10 | −0.197 | 0.254 | 0.438 | 0.821 | 0.499 | 1.351 |
| Ph | 0.795 | 0.330 | 0.016 | 2.214 | 1.159 | 4.229 |
|
B/T/uncertain-lineage | 0.361 | 0.304 | 0.234 | 1.435 | 0.791 | 2.601 |
| WBC | 0.003 | 0.001 | 0.012 | 1.003 | 1.001 | 1.006 |
| Hb | −0.002 | 0.006 | 0.737 | 0.998 | 0.987 | 1.009 |
| Pt | 0.001 | 0.003 | 0.774 | 1.001 | 0.995 | 1.006 |
Tables III–V demonstrate that Ph chromosome, MLL
status and WBC level were independent prognostic factors of OS.
Tables I and II indicate that MLL status and WBC
level were not significantly different in the pediatric and adult
ALL cases. This suggests that Ph+ is the primary reason for the
worse prognosis in adult ALL than in pediatric ALL.
The prognosis of the two groups in the present study
may differ so greatly as the pediatric and adult patients with ALL
have different biological features. Prognostic analysis of all
patients was stratified by the following biological features: i) In
Ph-negative (Ph-) and Ph+ patients the 3-year OS rate was 68.5 vs.
16.9% (HR=4.21; P<0.001) and the 3-year EFS rate was 60.0 vs.
6.7% (HR=4.33; P<0.001); ii) in MLL-negative and
-positive patients the 3-year OS rate was 62.5 vs. 51.8% (HR=1.34;
P=0.112) and the 3-year EFS rate was 56.5 vs. 34.5% (HR=1.59;
P=0.011); iii) in patients negative for trisomy 4/10 and
TEL-AML1, trisomy 4-positive, trisomy 10-positive,
TEL-AML1-positive patients, the 3-year OS rate was 52.6 vs.
60.6 vs. 82.6 vs. 87.2% (HR=0.587, P=0.004) and the 3-year EFS rate
was 42.6 vs. 65.2 vs. 78.3 vs. 71.3% (HR=0.646; P=0.003); iv) in B
lineage, T lineage or uncertain-lineage patients, the 3-year OS
rate was 48.8 vs. 61.8 vs. 47.6% (HR=1.24; P=0.423) and the 3-year
EFS rate was 35.8 vs. 53.7 vs. 40.0% (HR=1.29; P=0.131). Fig. 2 depicts the corresponding survival
curves. Fig. 3 depicts the
representative FACS results and morphological/histological images
of B cell ALL, T cell ALL and atypical B-ALL patients. Patient 1 is
a typical B-ALL patient. Immunologic cell markers of B-ALL show
CD19, CD22, CD34 and HLA-DR are positive; CD3, CD7 and CD33 are
negative. Wright's staining demonstrates the nuclei of B-ALL are
regular and not indented or twisted. The lymphoblast has a high
nuclear/cytoplasmic ratio. The POX staining is negative in all
lymphoblasts of B-ALL. Patient 2 is a typical T-ALL patient.
Immunologic cell markers of T-ALL indicate CD5, CD7, CD34 and CD38
are positive; CD19, CD20 and CD22 are negative. Wright's staining
of T-ALL demonstrates that the cells are mostly larger than in
B-ALL. There are more variations in cytologic features of the
lymphoblast. The POX staining is negative in all lymphoblasts of
T-ALL. Patient 3 is an atypical B-ALL patient accompanied by
myelogenous markers. Immunologic cell markers of atypical ALL
indicate CD10, CD19, CD22 and CD34 are positive; CD13 and CD33 are
partially positive; CD3, CD7 and CD56 are negative. Wright's
staining reveals that this cell type is larger than typical B-ALL.
These lymphoblasts have also a high nuclear/cytoplasmic ratio. The
POX staining is negative in almost all lymphoblasts.
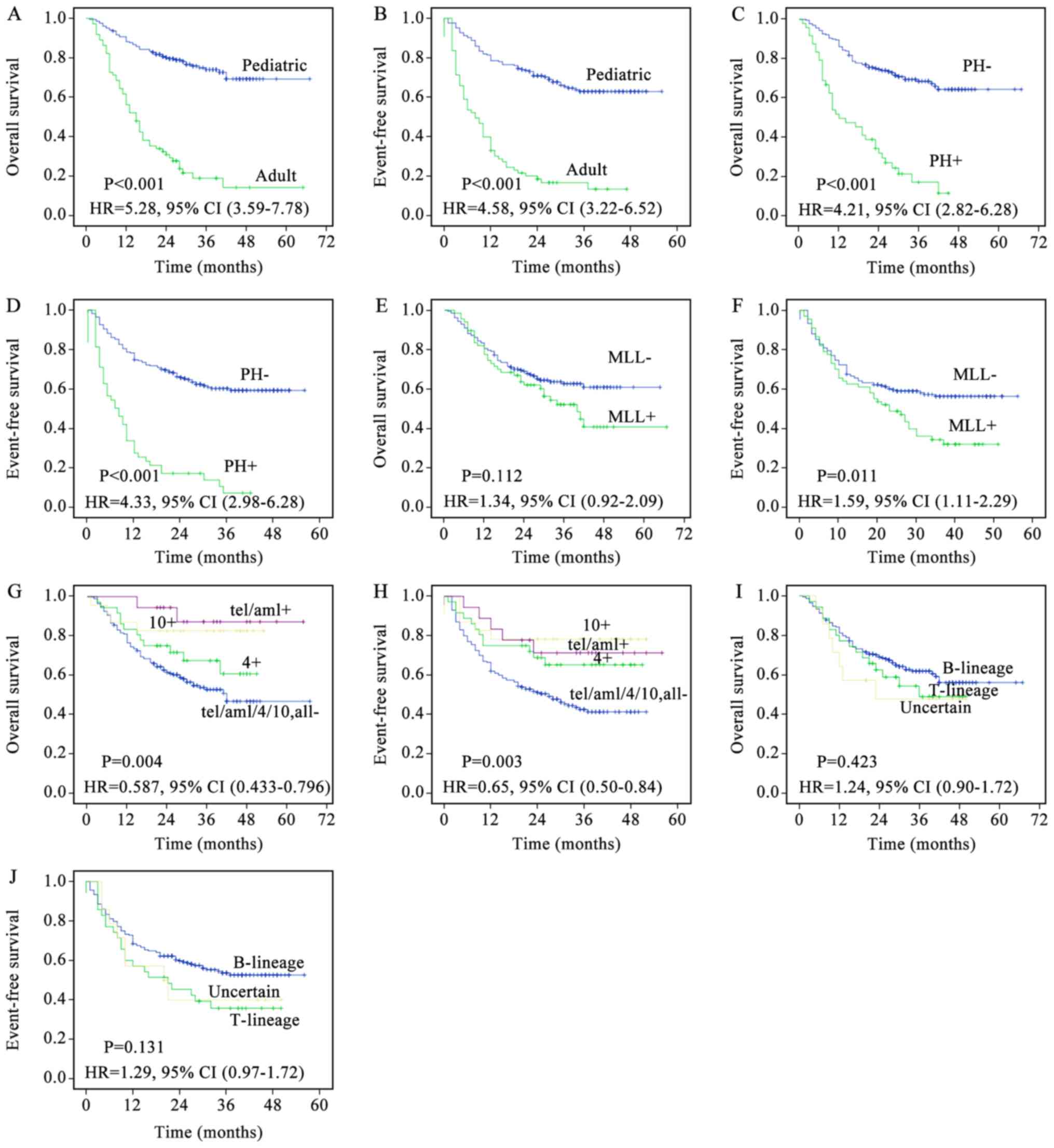 | Figure 2.Kaplan-Meier survival curves of 277
patients with ALL. (A) OS of pediatric and adult ALL. (B) EFS of
pediatric and adult ALL. (C) OS of Ph- and Ph+ ALL. (D) EFS of Ph-
and Ph+ ALL. (E) OS of MLL- and MLL+ ALL. (F) EFS of MLL- and MLL+
ALL. (G) OS of patients negative for trisomy 4/10 and
TEL-AML, and trisomy 4-positive, trisomy 10-positive, and
TEL-AML-positive patients. (H) EFS of patients negative for
trisomy 4/10 and TEL-AML, and trisomy 4-positive, trisomy
10-positive, and TEL-AML-positive patients. (I) OS of
T-lineage, B-lineage, and uncertain-lineage patients. (J) EFS of
T-lineage, B-lineage, and uncertain-lineage patients. P-values were
calculated using the unadjusted log-rank test; HR were calculated
using the unadjusted Cox proportional hazards model. ALL, acute
lymphoblastic leukemia; OS, overall survival; EFS, event-free
survival; Ph-, Philadelphia-negative; Ph+, Philadelphia-positive;
MLL-, mixed lineage leukemia-negative; MLL+, mixed lineage
leukemia-positive; TEL-AML1, translocation ETS leukemia-acute
myeloid leukemia 1; HR, hazard ratio; CI, confidence interval. |
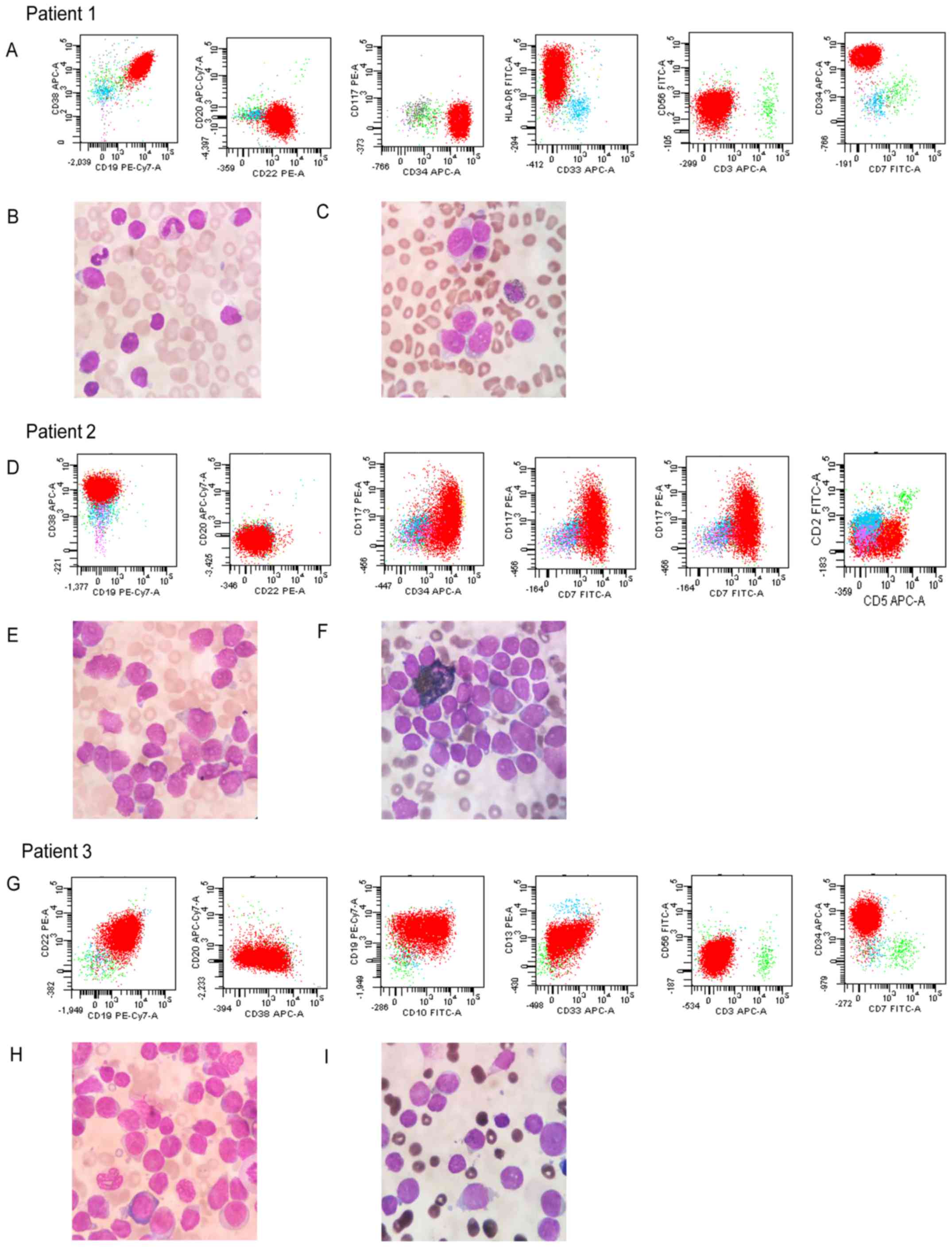 | Figure 3.Representative flow cytometry results
and morphological/histological images. Patient 1 (a B-ALL patient).
(A) Immunologic cell markers of B-ALL (CD19, CD22, CD34 and HLA-DR
are positive; CD3, CD7 and CD33 are negative). (B) Wright's
staining of B-ALL. Original magnification, ×1,000. The nuclei are
regular and not indented or twisted. The lymphoblast has a high
nuclear/cytoplasmic ratio. (C) POX staining of B-ALL. Original
magnification, ×1,000. The POX staining is negative in all
lymphoblasts of B-ALL. Patient 2 (a T-ALL patient). (D) Immunologic
cell markers of T-ALL (CD5, CD7, CD34 and CD38 are positive; CD19,
CD20 and CD22 are negative). (E) Wright's Staining of T-ALL.
Original magnification, ×1,000. The cell type prevails are larger
than B-ALL. There are more variations in cytologic feature of the
lymphoblast. (F) POX staining of B-ALL. Original magnification,
×1,000. The POX staining is negative in all lymphoblasts of this
type of ALL. Patient 3 (an atypical B-ALL patient). (G) Immunologic
cell markers of atypical ALL (CD10, CD19, CD22 and CD34 are
positive; CD13 and CD33 are partially positive; CD3, CD7 and CD56
are negative). (H) Wright's Staining of B-ALL. Original
magnification, ×1,000. The cell type prevails are larger and the
lymphoblast has also a high nuclear/cytoplasmic ratio. (I) POX
staining of atypical B-ALL. Original magnification, ×1,000. The
peroxidase staining is negative in almost all lymphoblasts of this
atypical B-ALL. ALL, acute lymphoblastic leukemia; B-ALL, B cell
ALL; CD, cluster of differentiation; POX, peroxidase; T-ALL, T cell
ALL; PE, phycoerythrin; Cy7, cyanine7; APC, allophycocyanin;
HLA-DR, human leukocyte antigen-antigen D related; FITC,
fluorescein isothiocyanate. |
Discussion
An estimated 6,000 new ALL cases (3,400 male and
2,600 female) are diagnosed annually in the USA (2). In China, this number is almost four
times higher due to the larger population (18). ALL occurs in both children and adults
but its incidence peaks between 2 and 5 years of age (3). The survival rate of childhood ALL is
~90%, but improvement is required for treatment in infants and
adults (8).
ALL affects infants, children, adolescents, and
adults. With current therapies, the vast majority of children with
ALL are now long-term survivors (19). However, the same positive results
have not been reported for adults with ALL (11). The present study confirms these
results.
The cause of the differing prognosis of ALL is
multifactorial, and largely includes genomic alterations, exogenous
or endogenous exposure to environmental toxins and chance. The
inferior prognosis of adult ALL is not fully understood but could
be attributed, in part, to genetic susceptibility as compared to
pediatric ALL (20,21). Significant differences were also
detected in immunophenotype, i.e., Ph chromosome, trisomy 4 and 10,
and the TEL-AML1 fusion gene, between pediatric and adult
patients. The differences in Ph chromosome status may be a leading
cause for the worse prognosis. It was also demonstrated that the OS
or EFS of Ph+ patients were lower than that of Ph- patients. The
same conclusion can be drawn from the multivariate analysis, in
that Ph+ is an independent poor prognostic factor in ALL
overall.
FISH is one of the most sensitive molecular methods
for detecting genetic abnormalities such as chromosome
translocation and submicroscopic chromosomal abnormalities with
specific DNA probes (22). Even in
non-mitotic cells or when cytogenetic studies are insufficient,
FISH may detect cryptic or rare chromosomal rearrangement (23). However, conventional cytogenetic
analysis cannot accurately detect non-dividing (interphase) cells,
which represent the most important fraction of bone marrow cells
(16). Therefore, FISH is more
sensitive and time-efficient than traditional chromosomal tests,
which was also confirmed previously (24). The inferior prognosis in adult ALL is
attributed, in part, to the higher rate of Ph+ detected by FISH in
adult patients as compared with pediatric patients.
The present retrospective study was designed
predominantly to elucidate the relevance of the prognosis in
pediatric and adult ALL and to define why the prognosis of these
two groups differs, and to clarify whether it can be explained
through the differing biological features detected by FISH.
Significant differences were identified between the biological
features and prognostic associations in adult and pediatric
patients with ALL.
According to the present findings, the incidence of
ALL decreases with age. A previous study of pediatric and adult
patients with ALL identified no significant differences between
sex, race/ethnic group and mean presenting WBC count (1).
Studies (25,26) have indicated that Ph+ ALL presents a
dismal prognosis, representing an independent prognostic factor not
only in pediatric patients, but also in adult patients. In the
majority of patients with chronic myeloid leukemia, the ABL
gene moves from chromosome 9 to the major breakpoint cluster region
on chromosome 22. This translocation results in a 210-kDa fusion
protein (p210). However, the ABL1 gene can also translocate
to the minor breakpoint cluster region on chromosome 22, resulting
in a 190-kDa fusion protein (p190) that occurs exclusively in ALL
(27).
Ph+ ALL is characterized by poor response to the
majority of chemotherapy combinations, short remission durations
and poor survival rates (28). The
findings suggested that the BCR-ABL fusion gene is an
independent unfavorable prognostic factor for adult patients with
ALL. Nevertheless, the development of allogeneic bone marrow
transplantation and specific tyrosine kinase inhibitors for Ph+ ALL
has potentially changed this (28).
In ALL, acute myeloid leukemia and
therapy-associated leukemia, the MLL gene is rearranged with
>70 partner genes and is located on the long arm of chromosome
11 (29). Reverse
transcriptase-polymerase chain reaction previously revealed that
this translocation was present in not only 40–50% of infants, but
also in 2–3% of children and ~10% of adults with ALL (25). In the present study, 24.2% of
pediatric patients and 16.0% of adult patients with ALL had
MLL translocation, suggesting the high incidence rate of
this location in China. Furthermore, the follow-up results indicate
that the MLL gene is not associated with the poor prognosis
of adult ALL, but that it is associated with poor prognosis of
pediatric ALL.
The TEL-AML1 fusion gene, generated by the
t(12;21)(p13;q22) chromosomal translocation, occurs in ~25% of
cases of B cell precursor ALL. It is one of the most common forms
of acute leukemia in children (30).
The TEL gene is an important regulator in hematopoietic cell
development, and the AML1 gene serves an important role in
definitive embryonic hematopoiesis (25). The presence of the TEL-AML1 fusion
protein in B-cell progenitors seems to be a hallmark of leukemic
lymphoblasts, and leads to disordered early B-lineage lymphocyte
development (26). The present
findings indicated that the frequency of TEL-AML1 fusion was
much higher in children than in adults, and is a favorable
prognostic factor in patients with ALL. The EFS in
TEL-AML1-positive patients was markedly longer than that of
TEL-AML1-negative patients. A previous Pediatric Oncology
Group study (31) revealed that
trisomy 4 and 10 are strongly indicative of favorable prognosis,
particularly in standard-risk B-precursor ALL. Although a number of
genetic abnormalities are associated with clinical outcome, only a
few are routinely used for treatment stratification (32,33). In
the present study, pediatric patients with combined chromosome 4
and 10 trisomies appeared to have more prognostically favorable
clinical features.
A limitation of the present study is that more
sophisticated techniques were not used for comparing the
disadvantages of FISH. Using second-generation sequencing
technology, Zhao et al (34)
previously revealed that 2,825 genes were upregulated and 1,952
were downregulated in the ALL group compared with the normal
control group. Based on the digital gene expression profiling data,
they investigated a further seven genes (WT1, RPS26, MSX1, CD70,
HOXC4, HOXA5, OXC6) predominantly associated with immune cell
differentiation, metabolic processes and programmed cell death.
Although FISH is widely used in diagnosis and prognosis prediction
of hematological malignancies, minimal residual disease (MRD)
diagnostics has proven to be the strongest prognostic factor that
may be used to guide treatment decisions. MRD techniques are
required to be sensitive, accurate, reliable and fast. Recently
developed high-throughput sequencing and next-generation
(multidimensional) flow cytometry have been demonstrated to have
greater potential means (35).
In conclusion, adult ALL has poorer prognosis than
pediatric ALL. Ph+ status is associated with the high-risk features
of increased age and is frequently observed and associated with
unfavorable prognosis. Trisomies 4 and 10 are also associated with
favorable prognosis but are not independent prognostic factors of
ALL. Ph+ ALL is an independent prognostic factor of ALL that is
frequently present in patients.
Acknowledgements
The author would like to thank the doctors of the
Hematology Department of Xiangya Hospital for providing clinical
data.
Funding
The present study was supported by the Hunan
Provincial Scientific Research Project of Traditional Chinese
Medicine (201798).
Availability of data and materials
All data generated or analyzed during this study are
included in this published. No any other data available for
supplementary materials.
Authors' contributions
The study presented here was performed as a
collaboration between all authors. PC, YY, WW and HX performed the
majority of the experiments. YH and PC made contributions to the
design, data analysis and interpretation and drafting of the
manuscript. PC, YY and WW collected and assembled the data. All
authors gave their final approval for publication of the
manuscript.
Ethics approval and consent to
participate
The Xiangya Hospital Ethics Committee approved the
present study (approval no. 201212478).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALL
|
acute lymphoblastic leukemia
|
|
FISH
|
fluorescence in situ
hybridization
|
|
MLL
|
mixed lineage leukemia
|
|
Ph
|
Philadelphia
|
|
TEL-AML1
|
translocation ETS leukemia-acute
myeloid leukemia 1
|
|
OS
|
overall survival
|
|
EFS
|
event-free survival
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lafage-Pochitaloff M, Baranger L, Hunault
M, Cuccuini W, Lefebvre C, Bidet A, Tigaud I, Eclache V, Delabesse
E, Bilhou-Nabéra C, et al: Impact of cytogenetic abnormalities in
adults with Ph-negative B-cell precursor acute lymphoblastic
leukemia. Blood. 130:1832–1844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pui CH, Crist WM and Look AT: Biology and
clinical significance of cytogenetic abnormalities in childhood
acute lymphoblastic leukemia. Blood. 76:1449–1463. 1990.PubMed/NCBI
|
|
6
|
Faderl S, O'Brien S, Pui CH, Stock W,
Wetzler M, Hoelzer D and Kantarjian HM: Adult acute lymphoblastic
leukemia: Concepts and strategies. Cancer. 116:1165–1176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aricò M, Schrappe M, Hunger SP, Carroll
WL, Conter V, Galimberti S, Manabe A, Saha V, Baruchel A,
Vettenranta K, et al: Clinical outcome of children with newly
diagnosed philadelphia chromosome-positive acute lymphoblastic
leukemia treated between 1995 and 2005. J Clin Oncol. 28:4755–4761.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassan R and Hoelzer D: Modern therapy of
acute lymphoblastic leukemia. J Clin Oncol. 29:532–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen S, Cai J, Chen J, Xue H, Pan C, Gao
Y, Tang Y, Wang J, Li B, Wang X, et al: Long-term results of the
risk-stratified treatment of childhood acute lymphoblastic leukemia
in China. Hematol Oncol. 2018.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Möricke A, Reiter A, Zimmermann M, Gadner
H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R,
et al: Risk-adjusted therapy of acute lymphoblastic leukemia can
decrease treatment burden and improve survival: Treatment results
of 2169 unselected pediatric and adolescent patients enrolled in
the trial ALL-BFM 95. Blood. 111:4477–4489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gökbuget N, Kneba M, Raff T, Trautmann H,
Bartram CR, Arnold R, Fietkau R, Freund M, Ganser A, Ludwig WD, et
al: Adult patients with acute lymphoblastic leukemia and molecular
failure display a poor prognosis and are candidates for stem cell
transplantation and targeted therapies. Blood. 120:1868–1876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sive JI, Buck G, Fielding A, Lazarus HM,
Litzow MR, Luger S, Marks DI, McMillan A, Moorman AV, Richards SM,
et al: Outcomes in older adults with acute lymphoblastic leukaemia
(ALL): Results from the international MRC UKALL XII/ECOG2993 trial.
Br J Haematol. 157:463–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoelzer D, Walewski J, Dohner H, Viardot
A, Hiddemann W, Spiekermann K, Serve H, Dührsen U, Hüttmann A,
Thiel E, et al: Improved outcome of adult Burkitt lymphoma/leukemia
with rituximab and chemotherapy: Report of a large prospective
multicenter trial. Blood. 124:3870–3879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas DA, O'Brien S, Faderl S,
Garcia-Manero G, Ferrajoli A, Wierda W, Ravandi F, Verstovsek S,
Jorgensen JL, Bueso-Ramos C, et al: Chemoimmunotherapy with a
modified hyper-CVAD and rituximab regimen improves outcome in de
novo Philadelphia chromosome-negative precursor B-lineage acute
lymphoblastic leukemia. J Clin Oncol. 28:3880–3889. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoelzer D, Bassan R, Dombret H, Fielding
A, Ribera JM and Buske C; ESMO Guidelines Committee, . Acute
lymphoblastic leukaemia in adult patients: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
Suppl 5:v69–v82. 2016.PubMed/NCBI
|
|
16
|
Chiaretti S, Zini G and Bassan R:
Diagnosis and subclassification of acute lymphoblastic leukemia.
Mediterr J Hematol Infect Dis. 6:e20140732014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown PA, Shah B, Fathi A, Wieduwilt M,
Advani A, Aoun P, Barta SK, Boyer MW, Bryan T, Burke PW, et al:
NCCN guidelines insights: Acute lymphoblastic leukemia, version
1.2017. J Natl Compr Canc Netw. 15:1091–1102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunger SP and Mullighan CG: Acute
lymphoblastic leukemia in children. N Engl J Med. 373:1541–1552.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szczepański T, Harrison CJ and van Dongen
JJ: Genetic aberrations in paediatric acute leukaemias and
implications for management of patients. Lancet Oncol. 11:880–889.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chilton L, Buck G, Harrison C J,
Ketterling RP, Rowe JM, Tallman MS, Goldstone AH, Fielding AK and
Moorman AV: High hyperdiploidy among adolescents and adults with
acute lymphoblastic leukaemia (ALL): Cytogenetic features, clinical
characteristics and outcome. Leukemia. 28:1511–1518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazloumi SH, Madhumathi DS, Appaji L and
Prasannakumari: Combined study of cytogenetics and fluorescence
in situ hybridization (FISH) analysis in childhood acute
lymphoblastic leukemia (ALL) in a tertiary cancer centre in South
India. Asian Pac J Cancer Prev. 13:3825–3827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moorman AV: The clinical relevance of
chromosomal and genomic abnormalities in B-cell precursor acute
lymphoblastic leukaemia. Blood Rev. 26:123–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao P, Li Y, Li X, Zhang G and Chen F:
Detecting chromosomal aberrations in myelodysplastic syndrome with
fluorescence in situ hybridization and conventional cytogenetic
analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 39:605–611. 2014.(In
Chinese). PubMed/NCBI
|
|
25
|
Moorman AV, Harrison CJ, Buck GA, Richards
SM, Secker-Walker LM, Martineau M, Vance GH, Cherry AM, Higgins RR,
Fielding AK, et al: Karyotype is an independent prognostic factor
in adult acute lymphoblastic leukemia (ALL): Analysis of
cytogenetic data from patients treated on the Medical Research
Council (MRC) UKALLXII/Eastern cooperative oncology group (ECOG)
2993 trial. Blood. 109:3189–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landau H and Lamanna N: Clinical
manifestations and treatment of newly diagnosed acute lymphoblastic
leukemia in adults. Curr Hematol Malig Rep. 1:171–179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Druker BJ, Sawyers CL, Kantarjian H, Resta
DJ, Reese SF, Ford JM, Capdeville R and Talpaz M: Activity of a
specific inhibitor of the BCR-ABL tyrosine kinase in the blast
crisis of chronic myeloid leukemia and acute lymphoblastic leukemia
with the Philadelphia chromosome. N Engl J Med. 344:1038–1042.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schrappe M, Hunger SP, Pui CH, Saha V,
Gaynon PS, Baruchel A, Conter V, Otten J, Ohara A, Versluys AB, et
al: Outcomes after induction failure in childhood acute
lymphoblastic leukemia. N Engl J Med. 366:1371–1381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Collins EC and Rabbitts TH: The
promiscuous MLL gene links chromosomal translocations to cellular
differentiation and tumour tropism. Trends Mol Med. 8:436–442.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cayuela JM, Baruchel A, Orange C, Madani
A, Auclerc MF, Daniel MT, Schaison G and Sigaux F: TEL-AML1 fusion
RNA as a new target to detect minimal residual disease in pediatric
B-cell precursor acute lymphoblastic leukemia. Blood. 88:302–308.
1996.PubMed/NCBI
|
|
31
|
Sutcliffe MJ, Shuster JJ, Sather HN,
Camitta BM, Pullen J, Schultz KR, Borowitz MJ, Gaynon PS, Carroll
AJ and Heerema NA: High concordance from independent studies by the
Children's Cancer Group (CCG) and Pediatric Oncology Group (POG)
associating favorable prognosis with combined trisomies 4, 10, and
17 in children with NCI Standard-Risk B-precursor Acute
Lymphoblastic Leukemia: A Children's Oncology Group (COG)
initiative. Leukemia. 19:734–740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yeoh EJ, Ross ME, Shurtleff SA, Williams
WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A,
et al: Classification, subtype discovery, and prediction of outcome
in pediatric acute lymphoblastic leukemia by gene expression
profiling. Cancer Cell. 1:133–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conter V, Bartram CR, Valsecchi MG,
Schrauder A, Panzer-Grümayer R, Möricke A, Aricò M, Zimmermann M,
Mann G, De Rossi G, et al: Molecular response to treatment
redefines all prognostic factors in children and adolescents with
B-cell precursor acute lymphoblastic leukemia: Results in 3184
patients of the AIEOP-BFM ALL 2000 study. Blood. 115:3206–3214.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao MY, Yu Y, Xie M, Yang MH, Zhu S, Yang
LC, Kang R, Tang DL, Zhao LL and Cao LZ: Digital gene expression
profiling analysis of childhood acute lymphoblastic leukemia. Mol
Med Rep. 13:4321–4328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Dongen JJ, van der Velden VH,
Brüggemann M and Orfao A: Minimal residual disease diagnostics in
acute lymphoblastic leukemia: Need for sensitive, fast, and
standardized technologies. Blood. 125:3996–4009. 2015. View Article : Google Scholar : PubMed/NCBI
|

















