Introduction
Steroid-induced avascular necrosis of femoral head
(SANFH) belongs to the non-traumatic type of avascular necrosis of
femoral head. It refers to the pathological process where the
high-dose glucocorticoids lead to death of active ingredients
(osteocytes, bone marrow hematopoietic cells and adipocytes) of
femoral heads (1). The main symptoms
are regular pains in hip joints, joint dysfunction, limp and lower
limb muscle atrophy, slow onset, long course of disease, high
disability. This orthopedic disease is considered as one of the
three major orthopedic problems (2).
SANFH ranks first (2/3) of the non-traumatic avascular necroses,
and the trend continues to increase (3), which has attracted great concerns of a
large number of medical workers and scientists.
Modern medical science can generally be divided into
the expectant and surgical treatment. Expectant treatments include
painkillers, brake limbs, restricted load-bearing, and
interventional therapy, but these methods do not acquire the
desired effect. Surgical treatments are divided into the hip
retention surgery and artificial joint replacement surgery
(4). In recent years, based on the
traditional Chinese medical theory and combined with the progress
of modern medicine research, traditional Chinese medicine proposed
the treatment based on syndrome differentiation from the overall
treatment for alleviating pain and improving functions. This
contributes to satisfactory results in the prevention and treatment
of SANFH. Thus, we should pay attention to it (5).
Epimedium is a traditional Chinese medicine. Modern
pharmacological experiments have shown that the main active
ingredients of epimedium include total flavonoids of epimedium
(TFE), and icariin (ICA). Epimedium participates in bone
metabolism, stimulates bone cell proliferation and inhibits the
activity of osteoclasts so as to prevent and cure osteoporosis
(6,7). In addition, it can also reduce the low,
medium and high shear viscosity of the whole blood, improve blood
rheology, and significantly reduce the platelet aggregation
function (8), as well as regulate
immune mechanism and improve immune system to prevent the primary
disease.
Autophagy was initially discovered by a transmission
electron microscopy. If cell autophagy occurs, vacuolar
bilayer-like structures can be observed under the transmission
electron microscopy. Sometimes autolysosomes and their residues can
also be observed (9). Various
cytokines and proteins such as Beclin1 and LC3 participate in cell
autophagy to varying degrees (10).
In recent years, with the deepening of research, many scholars have
found and believed that glucocorticoid-induced bone autophagy is
likely to play a role in the development of SANFH (11). Studies have shown that
glucocorticoids cannot only induce osteoblast apoptosis, but also
lead to the occurrence of bone autophagy (12). Excessive autophagy will cause harm to
the body. Therefore, we believe that bone cell autophagy plays an
important role in the pathogenesis of SANFH.
In this study, healthy Sprague-Dawley (SD) rats were
used, and the early SANFH model was induced by intramuscular
injection of prednisolone acetates. The mechanism of epimedium on
the disease was observed from bone mineral density (BMD),
immunohistochemical staining and other aspects, providing new drugs
for clinical prevention and treatment of SANFH.
Materials and methods
Experimental animals and drugs
Twenty-four healthy SD rats weighing 200±20 g,
provided by the Nanjing Qinglongshan Animal Experimental Center
(Nanjing, China) of general standard feed. The rats were housed in
a controlled room temperature (21±2°C) on a 12:12-h light/dark
cycle (lights on at 06:00). All rats had free access to food and
water.
This study was approved by the Animal Ethics
Committee of Soochow University Animal Center (Suzhou, China).
Acetic acid prednisolone injection was purchased
from Zhejiang Xianju Pharmaceutical Co., Ltd. (Zhejiang, China);
penicillin sodium injection was purchased from North China
Pharmaceutical Co., Ltd. (Shijiazhuang, China); epimedium
extracting solution was prepared by the liquid. A total of 1,080 g
epimedium tablets were boiled and concentrated to 720 ml solution
(containing 1.5 g/ml crude drugs). Primary mouse monoclonal B-cell
lymphoma-2 (Bcl-2) antibody (dilution, 1:100; cat. no. sc-56015)
and primary mouse monoclonal LC3 phosphatidylethanolamine conjugate
(LC3II) antibody (dilution, 1:100; cat. no. sc-271625) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The remaining reagents were purchased from Nanjing Keygen Biotech
Co., Ltd. (Nanjing, China).
Animal models and drug
administration
According to the conversion method, rats in the
glucocorticoid and epimedium groups were injected with 15
mg/kg/time prednisolone acetate injection in gluteal muscles twice
a week. In order to prevent infection, 100,000 U/kg/time penicillin
was injected intramuscularly after the injection of glucocorticoids
twice a week. At the same time, the rats in the control group were
given 10 ml/kg normal saline in gluteus muscles once a day for a
total of 6 weeks.
At the same time, the epimedium group was given 10
ml/kg of epimedium extract with crude drug concentration of 1.5
g/ml, and the control and glucocorticoid groups received 10 ml/kg
normal saline by gavage for 6 consecutive weeks. The rats received
anesthesia with 10% chloral hydrate (0.4 g/kg, i.p.) and then were
sacrificed by cervical dislocation.
Hematoxylin and eosin (H&E)
staining
The femoral head was put at one side, cut along the
coronal plane and put into 10% formalin solution for the fixation
for 1 week. Then the decalcification was performed by 5% nitric
acid, and the femoral head was rinsed with water for 24 h. After
that, a series of alcohols were used for dehydration and the
conventional paraffin embedding was performed. Afterwards, the head
was sliced up and H&E staining was conducted. Subchondral
regions were regarded as film reading areas in each group.
Pathological changes in bone trabeculae, bone marrow hematopoietic
tissues, osteoblasts, osteocytes and adipocytes in the femoral bone
and bone marrow tissues were observed under a light microscope
(BX-42; Olympus, Tokyo, Japan).
Detection of BMD
After anesthesia by 10% chloral hydrate, the anatomy
was conducted under sterile conditions, and the appearance, texture
and articular cartilage colour were observed when taking the
femoral head. We removed the surrounding muscles of the femoral
head at one side and placed the specimen on the BMD instrument with
a computer installed with bone density automatic measurement
software to detect its BMD.
Immunohistochemical staining
We took the femoral head at one side, cut along the
coronal plane and put it into 10% formalin solution for the
fixation at 4°C for 1 week. Then the decalcification was performed
by 5% nitric acid, and the femoral head was rinsed with water for
24 h. After that, a series of alcohols were used for dehydration
and the conventional paraffin embedding was performed. The head was
sliced up. Then, after the dewaxing, hydration and antigen repairs,
Bcl-2 or LC3-II antibodies were incubated at room temperature for 2
h, washed with phosphate-buffered saline (PBS) 3 times and
incubated for 10 min with biotin-labeled secondary antibodies. The
head was rinsed again, re-stained it with hematoxylin stain and
washed with running water for 1 min. Subchondral regions were
regarded as film reading areas in each group, and the staining
intensity was observed under a light microscope (BX-42;
Olympus).
Western blotting
Bone tissues (200 mg) were selected and weighed and
then cut into pieces. These tissue pieces were placed into the
pre-sterilization ceramic mortar, and the shredding tissues were
quickly crushed by a grinding hammer with liquid nitrogen. After
the liquid nitrogen fully volatilized, we added lysis solutions.
The added lysis solutions were prepared as follows:
radioimmunoprecipitation assay (RIPA) proteins containing
phenylmethylsulfonyl fluoride (PMSF) were added into 100 mg
tissues. The supernatant was extracted after centrifugation at
10,000 × g at 4°C for 20 min to determine the protein
concentration. After the installation of the electrophoresis plate,
we produced gels, and added proteins in the upper sample wells.
Then, we conducted the electrophoresis under constant pressure.
After the electrophoresis, we placed polyvinylidene difluoride
(PVDF) membranes covered by gels into the transfer buffer and
transferred membranes at 0°C under constant pressure. After the
transfer, the PVDF membrane was added with 5% skimmed milk powder
and closed at room temperature for 1 h. After that, the primary
antibody (LC3II) was added and the PVDF membranes were incubated
overnight at 4°C. The next day, the membranes were washed with
phosphate-buffered saline Tween-20 (PBST) 3 times, and then, the
secondary rabbit anti-mouse (HRP) IgG antibody (dilution, 1:2,000;
cat. no. ab6728; Abcam, Cambridge, MA, USA) was incubated at room
temperature. One hour later, the membranes were washed again 3
times. Finally, we added the developing liquid for exposure. The
developing liquid was visualised using ECL kit (Merck Millipore,
Billerica, MA, USA). The gray scale scan was conducted for the
developing bands, and data were analyzed. β-actin served as
internal control. Image J software (version 1.38; National
Institutes of Health, Bethesda, MA, USA) was used for quantitative
analysis of the blots.
Statistical analysis
Data statistical results were analyzed using
Statistical Product and Service Solutions (SPSS) 13.0 software
(SPSS, Inc., Chicago, IL, USA). Comparison between multiple groups
was done using one-way ANOVA test followed by post hoc test (Least
Significant Difference). P<0.05 indicated that the difference
was considered statistically significant.
Results
Glucocorticoid causes damage to BMD
reduced by epimedium
The BMDs in femoral tissues of rats were measured by
a BMD instrument. The results showed that there was significant
difference between the control and glucocorticoid groups
(P<0.05). There was no significant difference between the
control and epimedium groups (P>0.05), while there was
significant difference between the glucocorticoid and epimedium
groups (P<0.05) (Table I).
 | Table I.Rat BMD detection results at 6 weeks
(means ± SD). |
Table I.
Rat BMD detection results at 6 weeks
(means ± SD).
| Groups | N | BMD
(g/cm2) | F | P-value |
|---|
| Control group | 8 | 0.204±0.013 | 14.07 | <0.001 |
| Glucocorticoid
group | 8 |
0.170±0.012a |
|
|
| Epimedium group | 8 |
0.195±0.008b |
|
|
Glucocorticoid causes damage to bone
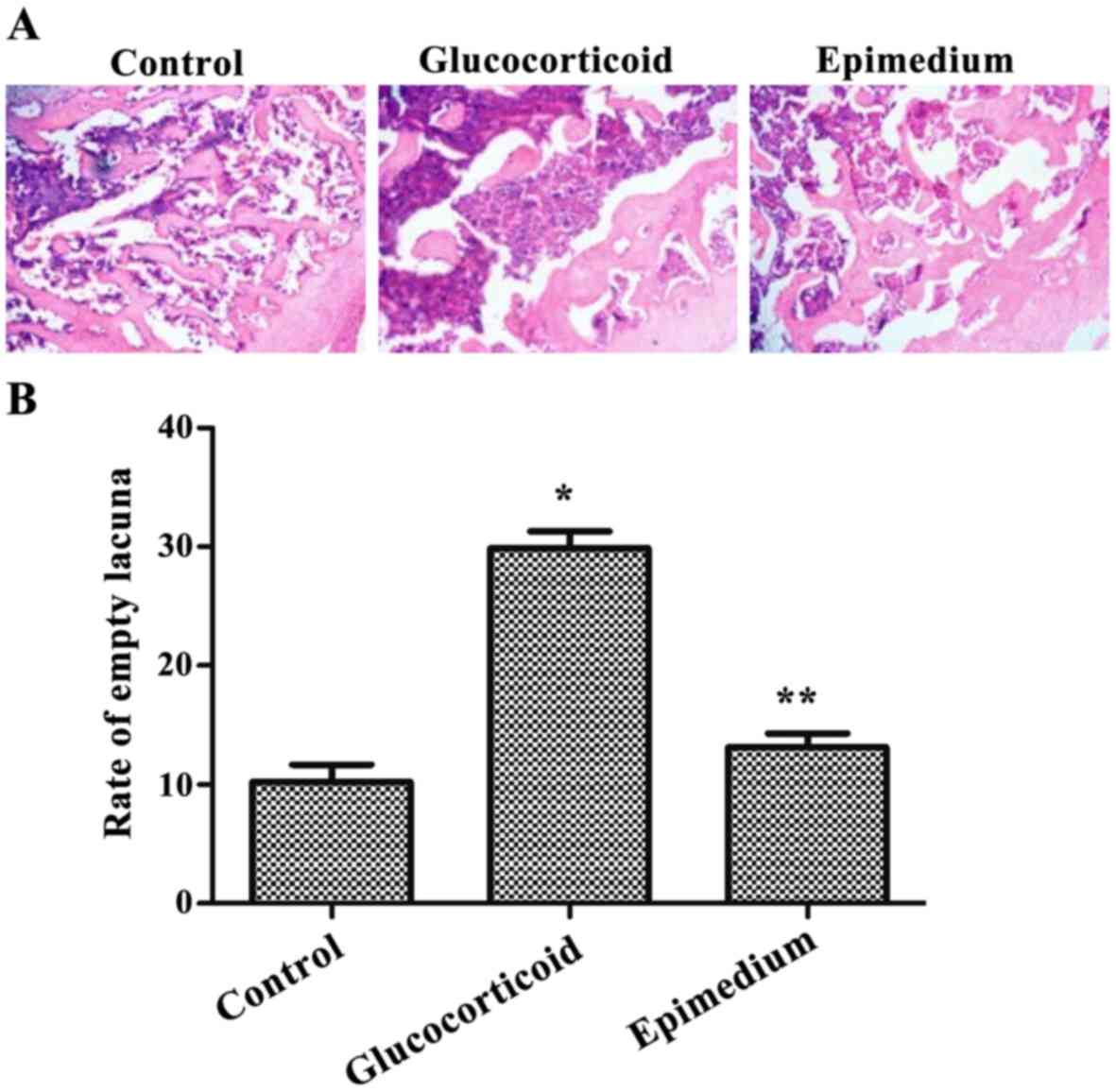
tissues reduced by epimedium
Bone tissue structures were detected by H&E
staining. The results showed that in the control group, most
trabecular bones were intact, thick, clear and regularly arranged
with low content of adipocytes, while the content of hematopoietic
cells was high. Some empty lacunae could be observed. In the
glucocorticoid group the trabecular bones were thin and loose, and
even the trabecular bone fractures were evident. Structures were
irregular and unclear, and abundant hypertrophic adipocytes were
observed in the medullary space. The content of hematopoietic cells
was decreased, and empty lacunae occupied the full vision. The
results of the epimedium group were very similar to those in the
control group (Fig. 1A). After
comparison between the control and glucocorticoid groups in empty
lacuna rate, the results showed that the empty lacuna rate of the
glucocorticoid group was 29.87±1.43%, which was significantly
higher than that in the control group (10.23±1.45%) (P<0.05).
The empty lacuna rate of the epimedium group was 13.11±1.22%, which
was significantly different from that of the glucocorticoid group
(P<0.05) (Fig. 1B). Therefore,
the above results pathologically confirmed the differences between
SANFH and normal femoral heads.
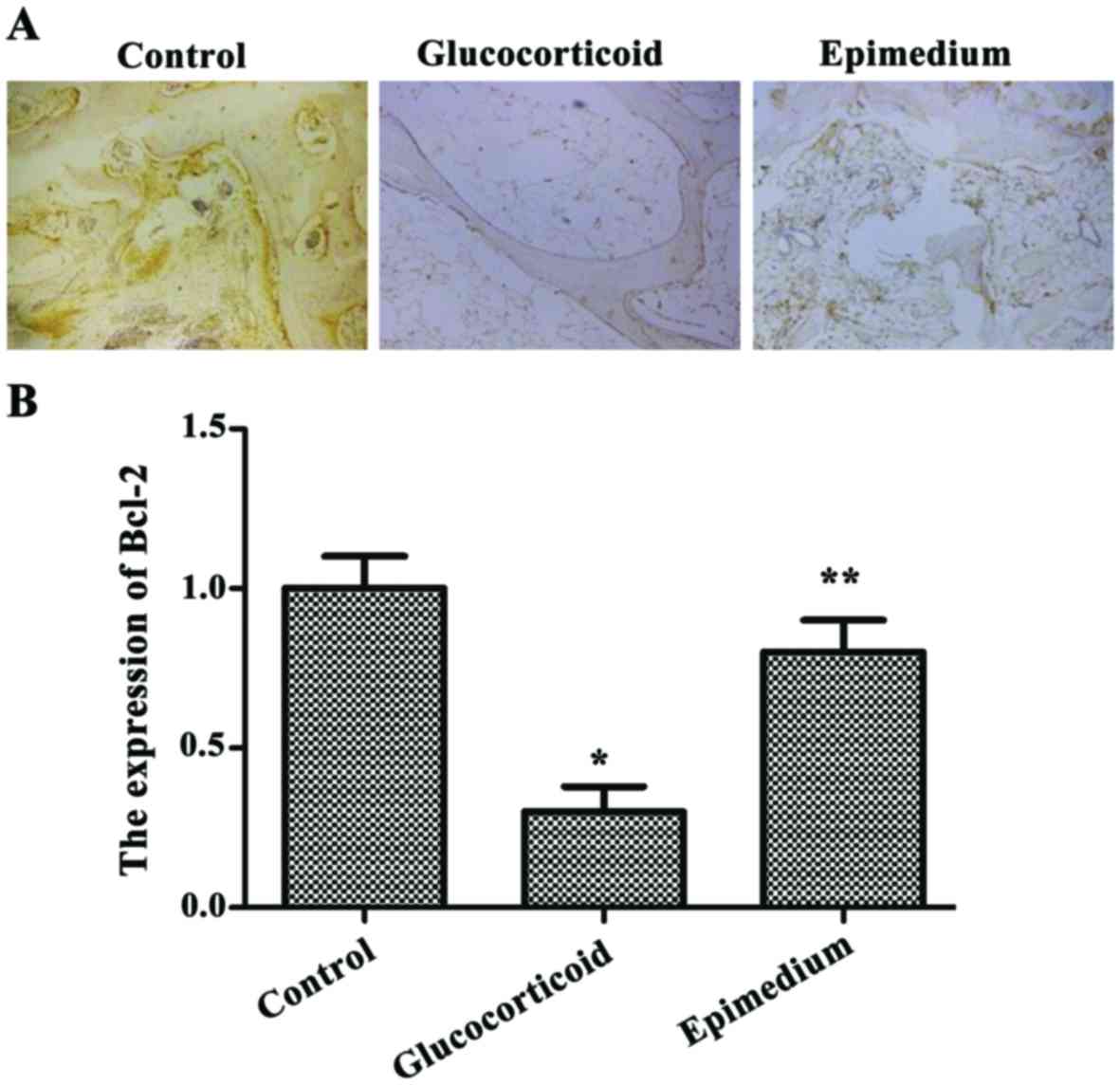
Glucocorticoid-induced bone cell
apoptosis is reduced by epimedium
Immunohistochemistry results of Bcl-2 showed that
the most positive results were located in the tan or pale brown
areas in the cytoplasm. The expression level of Bcl-2 in the
glucocorticoid group was significantly lower than that in the
control group, while the expression level of Bcl-2 in the epimedium
group was significantly higher than that in the glucocorticoid
group (P<0.05) (Fig. 2).
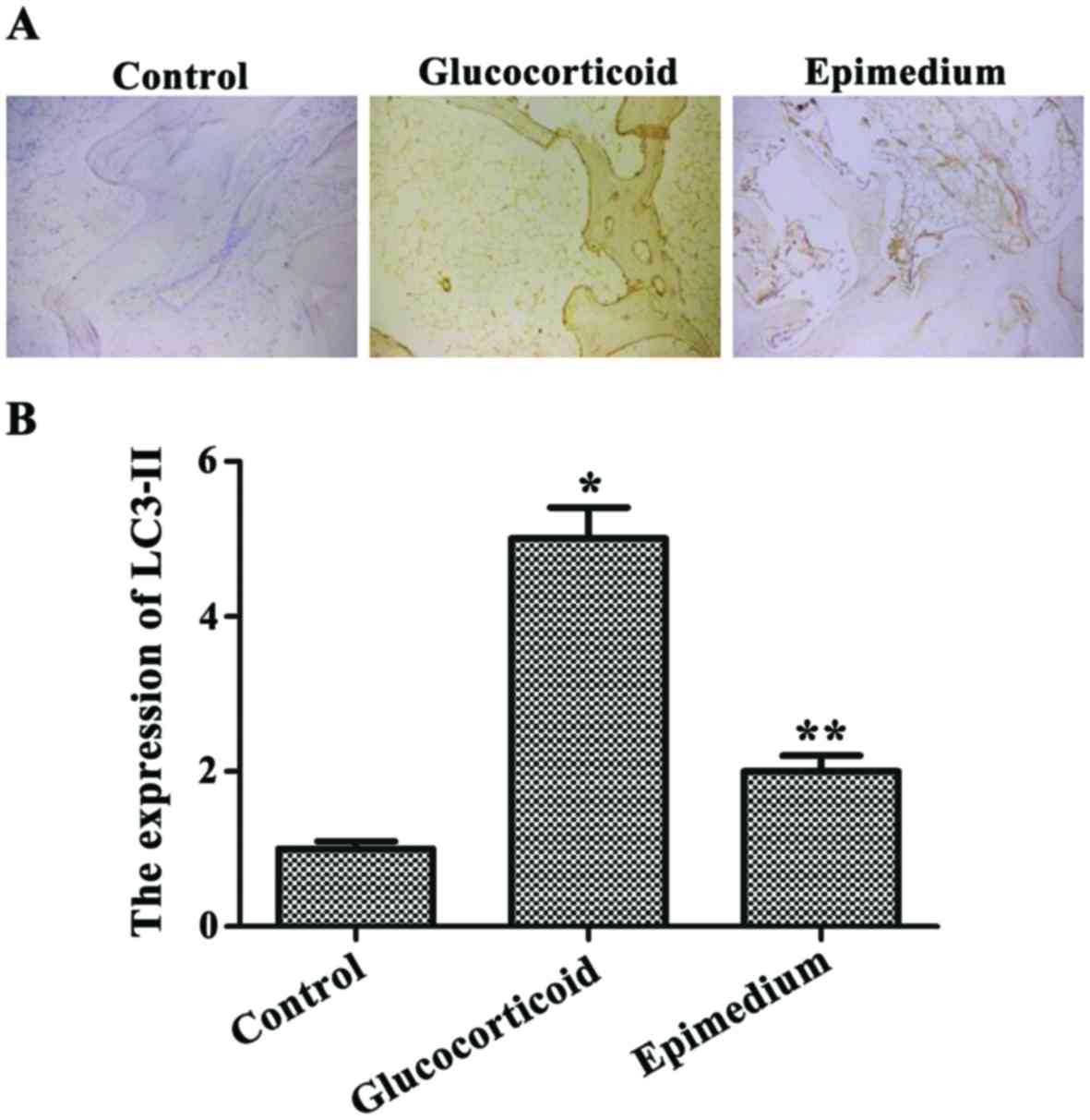
Glucocorticoid-induced
autophagy-associated protein expression level is reduced by
epimedium
Immunohistochemical results of LC3-II showed that
the most positive results were located in the tan or pale brown
areas in the cytoplasma, and there existed a small amount of
positive particles in a small area of the nuclei. The expression
level of LC3-II in the glucocorticoid group was significantly
higher than that in the control group. The expression level of
LC3-II was significantly lower in the epimedium group than that in
the glucocorticoid group, and the differences were statistically
significant (P<0.05) (Fig.
3).
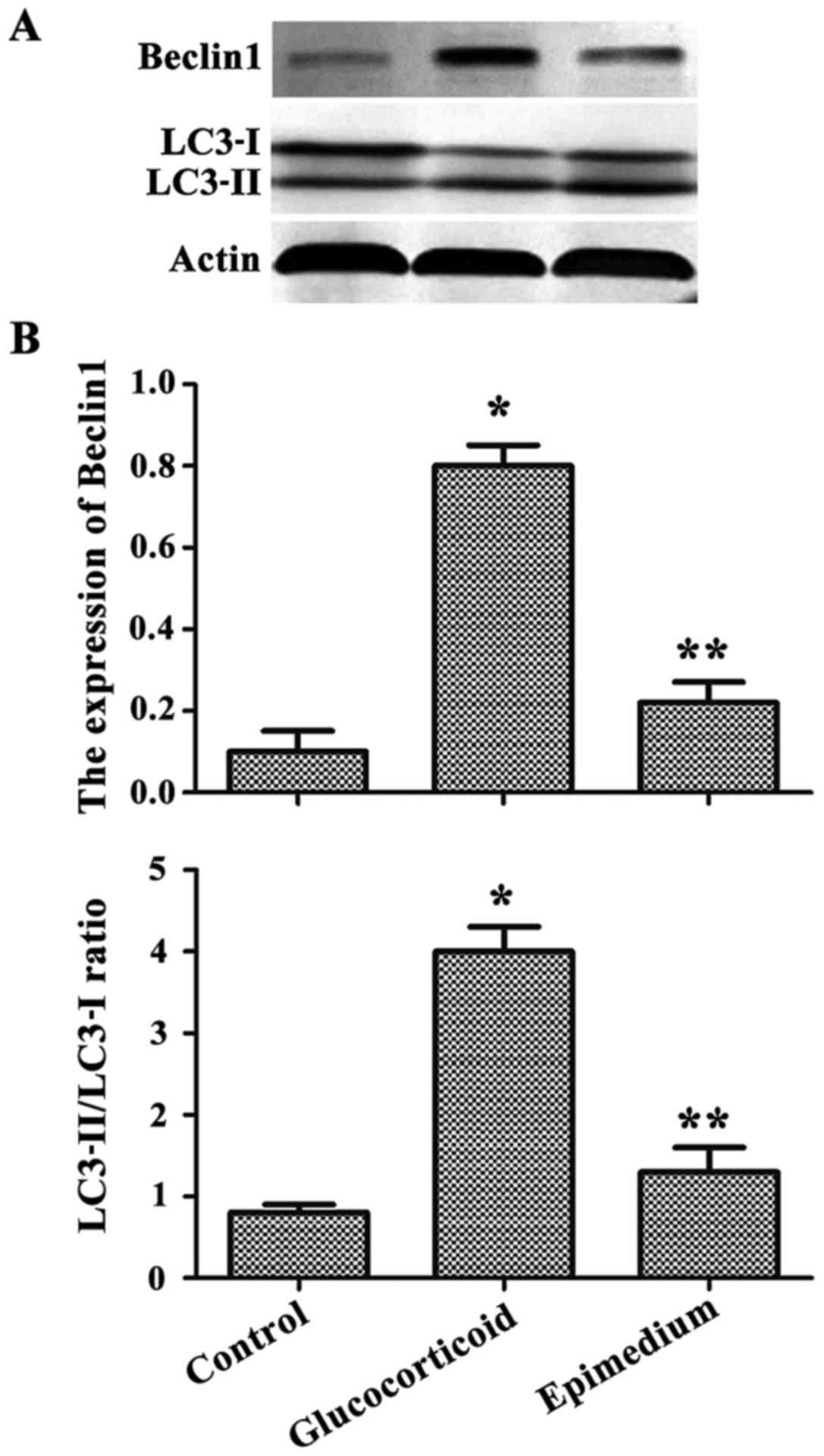
Western blotting results showed that the
concentration of autophagy-related protein Beclin1 and the ratios
of LC3-II/LC3-I were significantly increased, and in the
glucocorticoid group, the ratio of LC3-II/LC3-I was significantly
lower than that in the epimedium group (Fig. 4A). The results of gray scale scan
showed that the level of the autophagy-related protein was
significantly higher in the glucocorticoid group than that in the
control group, but the level of the autophagy-related protein in
the epimedium group was significantly lower in the glucocorticoid
group compared with the glucocorticoid group (P<0.05) (Fig. 4B).
Discussion
The first description of SANKF was recorded as early
as the 1970s. Potter et al (13) first described the symptoms of femoral
head necrosis after extensive use of glucocorticoids in kidney
transplant patients. Youm et al (14) studied 58 patients with different
types of femoral head necrosis. The study found that patients with
alcoholic avascular necrosis of femoral head and SANFH had high
expression of apoptosis in the bone cells, but patients with
idiopathic avascular necrosis of femoral head and traumatic
avascular necrosis of femoral head have low expression of
apoptosis. The present study supports the view that apoptosis plays
an important role in the pathogenesis of steroid- and
alcohol-induced avascular necroses of femoral heads. The H&E
staining results showed that the incidence rate of vacuolated
lacunae in SANFH was significantly higher than that in the normal
femoral head group. The results of immunohistochemistry showed that
the expression level of Bcl-2 protein in SANFH was significantly
lower than that in the control group. These data demonstrated that
apoptosis was involved in the pathogenesis of SANFH.
Autophagic cell death is a newly discovered method
of programmed cell death found in a molecular biology research. The
process mainly relies on the degrading pathway of lysosomes to
organelles or cytoplasmic components. It is a method by which cells
decompose their own constituents so as to maintain a stable
environment, and it is also a normal program of the body (9). Autophagy can be activated by various
forms of cellular stresses, and when the cells are subjected to the
above stresses, they initiate autophagy in order to survive
(15). In the metabolic stress
process, autophagy can produce adenosine triphosphates (ATPs) and
macromolecules to provide energy resources for cells, thus
enhancing cell viability, but when the cell stress is too strong or
lasts for a long time, cells will increase the possibility of
autophagic and programmed cell death (16). Autophagy not only plays an important
role in the survival of cells, but also can promote cell death
(17). Autophagy and apoptosis are
not irrelevant to each other's life process; instead, the two are
inextricably linked. Autophagy can cooperate with apoptosis to
induce cells to enter the death program. Studies have shown that
glucocorticoids can not only induce apoptosis in bone cells, but
also can induce autophagy (18).
These two processes are related to the dose of glucocorticoids. The
activation of relatively low doses of glucocorticoids can promote
autophagy, but that of relatively high doses of glucocorticoids
enhance apoptosis. Autophagy is likely to act as a protective
mechanism against cell death under stressful conditions with
relatively short duration or less stresses. On the contrary, if
stresses persist, autophagosomes accumulate and cause excessive
autophagy, thus resulting in cell death. This study showed that
autophagic apoptosis in SANFH exerted important effects on bone
losses.
Pharmacological tests of modern Chinese medicine
have confirmed that there are 74 active ingredients of epimedium,
mainly including TFE, epimedium polysaccharide (EPS) and ICA
(19). Modern pharmacological
studies have shown that epimedium has a certain pharmacological
effect on the blood system, immune system, cardiovascular and
cerebrovascular system, including anti-inflammatory,
anti-osteoporosis, and anti-aging (20). Epimedium has a unique advantage in
the prevention and treatment of osteoporosis and it improves BMD
(21). Studies have shown that
epimedium can increase the density of rat femora at mRNA and
protein level. It has been studied that different concentrations of
epimedium glycosides promote the growth and proliferation of
osteoblasts, and also enhance the osteoblastic activity of
osteoblasts (7). In addition, it has
been found that ICA can promote the expression of type I collagens
in osteoblasts and the synthesis of osteocalcins, and stimulates
osteoblast proliferation and differentiation cultured in
vitro (22). This study found
that epimedium could significantly alleviate SANFH and reduce
autophagic apoptosis.
In conclusion, the epimedium extracting solution can
significantly enhance BMD of the femoral heads, prevent the
collapse caused by osteoporosis, increase the expression of
apoptotic protective proteins and reduce the expression of
autophagy-related proteins, thus providing a preliminary
theoretical study for the prevention and treatment of SANFH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL and JG designed the study and performed the
experiments. SL, YH, ST and CW established the animal models. ST
and YX collected the data. YH and CW analyzed the data. SL and JG
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Soochow University Animal Center (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Drescher W, Schlieper G, Floege J and
Eitner F: Steroid-related osteonecrosis - an update. Nephrol Dial
Transplant. 26:2728–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Indira D, Snehal S, Sudha CR and Raja Babu
KK: Glucocorticosteroid-induced osteonecrosis: Lessons for the
dermatologist (CME). Indian J Dermatol Venereol Leprol. 66:173–181.
2000.PubMed/NCBI
|
|
3
|
Nowak DA and Yeung J: Steroid-Induced
osteonecrosis in dermatology: A review. J Cutan Med Surg.
19:358–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian ZJ, Liu BY, Zhang YT, Chen XZ, Qiao
GY, Wang S and Ma ZL: MiR-145 silencing promotes steroid-induced
avascular necrosis of the femoral head repair via upregulating
VEGF. Eur Rev Med Pharmacol Sci. 21:3763–3769. 2017.PubMed/NCBI
|
|
5
|
Tan XY, Gao FF, Gao ST, Liu YW, Chen XT
and Liu LY: Treatment of steroid-induced osteonecrosis of femoral
head by porous tantalum rod and gugutou huaisiyu capsule. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 36:40–43. 2016.(In Chinese). PubMed/NCBI
|
|
6
|
Xu F, Ding Y, Guo Y, Liu B, Kou Z, Xiao W
and Zhu J: Anti-osteoporosis effect of Epimedium via an
estrogen-like mechanism based on a system-level approach. J
Ethnopharmacol. 177:148–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang D, Zhang J, Fong C, Yao X and Yang
M: Herba epimedii flavonoids suppress osteoclastic differentiation
and bone resorption by inducing G2/M arrest and apoptosis.
Biochimie. 94:2514–2522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang WP, Bai XJ, Zheng XP, Xie XL and
Yuan ZY: Icariin attenuates the enhanced prothrombotic state in
atherosclerotic rabbits independently of its lipid-lowering
effects. Planta Med. 79:731–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen HM and Mizushima N: At the end of the
autophagic road: An emerging understanding of lysosomal functions
in autophagy. Trends Biochem Sci. 39:61–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Liu J, Tao Z, Wu P, Cheng W, Du Y,
Zhou N, Ge Y and Yang Z: Exogenous HGF prevents cardiomyocytes from
apoptosis after hypoxia via up-regulating cell autophagy. Cell
Physiol Biochem. 38:2401–2413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eberhardt AW, Yeager-Jones A and Blair HC:
Regional trabecular bone matrix degeneration and osteocyte death in
femora of glucocorticoid-treated rabbits. Endocrinology.
142:1333–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia X, Kar R, Gluhak-Heinrich J, Yao W,
Lane NE, Bonewald LF, Biswas SK, Lo WK and Jiang JX:
Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res.
25:2479–2488. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Potter DE, Genant HK and Salvatierra O:
Avascular necrosis of bone after renal transplantation. Am J Dis
Child. 132:1125–1129. 1978.PubMed/NCBI
|
|
14
|
Youm YS, Lee SY and Lee SH: Apoptosis in
the osteonecrosis of the femoral head. Clin Orthop Surg. 2:250–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boya P, Reggiori F and Codogno P: Emerging
regulation and functions of autophagy. Nat Cell Biol. 15:713–720.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kepp O, Galluzzi L, Lipinski M, Yuan J and
Kroemer G: Cell death assays for drug discovery. Nat Rev Drug
Discov. 10:221–237. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gurusamy N and Das DK: Is autophagy a
double-edged sword for the heart? Acta Physiol Hung. 96:267–276.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia J, Yao W, Guan M, Dai W, Shahnazari M,
Kar R, Bonewald L, Jiang JX and Lane NE: Glucocorticoid dose
determines osteocyte cell fate. FASEB J. 25:3366–3376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng S, Xiao G, Guo J, Fei Z, Xu Y, Roe BA
and Wang Y: Development of a EST dataset and characterization of
EST-SSRs in a traditional Chinese medicinal plant, Epimedium
sagittatum (Sieb. Et Zucc.) Maxim. BMC Genomics. 11:942010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai YK, Guo X, Pan YL, Niu YB, Li CR, Wu
XL and Mel QB: A systematic review of the efficacy and
pharmacological profile of Herba Epimedii in osteoporosis therapy.
Pharmazie. 68:713–722. 2013.PubMed/NCBI
|
|
21
|
Zhang DW, Cheng Y, Wang NL, Zhang JC, Yang
MS and Yao XS: Effects of total flavonoids and flavonol glycosides
from Epimedium koreanum Nakai on the proliferation and
differentiation of primary osteoblasts. Phytomedicine. 15:55–61.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen KM, Ma HP, Ge BF, Liu XY, Ma LP, Bai
MH and Wang Y: Icariin enhances the osteogenic differentiation of
bone marrow stromal cells but has no effects on the differentiation
of newborn calvarial osteoblasts of rats. Pharmazie. 62:785–789.
2007.PubMed/NCBI
|