Introduction
Bladder cancer is one of the most common urogenital
cancers worldwide and the 7th leading cause of cancer-associated
mortality (1). Approximately 79,000
new cases are diagnosed each year in the USA, of which there are an
estimated 16,800 mortalities (2).
During the last several decades, important advances have occurred
in the diagnosis and treatment of bladder cancer, which have led to
promising and significant improvements clinically; however the
survival rate of patients with late-stage disease remained
unfavorable (3). Additionally, the
underlying mechanisms of bladder cancer progression are largely
unknown (4). Therefore, studies have
focused on the molecular mechanisms of bladder cancer development
and the identification of novel molecular biomarkers (5,6).
LIM and SH3 Protein 2 (LASP2) is a member of the
nebulin family of actin-binding proteins characterized by a LIM
motif and a Src homology region 3 domain (7). LASP2 was originally identified in
chicken brains; the primary structure of LASP2 possesses a high
degree of similarity with another nebulin family member, LASP1
(8). Like LASP1, LASP2 contains an
N-terminal LIM domain, C-terminal SH3 domains and internal nebulin
repeats (8). LASP1 and LASP2 are
components of focal adhesions, and bind F-actin and zyxin (9). However, the sequence between the second
nebulin repeat and the SH3 domain of LASP2 is markedly different
from the sequence in the same area in LASP1 (8). The function of LASP2 has been linked to
its LIM and SH3 domains. The LIM domain has been identified in
diverse cellular functions in cell adhesion and signal transduction
(10). The SH3 domain is
characterized as a conserved sequence in the viral adaptor protein
v-Crk, which is associated with the dynamics of the actin
cytoskeleton (11). Therefore, the
role of LASP-2 has been focused on focal adhesion function and
organization. For example, Deng et al (12) revealed that LASP2 could increase the
rate of attachment and migration of fibroblasts on
fibronectin-coated surfaces, and may serve a role in cell
proliferation. Recently, attention has focused on the function of
LASP2 in tumorigenesis and tumor development (13,14).
However, the role of LASP2 in bladder cancer has not yet been
elucidated.
In the present study, it was revealed that LASP2 was
downregulated in bladder cancer, and that LASP2 expression was
associated with the clinicopathological features of patients with
bladder cancer. Overexpression of LASP2 effectively suppressed,
while LASP2 depletion promoted the proliferation, migration and
invasion of bladder cancer cells, and angiogenesis in bladder
cancer. Furthermore, it was demonstrated that the Wnt/β-catenin
signaling pathway is associated with the tumor-suppressing effects
of LASP2 in bladder cancer cells. Therefore, the data from the
present study highlighted the biological role of LASP2 in bladder
cancer progression, and suggested that it has potential value as a
therapeutic target for this disease.
Materials and methods
Cell lines
The bladder cancer cell line EJ (cat. no. CL-0274)
was obtained from Procell Life Science and Technology Co., Ltd.
(Wuhan, China), and 5637 (cat. no. TCHu 1), UM-UC-3 (cat. no. TCHu
217), T24 (cat. no. SCSP-536) and TCCSUP (cat. no. SCSP-571)
bladder cancer cell lines were obtained from the Cell Bank of
Chinese Academy of Sciences (Shanghai, China). The cells were
maintained as described previously (15). Normal urothelial cells (provided by
Huazhong University of Science and Technology, Wuhan, China) were
obtained from fresh human bladder samples. All the cancer cell
lines were cultured in RPMI 1640 medium (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a 5% CO2 atmosphere.
Tissue samples
A total of 196 bladder cancer RNA samples, which
were retrieved from the archives of The First People's Hospital of
Jingmen (Jingmen, China) were used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. A total of 60 pairs of snap-frozen bladder cancer and
normal adjacent-noncancerous tissues (48 male and 12 female, aged
from 43–70 years) for RT-qPCR analysis were obtained from The First
People's Hospital of Jingmen between January 2016 and February
2017. All patients were pathologically staged according to the
American Joint Committee on Cancer's classification system on TNM
staging (16). For the use of these
clinical materials for research purposes, informed consent from all
patients and approval from the Institutional Research Ethics
Committee of The First People's Hospital of Jingmen were
obtained.
Western blotting analysis
Western blotting was performed according to standard
methods as previously described (14). Cells and tissues were lysed with
radioimmunoprecipitation assay lysis buffer (Biyuntian
Biotechnology Research Institute, Nantong, China). The protein
concentration of each lysate was determined with the BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). Total proteins (30
µg/lane) were separated by 10% SDS-PAGE and transferred to a
polyvinylidene difluoride (PVDF) membrane. The blots were blocked
in 5% milk for 1 h at room temperature. PVDF membranes were
incubated using rabbit anti-LASP2 (1:100; cat. no. 260630;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), anti-cyclin D1
(1:10,000; cat. no. ab134175; Abcam, Cambridge, UK) and
anti-β-catenin (1:1,000; cat. no. ab22656; Abcam) antibodies
overnight at 4°C. Subsequently, membranes were incubated with
corresponding horseradish peroxidase-conjugated goat anti-rabbit
secondary antibodies (1:5,000; cat. no. ab6112; Abcam) for 2 h at
room temperature and detected using an enhanced chemiluminescence
reagent (ECL Prime; GE Healthcare, Chicago, IL, USA). The
anti-α-tubulin (1:5,000; cat. no. ab4074; Abcam,) and anti-P84
(1:10,000; cat. no. ab131268; Abcam) antibodies were used as
loading controls.
RNA extraction and RT-qPCR
Total RNA from cells and tissue samples were
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), followed by the reverse transcription of RNA
into cDNA using PrimeScript™ RT Master Mix (Takara
Biotechnology Co., Ltd., Dalian, China). The temperature protocol
used for reverse transcription was: 25°C for 10 min; 42°C for 50
min; and 70°C for 15 min. A SYBR PCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to amplify the
first-strand cDNA following the manufacturer's protocol.
Thermocycling was performed under the following conditions: 95°C
for 5 min followed by 40 cycles of amplification at 95°C for 15 sec
and 60°C for 60 sec. GAPDH was used as an endogenous control. All
samples were normalized to controls and fold changes were
calculated by the 2−ΔΔCq method (17). The sequences of the primers are
presented in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| LASP2 |
CATTCCCAAGGCTATGGCTA |
ATCGTACATGGCTCGGTAGG |
| c-Myc |
GGCTCCTGGCAAAAGGTCA |
CTGCGTAGTTGTGCTGATGT |
| c-Jun |
TCCAAGTGCCGAAAAAGGAAG |
CGAGTTCTGAGCTTTCAAGGT |
| CCND1 |
CAATGACCCCGCACGATTTC |
CATGGAGGGCGGATTGGAA |
| CTLA4 |
CATGATGGGGAATGAGTTGACC |
TCAGTCCTTGGATAGTGAGGTTC |
| LEF1 |
ATGTCAACTCCAAACAAGGCA |
CCCGGAGACAAGGGATAAAAAGT |
| TCF1 |
TTGATGCTAGGTTCTGGTGTACC |
CCTTGGACTCTGCTTGTGTC |
| AXIN2 |
ACTGCCCACACGATAAGGAG |
CTGGCTATGTCTTTGGACCA |
| GAPDH |
TCAAGAAGGTGGTGAAGCAG |
CGTCAAAGGTGGAGGAGTG |
Vectors construction and siRNA
transfection
T24 and EJ cells were seeded in 48-well plates at a
density of 2×105/well. The full-length human LASP2 cDNA
was amplified by PCR and cloned into pENTER vector (Shanghai
GenePharma Co., Ltd., Shanghai, China). LASP2 siRNAs and scramble
control siRNAs were synthesized from Shanghai GenePharma Co., Ltd.
The siRNA sequences used to knockdown LASP2 were as follows:
5′-GUCCUAUGCUAAACCAUGUTT-3′ and 5′-ACAUGGUUUAGCAUAGGACTT-3′, and
the scramble control siRNA sequences were:
5′-UUUAUAGGCAGCAUCGCUGAC-3′ and 5′-CAAUGCAGCAUUCACCAAATT-3′. The
plasmid and LASP2-siRNA were transfected into cells at a final
concentration of 1 mg/ml and 100 nM, respectively, by using
Lipofectamine 2000 reagents (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Knockdown was
assessed by RT-qPCR after 48 h of transfection.
Labeling with
5-ethynyl-2′-deoxyuridine (EdU)
Cell proliferation assays were carried out using EdU
labeling. Cells (1×105/well) were plated in 96-well
plates and cultured in RPMI 1640 medium. At 70–80% confluence,
cells were incubated with 50 µM EdU (cat. no. R11053; Guangzhou
RiboBio Co., Ltd., Guangzhou, China) for 3 h at 37°C and treated
with 100 µl 1X ApolloR reaction cocktail (Guangzhou RiboBio Co.,
Ltd.) according to the manufacturer's protocol. Finally, DAPI (1
µg/ml; Sigma-aldrich; Merck KGaA) was added to the slide, and
stained for 20 min at room temperature. All images were captured
via fluorescence microscopy (magnification, ×20). ImageJ 1.47
(National Institutes of Health, Bethesda, MD, USA) was used to
analyze the resulting images.
Wound healing assay
T24 and EJ cells (5×105 cells/well) were
seeded in 12-well plates and grown in RPMI 1640 medium at 37°C in a
5% CO2 atmosphere. A scratch was created on each
confluent monolayer using a 200-µl plastic pipette tip and the
cells were then incubated in serum-free RPMI 1640 medium at room
temperature for 24 h. The wound closure was calculated by examining
the distance between the opposite edges of the wound using a light
microscope (magnification, ×20). Each sample was assayed in
triplicate. ImageJ 1.47 was used to analyze the resulting
images.
Transwell cell migration assay
The migration of cells was assessed using 24-well
Transwell chambers with 8-µm pore membranes (Corning Life Sciences,
Corning, NY, USA). A total of 200 µl cell medium (1×105
cells/100 µl in RPMI 1640 medium) were added to the upper chamber
of each Transwell chamber. Medium containing 10% FBS (HyClone; GE
Healthcare Life Sciences) was added to the lower chamber. After
incubating the cells for 24 h, the cells were fixed in 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) at 0°C for 20 min and
stained with 0.1% crystal violet for 15 min at 25°C for
visualization using a light microscope (magnification, ×20).
Migrated cells were measured by image analysis software (ImagePro
Plus 6.0; Media Cybernetics, Inc., Rockville, MD, USA). Experiments
were performed in triplicate. For cell invasion experiments,
Matrigel (BD Biosciences) was plated inside Transwell culture
inserts, with the same protocol being followed.
Human umbilical vein endothelial cells
(HUVECs) tube formation assay
Precooled Matrigel (Corning Life Sciences) was
plated into each well of a 24-well plate and allowed to set into a
gel for 30 min at 37°C. HUVECs (5×104/well; Cell Bank of
the Chinese Academy of Sciences, Shanghai, China) in 200 µl F-12K
medium (Hyclone; GE Healthcare Life Sciences) were added to each
well and incubated at 37°C in 5% CO2 for 20 h. The
capillary tube structure was photographed using a light microscope
(magnification, ×20) and quantified by measuring the total length
of the completed tubes. ImageJ 1.47 was used to analyze the
resulting images.
Luciferase reporter assay
Cells (1×105 cells/well) were seeded in
96-well plates and allowed to settle for 12 h at 37°C. Reporter
plasmids (100 ng; EMD Millipore, Billerica, MA, USA) containing
wild-type (CCTTTGATC; TOP flash) or mutated (CCTTTGGCC; FOP flash)
T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) binding
sites used to determine β-catenin transcriptional activity
(18) plus 1 ng pRL-TK Renilla
plasmid were co-transfected using Lipofectamine 2000 according to
the manufacturer's protocol. Firefly and Renilla luciferase
activity were measured 48 h following transfection using the Dual
Luciferase Reporter Assay kit (Promega Corporation, Madison, WI,
USA) according to manufacturer's protocol. Luciferase activity was
normalized to Renilla luciferase activity. All experiments were
performed in triplicate.
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The one-way analysis of variance was
performed, followed by Student-Newman-Keuls post-hoc test. The
association between LASP2 expression and the clinicopathological
characteristics was analyzed via Pearson's χ2 test. The
overall survival was calculated as the time from the date of
primary surgery to the date of death or the date of the last
follow-up. Recurrent-free survival was defined as the time from the
date of primary surgery to the date of first recurrence or the date
of the last follow-up. Kaplan-Meier analysis and log-rank test were
used to perform survival analyses. The clinicopathological
characteristics was evaluated by two pathologists, and the
association between variables (sex, age, tumor grade, tumor size, T
classification using the TNM staging system and LASP2 expression
level) survival rates were analyzed by the Cox proportional hazards
model for univariate and multivariate analyses. P<0.05 indicated
that the difference between groups was statistically
significant.
Results
LASP2 is downregulated in patients
with bladder cancer
To reveal the function of LASP2 in bladder cancer,
RT-qPCR was performed to examine the LASP2 expression in bladder
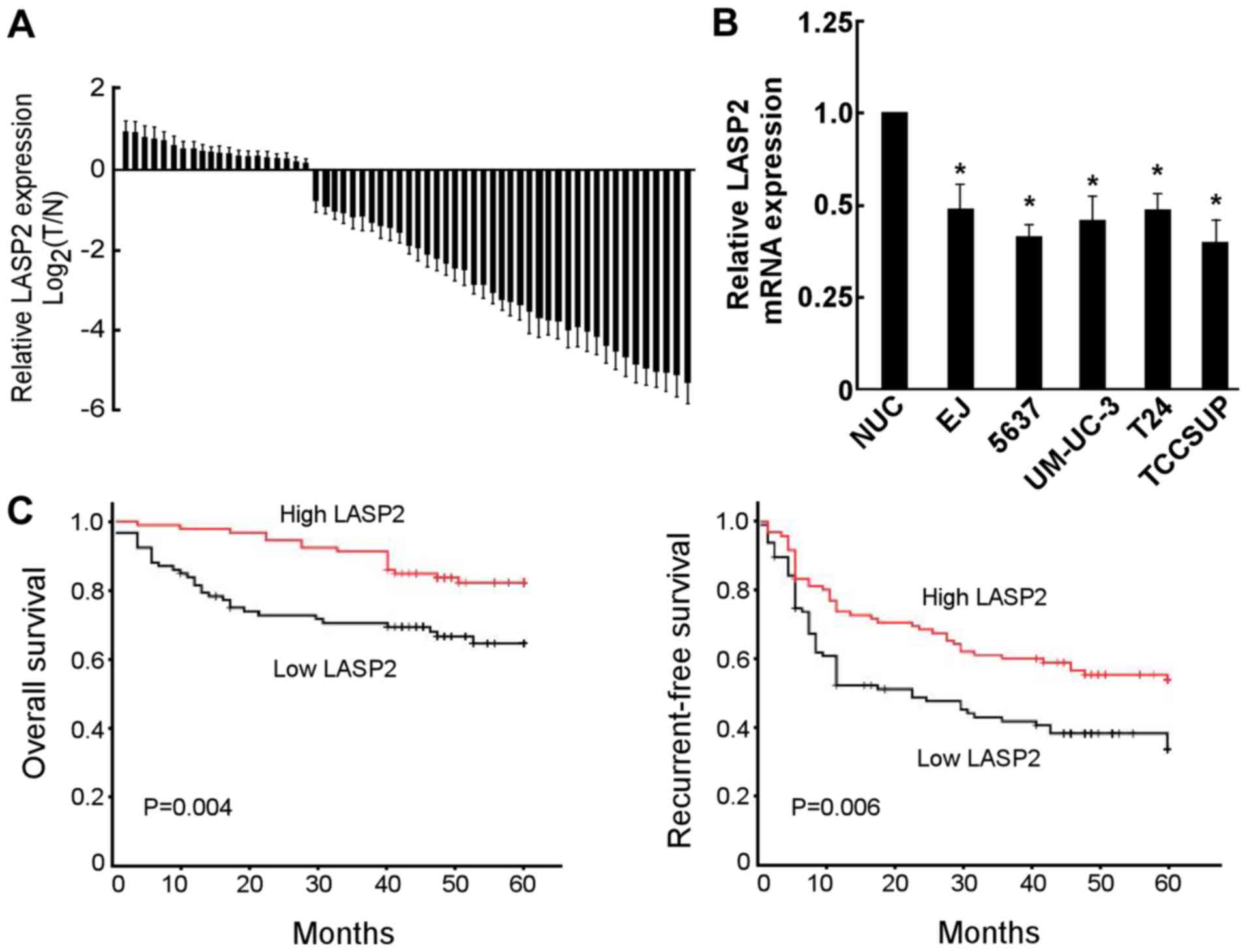
cancer tissues and cell lines. As presented in Fig. 1A, LASP2 mRNA expression was decreased
in 75.0% (45/60) of patients. Furthermore, LASP2 was significantly
decreased in bladder cancer cell lines compared with normal
urothelial cells (Fig. 1B).
LASP2 is associated with the
clinicopathological characteristics of patients with bladder
cancer
The association between LASP2 expression and
clinicopathological features was further investigated in bladder
cancer patients. Statistical analyses revealed that the expression
of LASP2 was significantly associated with tumor size and tumor
stage (Table II). In the
Kaplan-Meier analysis, the cut-off point (relative value, −2.34) of
LASP2 mRNA expression was defined as the median (low LASP2 group,
49 months; high LASP2 group, 48 months). The results revealed that
the overall and recurrent-free survival times of patients with low
LASP2 expression was significantly shorter compared with those with
high LASP2 expression (Fig. 1C).
Univariate and multivariate analyses revealed that LASP2 expression
was an independent prognostic factor for overall and recurrent-free
survival in patients with bladder cancer (Tables III and IV).
 | Table II.Association between LASP2 mRNA
expression and clinicopathological characteristics. |
Table II.
Association between LASP2 mRNA
expression and clinicopathological characteristics.
|
|
| LASP2 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | Patients (n) | Low | High | χ2 | P-value |
|---|
| Sex |
|
|
| 0.022 | 0.883 |
|
Male | 121 | 60 | 61 |
|
|
|
Female | 75 | 38 | 37 |
|
|
| Age (years) |
|
|
| 1.649 | 0.775 |
|
≤60 | 92 | 47 | 45 |
|
|
|
>60 | 104 | 51 | 53 |
|
|
| Tumor grade |
|
|
| 0.191 | 0.662 |
|
Low | 117 | 57 | 60 |
|
|
|
High | 79 | 41 | 38 |
|
|
| Tumor size |
|
|
| 5.819 | 0.016a |
| <3
cm | 143 | 64 | 79 |
|
|
| ≥3
cm | 53 | 34 | 19 |
|
|
| T
classification |
|
|
| 11.056 | 0.001a |
| Ta and
T1 | 130 | 54 | 76 |
|
|
|
T2-T4 | 66 | 44 | 22 |
|
|
| Total | 196 | 98 | 98 |
|
|
 | Table III.Univariate analysis in patients with
bladder cancer. |
Table III.
Univariate analysis in patients with
bladder cancer.
|
| Overall
survival | Recurrent-free
survival |
|---|
|
|
|
|
|---|
| Prognostic
variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex (male vs.
female) | 1.576
(0.832–2.986) | 0.163 | 1.353
(0.854–2.875) | 0.255 |
| Age (>60 vs. ≤60
years) | 1.343
(0.750–2.405) | 0.321 | 1.028
(0.695–1.520) | 0.892 |
| Tumor grade (high
vs. low) | 2.784
(1.383–5.640) | 0.004a | 2.569
(1.024–2.406) | 0.039a |
| Tumor size (≥3 vs.
<3 cm) | 1.275
(0.535–2.634) | 0.024a | 1.652
(0.936–2.909) | 0.082 |
| T classification
(T2-4 vs. T1 and Ta) | 1.333
(0.744–2.387) | 0.033a | 1.779
(0.509–2.195) | 0.025a |
| LASP2 (high vs.
low) | 0.421
(0.220–0.771) | 0.005a | 0.445
(0.734–0.782) | 0.012a |
 | Table IV.Multivariate analysis in patients
with bladder cancer. |
Table IV.
Multivariate analysis in patients
with bladder cancer.
|
| Overall
survival | Recurrent-free
survival |
|---|
|
|
|
|
|---|
| Prognostic
variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex (male vs.
female) | – | – | – | – |
| Age (>60 vs. ≤60
years) | – | – | – | – |
| Tumor grade (high
vs. low) | 2.861
(1.576–5.196) | 0.001 | – | – |
| Tumor size (≥3 vs.
<3 cm) | – | – | – | – |
| T classification
(T2-4 vs. T1 and Ta) | 1.924
(1.024–3.614) | 0.042 | – | – |
| LASP2 (high vs.
low) | 0.298
(0.154–0.579) | <0.001 | 0.585
(0.394–0.870) | 0.008 |
LASP2 inhibits bladder cancer cell
proliferation, migration and invasion, and angiogenesis in
vitro
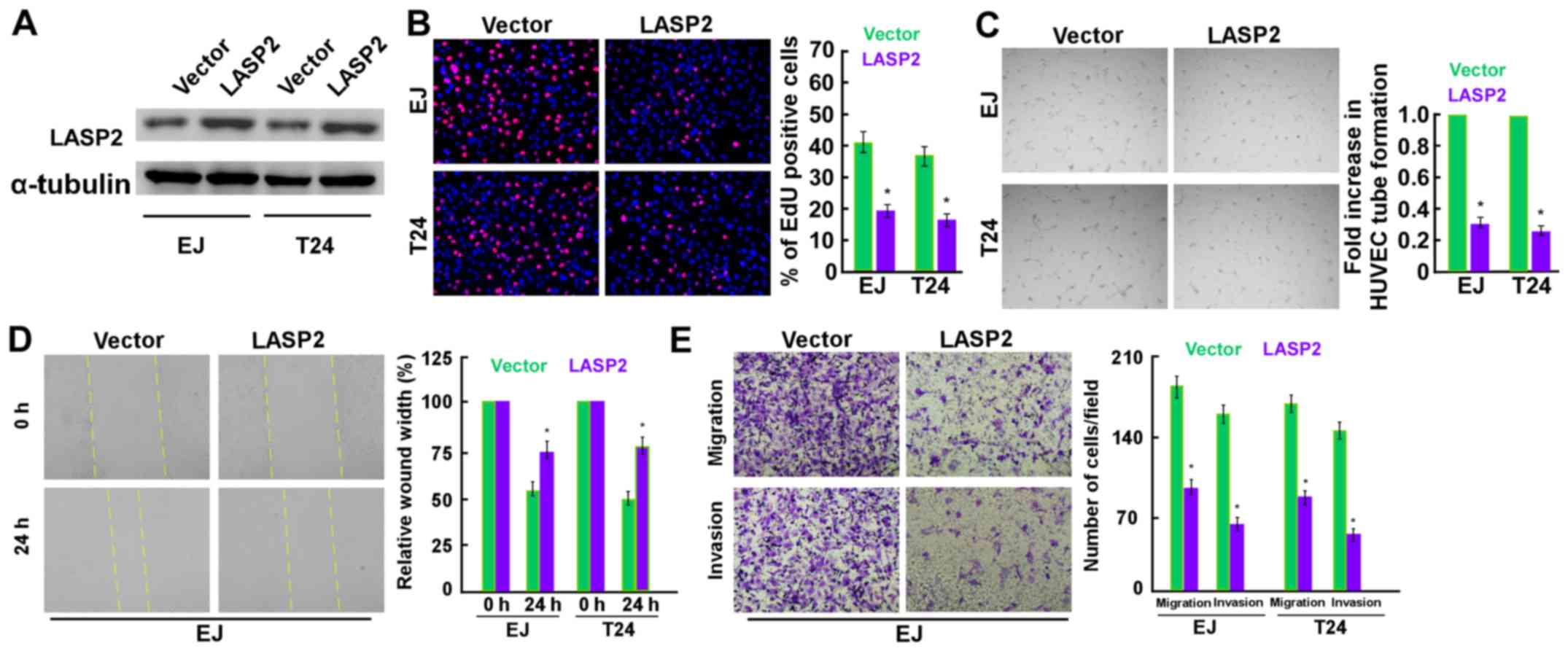
The effects of LASP2 on cell proliferation,
migration and invasion, and angiogenesis were then evaluated in EJ
and T24 cells through overexpressing and inhibiting LASP2
expression (Figs. 2 and 3). LASP2 overexpression was confirmed via
western blotting (Fig. 2A). The EdU
labeling assay revealed that upregulated LASP2 expression inhibited
the proliferation of the two cell lines (Fig. 2B). In addition, the overexpression of
LASP2 significantly decreased the ability of bladder cancer cells
to induce tube formation by HUVECs (Fig.
2C). The results of wound healing and Transwell migration assay
suggested that tumor migration was significantly decreased
following the transfection of LASP2 plasmids into EJ cells
(Fig. 2D and E). Also, the Matrigel
invasion assay suggested that tumor invasion decreased following
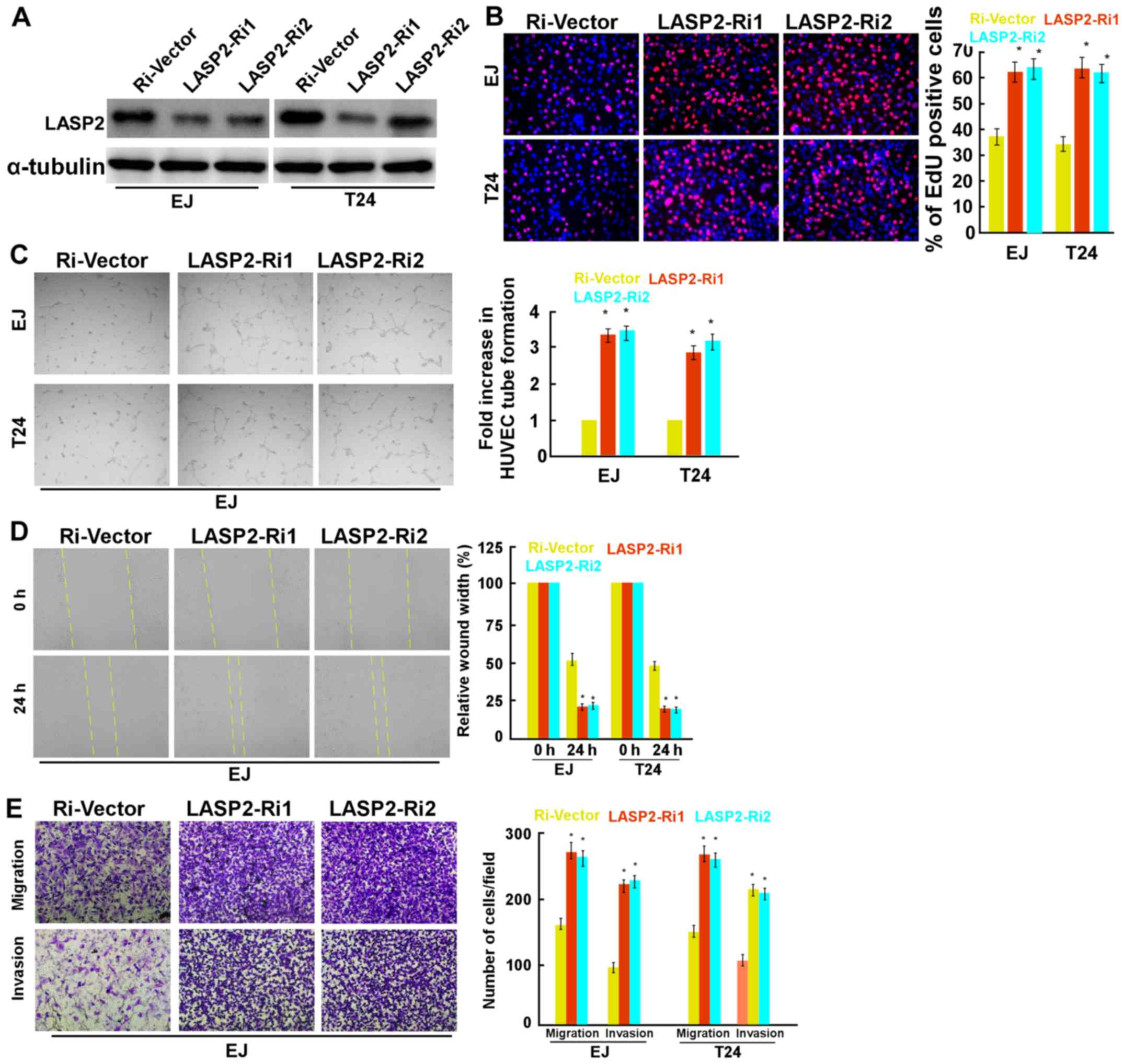
the transfection of LASP2 plasmids into EJ cells (Fig. 2E). In contrast, LASP2 inhibition
significantly promoted cell proliferation, invasion and migration
and angiogenesis (Fig. 3).
LASP2 mediates the Wnt/β-catenin
signaling pathway in bladder cancer cells
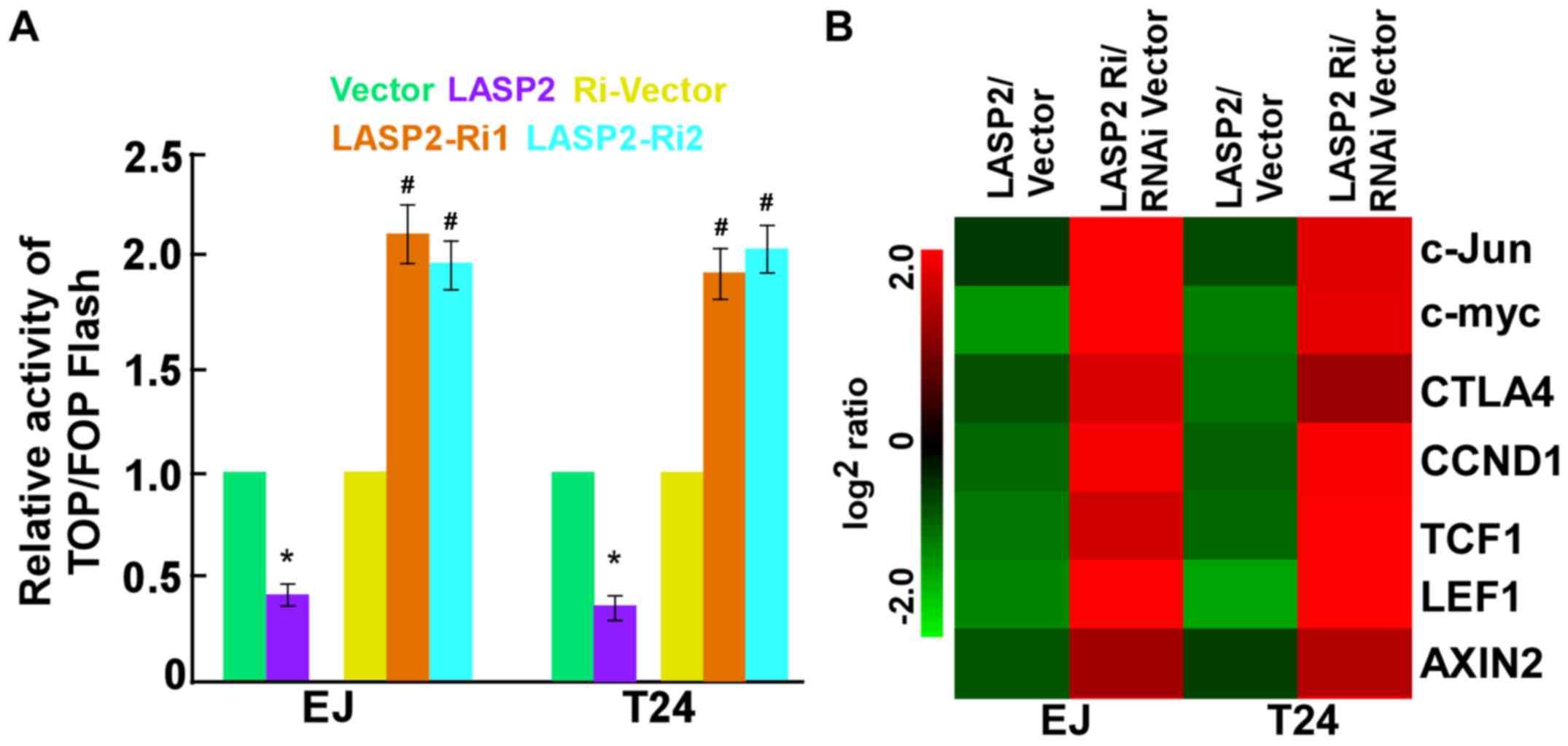
The molecular mechanism by which LASP2 exerts its
biological function was further explored. As presented in Fig. 4A, LASP2 upregulation significantly
inhibited, whereas LASP2 knockdown significantly increased the
transactivation activity of β-catenin/TCF in the two cell lines
using the luciferase reporter assay. Furthermore, the expression
levels of downstream targets of the Wnt/β-catenin signaling
pathway, including c-Myc, c-Jun, cyclin D1 (CCND1), cytotoxic T
lymphocyte associated protein 4, lymphoid enhancer binding factor
1, T cell factor 1 and AXIN2 were markedly lower in
LASP2-overexpressing tumor cells compared with that in
LASP2-silenced cells (Fig. 4B).
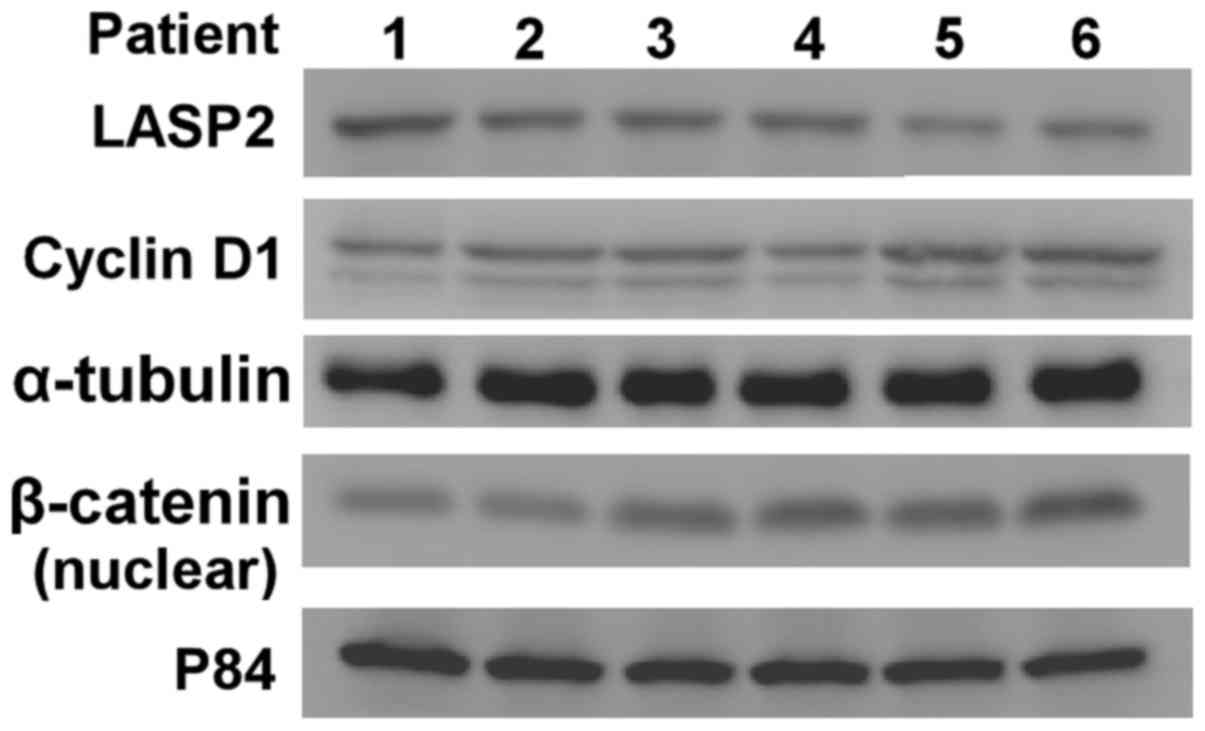
Western blotting assays were also performed to demonstrate that
LASP2 expression was negatively associated with CCND1expression and
nuclear β-catenin expression (Fig.
5).
Discussion
The results of the current study demonstrated that
LASP2 functions as a tumor suppressor in bladder cancer. Low LASP2
expression was frequently detected in bladder cancer, and was
associated with larger tumor size, advanced TNM staging (i.e. T
classification) and poor prognosis. Additionally, the
overexpression of LASP2 attenuates the proliferation, migration and
invasion of bladder cancer cells, and angiogenesis via the
regulation of Wnt/β-catenin signaling pathway. These findings
suggest that LASP2 may serve as a prognostic marker for patients
with bladder cancer and an important role in bladder cancer
progression.
Nebulin family members, with molecular weights
ranging from 34–900 kDa, have diverse expression patterns and
cellular functions (19). The
majority of nebulin family members contain the defining
characteristic of actin-binding domains referred to as ‘nebulin
repeats’ through which the members form multi-domain proteins to
interact with filamentous actin (20). The nebulin protein family has been
proposed to serve essential physiological roles in human diseases.
For example, mutations in nebulin were demonstrated to be the main
cause of the human muscle disorder nemaline myopathy, and
subsequent distal and core-rod myopathies (21). The abnormal expression of nebulette
or N-RAP genes have been associated with dilated cardiomyopathy
(22). As a shorter splice variant
of nebulette, LASP2 is a novel nebulin family member (23). In cardiomyocytes, LASP2 is localized
in different cytotskeletal structures, including the intercalated
discs, Z-discs and focal adhesions, implying that LASP2 may act as
a molecular scaffold that is important for cell migration and
adhesion, and focal adhesion turnover (24,25).
Recent data revealed that LASP2 is associated with
the development of human cancers. Wang et al (14) recently demonstrated that LASP2
functions as a tumor suppressor gene in colon cancer and inhibits
cancer progression via the c-Jun N-terminal kinase/p38
mitogen-activated protein kinase signaling pathway. In contrast,
Zhang et al (13) reported
that LASP2 acts as an oncogene in non-small-cell lung carcinoma
(NSCLC) and accelerates tumor invasion through facilitating the
phosphorylation of focal adhesion kinase. These studies indicate
the diverse roles of LASP2 in different cancers. In the present
study, the downregulation of LASP2 expression in bladder cancer was
detected. Additionally, LASP2 expression was associated with larger
tumor size and advanced TNM staging. In addition, functional
experiments indicated that LASP2 could attenuate bladder cancer
aggression. Together, these findings indicate that LASP2 may
perform different functions in different cancers.
Importantly, it was demonstrated that increased
expression of LASP2 was associated with the reduction in the
nuclear translocation of β-catenin in bladder cancer cells.
Additionally, it was revealed that LASP2 downregulates the
expression of downstream genes of the Wnt/β-catenin signaling
pathway. Together, the findings revealed that the tumor-suppressing
role of LASP2 in bladder cancer may be associated with
Wnt/β-catenin signaling pathway inactivation. The findings
highlighted the importance of the Wnt/β-catenin signaling pathway
in bladder cancer progression, and may partially explain the
different roles for LASP2 in different cancer types. Notably, it
was demonstrated that the upregulation of LASP2 inhibited, whereas
the knocking down of LASP2 induced HUVEC tube formation, revealing
the role of LASP2 in angiogenesis.
The clinical significance of nebulin family proteins
has been illustrated in previous studies. Qiu et al
(26) demonstrated that nebulette
overexpression in patients with colorectal cancer was associated
with increased overall survival. Yang et al (27) suggested that LASP1 may serve as a
prognostic biomarker for patients with clear cell renal cell
carcinoma. Zhang et al (13)
reported that the expression of LASP-2 was significantly correlated
with poor prognosis in patients with NSCLC. The current study
revealed that high LASP2 expression was associated with favorable
overall and recurrent-free survival rates in patients with bladder
cancer, further suggesting that LASP2 may be a marker used to
predict the prognosis of patients with cancer.
The present study has several limitations. First,
the retrospective, observational nature of the current study with
unknown factors may affect the studied outcomes and were not
captured in the present data collection. Second, only 196 patients
were included; further studies on larger populations are required
to confirm the preliminary results of the current study.
In summary, the present study has demonstrated that
LASP2 has an important role in bladder carcinogenesis. LASP2 was
revealed to mediate bladder cancer cell proliferation and
metastasis, and angiogenesis in bladder cancer via the
Wnt/β-catenin signaling pathway. In addition, LASP2 may function as
an independent prognostic marker for bladder cancer. Thus, LASP2
could be a promising therapeutic target for the future treatment of
bladder cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
Fund of The First People's Hospital of Jingmen (grant no.
20154567).
Availability of data and materials
All data analyzed during the present study are
included in this manuscript.
Authors' contributions
RY participated in data collection and drafted the
manuscript. ZL performed the statistical analysis. YC and JK
participated in the design of the study. All authors analyzed and
interpreted the patient data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Research Ethics Committee of the The First People's Hospital of
Jingmen (Jingmen, China). All patients provided written informed
consent.
Patient consent for publication
Written informed consent was obtained from each
patient for the use of the tissue samples for paper
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madersbacher S, Hochreiter W, Burkhard F,
Thalmann GN, Danuser H, Markwalder R and Studer UE: Radical
cystectomy for bladder cancer today-a homogeneous series without
neoadjuvant therapy. J Clin Oncol. 21:690–696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Volanis D, Papadopoulos G, Doumas K,
Gkialas I and Delakas D: Molecular mechanisms in urinary bladder
carcinogenesis. J BUON. 16:589–601. 2011.PubMed/NCBI
|
|
5
|
Chen Z, Zhou L, Liu X, Wang L, Kazobinka
G, Zhang X and Hou T: Loss of Fezf2 promotes malignant progression
of bladder cancer by regulating the NF-κB signaling pathway. Lab
Invest. 98:1225–1236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soria F, D'Andrea D, Pohar K, Shariat SF
and Lotan Y: Diagnostic, prognostic and surveillance urinary
markers in nonmuscle invasive bladder cancer: Any role in clinical
practice? Curr Opin Urol. 27–Aug;2018.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Chew CS, Chen X, Parente JA Jr, Tarrer S,
Okamoto C and Qin HY: Lasp-1 binds to non-muscle F-actin in vitro
and is localized within multiple sites of dynamic actin assembly in
vivo. J Cell Sci. 115:4787–4799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terasaki AG, Suzuki H, Nishioka T,
Matsuzawa E, Katsuki M, Nakagawa H, Miyamoto S and Ohashi K: A
novel LIM and SH3 protein (lasp-2) highly expressing in chicken
brain. Biochem Biophys Res Commun. 313:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Zhuang L and Trueb B: Zyxin
interacts with the SH3 domains of the cytoskeletal proteins
LIM-nebulette and Lasp-1. J Biol Chem. 279:20401–20410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kadrmas JL and Beckerle MC: The LIM
domain: From the cytoskeleton to the nucleus. Nat Rev Mol Cell
Biol. 5:920–931. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stradal TE and Scita G: Protein complexes
regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol.
18:4–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng XA, Norris A, Panaviene Z and Moncman
CL: Ectopic expression of LIM-nebulette (LASP2) reveals roles in
cell migration and spreading. Cell Motil Cytoskeleton. 65:827–840.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Cai L, Zhou H, Liu Y, Fan C, Wang
L, Li A, Miao Y, Li Q, Qiu X and Wang E: Lasp2 enhances tumor
invasion via facilitating phosphorylation of FAK and predicts poor
overall survival of non-small cell lung cancer patients. Mol
Carcinog. 56:2558–2565. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Zhang L and Zhao L, Zhou R, Ding
Y, Li G and Zhao L: LASP2 suppresses colorectal cancer progression
through JNK/p38 MAPK pathway meditated epithelial-mesenchymal
transition. Cell Commun Signal. 15:212017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang C, Zhang W, Wang L, Kazobinka G, Han
X, Li B and Hou T: Musashi-2 promotes migration and invasion in
bladder cancer via activation of the JAK2/STAT3 pathway. Lab
Invest. 96:950–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G and McKenney JK: Urinary bladder
pathology: World Health Organization (WHO) classification and
american joint committee on cancer (AJCC) staging update. Arch
Pathol Lab Med. 25–Jul;2018.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kazmierski ST, Antin PB, Witt CC, Huebner
N, McElhinny AS, Labeit S and Gregorio CC: The complete mouse
nebulin gene sequence and the identification of cardiac nebulin. J
Mol Biol. 328:835–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pappas CT, Bliss KT, Zieseniss A and
Gregorio CC: The Nebulin family: An actin support group. Trends
Cell Biol. 21:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romero NB, Lehtokari VL, Quijano-Roy S,
Monnier N, Claeys KG, Carlier RY, Pellegrini N, Orlikowski D,
Barois A, Laing NG, et al: Core-rod myopathy caused by mutations in
the nebulin gene. Neurology. 73:1159–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ehler E, Horowits R, Zuppinger C, Price
RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM and
Perriard JC: Alterations at the intercalated disk associated with
the absence of muscle LIM protein. J Cell Biol. 153:763–772. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katoh M and Katoh M: Identification and
characterization of LASP2 gene in silico. Int J Mol Med.
12:405–410. 2003.PubMed/NCBI
|
|
24
|
Zieseniss A, Terasaki AG and Gregorio CC:
Lasp-2 expression, localization, and ligand interactions: A new
Z-disc scaffolding protein. Cell Motil Cytoskeleton. 65:59–72.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panaviene Z and Moncman CL: Linker region
of nebulin family members plays an important role in targeting
these molecules to cellular structures. Cell Tissue Res.
327:353–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu X, Feng JR, Wang F, Chen PF, Chen XX,
Zhou R, Chang Y, Liu J and Zhao Q: Profiles of differentially
expressed genes and overexpression of NEBL indicates a positive
prognosis in patients with colorectal cancer. Mol Med Rep.
17:3028–3034. 2018.PubMed/NCBI
|
|
27
|
Yang F, Zhou X, Du S, Zhao Y, Ren W, Deng
Q, Wang F and Yuan J: LIM and SH3 domain protein 1 (LASP-1)
overexpression was associated with aggressive phenotype and poor
prognosis in clear cell renal cell cancer. PLoS One. 9:e1005572014.
View Article : Google Scholar : PubMed/NCBI
|