Introduction
The success of CD19-targeted chimeric antigen
receptor (CAR) T-cells in hematopoietic malignancies has led to
further research surrounding the use of adoptive immunotherapy in
the treatment of solid tumors (1).
Carcinoembryonic antigen (CEA)-targeted CAR T-cells have previously
been used to treat liver and peritoneal metastases of colorectal
cancer (CRC) (2–4). However, the application of CAR T-cells
for the treatment of solid tumors is limited due to the inherent
immunosuppressive tumor microenvironment (5).
Currently, several studies have demonstrated that
when tumor cells are exposed to hypoxic conditions,
immunosuppressive molecules including programmed cell death
ligand-1 (PD-L1), vascular endothelial growth factor (VEGF),
cyclooxygenase-2 (COX-2), galectin-1, interleukin (IL)-10 and
transforming growth factor-1 (TGF-β1) are upregulated (6–10). These
immunosuppressive factors serve an important role in the
immunosuppressive tumor microenvironment inducing T-cell apoptosis,
suppressing the maturation of dendritic cells and influencing the
differentiation of immune cells (11). In addition, hypoxia-inducible
factor-1 (HIF-1) is an important oxygen-sensitive response factor
that drives the transcription of several immunosuppressive
molecules, which include PD-L1, VEGF, COX-2 and galectin-1
(6,11).
Rhein is a lipophilic anthraquinone mainly extracted
from rhizomes of several traditional medicinal plants (12). A previous study revealed that rhein
decreased the level of HIF-1α (a subunit of HIF-1) in tumor cells
under hypoxic conditions (13). The
present study identified correlations between HIF-1α and the
immunosuppressive molecules in tissue samples from patients with
CRC. This study also demonstrated that rhein induces an
immunoregulatory effect, downregulating the expression of HIF-1α
and immunosuppressive molecules in CRC cell lines under hypoxic
conditions. In addition, rhein enhanced the cytotoxicity of
effector lymphocytes toward CRC tumor cells under hypoxic
conditions. It may be hypothesized that rhein downregulates the
expression of immunosuppressive molecules by inhibiting the
expression of HIF-1α under hypoxic conditions, and as a result this
may enhance the cytotoxicity of effector lymphocytes.
Materials and methods
Patient samples
Fresh frozen tissue samples from 12 patients (8
males and 4 females aged from 49–83 years old) with CRC were
provided by the Department of Colorectal Surgery, Tianjin Union
Medical Center (Tianjin, China). All the tissue samples were
collected from patients pathologically diagnosed with CRC between
Janunary and March 2018, and the characteristics of each patient
are summarized in Table I. This
study was approved by the Tianjin Nankai Hospital ethics commission
(Tianjin, China; approval no. NKYY_YX_IRB_044_01).
 | Table I.Patient information. |
Table I.
Patient information.
| Patient no. | Sex | Age, years | Tumor location |
|---|
| 1 | Female | 57 | Sigmoid colon |
| 2 | Male | 83 | Ascending colon |
| 3 | Male | 61 | Ascending colon |
| 4 | Male | 64 | Ascending colon |
| 5 | Female | 50 | Descending colon |
| 6 | Male | 62 | Sigmoid colon |
| 7 | Female | 69 | Ascending colon |
| 8 | Male | 67 | Transverse colon |
| 9 | Male | 58 | Descending colon |
| 10 | Male | 62 | Ileocecus |
| 11 | Male | 70 | Descending colon |
| 12 | Female | 49 | Ascending colon |
Cell culture
The human CRC cell lines, HT29, HCT116, Colo205 and
SW620 were obtained from the Tianjin Institute of Integrative
Medicine for Acute Abdominal Diseases, Tianjin Nankai Hospital and
the 293T cell line was provided by Professor Xiong Dongsheng from
the Institute of Hematology & Blood Diseases Hospital, Chinese
Academy of Medical Sciences & Peking Union Medical College
(Tianjin, China). Cells were maintained in Dulbecco's modified
Eagle medium (DMEM; cat. no. 1791922; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; cat. no. 25030-081; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C. Cells cultured under hypoxic conditions
were placed in a hypoxic chamber as previously described (14,15). The
air inside the chamber was flushed with a mixture of N2
(95%) and CO2 (5%) and when the oxygen concentration
decreased to 1%, the chamber was sealed and kept at 37°C.
Peripheral blood lymphocytes (PBLs) were isolated
from the blood of healthy donors from the Tianjin Blood Center
(Tianjin, China; dataset no. ISCP17021885; tjbc.org.cn) by Ficoll® solution (cat. no.
LTS10771; TBD Science, Tianjin, China). The cells were maintained
in Roswell Park Memorial Institute-1640 medium (cat. no. 1721503;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
and 100 U/ml recombinant human IL-2 (cat. no. 202-IL-010; R&D
Systems, Inc., Minneapolis, MN, USA) at 37°C every 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells or tissue samples
using TRIzol® reagent (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Total RNA (4 µg) was reverse transcribed into cDNA using
the RevertAid First Strand cDNA Synthesis kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was subsequently performed using the
SYBR® Premix Ex Taq™ kit (cat. no. RR420L; Takara
Biotechnology Co., Ltd., Dalian, China). The primer pairs used are
presented in Table II, and β-actin
was used as a reference gene for normalization. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5 sec and
60°C for 34 sec. To analyze the correlation of gene expression in
tumor tissues of patients with CRC, the Cq values from patient 1
were used as a baseline to correlate the Cq values of other
patients, and the Pearson product-moment correlation coefficient
(Pearson's R) was measured. Relative gene expression in CRC cell
lines was quantified using the 2−∆∆Cq method (16). These experiments were repeated in
triplicate.
 | Table II.Quantitative polymerase chain reaction
primer sequences. |
Table II.
Quantitative polymerase chain reaction
primer sequences.
|
| Primer sequence
(5′→3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| HIF-1α |
TGATTGCATCTCCATCTCCTACC |
GACTCAAAGCGACAGATAACACG |
| PD-L1 |
TGTACCGCTGCATGATCAG |
AGTTCATGTTCAGAGGTGACTG |
| VEGF |
ACTGAGGAGTCCAACATCAC |
GTCTGCATTCACATTTGTTG |
| COX-2 |
GGTCTGGTGCCTGGTCTGAT |
TCCTGTTTAAGCACATCGCATACT |
| Galectin-1 |
CGCTAAGAGCTTCGTGCTGAAC |
CACACCTCTGCAACACTTCCAG |
| IL-10 |
GCCTTGTCTGAGATGATCCAGTT |
TCACATGCGCCTTGATGTCT |
| TGF-β |
GGGAAATTGAGGGCTTTCG |
GAACCCGTTGATGTCCACTTG |
| β-actin |
GGACATCCGCAAAGACCTGTA |
GCATCCTGTCGGCAATGC |
Cell viability assay
Cells were seeded onto 96-well plates at a density
of 1×104 cells/well and treated with 0, 12.5, 25, 50,
100 or 200 µM rhein (cat. no. SG8100; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) for 48 h. Then the
purple formazan were dissolved in DMSO (cat. no. D8371-50; Beijing
Solarbio Science & Technology Co., Ltd.) and measured at a
wavelength of 492 nm. In CRC cell lines, cell viability was
measured by MTT reduction assay (cat. no. M2128; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany). In PBLs, cell viability was
measured by cell counting kit-8 (cat. no. CK04; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) as manufacturer's protocol.
Untreated cells were used as a negative control and cell viability
was set as 100%.
Establishment of a cell line stably
expressing the membrane-bound single chain Fv against CD3
(mCD3scfv)
The mCD3scfv sequence was provided by Professor
Xiong Dongsheng and amplified by PCR using Pyrobest™ DNA
Polymerase (cat. no. R005A; Takara Biotechnology Co., Ltd.) and the
following primers: 5′-CGTAGAATTCGCCACCATGGAGACAGACACACTCCTG-3′ and
5′-CGTAGGATCCCTAACGTGGCTTCTTCTCGT0-3′. The thermocycling condition
was as follows: Initial denaturation at 95°C for 5 min; 35 cycles
of 95°C for 10 sec, 60°C for 60 sec and 72°C for 60 sec. The PCR
product was purified and cloned into the lentiviral expression
vector pCDH1-CMV-MSC-EF1 α-Puro (cat. no. CD510B-1; System
Biosciences, LLC., Palo Alto, CA, USA) using EcoRI and BamHI
restriction sites. The lentiviral expression construct was
co-transfected with backbone plasmids [5 µg MD2G, 3 µg PAX2
(Invitrogen; Thermo Fisher Scientific, Inc.) and 7 µg pCDH1] in
293T cells using X-tremeGENE™ HP DNA transfection
reagent (cat. no. 06366236001; Roche Molecular Diagnostics,
Pleasanton, CA, USA) to produce the lentivirus (17). HT29 cells were transduced with the
lentivirus for 48 h and selected with 30 µg/ml puromycin (cat. no.
P8230; Beijing Solarbio Science & Technology Co., Ltd.) for 2
weeks. The HT29 cell line stably expressing mCD3scfv, was termed
HT29-CD3scfv.
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation lysis buffer (cat. no. R0010) and the
protein concentration was determined by BCA Protein Assay kit (cat.
no. PC0020; both Beijing Solarbio Science & Technology Co.,
Ltd.). The proteins were separated using SDS-PAGE (10%
polyacrylamide gel; 40 µg total protein per lane) and transferred
onto polyvinylidene difluoride membranes (cat. no. IPVH00010; EMD
Millipore, Billerica, MA, USA). Membranes were blocked by 5% skim
milk at room temperature for 4 h and then incubated with primary
antibodies (1:500) against HIF-1α (cat. no. 100-449), β-tubulin
(cat. no. 66240-1; both 1:500; Wuham Sanying Biotechnology, Inc.,
Wuhan, China), and HA-tag (cat. no. ab1818; 1:500; Abcam,
Cambridge, MA, USA) overnight at 4°C. Following primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse (cat. no. SE12) or anti-rabbit (cat. no. SE13)
secondary antibodies (both 1;2,000; Beijing Solarbio Science &
Technology Co., Ltd.) for 1 h at room temperature. Protein bands
were visualized using the BioVision ECL Western Blotting Substrate
kit (cat. no. K820-500; BioVision, Inc., Milpitas, CA, USA).
Immunofluorescence
HT29-CD3scfv or HT29-control cells were fixed with
4% paraformaldehyde for 10 min and blocked with 1% bovine serum
albumin (cat. no. A8010; Beijing Solarbio Science & Technology
Co., Ltd.) for 1 h. Cells were incubated with mouse anti-HA-tag
primary antibody (cat. no. ab18181) for 1 h, followed by incubation
with allophycocyanin (APC)-conjugated goat anti-mouse IgG (cat. no.
ab130782; both 1:1,000; Abcam) secondary antibody for 1 h. Cells
were stained with DAPI (Sigma-Aldrich, Merck KGaA) for 5 min. All
the steps were performed at room temperature. Images were captured
using a two-photon laser scanning confocal microscope (Olympus
Corporation, Tokyo, Japan; FV1200 MPE).
Flow cytometry
HT29-CD3scfv or HT29-control cells were incubated
with mouse anti-HA tag primary antibody (cat. no. ab18181; 1:1,000;
Abcam) for 30 min at room temperature. Following primary
incubation, cells were washed twice using PBS and incubated with
APC-conjugated goat anti-mouse IgG (cat. no. ab130782; 1:1,000;
Abcam) secondary antibody for 30 min at room temperature. Following
one wash by PBS, cells were analyzed using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA; FACS LSRII). The data were
analyzed by FlowJo software (version 7.6; Tree Star, Inc., Ashland,
OR, USA).
Cytotoxicity assays of PBLs
The specific lysis of target cells was measured by
lactate dehydrogenase release assay using the CytoTox96®
Non-Radioactive Cytotoxicity Assay kit (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. HT29
and HT29-CD3scfv cells were seeded onto 96 well plates
(1×104 cells/well) as target cells. PBLs were added at
different effector-to-target (E:T) cell ratios 5:1, 10:1 and 20:1
to 96-well culture plates and co-cultured with target cells for 16
h.
For assessment under hypoxic conditions,
HT29-CD3scfv cells were pretreated with or without 50 µM rhein for
8 h. Untreated cells in normoxia were used as the negative control.
PBLs were subsequently added at different E:T cell ratios and
co-cultured with target cells for 16 h, and specific cytotoxicity
was detected as described above.
Statistical analysis
The results are reported as the mean ± standard
deviation of at least three independent experiments. All
experimental data were analyzed using one-way analysis of variance
(ANOVA) with Dunnett's post-test. The strength of a linear
association between two variables was calculated with the Pearson
product-moment correlation coefficient (Pearson's R coefficient).
Pearson's R coefficient and ANOVA were performed using GraphPad
Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
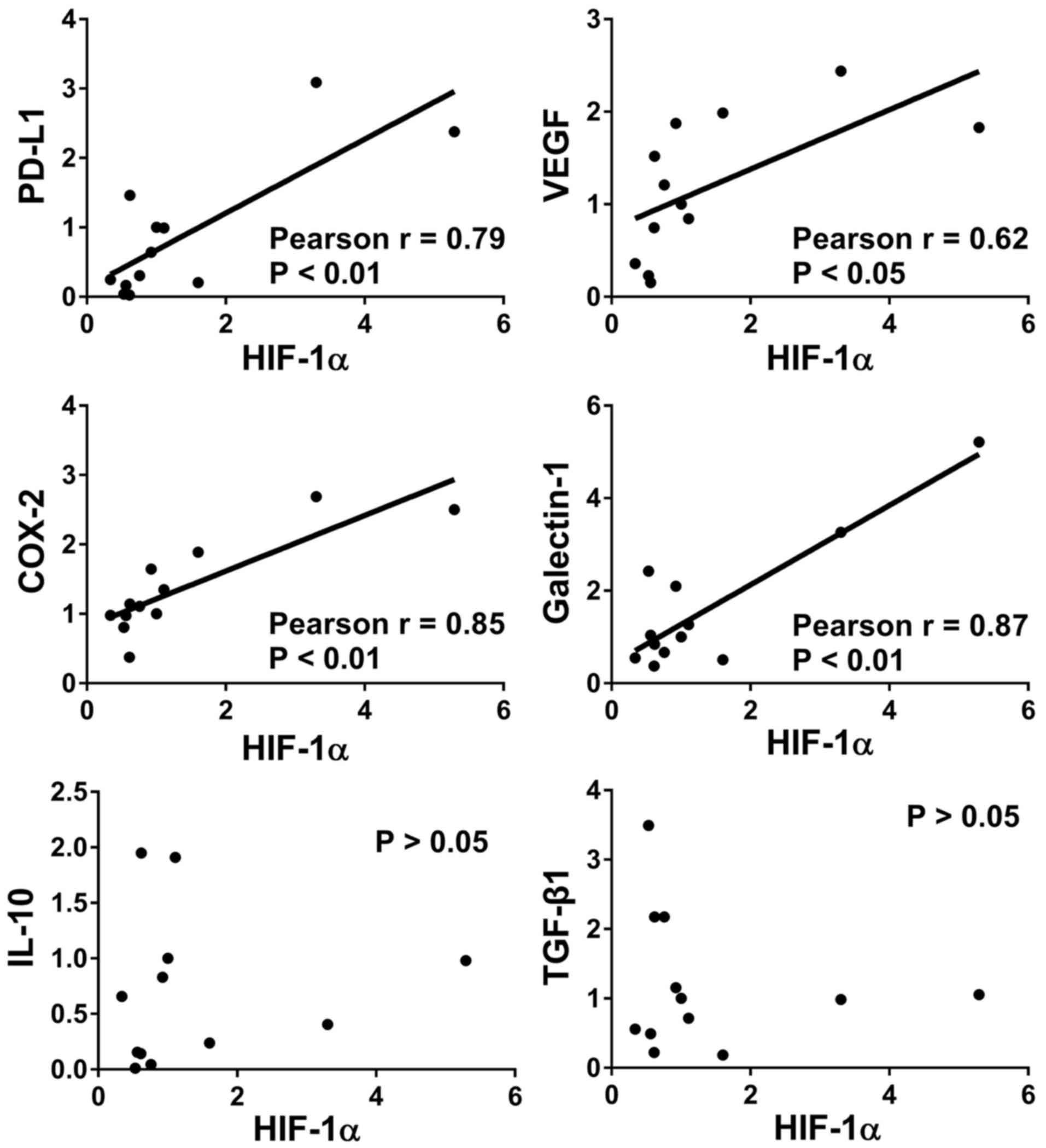
HIF-1α correlates with several
immunosuppressive factors in CRC
To determine the effect of HIF-1α in CRC, the mRNA
expression levels of HIF-1α and six immunosuppressive molecules
were determined by RT-qPCR from 12 CRC tumor tissue samples. The
correlation between the mRNA expression level of HIF-1α and the six
immunosuppressive molecules was analyzed by Pearson's R
coefficient. The mRNA expression levels of PD-L1, VEGF, COX-2, and
galectin-1 were positively correlated with that of HIF-1α in CRC
tissues (Fig. 1). However, there was
no significant correlation between HIF-1α and IL-10 or TGF-β1 in
the CRC tissue samples analyzed.
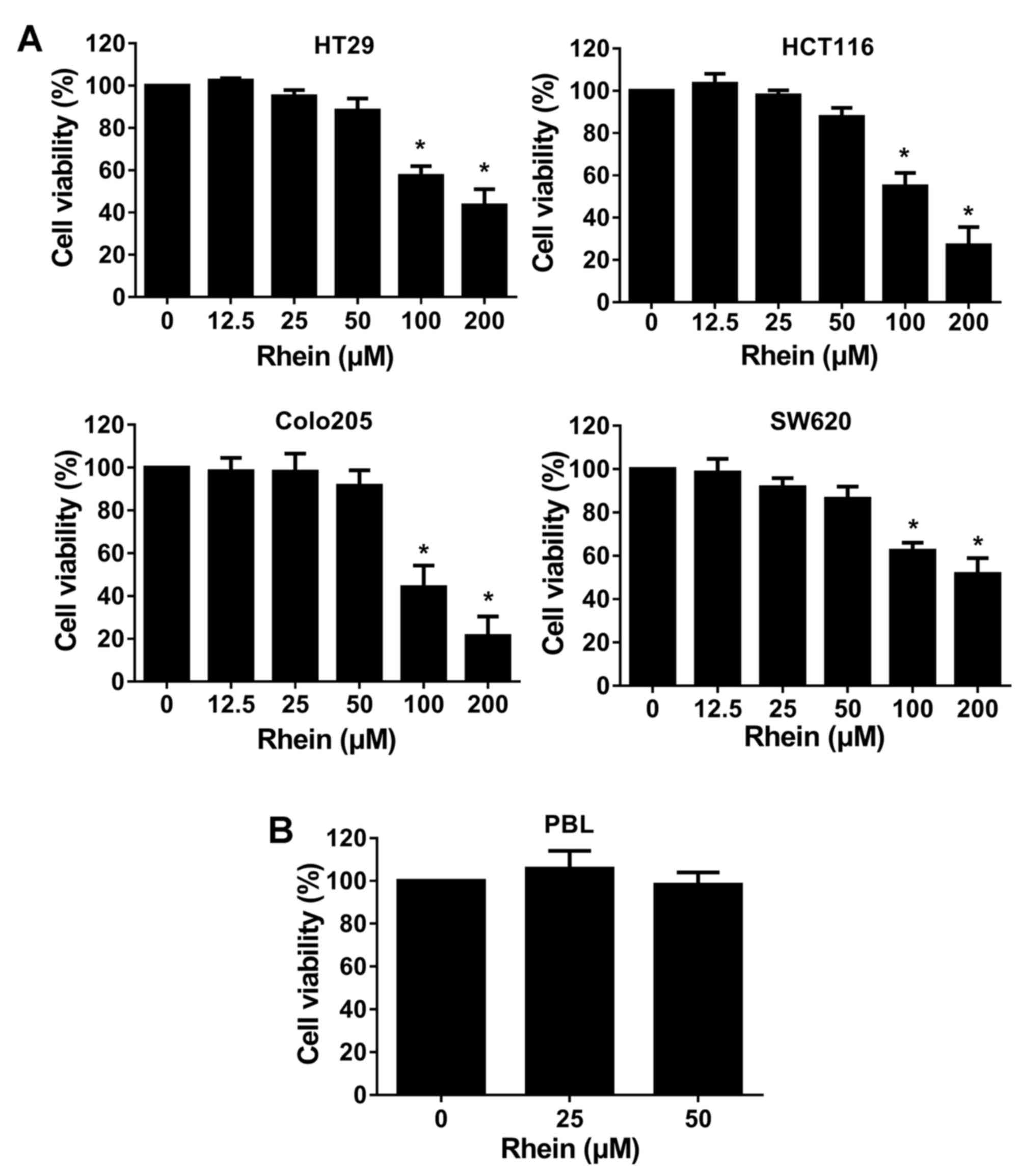
Rhein cytotoxicity
To determine the effect of rhein in CRC, cell
viability was examined using the MTT and CCK-8 assays. The MTT
assay was performed in CRC cell lines (HT29, HCT116, Colo205,
SW620) following treatment with rhein (12.5, 25, 50, 100 and 200
µM) for 48 h. Rhein (100 and 200 µM) significantly decreased cell
viability in a what appeared to be a dose-dependent manner in all
four CRC cell lines (P<0.05; Fig.
2A). The CCK-8 assay was performed in PBLs following treatment
with rhein (25 or 50 µM) for 48 h. Rhein had no affect on PBL cell
viability (Fig. 2B).
Rhein downregulates the expression of
HIF-1α and immunosuppressive molecules in hypoxic conditions
To determine the inhibitory effect of rhein in
hypoxia, the expression levels of HIF-1α and six immunosuppressive
molecules were examined using western blotting in HT29 cells
cultured in 4 conditions: Normoxia, hypoxia, hypoxia + 25 µM rhein,
and hypoxia + 50 µM rhein for 16 h. The protein expression level of
HIF-1α was increased in hypoxia, compared with normoxia.
Additionally, rhein markedly decreased the protein expression level
of HIF-1α under hypoxic conditions in a dose-dependent manner
(Fig. 3A). The mRNA levels of six
immunosuppressive molecules were detected by RT-qPCR. Similar to
HIF-1α protein expression, the mRNA expression levels of PD-L1,
VEGF, COX-2, galectin-1, IL-10 and TGF-β1 were upregulated in
hypoxia, compared with normoxia. Rhein significantly decreased the
mRNA expression levels under hypoxic conditions in a dose-dependent
manner (Fig. 3B). These results
demonstrated that the mechanism underlying the inhibitory effect of
rhein may be HIF-1α-dependent. Although the mRNA expression levels
of IL-10 and TGF-β1 were also downregulated following treatment
with rhein under hypoxic conditions (Fig. 3B), there was no correlation between
HIF-1α and IL-10 or TGF-β1 observed (Fig. 1). These results identified a
potential HIF-1α-independent mechanism underlying the inhibitory
effect of rhein.
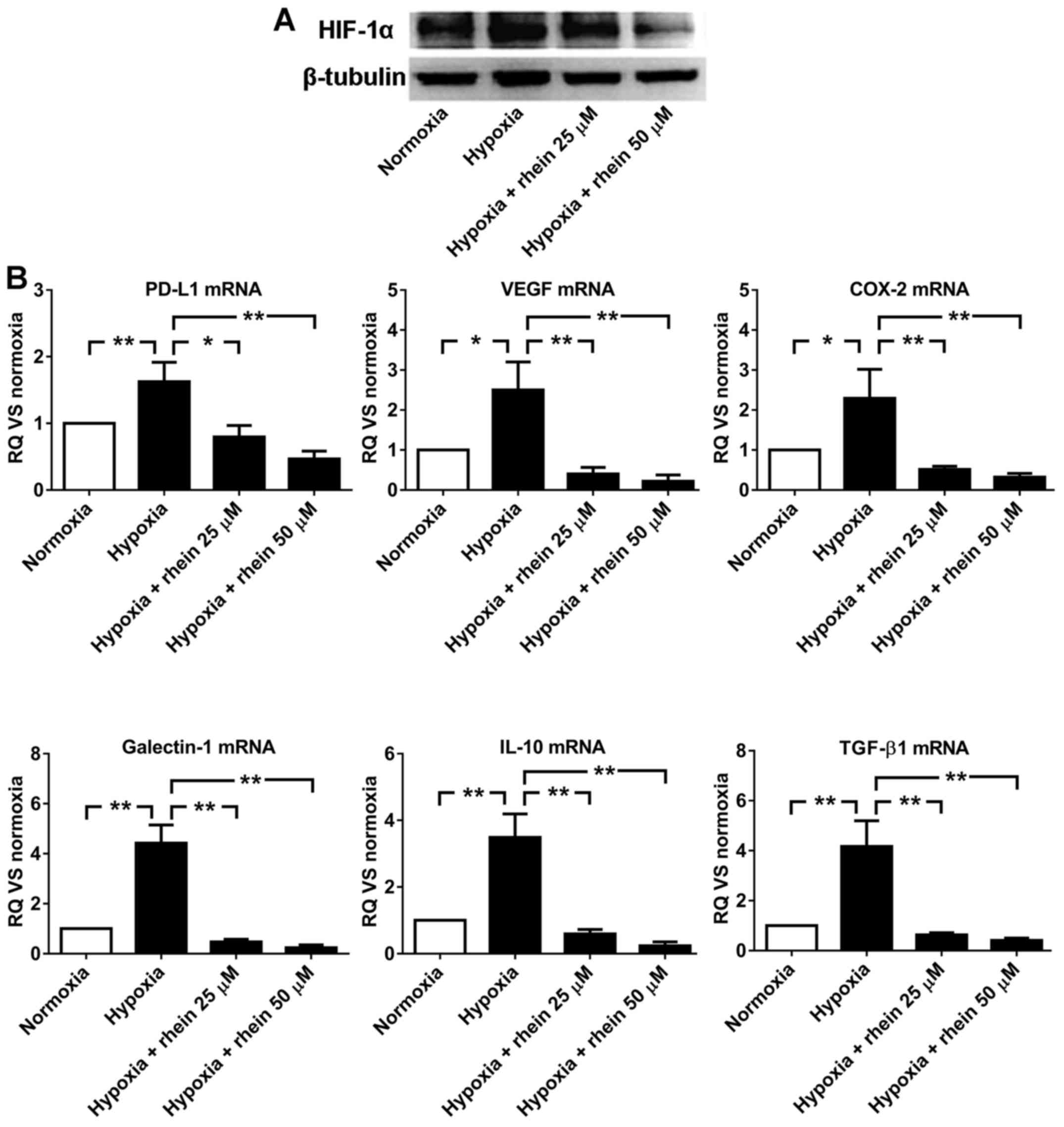 | Figure 3.Rhein downregulates the expression of
HIF-1α and six immunosuppressive molecules. (A) The protein
expression levels of HIF-1α were determined by western blot
analysis in HT29 cells cultured under conditions of normoxia,
hypoxia, hypoxia + 25 µM rhein, and hypoxia + 50 µM rhein for 16 h.
(B) The mRNA expression levels of PD-L1, VEGF, COX-2, galectin-1,
IL-10 and TGF-β1 were determined by reverse
transcription-quantitative polymerase chain reaction in HT29 cells
treated as above. The mRNA expression levels were normalized to
that of cells under normoxia. The data are presented as the mean ±
standard deviation from three independent experiments. *P<0.05
and **P<0.01 as indicated. RQ, relative quantification; CRC,
colorectal cancer; HIF-1α hypoxia-inducible factor 1 subunit α;
PD-L1, programmed cell death 1 ligand 1; COX-2, cyclooxygenase-2;
IL-10, interleukin 10; VEGF, vascular endothelial growth factor;
TGF-β1, transforming growth factor-β1; HT29, human CRC cell
line. |
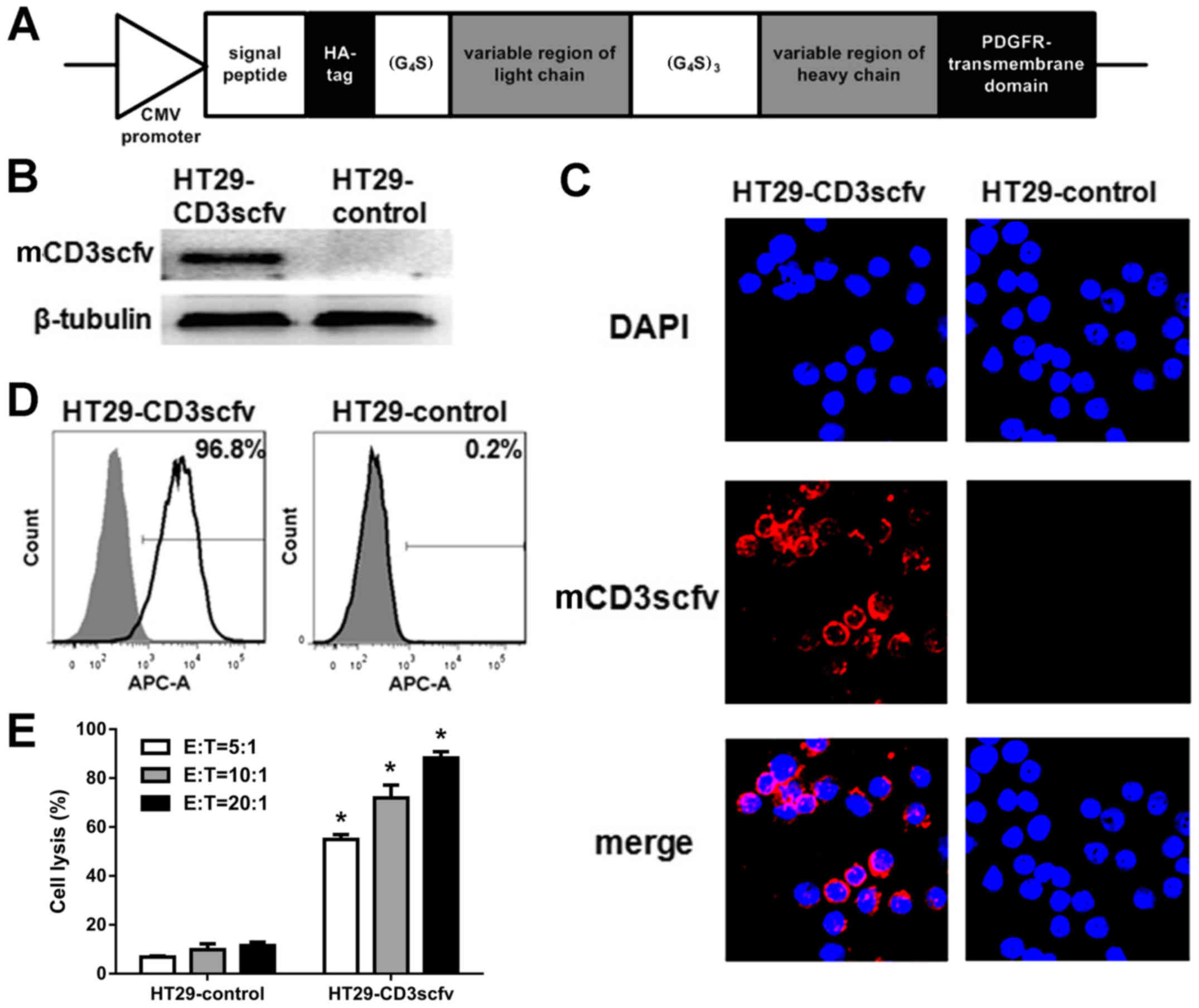
Cytotoxicity of PBLs toward
HT29-CD3scfv cells
To examine the cytotoxicity of PBLs in CRC, a CRC
cell model specifically recognized by PBLs was established using a
lentivirus containing the membrane-bound anti-CD3scFv expression
cassette (Fig. 4A). HT29 cells were
transduced with the lentiviral vector or blank control for 48 h and
then selected with puromycin for an additional 2 weeks. To identify
HT29 cells stably expressing mCD3scfv, western blot analysis,
confocal microscopy and flow cytometry were used to detect the
N-terminal HA-tag of the fusion protein. The results revealed that
mCD3scfv was expressed in HT29-CD3scfv cells and mCD3scfv localized
on the cell membrane (Fig. 4B and
C). Furthermore, HT29 cells expressing the fusion protein
accounted for 96.8% of the total HT29-CD3scfv cells examined
(Fig. 4D). Finally, HT29-CD3scfv and
HT29-control target cells were co-cultured with PBLs at different
E:T cell ratios, respectively for 16 h. CytoTox96®
non-radioactive cytotoxicity assays revealed that PBLs were
cytolytic toward HT29-CD3scfv cells. Their cytotoxic effect
increased as the E:T ratio using PBLs as effector cells and
HT29-CD3scfv cells as target cells increased. PBLs were slightly
cytolytic toward HT29-control cells. Furthermore, the cytotoxity of
HT29-CD3scfv singicantly increase compared with HT29-control cells
at the corresponding E:T ratio (P<0.05; Fig. 4E).
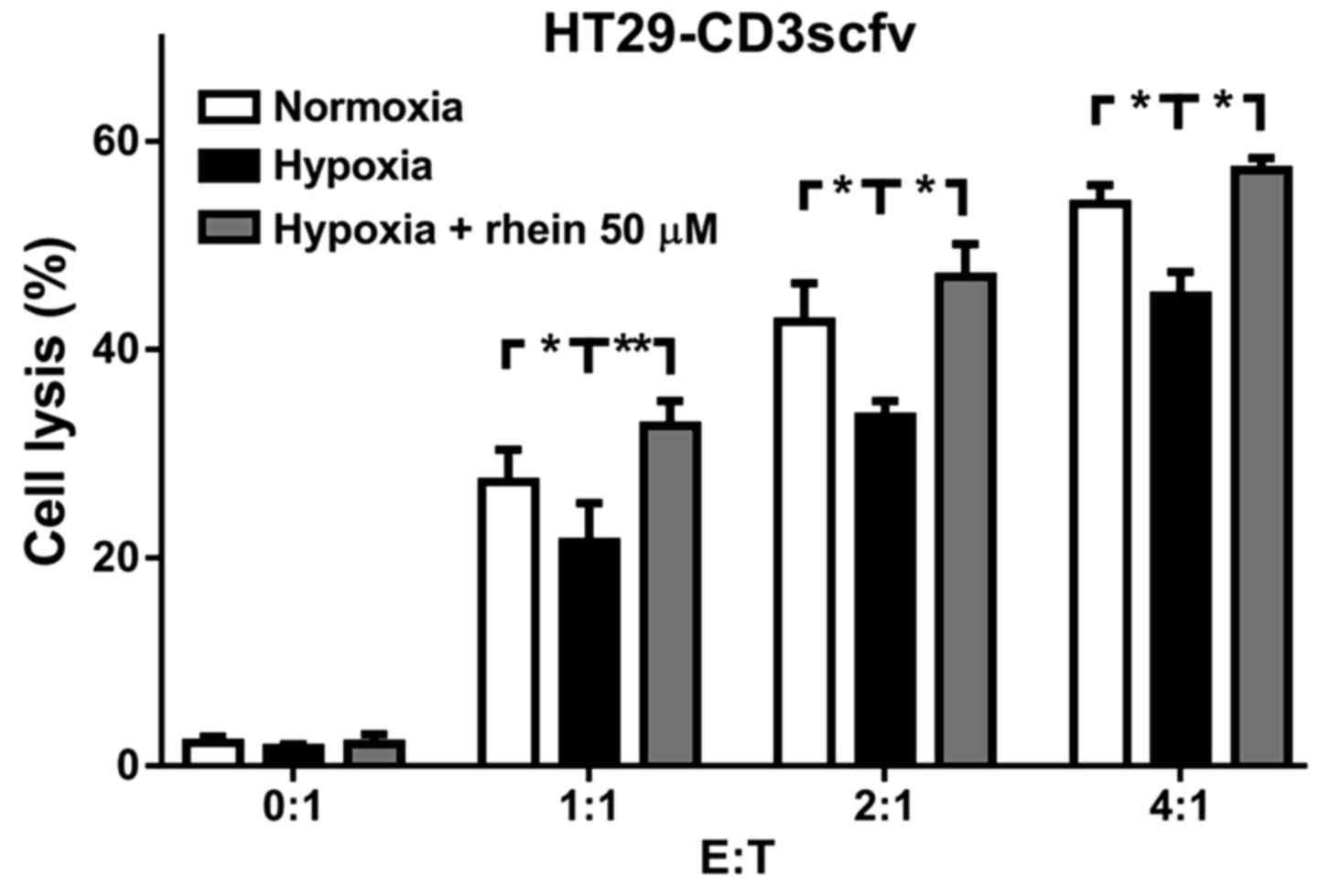
Rhein enhances the cytotoxicity of
PBLs in hypoxia
To further examine the inhibitory effect of rhein in
hypoxia, the cytotoxicity of PBLs toward CRC cells was examined
following treatment with rhein under hypoxic conditions.
HT29-CD3scfv cells were cultured under conditions of normoxia,
hypoxia and hypoxia + 50 µM rhein, followed by co-culture with PBLs
at different effector-to-target cell ratios (5:1, 10:1 and 20:1).
CytoTox96® non-radioactive cytotoxicity assays revealed
that PBLs are cytolytic toward HT29-CD3scfv cells, and their
cytotoxic effect was inhibited by hypoxia. Treatment with rhein
enhanced the cytotoxicity of PBLs in hypoxia, compared with
normoxia (Fig. 5).
Discussion
Since the expression of HIF-1α is inhibited by rhein
(13), it may be hypothesized that
rhein downregulates immunosuppressive molecules driven by HIF-1 and
further enhances the anti-tumor effect of PBLs in hypoxia. The
present study investigated the correlation between the mRNA levels
of HIF-1α and the six immunosuppressive molecules in tumor tissue
samples from patients with CRC. The mRNA expression of four
immunosuppressive molecules positively correlated with HIF-1α,
however IL-10 and TGF-β1 did not correlate with HIF-1α expression.
The expression levels of HIF-1α and the six immunosuppressive
molecules were upregulated in hypoxia, however the addition of
rhein significantly decreased the mRNA expression levels under
hypoxic conditions. These results indicated that the mechanism
underlying the inhibitory effect of rhein may be HIF-1α-dependent.
However, the mRNA expression levels of IL-10 and TGF-β1 were
downregulated following treatment with rhein under hypoxic
conditions without the observed correlation with HIF-1α, which
indicated a potential HIF-1α-independent mechanism underlying the
inhibitory effect of rhein in hypoxia. Furthermore, the current
study demonstrated that hypoxic conditions reduced the anti-tumor
effect of PBLs. However, the addition of rhein under these
conditions enhanced the anti-tumor effect of PBLs in CRC cells.
Although the present study demonstrated that rhein
enhanced the cytotoxicity of PBLs on CRC cells in hypoxia, there
are several limitations that should be noted. Despite having no
effect on the viability of PBLs, other regulatory functions of
rhein on effector lymphocytes were not addressed in this study and
will need further investigation. Furthermore, the role of HIF-1α in
T-cells is controversial and needs further investigation. Some
studies suggested that the activity of T-cells in the tumor
microenvironment is dependent on the energy supplied by the
increased expression of HIF-1 (18,19),
however, other studies have demonstrated that the inhibition of
HIF-1α may transform T-cells into memory cells, as well as
enhancing the effect of cancer vaccines (20–22). The
effect of rhein on metabolism and effector T-cell activity is under
investigation. Additionally, immunosuppressive molecules and
immunosuppressive cells, which include tumor-associated
macrophages, myeloid-derived suppressor cells and regulatory
T-cells, are components of the immunosuppressive tumor
microenvironment and the immunosuppressive function of these cells
is dependent on immunosuppressive molecules and HIF (23–25).
Additional in vivo studies investigating the effects of
rhein with respect to the proportion of immunosuppressive cells in
the tumor and infiltration of effector lymphocytes are
necessary.
In conclusion, the current study validated the
hypothesis that rhein, a down-regulator of HIF-1α, inhibited the
expression of immunosuppressive molecules in CRC cells and enhance
the cytotoxicity of effector lymphocytes under hypoxic conditions.
It was revealed that HIF-1α positively correlated with
immunosuppressive molecules in CRC tissues, and rhein decreased the
mRNA levels of HIF-1α and immunosuppressive molecules in CRC cell
lines, and increased the cytotoxicity of effector lymphocytes to
HT29-CD3scfv cells in hypoxia. These results indicate that rhein
may have great potential to combine with effector T lymphocytes for
the treatment of CRC.
Acknowledgements
The authors would like to thank Professor Xiong
Dongsheng from the Institute of Hematology & Blood Diseases
Hospital, Chinese Academy of Medical Sciences & Peking Union
Medical College (Tianjin, China) for providing the 293T cell line
and the mCD3scfv sequence.
Funding
National Natural Science Foundation of China (Grant
no. 81572318).
Availability of data and materials
Not applicable.
Authors' contributions
XY and FW designed the study. XY, WT and LH
performed the experiments. JX and JH analyzed the data. YH and JY
collected the human tissue samples.
Ethics approval and consent to
participate
The current study was approved by the Tianjin Nankai
Hospital ethics commission (approval no. NKYY_YX_IRB_044_01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davila ML, Bouhassira DC, Park JH, Curran
KJ, Smith EL, Pegram HJ and Brentjens R: Chimeric antigen receptors
for the adoptive T cell therapy of hematologic malignancies. Int J
Hematol. 99:361–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burga RA, Thorn M, Point GR, Guha P,
Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Joseph Espat N,
Junghans RP and Katz SC: Liver myeloid-derived suppressor cells
expand in response to liver metastases in mice and inhibit the
anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother.
64:817–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katz SC, Point GR, Cunetta M, Thorn M,
Guha P, Espat NJ, Boutros C, Hanna N and Junghans RP: Regional
CAR-T cell infusions for peritoneal carcinomatosis are superior to
systemic delivery. Cancer Gene Ther. 23:142–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katz SC, Burga RA, McCormack E, Wang LJ,
Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al:
Phase I hepatic immunotherapy for metastases study of
intra-arterial chimeric antigen receptor-modified T-cell therapy
for CEA+ liver metastases. Clin Cancer Res. 21:3149–3159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Y, Wang Y and Han W: Chimeric antigen
receptor-modified T cells for solid tumors: Challenges and
prospects. J Immunol Res. 2016:38508392016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barsoum IB, Smallwood CA, Siemens DR and
Graham CH: A mechanism of hypoxia-mediated escape from adaptive
immunity in cancer cells. Cancer Res. 74:665–674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gabrilovich D: Mechanisms and functional
significance of tumourinduced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greenhough A, Smartt HJ, Moore AE, Roberts
HR, Williams AC, Paraskeva C and Kaidi A: The COX-2/PGE2 pathway:
Key roles in the hallmarks of cancer and adaptation to the tumour
microenvironment. Carcinogenesis. 30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue
F, Jiang Y, Chen GQ and Zhao KW: Hypoxia inducible factor-1
mediates expression of galectin-1: The potential role in
migration/invasion of colorectal cancer cells. Carcinogenesis.
31:1367–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barsoum IB, Koti M, Siemens DR and Graham
CH: Mechanisms of hypoxia-mediated immune escape in cancer. Cancer
Res. 74:7185–7190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun H, Luo G, Chen D and Xiang Z: A
comprehensive and system review for the pharmacological mechanism
of action of rhein, an active anthraquinone ingredient. Front
Pharmacol. 7:2472016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu L, Cui R, Liu H and Wang F: Emodin and
rhein decrease levels of hypoxia-inducible factor-1α in human
pancreatic cancer cells and attenuate cancer cachexia in athymic
mice carrying these cells. Oncotarget. 8:88008–88020. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma F, Hu L, Yu M and Wang F: Emodin
decreases hepatic hypoxia-inducible factor-1α by inhibiting its
biosynthesis. Am J Chin Med. 44:997–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Li SS, Segersvärd R, Strömmer L,
Sundqvist KG, Holgersson J and Permert J: Hypoxia inducible
factor-1 mediates effects of insulin on pancreatic cancer cells and
disturbs host energy homeostasis. Am J Pathol. 170:469–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Yang Y, Zhang L, Lu Y, Zhang Q,
Fan D, Zhang Y, Zhang Y, Ye Z and Xiong D: Mesenchymal stromal
cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for
the treatment of B cell lymphoma combined with IDO pathway
inhibitor D-1-methyl-tryptophan. J Hematol Oncol. 10:562017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Chaudhury A, Zhang M, Savoldo B,
Metelitsa LS, Rodgers J, Yustein JT, Neilson JR and Dotti G:
Glycolysis determines dichotomous regulation of T cell subsets in
hypoxia. J Clin Invest. 126:2678–2688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ottensmeier CH, Perry KL, Harden EL,
Stasakova J, Jenei V, Fleming J, Wood O, Woo J, Woelk CH, Thomas GJ
and Thirdborough SM: Upregulated glucose metabolism correlates
inversely with CD8+ T-cell infiltration and survival in squamous
cell carcinoma. Cancer Res. 76:4136–4148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao JH, Barbi J and Pan F:
Hypoxia-inducible factors in T lymphocyte differentiation and
function. A review in the theme: Cellular responses to hypoxia. Am
J Physiol Cell Physiol. 309:C580–C589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sukumar M, Liu J, Ji Y, Subramanian M,
Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly
ED, et al: Inhibiting glycolytic metabolism enhances CD8+ T cell
memory and antitumor function. J Clin Invest. 123:4479–4488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kheshtchin N, Arab S, Ajami M, Mirzaei R,
Ashourpour M, Mousavi N, Khosravianfar N, Jadidi-Niaragh F, Namdar
A, Noorbakhsh F, et al: Inhibition of HIF-1α enhances anti-tumor
effects of dendritic cell-based vaccination in a mouse model of
breast cancer. Cancer Immunol Immunother. 65:1159–1167. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doedens AL, Stockmann C, Rubinstein MP,
Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW and
Johnson RS: Macrophage expression of hypoxia-inducible factor-1
alpha suppresses T-cell function and promotes tumor progression.
Cancer Res. 70:7465–7475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clambey ET, McNamee EN, Westrich JA,
Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC,
Stenmark KR, Colgan SP and Eltzschig HK: Hypoxia-inducible factor-1
alpha-dependent induction of FoxP3 drives regulatory T-cell
abundance and function during inflammatory hypoxia of the mucosa.
Proc Natl Acad Sci USA. 109:E2784–2793. 2012. View Article : Google Scholar : PubMed/NCBI
|