Introduction
Skin cancer is one of the most common malignancies
in the world, and its morbidity is increasing year by year. It has
become a major disease that is detrimental to human health. Skin
cancers can be divided into basal cell carcinoma, squamous cell
carcinoma and melanoma. It has intricate pathogenesis, which is
currently considered to be attributed to environmental factors,
gene mutation and viral infection. Skin malignancies, such as
squamous cell carcinoma and malignant melanoma have no effective
prevention and treatment at present. Therefore, the study of the
occurrence and development mechanism of skin cancers is imperative
(1).
Crocin is a less common water-soluble carotenoid
(dicarboxylic acid monoglyceride) extracted from saffron (2,3).
Research has shown that cytoplasmic membrane rupture, nuclear
pyknosis and cell apoptosis were observed in cervical carcinoma
cells after the cells were treated with crocin (4). Crocin inhibited the growth of tumor
cells, the mechanism of which may be related to its strong
antitumor cytotoxicity (5).
Tumor development is a multi-gene, multi-step,
multi-stage sophisticated process. The biological characteristics
of tumor cells were mainly manifested as uncontrolled
proliferation, blocked apoptosis and strong invasiveness. In normal
tissues, cell proliferation and apoptosis is under a precisely
regulated dynamic balance status. Nevertheless, this balance is
broken in tumor tissues. Tumor cells begin to resist apoptosis,
immune destruction and other mechanisms of elimination. As a
result, tumor cells cannot be cleared in time, which is the
determinant of unlimited tumor proliferation (6).
The purpose of this study was to investigate the
effects of crocin on proliferation and apoptosis of human skin
cancer cells A431 and SCL-1, also to preliminarily explore its
underlying mechanism.
Materials and methods
Materials and reagents
Human skin cancer cells A431 and SCL-1 were provided
by the Dermatology Laboratory of Nanjing Medical University First
Affiliated Hospital (Nanjing, China). RPMI-1640 medium was
purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT,
USA). Fetal bovine serum, trypsin, penicillin and streptomycin were
purchased from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA. Crocin and methyl thiazolyl tetrazolium (MTT) were purchased
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany. Annexin V-FITC
apoptosis detection kit was purchased from Bender MedSystems
(Thermo Fisher Scientific, Inc.). Bid, procaspase-3, Jak2, Stat3
and Bcl-2 antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Polyclonal goat anti-rabbit IgG-HRP
secondary antibody (cat. no. sc-2004; dilution, 1:500) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Preparation of crocin solution
Under sterile condition, 20 mg of crocin and 12.5 mg
of EDTA was dissolved into 4 ml of 3-fold distilled water for stock
solution with a concentration of 50 mmol/l and stored at 4°C.
Cell culture
A431 and SCL-2 cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (FBS), 100 U/l penicillin
and 100 µg/ml streptomycin in incubator with 5% CO2 at
37°C. The cells were subcultured routinely with trypin digestion
containing 0.02% EDTA.
Cell transfection
A431 and SCL-1 cells in the logarithmic growth phase
were inoculated into cell culture plates according to the
appropriate cell numbers and cultured overnight. The following day,
the cells were treated with different concentrations (0, 0.4 and
0.8 mM) of crocin to detect the cell phenotypes. Alternatively,
according to the instructions of Lipofectamine 2000, the cells were
transfected with Jak2 overexpression plasmid (pcDNA-Jak2) for 8 h,
and then replaced with complete medium for further culture. The
related phenotypes were detected after the transfection
process.
Cell viability assay
Cell viability was determined by MTT assay. A431 and
SCL-1 cells in logarithmic growth phase were harvested for cell
counting. The cell density was adjusted for a concentration of
2×104cells/ml by Dulbecco's modified Eagle's medium
(DMEM), then seeded into 96-well plates and incubated at 37°C in a
5% CO2 saturated humidity incubator. After overnight
adherence, the supernatant was aspirated and different
concentrations of crocin (concentration 0, 0.2, 0.4, 0.8 and 1.0
mmol/l) were added to corresponding treatments for another 24 h
incubation. On the other hand, the same concentration (0.8 mmol/l)
of crocin was added and cultured for 0, 6, 12, 24, 48 and 72 h,
respectively. A total of 20 µl MTT (5 g/l) solution was added to
each well for 4 h and centrifuged at 1,750 × g for 10 min at 4°C.
The reaction was terminated by discarding the supernatant and
adding 150 µl dimethylsulphoxide (DMSO) per-well. The sample was
placed on a shaker at shaking speed for 10 min. The OD value was
measured using spectrophotometer (Hitachi, Ltd., Tokyo, Japan) at
570 nm wavelength. Each treatment was set up in triplicates.
Inhibition rate was calculated as follows: Inhibition rate (%) =
(control group A - experimental group A)/control group A ×100%.
Colony formation assay
Cells in logarithmic growth phase were converted
into cell suspension status by using conventional digestion and
passage method. The cells were dissociated thoroughly and
repeatedly to make the cell percentage of each single cell above
95%. A total of 200 cells were taken and seeded in 6-well plates
with 2 ml medium and shaken gently on a shaker. Afterwards the
plate was placed and incubated at 37°C in a 5% CO2
saturated humidity incubator for another 2 weeks. The formation of
clones was monitored under a microscope (BX-42; Olympus
Corporation, Tokyo, Japan). The culture medium was removed when
clones were grown to the appropriate number and size. Cells were
fixed by adding 4% polyoxymethylene for 10 min, and then rinsed
with phosphate-buffered saline (PBS) twice. After stained with
hematoxylin for 10 min, the cells were rinsed by PBS and air dried.
After that the cells were photographed and counted under the
microscope (BX-42; Olympus Corporation).
EDU staining experiment
EDU kit was used for the experiment. Cells in
logarithmic growth phase were collected for routine digestion,
centrifugation, resuspension and counting. Harvested cells were
seeded into 96-well plates at a density of 4×103
cells/well. After the cells grew adherently and in proper
concentration, EDU staining procedure was performed following the
kit instructions. After staining the cells were photographed and
counted under the fluorescence microscope (IX70; Olympus
Corporation). Samples that appeared in >3 random sights were
selected and calculated utilizing IPWIN60 software for the number
of cells in S phase out of every 200 cells. The ratio was
calculated by dividing the number of cells in S phase by 200, and
then by further statistical analysis.
Flow cytometric analysis for
evaluating apoptosis index and the cell cycle
The human skin cancer cells were collected and
adjusted to a concentration of 1×105/ml, and then seeded
on 6-well plates with 2 ml per well. After the cells were incubated
for 24 h, supernatant was removed and serum-free medium containing
0, 0.4, 0.8 mmol/l of crocin was added. The 6-well plates were
placed into the incubator again for another 24 h culture for
following tests. For apoptosis assay, the cells were washed twice
with PBS, then collected at a density of 5×105. After
centrifugation at 1,500 × g for 10 min at 20°C, 500 µl of binding
buffer was added to resuspend cells. A total of 5 µl of Annexin
V-FITC and 5 µl of pro-pidium iodide (PI) was added and mixed. The
reaction was protected from light at room temperature for 10 min.
Finally, apoptosis index was evaluated utilizing the flow
cytometer. To detect the cell cycle phrase distribution, the cells
were washed once with PBS and then collected and adjusted to a
concentration of 1×106/ml after centrifugation. The
sample was stabilized with 70% ethanol and preserved at 4°C. The
fixative was then washed with PBS before staining. A total of 100
µl RNase A was added and heated up to 37°C in water bath for
approximately 30 min. Then the sample was treated with 400 µl PI
staining mix and incubated in the dark at 4°C. After 30 min, the
red fluorescence at 488 nm wavelength was evaluated and recorded
utilizing the flow cytometer.
Western blot analysis
According to the amount of cells, appropriate amount
of RIPA cell lysis solution was added. RIPA lysis and extraction
buffer (cat. no. 89900; Thermo Fisher Scientific, Waltham, MA, USA)
was used for the western blot analysis. Cell lysate was collected,
sonicated on ice for 1 min, and centrifuged. After centrifugation
at 10,000 × g for 10 min at 4°C, the supernatant was collected to
determine the protein concentration by bicincho-ninic acid (BCA)
method. A total of 30 µg protein sample was loaded on 10% sodium
dodecyl sulphate (SDS) gel for each well. After the
electrophoresis, the gel was transferred to the polyvinylidene
fluoride (PVDF) membrane and blocked using 5% fat-free milk for 1
h. After that, corresponding primary antibodies was added to each
corresponding sample and incubated at 4°C overnight. Then the
corresponding secondary antibody were added for 1 h incubation at
room temperature. Primary mouse monoclonal B-cell lymphoma-2
(Bcl-2) antibody (cat. no. ab59348; dilution, 1:500); rabbit
polyclonal Caspase-3 antibody (cat. no. ab13847; dilution, 1:500);
rabbit monoclonal Bid antibody (cat. no. ab32060; dilution, 1:500);
rabbit monoclonal JAK2 antibody (cat. no. ab108596; dilution,
1:500); mouse monoclonal STAT3 antibody (cat. no. ab119352;
dilution, 1:500); rabbit polyclonal GAPDH antibody (cat. no.
ab37168; dilution, 1:500) and secondary goat anti-rabbit (HRP) IgG
antibody (cat. no. ab6721; dilution, 1:2,000) were all purchased
from Abcam (Cambridge, MA, USA). Lastly enhanced chemiluminescent
method was used to expose the protein band. The experiment was
repeated three times independently.
Statistical analysis
The measurement data were expressed as mean ±
standard deviation, utilizing SPSS 11.0 (SPSS Inc., Chicago, IL,
USA) software for statistical analysis. t-test was used to compare
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
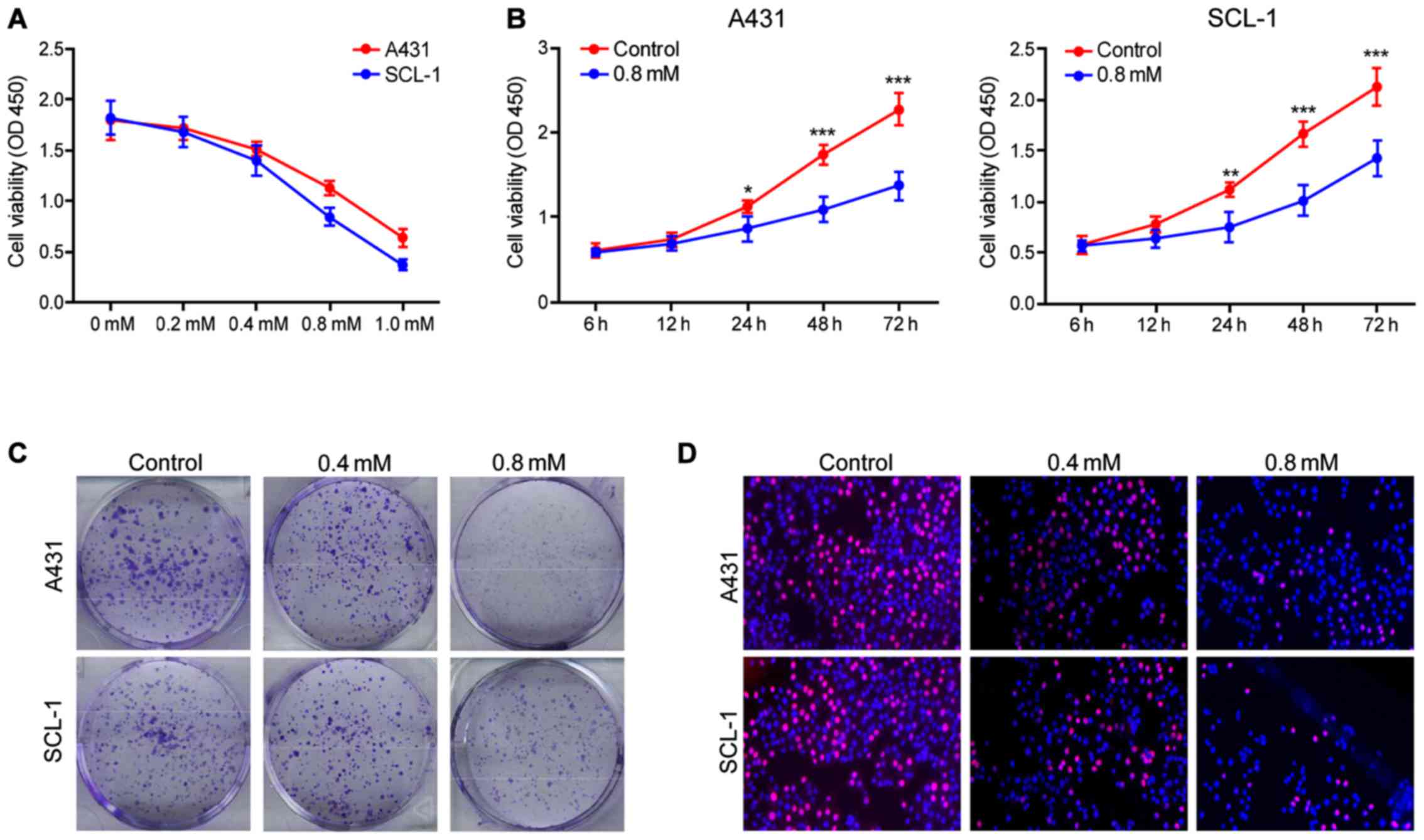
Crocin inhibits the proliferation of
human skin cancer cells A431 and SCL-1
Crocin significantly inhibited the proliferation of
A431 and SCL-1 cells and in a dose-dependent manner. The maximum
inhibitory effect appeared at 1 mM (Fig.
1A), and the effect increased with the training time (Fig. 1B). Crocin was also found capable of
constraining the clonogenic capacity of human skin cancer cells.
Also, the group using the concentration of 0.8 mmol/l crocin had
enhanced inhibitory effects compared to 0.4 mmol/l (Fig. 1C). Furthermore, EDU experimental
results showed that crocin can inhibit the viability of human skin
cancer cells in a concentration-dependent manner (Fig. 1D). In conclusion, the above results
showed that crocin has potential therapeutic effect on skin
cancer.
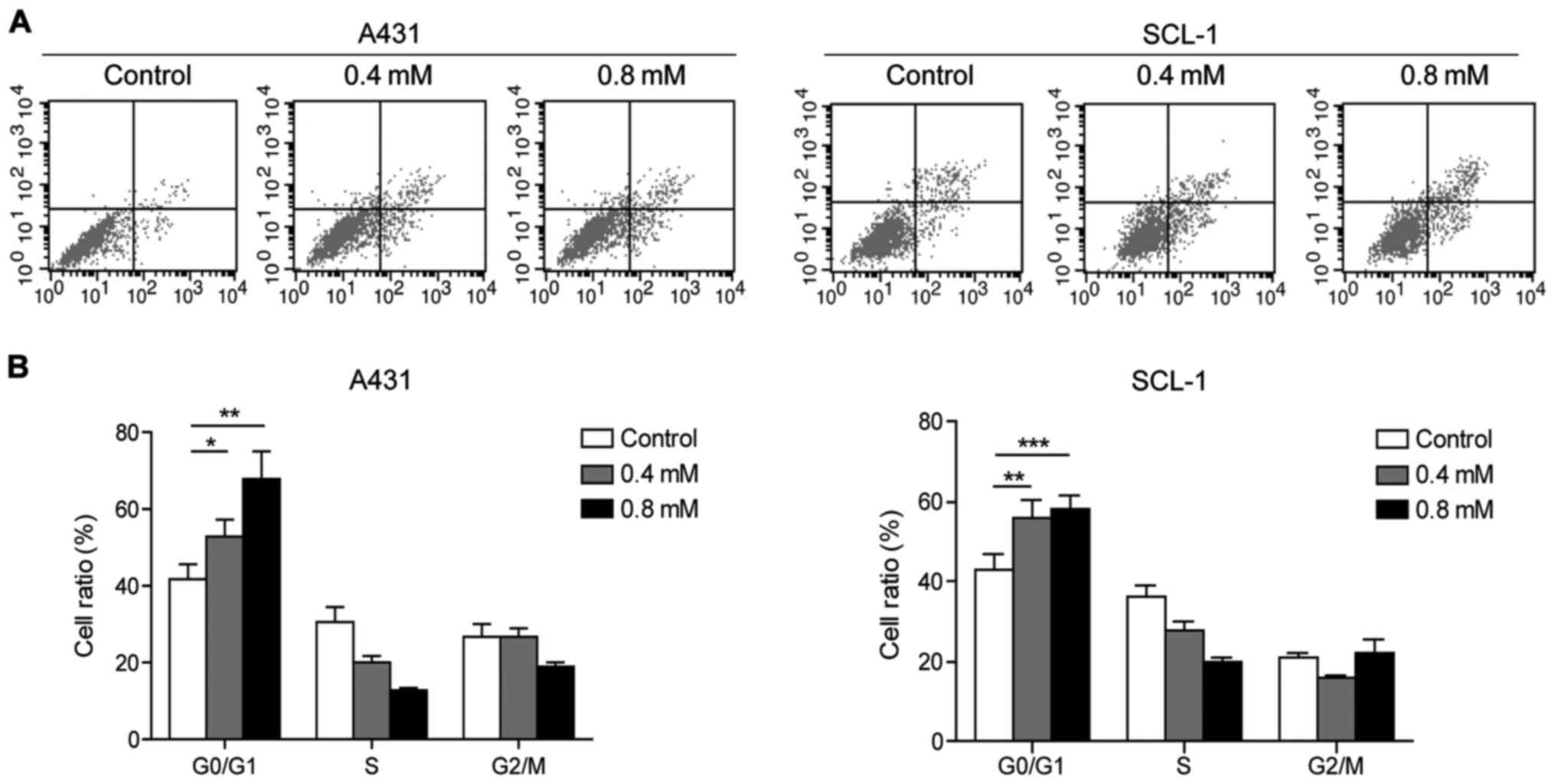
Crocin inhibits the cell cycle
transition and cell proliferation of A431 and SCL-1 cells
Flow cytometry analysis showed that crocin could
induce apoptosis of A431 and SCL-1 cells to a certain extent, which
was positively correlated with the concentration (Fig. 2A). Moreover, compared with the
control group, it was found that crocin could arrest A431 and SCL-1
cells in G0/G1 phase (Fig. 2B).
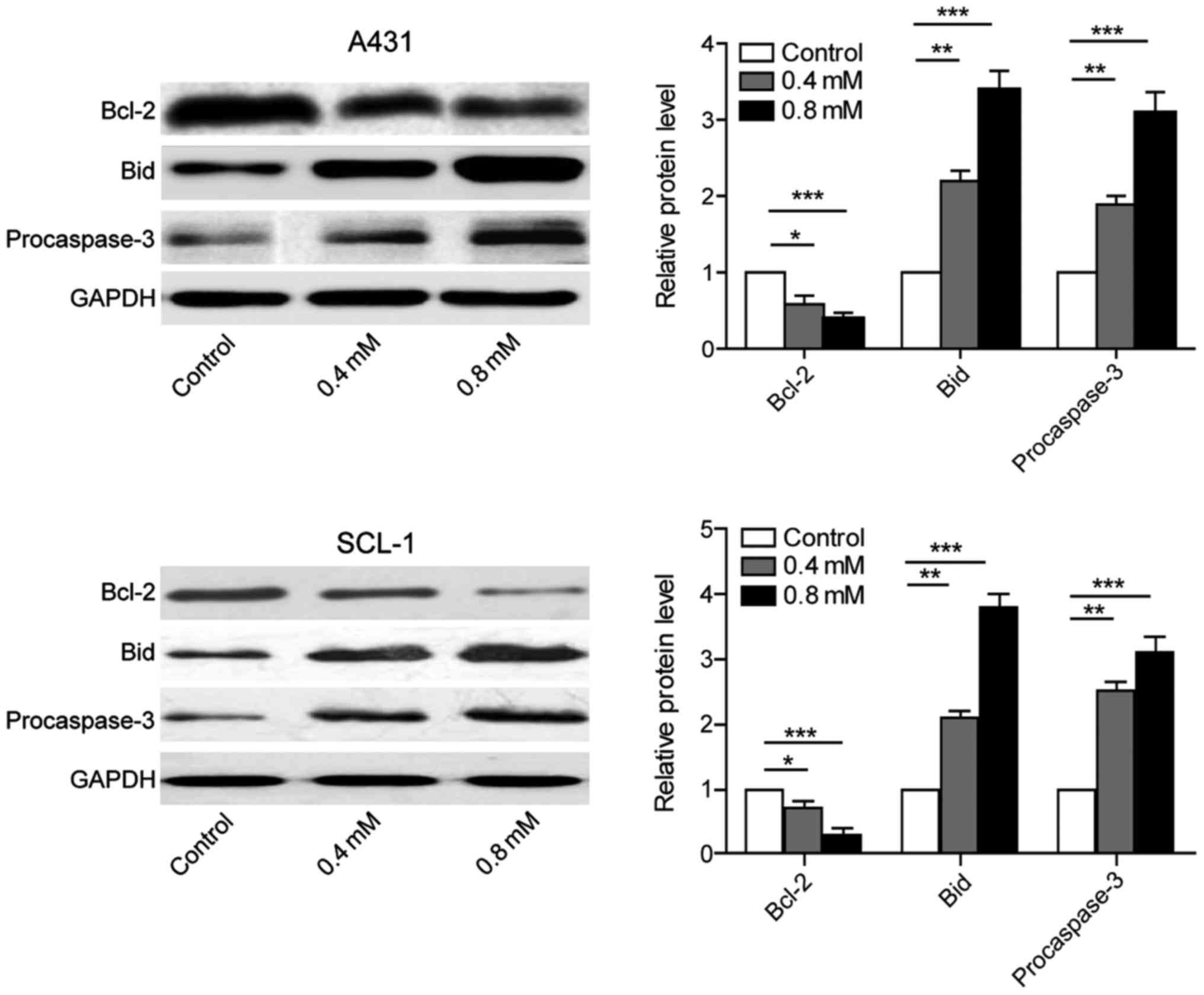
Crocin inhibits the expression of
anti-apoptotic proteins and promotes the expression of
pro-apoptotic proteins
A431 and SCL-1 cells were cultured individually with
0, 0.4, 0.8 mmol/l crocin. The proteins were collected after 24 h
to evaluate the expression of anti-apoptotic protein Bcl-2,
pro-apoptotic protein Bid and procaspase-3. The results showed that
the expression of anti-apoptotic protein was downregulated, while
the expression of pro-apoptotic protein was upregulated. Also, the
group using the concentration of 0.8 mmol/l crocin exerted better
effects than that of 0.4 mmol/l. These results suggested that
crocin induced apoptosis of skin cancer cells A431 and SCL-1 by
regulating apoptosis pathway (Fig.
3).
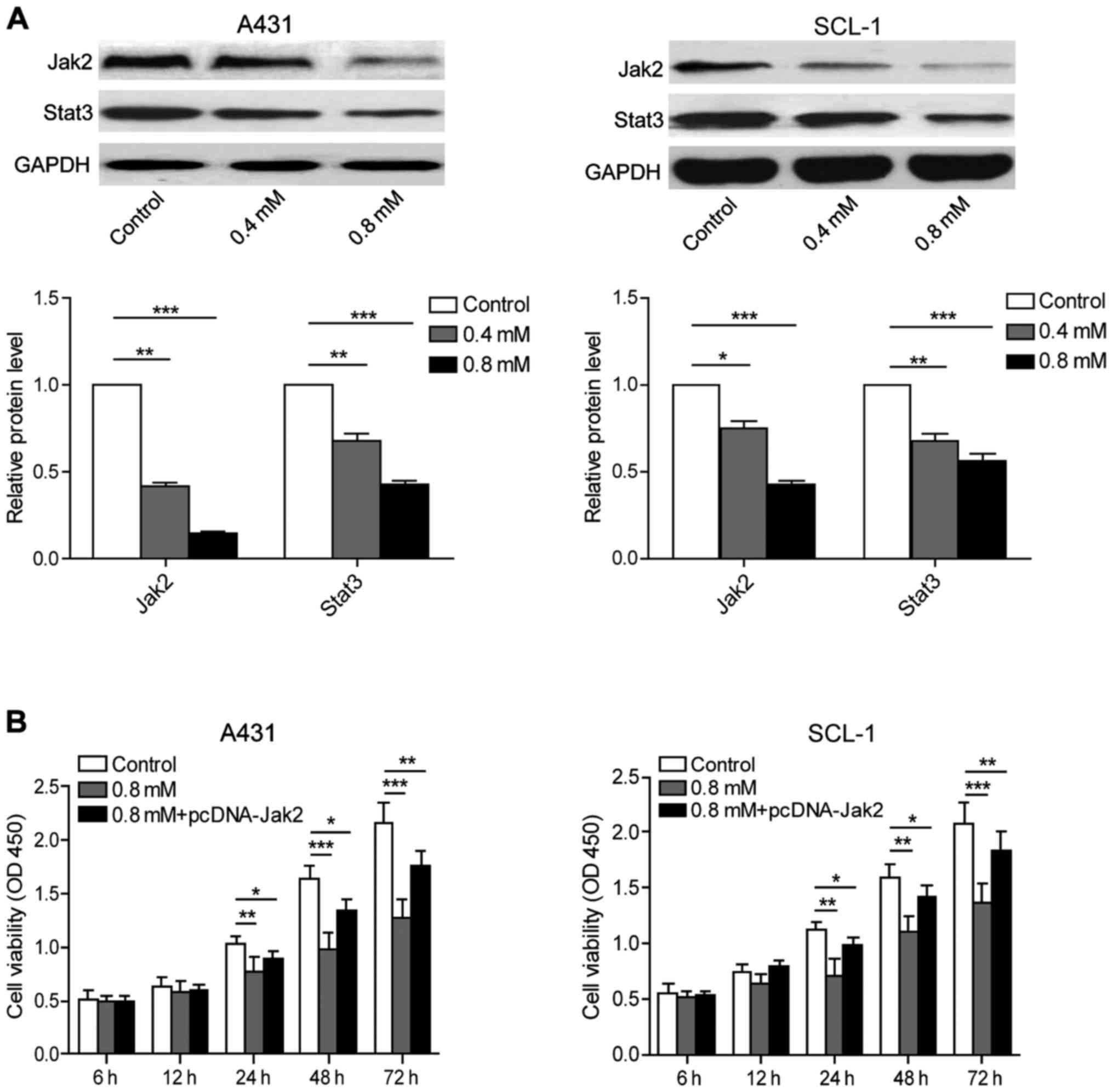
Crocin has inhibitory effects on
JAK/STAT signaling pathway in human skin cancer cells
A431 and SCL-1 cells were cultured in medium
containing 0, 0.4 and 0.8 mmol/l crocin respectively for the
purpose of evaluating the expression of Jak2 and Stat3 proteins
using western blot analysis. The results showed that crocin can
inhibit the expression of Jak2 and Stat3 protein in a
dose-dependent pattern (Fig. 4A).
After crocin treatment and overexpressing Jak2 at the same time,
cell viability result by MTT assay showed that the cell viability
of 0.8 mmol/l crocin group showed a significant decrease at 24, 48
and 72 h compared with the control group. The overexpression of
Jak2 partially reversed the inhibitory effects of crocin, further
suggesting that crocin could inhibit the Jak2/Stat3 pathway in
human skin cancer cells to facilitate apoptosis (Fig. 4B).
Discussion
In recent years, traditional Chinese medicine
treatment for various tumors has become a hotspot and focus of
oncology field, considering its benefit of reduced toxicity and
fewer side effects. Previous studies indicated that the traditional
Chinese medicine treatment could inhibit proliferation, promote
apoptosis and exert antitumor effects using other mechanisms
(3,7). Studies have demonstrated that crocin
extracted from saffron has prominent inhibitory effects on a
variety of cancer cell proliferation (5,8,9) by acting on various pathways, including
apoptosis pathway (10–12).
Apoptotic pathways are mainly regulated by
endogenous pathways, exogenous pathways and endoplasmic reticulum
stress pathways. The endogenous pathway is also a
mitochondrial-dependent pathway, in which the mitochondrial
membrane potential changes after the cells receive the exogenous
signal. The increased mitochondrial membrane permeability enables
the release of mitochondrial protein into the cytoplasm, thereby
inducing apoptosis. Exogenous pathway is induced by the binding of
death ligand and death receptor, which recruits adapter protein and
forms a death-inducing signaling complex (DISC) with the caspase-8
precursor, thus activating caspase-3 for apoptotic induction. The
cellular endoplasmic reticulum stress signal is activated when the
cellular misfolded protein accumulates excessively, triggering
apoptotic signals and promoting apoptosis through a series of
apoptosis-related molecules (13).
The Bcl-2 family is a type of important regulatory protein in the
process of apoptosis. The balance of pro-apoptotic proteins and
anti-apoptotic proteins in this family affects whether the cells
survive or die. When cells receive a survival signal,
anti-apoptotic molecules such as Bcl-2, Mcl-1 and Bcl-w are
activated. In contrast, the pro-apoptotic protein such as Bid, Bax
would be activated to induce apoptosis of cells, when the cells
receive the pro-apoptotic signal (14). In this study, we preliminary
demonstrated that saffron can inhibit the proliferation of skin
cancer cells as well as promote apoptosis. While detecting
apoptosis-related proteins, we found out that the expression of
anti-apoptotic protein Bcl-2 was downregulated, while the
expression of pro-apoptotic protein Bid and procaspase-3 was
upregulated.
Recent studies have found that JAK/STAT signaling
pathway was activated abnormally in a variety of tumor tissues,
which has an immense influence on tumor progression (15,16).
JAK/STAT signaling pathway is an important intracellular signal
transduction pathway involved in a variety of physiological
processes such as cell growth, differentiation and apoptosis
(17). STAT persistent specific
signals, especially STAT3 and STAT5, can encode apoptosis
inhibitors. It can also stimulate cell proliferation and inhibit
apoptosis by upregulating the effect of Bcl-xl, Bcl-1 cyclin D1/D2
and c-Myc genes, thereby participating in the formation of tumors
(18,19). The continuous activation of STAT3,
which is capable of promoting malignant transformation, has been
corroborated as an oncogene (20).
Under the pathological conditions, the downstream target genes of
STAT signals, such as Bcl-2, caspases, survivin, Bcl-xl, cyclin D1,
p21 and VEGF, Mcl-1, c-Myc, c-Jun and Fas, are abnormally activated
and involved in a variety of pathophysiological processes such as
cell proliferation, differentiation, malignant transformation, and
apoptosis inhibition (21–23). In this study, the expression of Jak2
and Stat3 was downregulated after crocin treatment of human skin
cancer cells. In the combination group with both Jak2 expression
and crocin treatment, there was no evidence of any tumor-promoting
effect in JAK/STAT pathway. This illustrated that crocin can play a
tumor-promoting role by inhibiting Jak2/Stat3 pathway in human skin
cancer.
In conclusion, crocin could inhibit the
proliferation of A431 and SCL-1 skin cells and promote apoptosis.
The possible mechanism of apoptosis is to inhibit the Jak2/Stat3
pathway, downregulate the anti-apoptotic protein Bcl-2 expression
and enhance the levels of pro-apoptotic protein Bid and
procaspase-3.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GW and BZ designed the study and performed the
experiments, GW, YW and SH cultured the cells, BZ and CW collected
the data, GW and YW analyzed the data, GW prepared the manuscript.
All authors read and approved the final study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eisemann N, Waldmann A, Geller AC,
Weinstock MA, Volkmer B, Greinert R, Breitbart EW and Katalinic A:
Non-melanoma skin cancer incidence and impact of skin cancer
screening on incidence. J Invest Dermatol. 134:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jagadeeswaran R, Thirunavukkarasu C,
Gunasekaran P, Ramamurty N and Sakthisekaran D: In vitro studies on
the selective cytotoxic effect of crocetin and quercetin.
Fitoterapia. 71:395–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jnaneshwari S, Hemshekhar M, Santhosh MS,
Sunitha K, Thushara R, Thirunavukkarasu C, Kemparaju K and Girish
KS: Crocin, a dietary colorant, mitigates cyclophosphamide-induced
organ toxicity by modulating antioxidant status and inflammatory
cytokines. J Pharm Pharmacol. 65:604–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escribano J, Alonso GL, Coca-Prados M and
Fernandez JA: Crocin, safranal and picrocrocin from saffron
(Crocus sativus L.) inhibit the growth of human cancer cells
in vitro. Cancer Lett. 100:23–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdullaev FI and Espinosa-Aguirre JJ:
Biomedical properties of saffron and its potential use in cancer
therapy and chemoprevention trials. Cancer Detect Prev. 28:426–432.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishizuka F, Shimazawa M, Umigai N,
Ogishima H, Nakamura S, Tsuruma K and Hara H: Crocetin, a
carotenoid derivative, inhibits retinal ischemic damage in mice.
Eur J Pharmacol. 703:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdullaev FI: Cancer chemopreventive and
tumoricidal properties of saffron (Crocus sativus L.). Exp
Biol Med (Maywood). 227:20–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Olmo DC, Riese HH, Escribano J,
Ontañón J, Fernandez JA, Atiénzar M and García-Olmo D: Effects of
long-term treatment of colon adenocarcinoma with crocin, a
carotenoid from saffron (Crocus sativus L.): An experimental
study in the rat. Nutr Cancer. 35:120–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawkins RE, Russell SJ and Winter G:
Selection of phage antibodies by binding affinity. Mimicking
affinity maturation. J Mol Biol. 226:889–896. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith GP: Filamentous fusion phage: Novel
expression vectors that display cloned antigens on the virion
surface. Science. 228:1315–1317. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davies J and Riechmann L: An antibody VH
domain with a lox-Cre site integrated into its coding region:
Bacterial recombination within a single polypeptide chain. FEBS
Lett. 377:92–96. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azmi AS, Wang Z, Philip PA, Mohammad RM
and Sarkar FH: Emerging Bcl-2 inhibitors for the treatment of
cancer. Expert Opin Emerg Drugs. 16:59–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Q, Lai R, Chirieac LR, Li C, Thomazy
VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K,
et al: Constitutive activation of JAK3/STAT3 in colon carcinoma
tumors and cell lines: Inhibition of JAK3/STAT3 signaling induces
apoptosis and cell cycle arrest of colon carcinoma cells. Am J
Pathol. 167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao B, Shen X, Kunos G, Meng Q, Goldberg
ID, Rosen EM and Fan S: Constitutive activation of JAK-STAT3
signaling by BRCA1 in human prostate cancer cells. FEBS Lett.
488:179–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grandis JR, Drenning SD, Zeng Q, Watkins
SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y and Kim JD:
Constitutive activation of Stat3 signaling abrogates apoptosis in
squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA.
97:4227–4232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dell'Albani P, Kahn MA, Cole R, Condorelli
DF, Giuffrida-Stella AM and de Vellis J: Oligodendroglial survival
factors, PDGF-AA and CNTF, activate similar JAK/STAT signaling
pathways. J Neurosci Res. 54:191–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leong PL, Andrews GA, Johnson DE, Dyer KF,
Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, et
al: Targeted inhibition of Stat3 with a decoy oligonucleotide
abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA.
100:4138–4143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia L, Wang L, Chung AS, Ivanov SS, Ling
MY, Dragoi AM, Platt A, Gilmer TM, Fu XY and Chin YE:
Identification of both positive and negative domains within the
epidermal growth factor receptor COOH-terminal region for signal
transducer and activator of transcription (STAT) activation. J Biol
Chem. 277:30716–30723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|