Introduction
Asthma is a chronic airway inflammatory disease,
which is mainly regulated by airway inflammatory cells and
dominated by bronchial asthma (1,2). The
incidence of asthma is affected jointly by the environment,
genetics and immune function, whose main clinical manifestations
are recurrent asthma and cough. Due to changes in time and solar
terms, it occurs regularly, and is often aggravated at night and in
the early morning. This means that for children who are sensitive,
an asthma attack may occur, seriously affecting the development of
children (3,4).
Previous findings suggest that the inflammatory
response produced after lymphocyte activation and airway
infiltration is an important mechanism of asthma (5,6). Vitamin
D is a steroid derivative with a strong effect of regulating blood
calcium and bone metabolism. Research data have shown that vitamin
D can also regulate the hormone level of human immunity (7). Ali and Nanji (8) found that the level of serum vitamin D
is closely related to the incidence of asthma in children, and the
level of vitamin D also affects the lung function of children.
However, the correlations of vitamin D receptor (VDR) level in
asthma patients with the pathogenesis of asthma and airway
inflammation have not been reported yet.
This study aimed to clarify the role of VDR in
asthma by comparing the level of VDR between asthma patients and
healthy controls, and study the relationships of VDR with the
prognoses of inflammation and asthma to provide new ideas for a
clearer understanding and effective treatment of asthma.
Patients and methods
Patients and grouping
Patients with bronchial asthma treated at the
Tianjin Hospital of ITCWM Nankai Hospital (Tianjin, China) from
March, 2015 to March, 2016 were selected and screened according to
the criteria in the Guidelines on Diagnosis and Prevention of
Children's Bronchial Asthma (9). A
total of 30 patients meeting the criteria were selected as the
experimental group, while 30 healthy individuals were selected
during the same period as the control group. The experimental group
comprised 16 males and 14 females, aged 8–16 years. The control
group comprised 15 males and 15 females, aged 9–16 years. There
were no statistically significant differences in age and sex
between the two groups. Inclusion criteria of bronchial asthma
patients were: i) patients with recurrent asthma induced by
exercise and other factors; ii) the expiratory phase-based wheezing
rale could be heard in lungs during the onset; iii) patients who
did not take other drugs and vitamin D-related drugs during the
same period. Patients with other consumptive diseases were excluded
from the study. The study was approved by the Ethics Committee of
Tianjin Hospital of ITCWM Nankai Hospital, and the patients had
complete clinical and pathological data and the complete
therapeutic regimen. Informed consents were signed by the parents
and/or guardians of the patients.
Detection of VDR expression in vivo
via reverse transcription-quantitative polymerase chain reaction
(RT-PCR)
Fasting blood (5 ml) was drawn from the patients and
controls and centrifuged at 2,300 × g for 10 min at 4°C to collect
the supernatant. After the TRIzol reagent (volume ratio of 1:1)
(TRIzol kit; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
added, chloroform was added and the mixture was repeatedly shaken
10 times, followed by centrifugation at 11,000 × g for 10 min at
4°C. The supernatant was transferred to a new centrifuge tube and
isopropanol was added. After centrifugation at 9,060 × g for 10 min
at 4°C, the supernatant was discarded. Then, 1 ml freshly-prepared
75% ethanol was added, and the mixture was repeatedly shaken and
washed, followed by centrifugation at 11,000 × g for 10 min at 4°C.
The supernatant was discarded and the tube cap was opened to dry
naturally at room temperature, and 50 µl diethylpyrocarbonate
(DEPC) was dissolved to obtain the RNA samples.
After the determination of
A260/A280 and the optical density (OD) value,
10% agarose gel was used to confirm that the extracted RNA was not
degraded. According to the instructions of kit, the strand of
complementary DNA (cDNA) was obtained, based on which primers, Taq
polymerase, Taq buffer, deoxyribonucleoside triphosphate (dNTP)
mixture and double-distilled water (ddH2O) were added
for PCR amplification on a PCR instrument. Finally, the products
were placed on a quantitative PCR instrument to detect the mRNA
expression of target genes. The size of amplified product was 233
bp, and the PCR conditions are as follows: pre-denaturation at 92°C
for 45 sec, denaturation at 95°C for 5 sec, and annealing at 60°C
for 30 sec; a total of 35 cycles. The above primer sequences were
synthesized by Tiangen Biotech Co., Ltd. (Beijing, China) and the
sequences are shown in Table I.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene name | Primer sequences |
|---|
| VDR | F:
5′-TCGTATGGACGGAAGTACAGG-3′ |
|
| R:
5′-AAGACTGGTTGGAGCGTAACA-3′ |
|
Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) | F:
5′-GGTGAAGGTCGGTGTGAACG-3′ |
|
|
R:5′-CTCGCTCCTGGAAGATGGTG-3′ |
Detection of expression of related
proteins via western blot analysis
Peripheral blood (5 ml) was collected from patients
in each group, and added with lysis buffer (volume ratio of 1:1),
followed by centrifugation at 1,100 × g at 4°C for 10 min. The
supernatant was the total protein, and the total protein
concentration in the blood sample in each group was detected using
the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford,
IL, USA). The loading buffer was prepared according to the
concentration of samples, and the total protein level in loading
buffer in each group was made equal. After the gel preparation, the
samples were loaded for sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) under the constant voltage of 80 V for
100 min. The protein was transferred onto a polyvinylidene fluoride
(PVDF) membrane (IPVH00010; Millipore, Billerica, MA, USA) at 90 V
for 100 min. The target bands were cut and sealed in 5% skimmed
milk powder for 2 h, after which the mouse anti-human VDR and GAPDH
polyclonal antibodies (1:800; cat. nos. SAB1406580 and SAB1405848,
respectively; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were
incubated at 4°C overnight. The bands were washed with
phosphate-buffered saline (PBS) 3 times (5 min each time) and
incubated with the rabbit anti-mouse horseradish
peroxidase-conjugated secondary polyclonal antibody (1:1,000; cat.
no. A9044; Sigma-Aldrich; Merck KGaA) at room temperature for 2 h.
The bands were washed again with PBS 3 times and added with the
electrochemiluminescence (ECL) solution to obtain the image using
the fluorescence developing technique. The relative expression
level of VDR was presented as VDR/GAPDH.
Detection of contents of inflammatory
factors and 25-hydroxyvitamin D3 [25-(OH) D3]
in vivo via enzyme-linked immunosorbent assay (ELISA)
Fasting blood (5 ml)was drawn from patients in each
group and centrifuged to obtain the serum. The contents of
interleukin-6 (IL-6), IL-10, transforming growth factor-β (TGF-β),
tumor necrosis factor-α (TNF-α) and 25-(OH) D3 were
detected using the ELISA kit: The standard curves of IL-6, IL-10,
TGF-β, TNF-α and 25-(OH) D3 were drawn using the
supporting standard samples in the ELISA kit, and the above
standard curves were used to quantify the corresponding proteins.
Then, 100 µl serum samples in each group were diluted 10-fold using
the sample diluent that was added into the sample wells, and the
plate was sealed with the sealing membrane for reaction under the
constant temperature at 37°C for 60 min. The liquid in the plate
was patted dry and added with the corresponding biotin-labeled
antibody for reaction at 37°C for 60 min. After that, the liquid in
the plate was patted dry again and washed with liquid 3 times (1
min each time). Then, 100 µl avidin-peroxidase complex was added
for reaction at 37°C for 30 min. The liquid in the plate was patted
dry again 5 times (2 min each time), and 100 µl of stop buffer was
added to terminate the reaction. The OD value of each well was
measured at 450 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA), and the serum IL-6, IL-10, TGF-β, TNF-α
25-(OH) D3 levels were calculated using the standard
curve.
Determination of lung function
The lung function of patients in each group was
detected using an EasyOne Spirometer lung function apparatus (NDD,
Zurich, Switzerland) in the Pneumology Department of Tianjin
hospital. The following parameters of patients in each group were
recorded to evaluate the lung function: the percentage of forced
vital capacity in predicted value (FVC%), the percentage of forced
expiratory volume in 1 sec in predicted value (FEV1%), and 25% peak
expiratory flow (PEF25).
Assessment of asthma prognosis
Following treatment with the same regimen for 1
year, the prognosis of asthma patients in each group was evaluated
using the child asthma control test (C-ACT) questionnaire with a
total score of 27 points. Score <19 points: no asthma control;
score 19–22 points: partial control of asthma; score ≥23 points,
full control of asthma.
Statistical analysis
The data in this study are presented as mean ±
standard deviation and analyzed using Statistical Product and
Service Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL,
USA). The t-test was used for the intergroup comparison, Chi-square
test was used for the enumeration data, and analysis of variance
was used for the comparison among groups. The homogeneity test of
variance was performed; if the variance was homogeneous, Bonferroni
method was used for the pairwise comparison; if the variance was
heterogeneous, Welch method was used for analysis. Dunnett's T3
method was adopted for multiple comparisons. Pearson's analysis was
used for the correlation among factors. P<0.05 suggested that
the difference was statistically significant.
Results
Patient characteristics
General data, including sex, age and body mass index
(BMI), of experimental group and control group were recorded in
detail. The results (Table II)
showed that the differences in sex, age and BMI of patients were
not statistically significant between the experimental and control
groups (P>0.05).
 | Table II.General data of patients in each
group. |
Table II.
General data of patients in each
group.
|
| Experimental
group | Control group | P-value |
|---|
| Sex
(male/female) | 16/14 | 15/15 | >0.05 |
| Age (years) | 13.7±3.9 | 14.1±2.8 | >0.05 |
| BMI |
|
Normal | 21 | 20 | >0.05 |
|
Overweight | 5 | 7 | >0.05 |
|
Obese | 4 | 3 | >0.05 |
mRNA and protein expression levels of
VDR
The mRNA and protein expression levels of VDR were
detected via reverse transcription-quantitative PCR and western
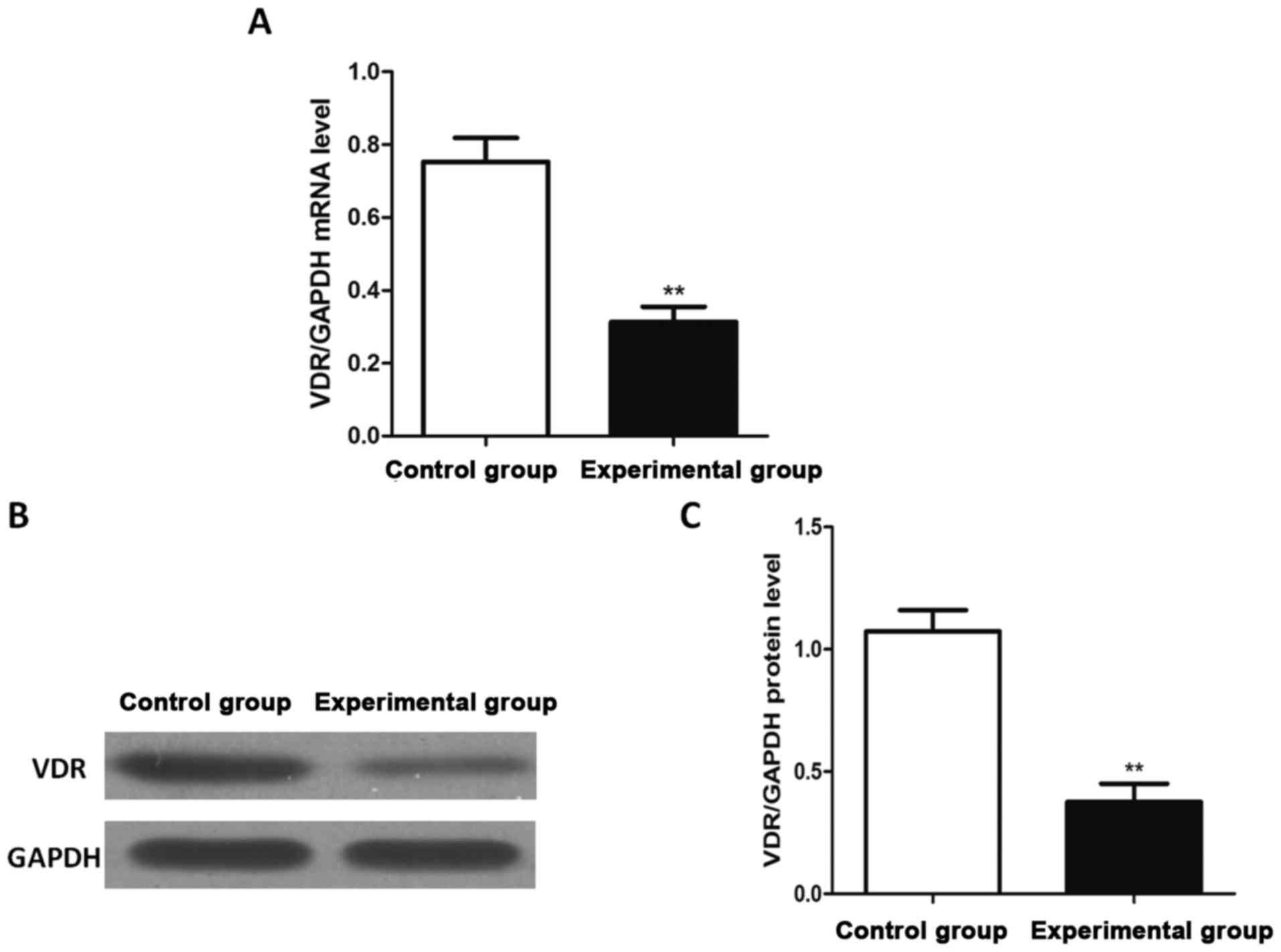
blot analysis, respectively. The results (Fig. 1) revealed that the mRNA and protein
expression levels of VDR in experimental group were significantly
lower than those in control group, and the differences were
statistically significant (P<0.01).
Contents of inflammatory factors and
25-(OH) D3
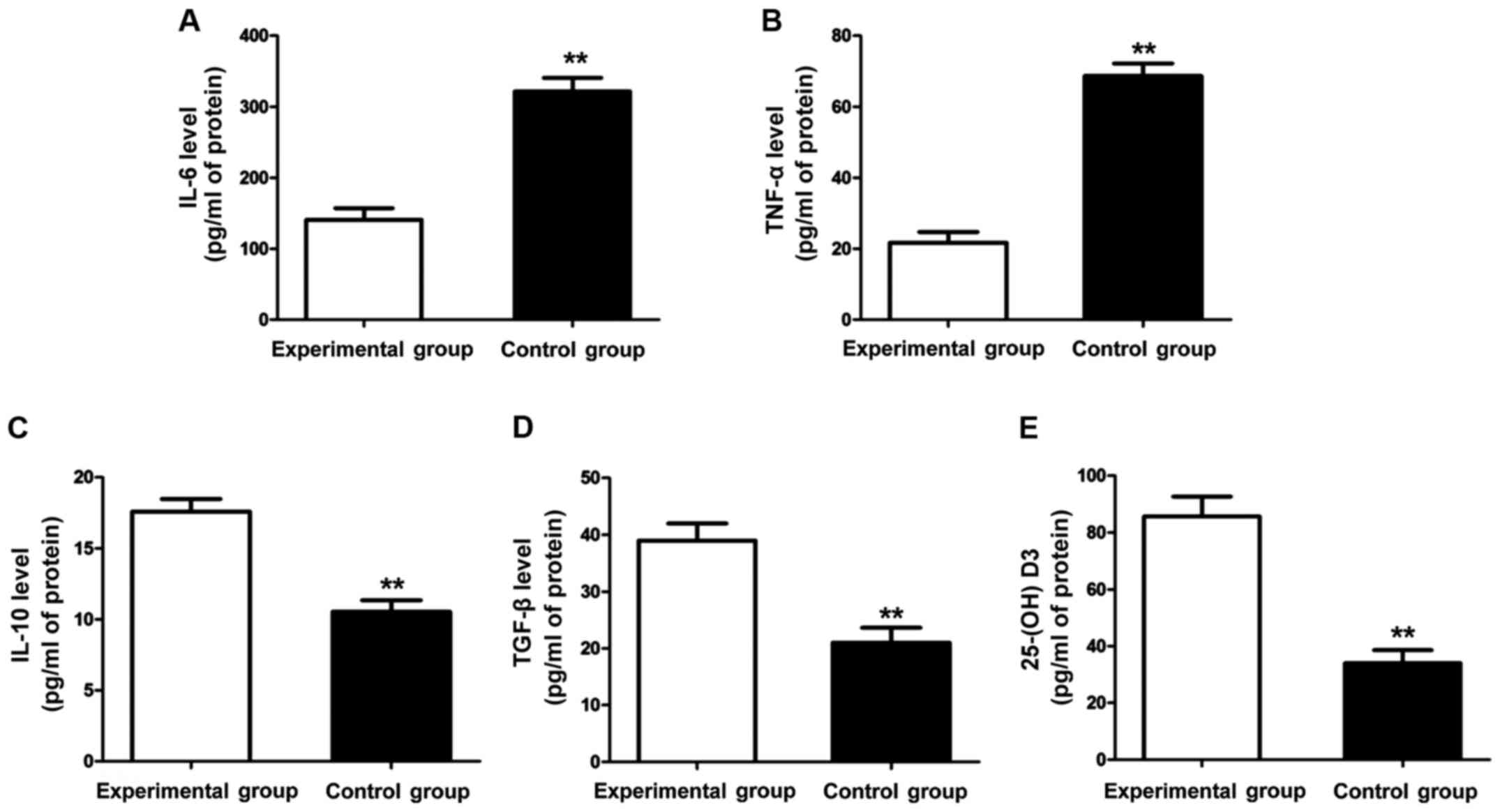
The contents of inflammatory factors and 25-(OH)
D3 in each group were detected using ELISA kit. The
results (Fig. 2) showed that the
contents of inflammatory factors (IL-6 and TNF-α) in the control
group were significantly lower than those in the experimental group
(P<0.01), but the contents of anti-inflammatory factors (TGF-β
and IL-10) were significantly higher than those in the experimental
group (P<0.01). Additionally, the content of 25-(OH)
D3 in the experimental group was obviously lower than
that in the control group, and the difference was statistically
significant (P<0.01).
Analysis of correlations between
inflammatory factors and VDR expression level
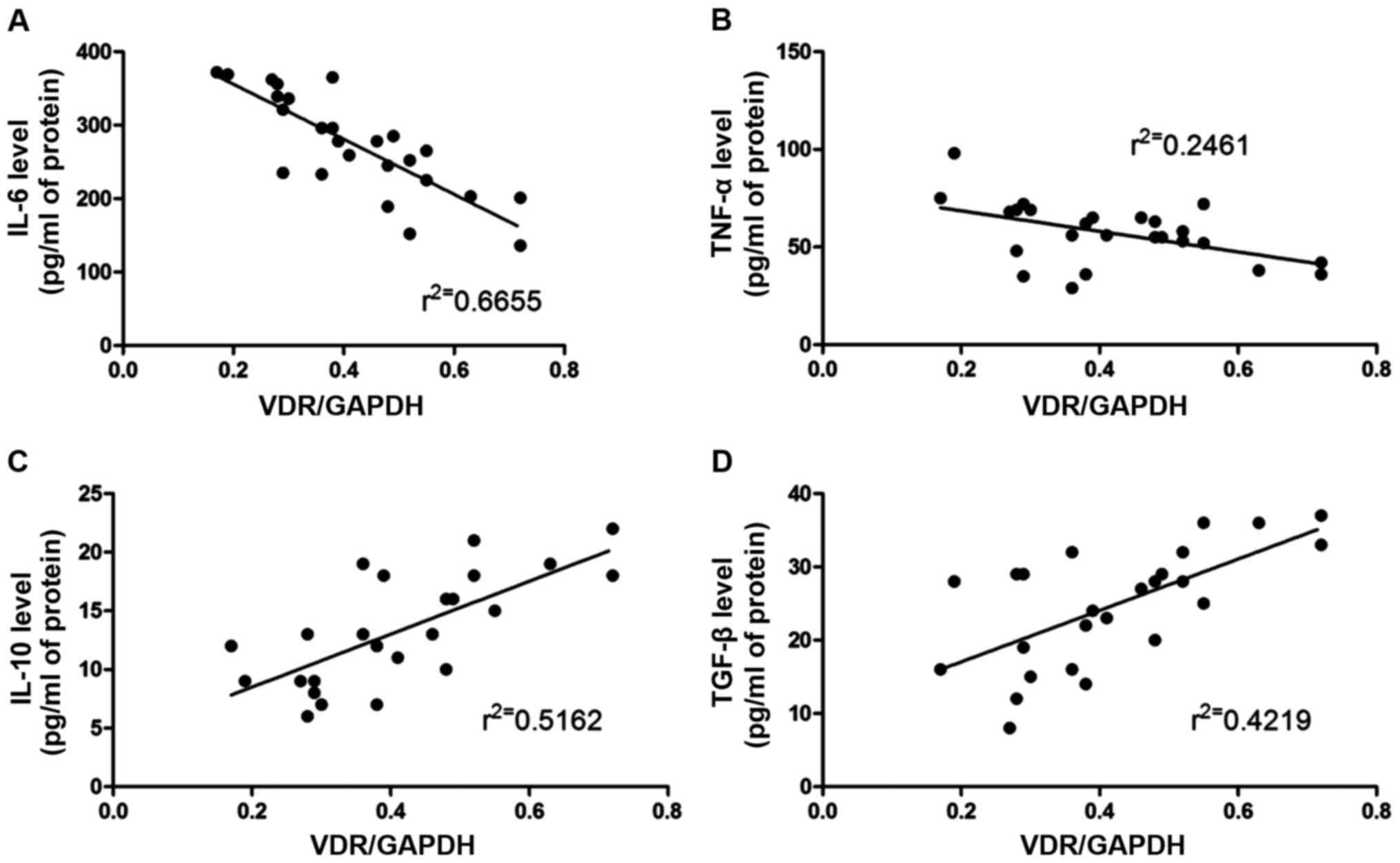
The correlation between the inflammatory factors
(IL-6, IL-10, TNF-α and TGF-β) and the VDR expression level was
analyzed via Pearson's analysis. The results (Fig. 3) revealed that the VDR expression
level was negatively correlated with IL-6 and TNF-α, but positively
correlated with IL-10 and TGF-β.
Correlation between VDR expression and
lung function of asthma patients
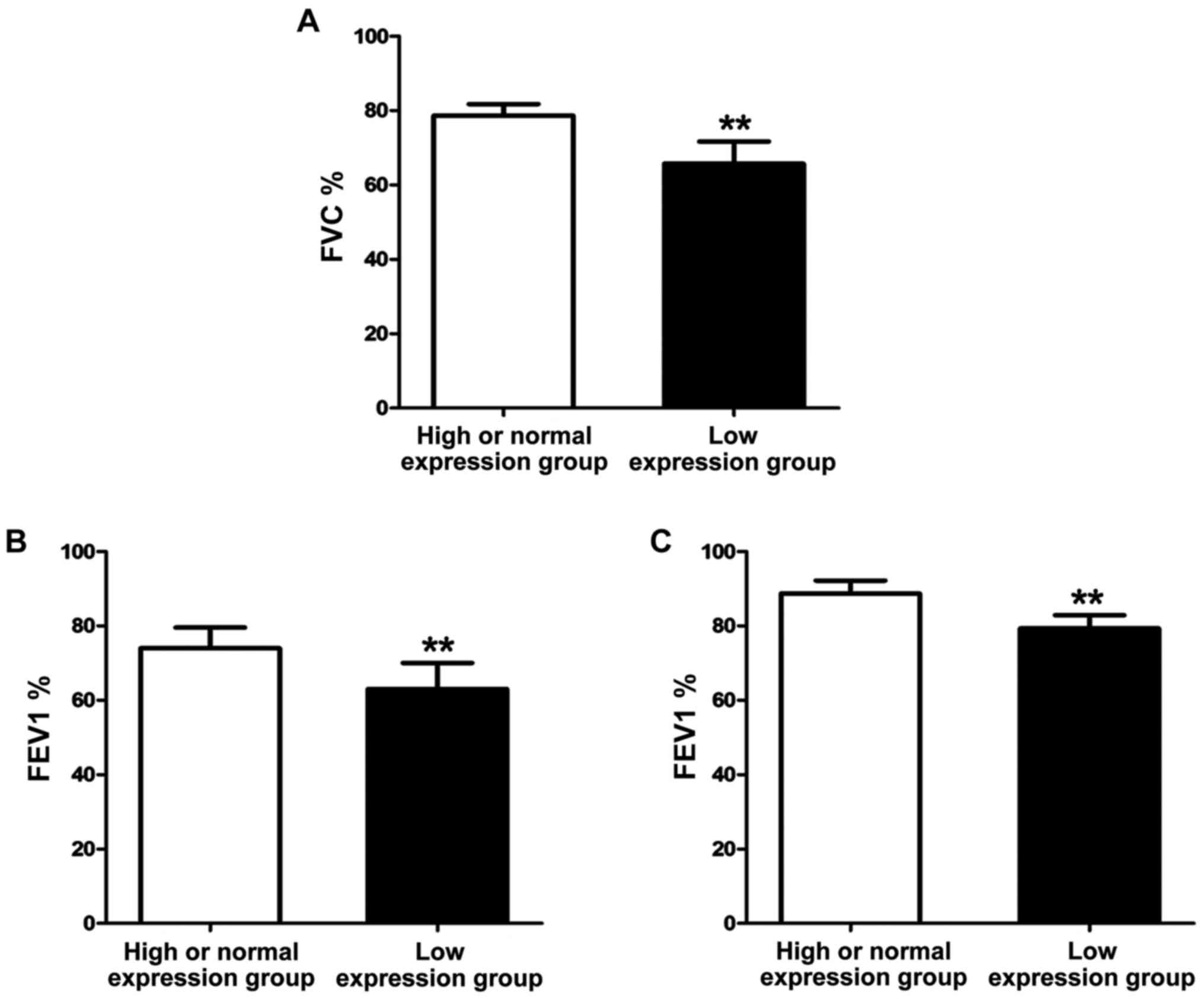
After treatment with the same regimen for 1 year,
the lung function of asthma patients was detected using the lung
function monitor. The results (Fig.
4) showed that FVC%, FEV1% and PEF25 of patients in high or
normal expression group were significantly higher than those of
patients in low expression group, and the differences were
statistically significant (P<0.01).
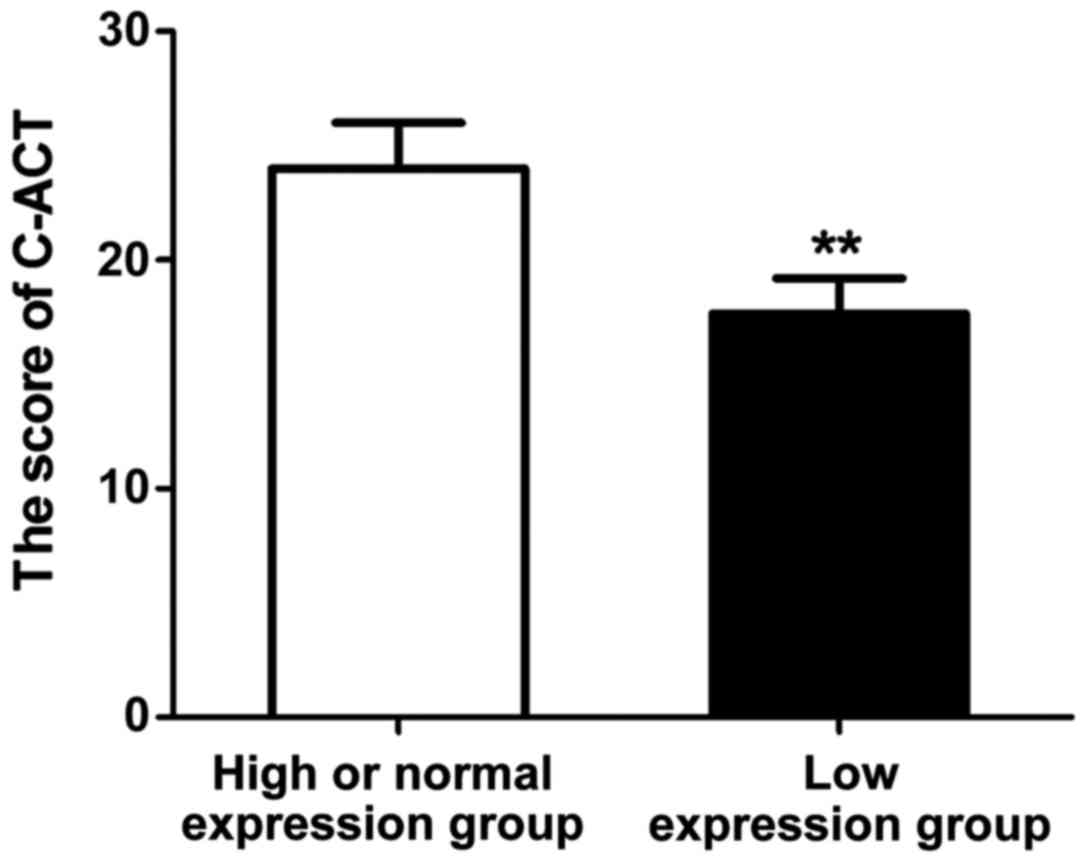
Correlation between VDR expression and
prognosis of asthma
After treatment with the same regimen for 1 year,
the prognosis of asthma patients in each group was evaluated using
the C-ACT questionnaire, and the score of each patient was
recorded. The results (Fig. 5 and
Table III) showed that the C-ACT
score of patients in the high or normal expression group was
significantly higher than that of patients in low expression group,
and the difference was statistically significant (P<0.01), and
the asthma control rate of patients in the high or normal
expression group was obviously higher than that of patients in low
expression group (P<0.01).
 | Table III.Asthma control rate. |
Table III.
Asthma control rate.
|
| High or normal
expression group | Low expression
group | P-value |
|---|
| Asthma control
rate | 85.6% | 53.7% | <0.01 |
Discussion
Environmental pollution problem has been aggravated
annually, and a large number of dusts in the air and a variety of
allergens lead to the increased incidence of bronchial asthma
(10,11). Bronchial asthma is mainly the
immunoglobulin E (IgE)-mediated type I allergic reaction, and the
Th1/Th2 ratio imbalance will promote the production of IgE, and
induce and aggravate the occurrence of bronchial asthma (12). In recent years, vitamin D has been
found to be an immunomodulator that can mediate the balance of Th
cells, thus affecting asthma (13,14).
Vitamin D can also regulate a variety of signaling pathways
produced by inflammatory factors, and affect the expression levels
of inflammatory factors, so vitamin D is closely related to asthma
(15).
Studies have found that the content of active
component of vitamin D, 25-(OH) D3, can regulate the
occurrence of asthma (16). In this
study, the content of 25-(OH) D3 in patients with
bronchial asthma was detected with the normal people as the
control, and it was found that the content of 25-(OH) D3
in asthma patients was significantly decreased, which was
consistent with the finding of Jensen et al (17) that the content of 25-(OH)
D3 in asthma children was decreased, leading to the
deficiency of vitamin D. Moreover, the mRNA and protein expression
of VDR in asthma patients were studied via real-time quantitative
PCR and western blotting in this study. The results showed that the
expression level of VDR in asthma patients was obviously decreased,
suggesting that the VDR expression level can also affect the
progression of asthma. Brehm et al (18) found that 25-(OH) D3 can
exert a regulatory effect and affect the development of Th cells
only when binding to VDR. Boonpiyathad et al (19) established the
lipopolysaccharide-induced asthma model using VDR-knockout mice and
wild-type mice, and found that the lung inflammation and asthma
symptoms of VDR-knockout mice are not significant compared with
those of wild-type mice. In this study, the correlations of VDR
expression level in asthma patients with inflammatory factors were
analyzed. The results revealed that the expression level of VDR was
negatively correlated with the levels of pro-inflammatory factors,
but positively correlated with the levels of anti-inflammatory
factors; in other words, the VDR expression can affect the
inflammatory response in the body. The study of Cantorna et
al (20) found that vitamin D
can regulate the immune responses in alveolar epithelial cells and
alveolar macrophages and reduce the production of pulmonary
inflammation, whereas 25-(OH) D3 can exert corresponding
effects only when binding to its receptor. Besides, it was also
found in this study that the expression level of VDR affected the
prognosis and lung function of asthma patients, and the recovery of
lung function and asthma control in patients with high VDR
expression were better after treatment. The increased VDR
expression level could help effectively regulate the metabolic
process in the body, and the utilization level of vitamin D was
also significantly increased (21).
In conclusion, the expression level of VDR is
positively correlated with the levels of inflammatory factors in
patients with asthma, and the expression of VDR can significantly
affect the prognosis and recovery of lung function of patients with
asthma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YC wrote the manuscript. YC and TX performed PCR and
western blot analysis. TX assisted in the statistical analysis.
Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Tianjin Hospital of
ITCWM Nankai Hospital (Tianjin, China). Informed consents were
signed by the parents and/or guardians of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roxbury CR and Lin SY: Efficacy and safety
of subcutaneous and sublingual immunotherapy for allergic
rhinoconjunctivitis and asthma. Otolaryngol Clin North Am.
50:1111–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Yu Q, Zhao W, Zhang J, Liu W, Huang
M and Zeng X: Oligomeric proanthocyanidins attenuate airway
inflammation in asthma by inhibiting dendritic cells maturation.
Mol Immunol. 13:226–232. 2012.
|
|
3
|
Chen G, Chiang WL, Shu BC, Guo YL, Chiou
ST and Chiang TL: Associations of caesarean delivery and the
occurrence of neurodevelopmental disorders, asthma or obesity in
childhood based on Taiwan birth cohort study. BMJ Open. 3:622–635.
2015.
|
|
4
|
Shin E, Lee YC, Kim SR, Kim SH and Park J:
Drug signature-based finding of additional clinical use of
LC28-0126 for neutrophilic bronchial asthma. Sci Rep. 5:177842015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaur S, Gupta VK, Shah A, Thiel S, Sarma
PU and Madan T: Elevated levels of mannan-binding lectin
[corrected] (MBL) and eosinophilia in patients of bronchial asthma
with allergic rhinitis and allergic bronchopulmonary aspergillosis
associate with a novel intronic polymorphism in MBL. Clin Exp
Immunol. 143:414–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Zhong N and Lai K: Re-challenge
with ovalbumin failed to induce bronchial asthma in mice with
eosinophilic bronchitis. PLoS One. 8:e751952013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Checkley W, Robinson CL, Baumann LM,
Hansel NN, Romero KM, Pollard SL, Wise RA, Gilman RH, Mougey E and
Lima JJ; PURA Study Investigators, . 25-hydroxy vitamin D levels
are associated with childhood asthma in a population-based study in
Peru. Clin Exp Allergy. 45:226–232. 2015. View Article : Google Scholar
|
|
8
|
Ali NS and Nanji K: A Review on the role
of vitamin D in asthma. Cureus. 9:e12882017.PubMed/NCBI
|
|
9
|
Staticescu S, Chereches-Panta P, Ichim G,
Valeanu M and Nanulescu MV: The value of induced sputum in the
diagnosis and management of children with bronchial asthma. Clujul
Med. 87:171–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berenguer AG, Fernandes AT, Oliveira S,
Rodrigues M, Ornelas P, Romeira D, Serrão T, Rosa A and Câmara R:
Genetic polymorphisms and asthma: Findings from a case-control
study in the Madeira island population. Biol Res. 47:402014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pásztor D, Kolozsvári BL, Csutak A, Berta
A, Hassan Z, Kettesy BA, Gogolák P and Fodor M: Scheimpflug imaging
parameters associated with tear mediators and bronchial asthma in
keratoconus. J Ophthalmol. 2016:93926402016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim SY, Jo YJ and Chun EM: The correlation
between the bronchial hyperresponsiveness to methacholine and
asthma like symptoms by GINA questionnaires for the diagnosis of
asthma. BMC Pulm Med. 14:1612014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Liu D and Liu CT: Can vitamin D
supplementation in addition to asthma controllers improve clinical
outcomes in patients with asthma? A Meta-Analysis. Medicine
(Baltimore). 94:e21852015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Somashekar AR, Prithvi AB and Gowda MN:
Vitamin D levels in children with bronchial asthma. J Clin Diagn
Res. 8:4–7. 2014.
|
|
15
|
Augusto A: Vitamin D deficiency as a risk
factor for childhood allergic disease and asthma. Curr Opin Allergy
Clin Immunol. 12:86–91. 2012.
|
|
16
|
Jat KR and Khairwa A: Vitamin D and asthma
in children: A systematic review and meta-analysis of observational
studies. Lung India. 34:1787–1799. 2017. View Article : Google Scholar
|
|
17
|
Jensen ME, Mailhot G, Alos N, Rousseau E,
White JH, Khamessan A and Ducharme FM: Vitamin D intervention in
preschoolers with viral-induced asthma (DIVA): A pilot randomised
controlled trial. Trials. 17:3532016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brehm JM, Schuemann B, Fuhlbrigge AL,
Hollis BW, Strunk RC, Zeiger RS, Weiss ST and Litonjua AA;
Childhood Asthma Management Program Research Group, . Serum vitamin
D levels and severe asthma exacerbations in the Childhood Asthma
Management Program study. J Allergy Clin Immunol. 126:52–8.e5.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boonpiyathad T, Chantveerawong T,
Pradubpongsa P and Sangasapaviliya A: Serum vitamin D levels and
vitamin D supplement in adult patients with asthma exacerbation. J
Allergy (Cairo). 2016:40706352016.PubMed/NCBI
|
|
20
|
Cantorna MT, Zhao J and Yang L: Vitamin D,
invariant natural killer T-cells and experimental autoimmune
disease. Proc Nutr Soc. 71:62–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain SK, Micinski D, Huning L, Kahlon G,
Bass PF and Levine SN: Vitamin D and L-cysteine levels correlate
positively with GSH and negatively with insulin resistance levels
in the blood of type 2 diabetic patients. Eur J Clin Nutr.
68:1148–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|