Introduction
Diabetes is one of the most important
non-communicable diseases worldwide (1). According to the International Diabetes
Federation statistics, the number of patients with diabetes
worldwide in 2011 reached 370 million, and this number will be
nearly 550 million by 2030. T2DM is a progressive disease caused by
insulin resistance and/or beta cell dysfunction, which results in
relative insulin deficiency. The reduction in beta cell mass and
beta cell dysfunction contribute toward the pathological process
(2). A previous study has suggested
that beta cell dysfunction in patients with clinical manifestations
of T2DM may have begun 15 years previously (2). Increased levels of circulating free
fatty acids (FFAs) have been indicated to cause defective beta cell
proliferation and increased beta cell apoptosis. Therefore,
lipotoxicity has an important role in the pathogenesis of T2DM
(3).
THBS-1 is an extracellular matrix-bound factor and
was reported as the first naturally occurring inhibitor of
angiogenesis (4). It was also
revealed to be involved in other processes, including regulation of
extracellular matrix function, blood clot formation and the immune
response (5–7). Recently, accumulating research has
suggested that THBS-1 is associated with T2DM and beta cell
function (8–10).
MicroRNAs (miRs) are 19 to 22-nucleotide noncoding
RNAs that can regulate cell survival, cell function, apoptosis and
differentiation by suppressing the transcription of mRNA (11–13).
Currently, extensive research has suggested that miRs are involved
in fatty acid-induced beta cell dysfunction (14–16).
miR-182-5p, which has been predicted to target THBS-1, has been
confirmed to participate in the progression of various diseases,
including cancer (17),
ischemia-reperfusion injury (18)
and leukemia (19). However, to the
best of our knowledge miR-182-5p has not been reported to be
associated with T2DM or beta cell function.
The present study aimed to identify whether
palmitate (Pal) impacted the viability and apoptosis of INS-1
cells. Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was performed to assess the THBS-1 mRNA expression levels
in Pal-treated cells compared with the control cells. Subsequent
MTT and flow cytometry assays were also performed. The present
study provided an insight into whether miR-182-5p may participate
in the protective effects of THBS-1 against lipotoxicity in INS-1
cells, and whether it may be a novel biomarker for the diagnosis
and treatment of T2DM.
Materials and methods
Cell culture
Rat INS-1 cells (Shanghai Fushan Industrial Co.,
Ltd, Shanghai, China) were cultured in RPMI 1640 medium (Gibco,
Thermo Fisher Scientific, Inc., Waltham, MA, USA), containing 11
mmol/l glucose, 10% fetal bovine serum (Gibco, Thermo Fisher
Scientific, Inc.), 1 mmol/l sodium pyruvate, 2 mmol/l glutamine, 10
mmol/l HEPES, 55 µmol/l beta-mercaptoethanol, 100 IU/ml penicillin
and 100 µg/ml streptomycin at 37°C containing 5%
CO2.
Vector constructions and miR
transfection
The coding sequence of THBS-1 was cloned into a
pcDNA3 vector, prior to the construction of the THBS-1 expression
plasmid, pcDNA3-THBS-1. The enhanced green fluorescent (EGFP)
coding region from the pEGFP-N2 vector was cloned into pcDNA3 to
form pcDNA3-EGFP, and the wild-type or mutant-type THBS-1 3′-UTR
was amplified and cloned into the pcDNA3-EGFP vector. A total of 20
µM miR-182-5p mimics, inhibitors and the corresponding control
(miR-NC) (Shanghai GenePharma Co., Ltd., Shanghai, China) were
transfected into INS-1 cells using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The sequences used were as follows:
miR-182-5p, 5′-UUUGGCAAUGGUAGAACUCACACCG-3′; miR-182-5p inhibitor,
5′-CGGUGUGAGUUCUACCAUUGCCAAA-3′; and miR-NC,
5′-UUGUACUACACAAAAGUAGUC-3′. Following 24 h of transfection, the
subsequent experiments were carried out.
FFA treatment
Pal (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
which was used as the FFA, was dissolved into 0.01 mol/l NaOH to a
concentration of 100 mmol/l. Subsequently, the solution was diluted
in RPMI-1640 medium and mixed with 0.5% FFA-free bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) for 30 min at 55°C.
INS-1 cells were cultured and exposed at 37°C to Pal (0.25, 0.5,
and 1.0 mol/l) for 8, 24 or 48 h. The control cells were treated
with equivalent concentrations of NaOH.
MTT assay
INS-1 cells were transfected with plasmids,
miR-182-5p mimics or inhibitors. A total of 8×103 cells
were placed into 96-well culture plates for 24 h at 37°C. Cells
were incubated with different concentrations of Pal (0.25, 0.5 and
1.0 mmol/l) for a further 24 h. A total of 10 µl of MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added into the wells and the cells
were incubated for 4 h at 37°C. The culture plate was centrifuged
at 225 × g for 5 min at room temperature and the supernatant was
removed. Following this, 150 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA) was added to the medium of each well and the plate was
placed on a shaker in the dark for 20 min at room temperature.
Finally, the absorbance value of each well at the wavelength of 570
nm (A570) was measured.
Apoptosis assay
An annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (BD
Biosciences Medical Devices Shanghai Co., Ltd., Shanghai, China)
was used to detect INS-1 cell apoptosis. Cells were collected
following centrifugation at room temperature for 10 min at 225 × g,
washed with phosphate buffered saline and resuspended in binding
buffer (300 µl). A total of 5 µl annexin V-FITC solution was added,
followed by incubation at room temperature for 15 min in the dark.
Finally, 5 µl PI was added to the cell suspension. A flow cytometer
(BD Bioscience, Shanghai, China) was used to analyze cell
apoptosis. Among the analyzed cells,
FITC−/PI− cells represented healthy living
cells, FITC+/PI− cells indicated early
apoptotic cells and FITC+/PI+ cells
represented necrosis and late apoptotic cells.
RT-qPCR analysis
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc) and the mirVana miRNA Isolation Kit (Ambion;
Thermo Fisher Scientific.) were used to extract total RNA and miRs.
The oligo-dT or stem-loop reverse transcriptase primers were used
to obtain cDNA. PCR was performed using the SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd., Dalian, China) to detect the
expression levels of THBS-1 mRNA and miR-182-5p. β-actin and U6
were used as the corresponding controls. The primer sequences used
in the present study are indicated in Table I. The reaction conditions used were
as follows: 94°C for 5 min, followed by 40 cycles of 94°C for 1
min, 56°C for 1 min and 72°C for 1 min. The relative expression
levels were calculated using the 2−ΔΔCq method (20).
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Name | Primer sequence |
|---|
| miR-182-5p RT
primer |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAGTGTG-3′ |
| U6 RT primer |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC-3′ |
| miR-182-5p
forward |
5′-TCGGGTTTGGCAATGGTAGAAC-3′ |
| U6 forward |
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ |
| Reverse |
5′-CCAGTGCAGGGTCCGAGGT-3′ |
| THBS1 forward |
5′-TTTGCTGCGTTTGTGGAA-3′ |
| THBS1 reverse |
5′-GAGGAGGTATCTGTAATGC-3′ |
| β-actin forward |
5′-CGTGACATTAAGGAGAAGCTG-3′ |
| β-actin reverse |
5′-CTAGAAGCATTTGCGGTGGAC-3′ |
Western blot analysis
Cells were transfected with miR-182-5p mimics or
inhibitors. Protein concentration was determined by bicinchoninic
acid assay. Cell protein samples were obtained using
radioimmunoprecipitation assay lysis buffer (Beijing BLKW
Biotechnology Co., Ltd, Beijing, China), separated by 8% SDS-PAGE
(20 µg/lane) and transferred to nitrocellulose membranes. Following
this, samples were blocked with 5% skimmed milk for 2 h at room
temperature. Membranes were incubated with anti-THBS-1 antibody
(ab88529; 1:200; Abcam, Cambridge, MA, USA) and anti-GAPDH antibody
(ab8245; 1:500; Abcam) overnight at 4°C. Subsequently, the
horseradish peroxidase-conjugated corresponding immunoglobulin G
(ab7090; 1:1,000; Abcam) was added for incubation at room
temperature for 2 h. The protein expression level was assessed
using an enhanced chemiluminescence regent (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). GAPDH was used as the
corresponding control. The relative protein expression levels were
determined relative to GAPDH.
miR target prediction
The possible miRs that can target THBS-1 were
predicted using miRanda (http://microrna.sanger.ac.uk/), TargetScan (genes.mit.edu/targetscan) and PicTar (http://pictar.mdc-berlin.de).
EGFP reporter assay
INS-1 cells were transfected with miR-182-5p mimics,
miR-182-5p inhibitor and reporter vectors bearing either THBS 1
3′UTR wild-type or THBS 1 3′UTR mutant-type. An F-4500 fluorescence
spectrophotometer (Hitachi, Ltd., Tokyo, Japan) was used to detect
the intensities of fluorescence. Red fluorescent protein (RFP)
expression vector was used as a reporter control. Relative EGFP
fluorescence intensity was normalized to the RFP fluorescence
intensity.
Statistical analysis
Data were analyzed using GraphPad Prism 6.0
statistical software (GraphPad Software, Inc., La Jolla, CA, USA)
and were presented as the mean ± standard deviation. A two-tailed
Student's t-test was performed to compare differences between two
groups. One-way analysis of variance followed by Tukey's post hoc
test was used to compare differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
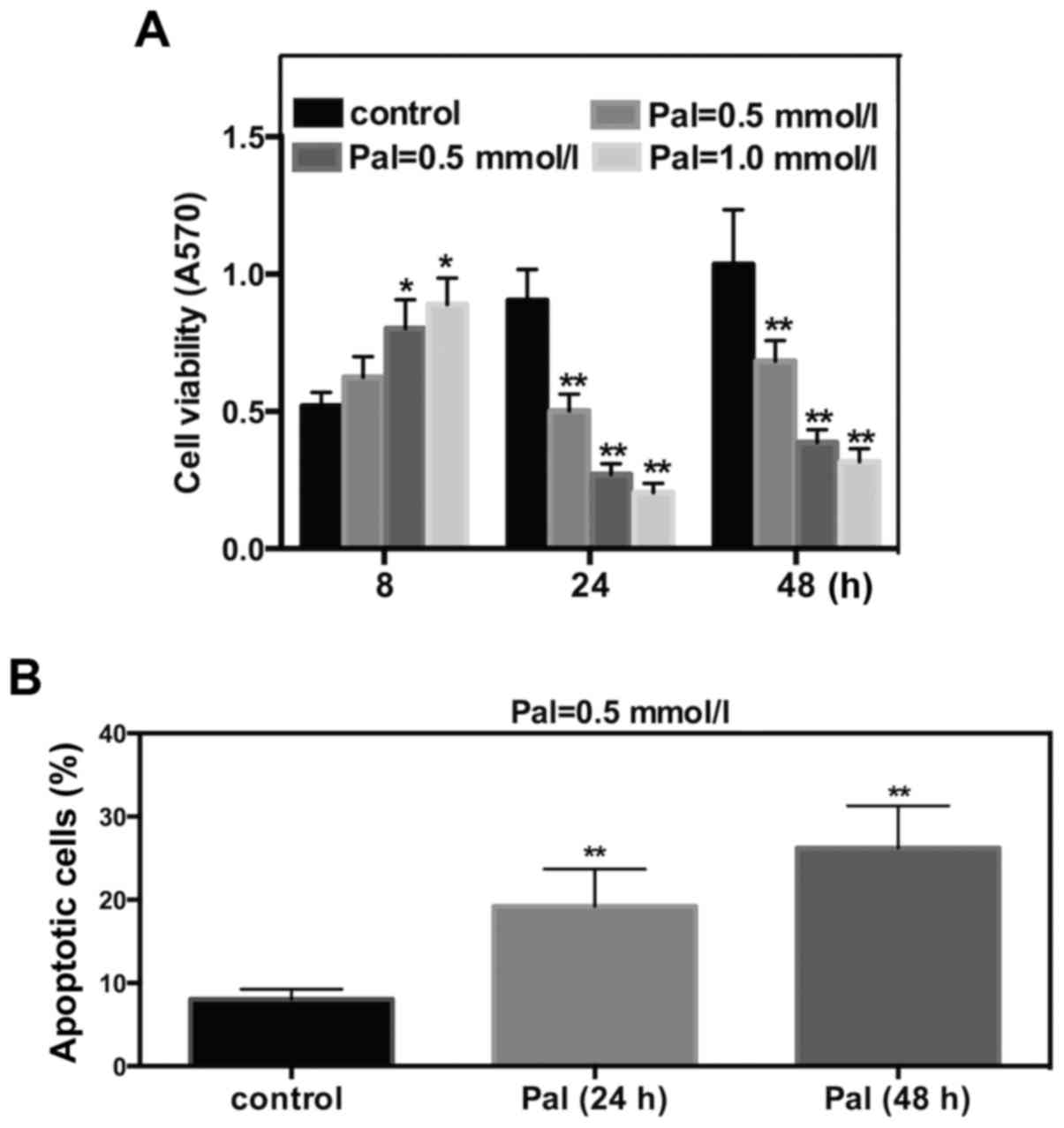
FFA induces cell toxicity in INS-1
cells
To determine the effect of FFA on cell viability and
apoptosis, Pal was selected as the FFA. INS-1 cells were cultured
and incubated with different concentrations of Pal. The subsequent
MTT assay revealed that Pal increased INS-1 cell viability
following 8 h of incubation and significantly decreased cell
viability at 24 or 48 h of incubation. These effects occurred in a
dose-dependent manner (Fig. 1A).
Results of flow cytometry suggested that incubations for 24 or 48 h
with Pal (0.5 mmol/l) increased the cell apoptosis rate by 2.8- or
3.2- fold, respectively (Fig. 1B).
These results suggested that Pal can induce cell toxicity in INS-1
cells.
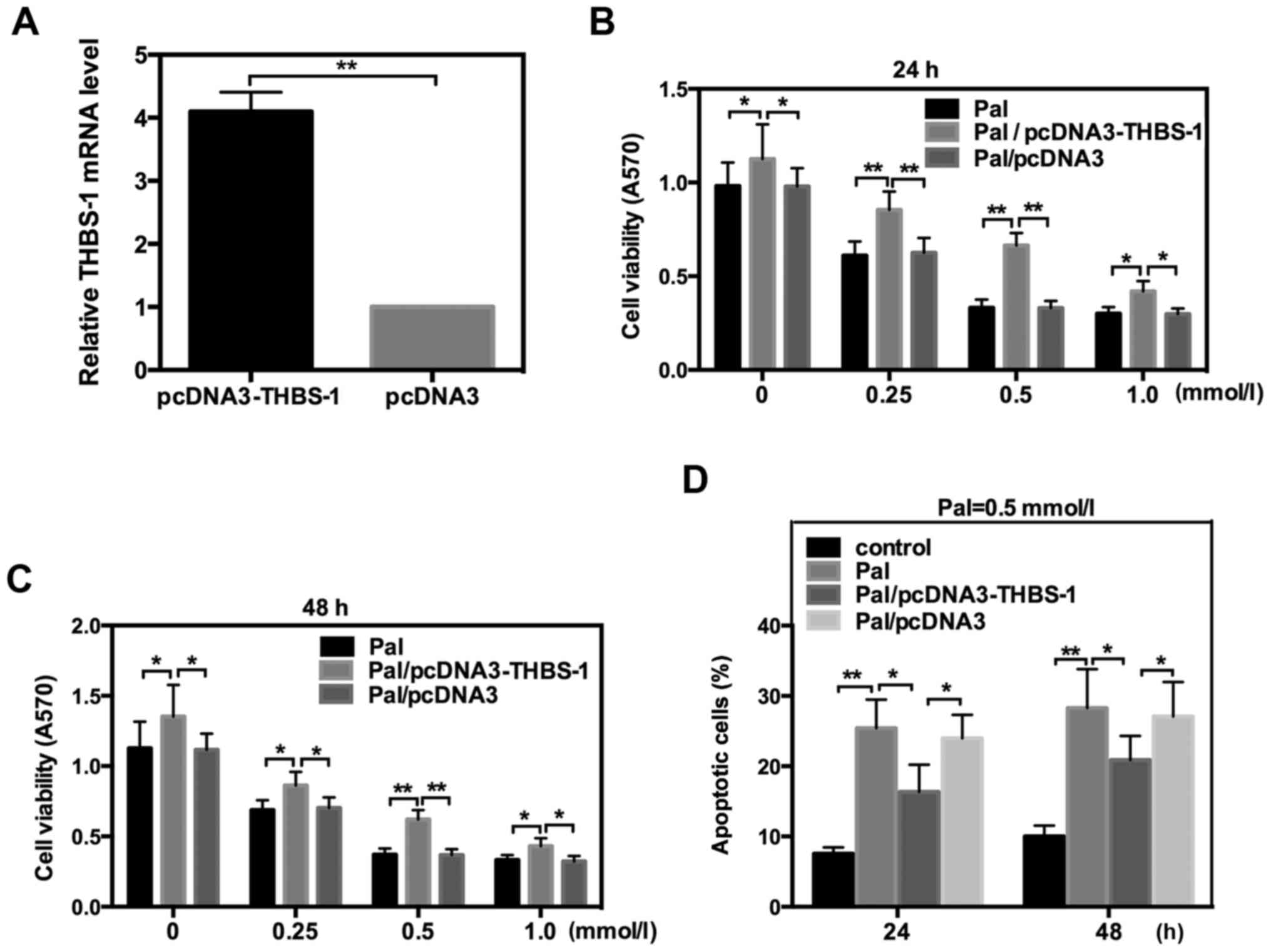
THBS-1 protects INS-1 cells from
Pal-induced cell toxicity
It has previously been indicated that THBS-1 is
associated with beta cell function and T2DM (8). To further explore whether THBS-1 is
involved in the lipotoxicity of INS-1 cells, the THBS-1 expression
plasmid, pcDNA3-THBS-1, was transfected into INS-1 cells, which
were incubated with different concentrations of Pal. The efficiency
of pcDNA3-THBS 1 was detected by RT-qPCR (Fig. 2A). Data indicated that THBS-1
significantly reversed the changes in cell viability and apoptosis
that were induced by Pal following incubation for 24 or 48 h. These
data suggested that THBS 1 protects INS-1 cells from Pal-induced
lipotoxicity (Fig. 2).
Bioinformatics prediction
Previous studies have reported that a number of miRs
are involved in regulating the occurrence and progression of T2DM
(12–16). To further determine whether THBS-1 is
regulated by other molecules in its actions on the lipotoxicity of
INS-1 cells, miRanda, Targetscan and PicTar were used to predict
the possible miRs that may target THBS-1. The prediction results
from these bioinformatics software were combined and several miRs
were selected. Among these, miR-182-5p was of interest. To the best
of our knowledge, miR-182-5p has not been reported to be associated
with T2DM. A total of 7 bases from the 3′-UTR region of THBS-1 were
identified to be complementary to the seed sequence of miR-182-5p
(Fig. 3). Considering these
observations, miR-182-5p was selected for further study.
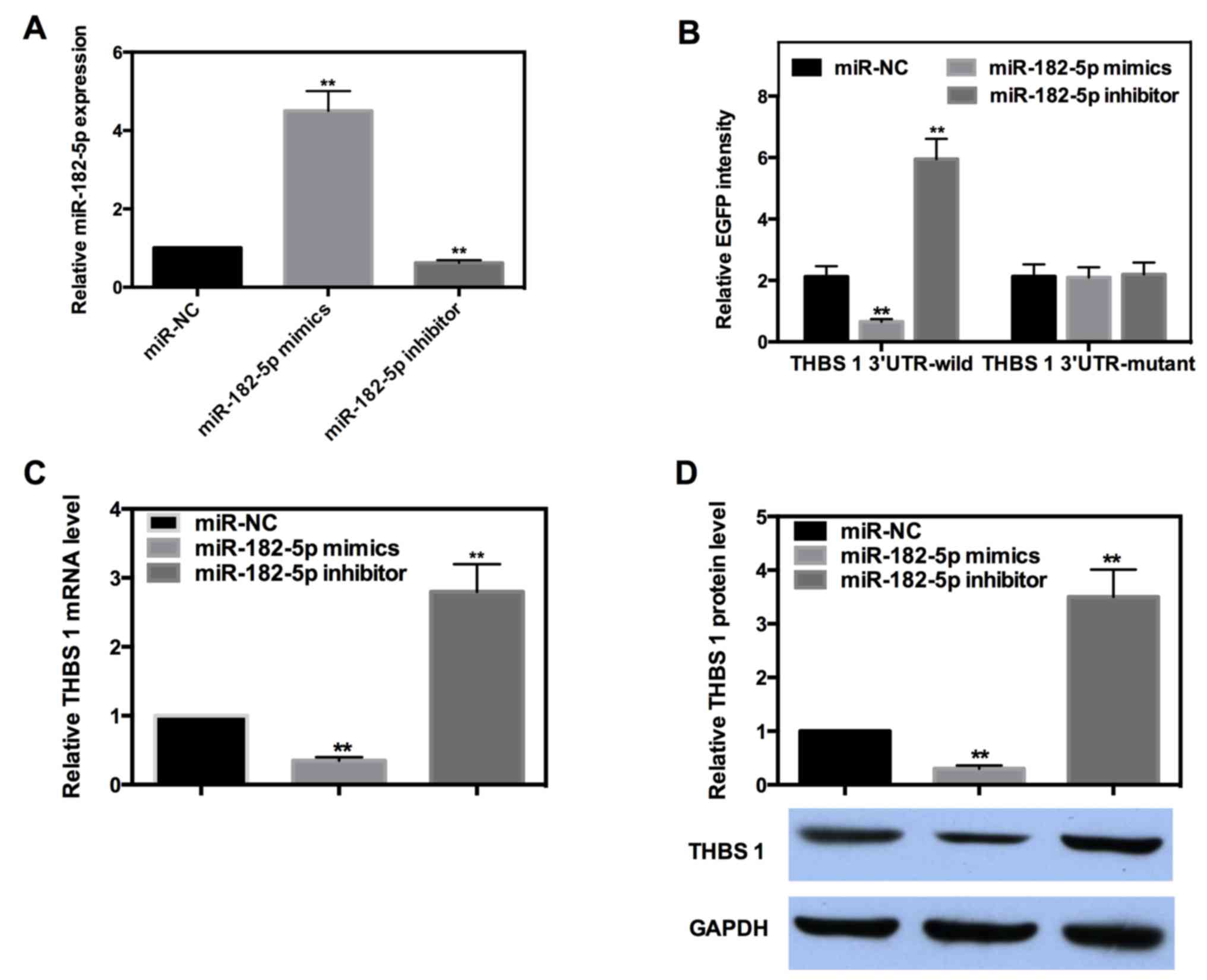
miR-182-5p directly targets the 3′-UTR
of THBS-1 and downregulates its expression
miRs are well known to exert their function by
targeting the 3′-UTR of target genes. To determine whether or not
miR-182-5p directly targets THBS-1, EGFP reporter analysis was
performed. Reporter vectors bearing either wild-type or mutant-type
THBS-1 3′-UTR were co-transfected with miR-182-5p mimics or
inhibitor into INS-1 cells. The efficiency of miR-182-5p mimics and
inhibitors were detected by RT-qPCR (Fig. 4A). The fluorescence intensity of each
group was measured and the results indicated miR-182-5p decreased
the intensity of wild-type 3′-UTR by ~69%, while the intensity of
the wild-type 3′-UTR was increased by ~1.8-fold when miR-182-5p was
inhibited. However, the intensity of mutant-type 3′-UTR was not
significantly affected when miR-182-5p was overexpressed or
inhibited (Fig. 4B).
The majority of miRs can negatively regulate their
target genes by targeting the 3′-UTR. To further determine the
regulation mode of miR-182-5p in the present study, RT-qPCR and
western blot analysis were performed. The results demonstrated that
miR-182-5p mimics decreased the mRNA and protein expression level
of THBS-1 by ~65 or 70%, respectively, whereas the expression of
THBS-1 was significantly increased by ~1.8- or 2.5-fold when
miR-182-5p was inhibited (Fig. 4C and
D). These results suggested that miR-182-5p can directly bind
to the 3′-UTR of THBS-1 and negatively regulate its expression at
mRNA and protein levels.
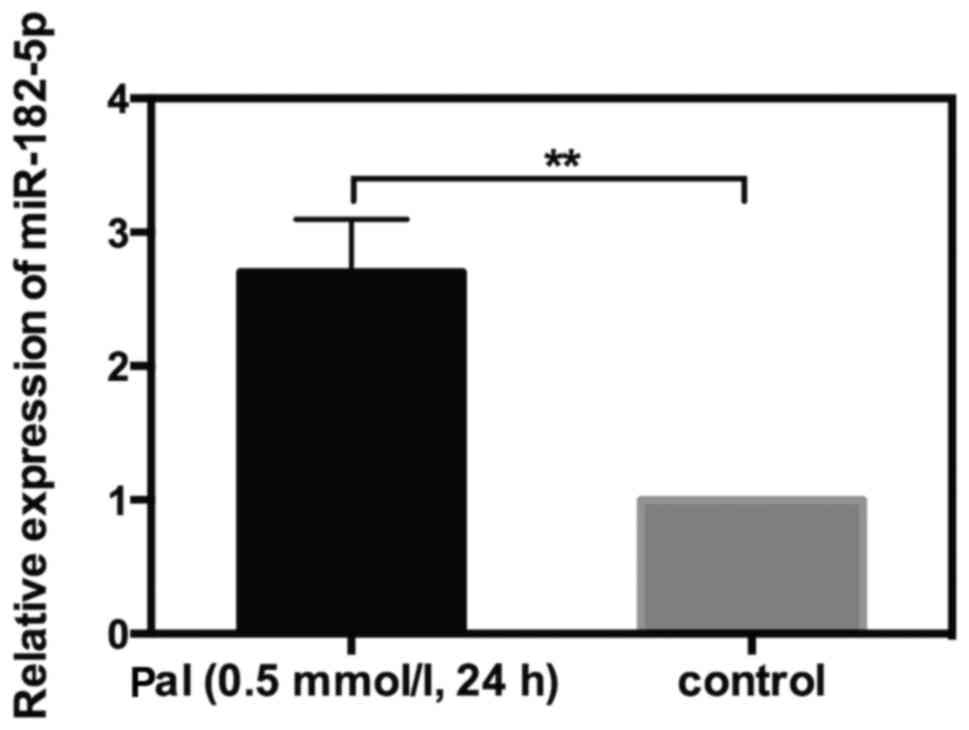
Upregulation of miR-182-5p in
Pal-treated cells
The aforementioned results indicated that miR-182-5p
could target THBS-1 in INS-1 cells. To further investigate whether
or not miR-182-5p was involved in Pal-induced cytotoxicity, RT-qPCR
was used to detect the alteration in miR-182-5p expression in
response to Pal. INS-1 cells were incubated with Pal (0.5 mmol/l)
for 24 h. The results of RT-qPCR indicated that miR-182-5p
expression level was increased by 1.7-fold in Pal-amended cells
compared with the control cells (Fig.
5). This result suggested that miR-182-5p may serve a role in
Pal-induced lipotoxicity in INS-1 cells.
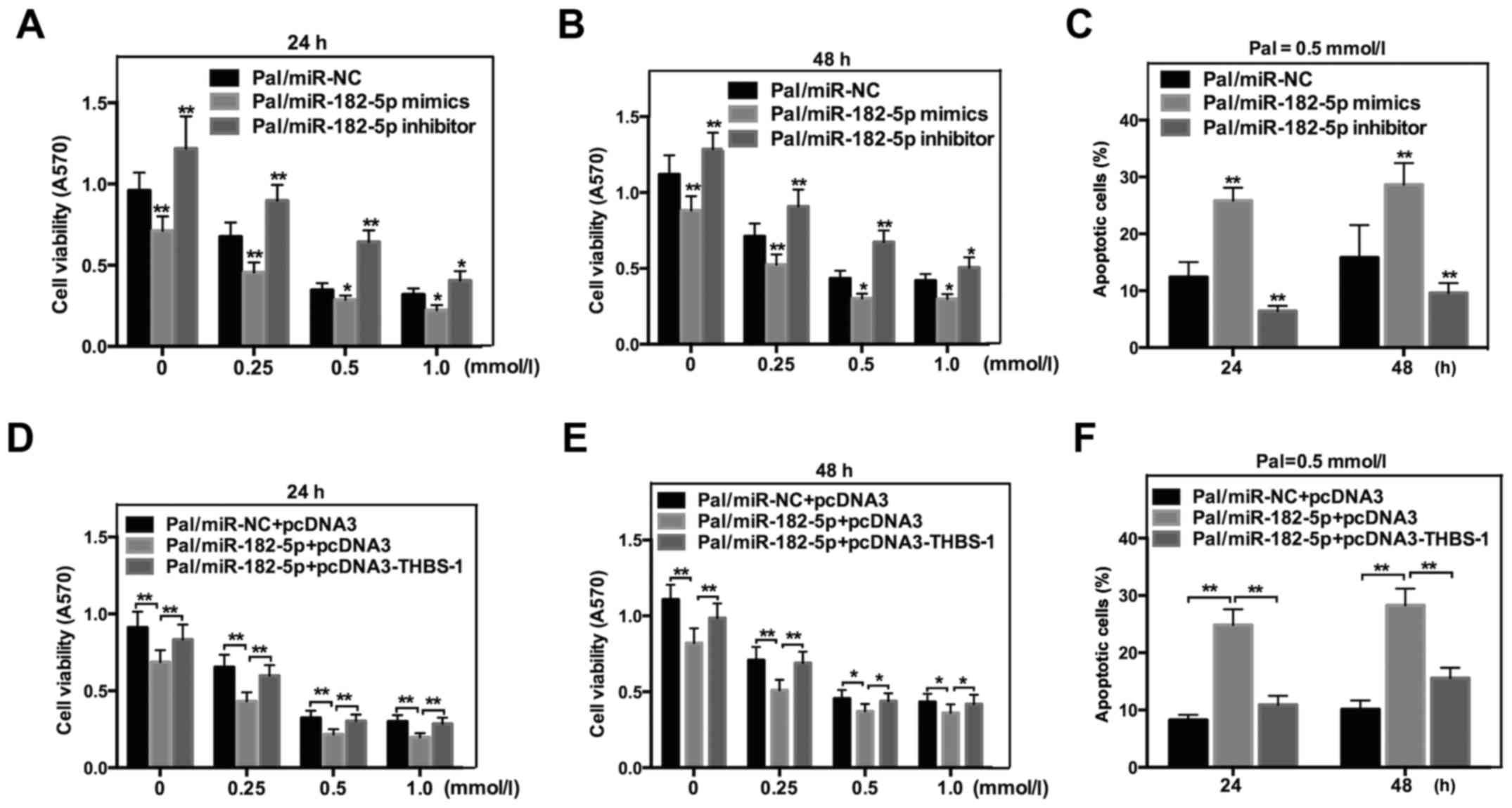
miR-182-5p promotes Pal-induced
lipotoxicity of INS-1 cells by directly targeting THBS-1
To determine the exact effect of miR-182-5p on
Pal-induced cytotoxicity in INS-1 cells, MTT and flow cytometric
analysis were also performed. As indicated in Fig. 6, miR-182-5p mimics significantly
decreased THBS-1 cell viability (Fig. 6A
and B), while increasing Pal-induced apoptosis in INS-1 cells
following incubation with Pal (Fig.
6C). However, the cell viability was increased (Fig. 6A and B) and Pal-induced apoptosis was
decreased (Fig. 6C) in cells that
were transfected with miR-182-5p inhibitors. To further confirm
that miR-182-5p affected Pal-induced lipotoxicity by regulating
THBS-1 directly, a rescue experiment was performed. miR-182-5p and
THBS-1 expression plasmid without the 3′-UTR (pcDNA3-THBS 1) were
co-transfected into INS-1 cells. As expected, overexpression of
THBS-1 counteracted the effect of miR-182-5p on cell viability
(Fig. 6D and E) and apoptosis
(Fig. 6F). Taken together, these
results indicated that miR-182-5p affects Pal-induced lipotoxicity
by directly regulating THBS-1.
Discussion
More than 415 million people worldwide are estimated
to have diabetes, with an ever-increasing morbidity and mortality
(21). It is reported that ~95% of
all diabetes cases are T2DM (22,23).
T2DM is a complex disorder characterized by insulin resistance and
defects in pancreatic beta cell function. Numerous experiments have
reported that the failure of beta cells to increase mass and
function is central in T2DM (24,25).
Nutrients are essential for the maintenance of beta cell function
and mass. However, an excess of nutrients such as glucose or FFAs
can induce injurious effects on beta cell mass and function that
contribute toward the progressive loss of functional beta cell mass
(26).
In the present study, Pal, which was used as an FFA,
increased INS-1 cell viability after a short exposure time (8 h).
However, Pal significantly decreased cell viability following 24 or
48 h of incubation. These results indicated that FFAs exert
stimulatory effects acutely, but inhibitory effects chronically on
beta cell survival. The subsequent flow cytometry assay suggested
that Pal (0.5 mmol/l) markedly increased the cell apoptosis rate
following 24 or 48 h of exposure.
THBS-1 is a multifunctional glycoprotein released
from various types of cell and exerts its diverse biological
effects through binding to extracellular matrix proteins and cell
surface receptors (8). Recent
studies have suggested that THBS-1 is involved in the pathogenesis
of insulin resistance and is important for beta cell function
(27,28). However, the exact effect of THBS 1 on
FFA-induced lipotoxicity is not thoroughly understood. The present
study revealed that THBS-1 significantly reversed the changes of
cell viability and apoptosis that were induced by Pal exposure.
These results suggested that THBS-1 can protect INS-1 cells from
Pal-induced lipotoxicity.
Additionally, the precise mechanisms of THBS-1 that
caused lipotoxic effects, and whether or not THBS-1 is regulated by
other molecules, were further investigated. In recent years, it has
been determined that an increasing number of miRs participate in
various pathological processes (29–31),
including lipotoxicity (32,33). Subsequent experiments focused on the
potential participation of miRs in Pal-induced lipotoxicity. To
begin with, combining the prediction results of three bioinformatic
analyses, miR-182-5p was selected. To further identify whether or
not miR-182-5p directly targets THBS-1, an EGFP reporter assay was
performed, which revealed that miR-182-5p could negatively regulate
the intensity of wild-type 3′-UTR. However, miR-182-5p could not
affect the intensity of mutant-type 3′-UTR. Furthermore, miR-182-5p
was indicated to negatively regulate the expression of THBS-1 at
the mRNA and protein levels.
The aforementioned results have indicated that
miR-182-5p could target THBS-1 in INS-1 cells. To further
investigate whether or not miR-182-5p was involved in Pal-induced
cytotoxicity, miR-182-5p expression was increased in Pal-amended
cells and it was hypothesized that miR-182-5p may participate in
Pal-induced lipotoxicity. The subsequent cell phenotype experiments
suggested that miR-182-5p decreased cell viability and increased
Pal-induced apoptosis in INS-1 cells that were incubated with Pal.
To further confirm that THBS-1 was a direct functional target gene
of miR-182-5p, a rescue experiment was designed. The results
suggested that restoration of THBS-1 could counteract the effects
of miR-182-5p on cell viability and apoptosis. Taken together, the
results of the present study suggested that miR-182-5p affects
Pal-induced cell viability and apoptosis by directly targeting
THBS-1.
In conclusion, the present findings suggested that
THBS-1 can protect INS-1 cells from FFA-induced lipotoxicity.
Furthermore, miR-182-5p, which is able to directly target the
3′-UTR of THBS-1, can increase FFA-induced cytotoxicity. These
findings demonstrated that miR-182-5p may be useful for identifying
novel therapeutic approaches that may improve beta cell mass and
function. Additionally, miR-182-5p may be a novel biomarker for the
pathogenesis and progression of T2DM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RB conceived and designed the study, acquired data
and had an important role in interpreting the results. LY performed
the experiments, contributed significantly to analysis and
manuscript preparation. DJ assisted in performing the data analysis
with constructive discussions. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRs
|
microRNAs
|
|
T2DM
|
type 2 diabetes
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
FFAs
|
free fatty acids
|
References
|
1
|
Camlio A, Cecilia SP, Valeria H and Marcos
S: Analysis of the relationship between the referral and evolution
of patients with type 2 diabetes mellitus. Int J Environ Res Public
Health. 15:15342018. View Article : Google Scholar
|
|
2
|
Mashitisho ML and Mashitisho BG: Early
insulin therapy in patients with type 2 diabetes mellitus. J
Endocrinol Metab Diab South Africa. 21:13–15. 2016. View Article : Google Scholar
|
|
3
|
Gu J, Wei Q, Zheng H, Meng X, Zhang J and
Wang D: Exendin-4 promotes survival of mouse pancreatic β-cell line
in lipotoxic conditions, through the extracellular signal-related
kinase 1/2 pathway. J Diabetes Res. 2016:52940252016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Good DJ, Polverini PJ, Rastinejad F, Le
Beau MM, Lemons RS, Frazier WA and Bouck NP: A tumor
suppressor-dependent inhibitor of angiogenesis is immunologically
and functionally indistinguishable from a fragment of
thrombospondin. Proc Natl Acad Sci USA. 87:6624–6628. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams JC and Lawler J: The
thrombospondins. Int J Biochem Cell Biol. 36:961–968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savill J, Fadok V, Henson P and Haslett C:
Phagocyte recognition of cells undergoing apoptosis. Immunol Today.
14:131–136. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ribeiro SM, Poczatek M, Schultz-Cherry S,
Villain M and Murphy-Ullrich JE: The activation sequence of
thrombospondin-1 interacts with the latency-associated peptide to
regulate activation of latent transforming growth factor-beta. J
Biol Chem. 274:13586–13593. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuo Y, Tanska M, Yamakage H, Sasaki Y,
Muranaka K, Hata H, Ikai I, Shimatsu A, Inoue M, Chun TH and
Satoh-Asahara N: Thrombospondin 1 as a novel biological marker of
obesity and metabolic syndrome. Metabolism. 64:1490–1499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delić D, Eisele C, Schmid R, Baum P, Wiech
F, Gerl M, Zimdahl H, Pullen SS and Urquhart R: Urinary exosomal
miRNA signature in type II diabetic nephropathy patients. PLoS One.
11:e01501542016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olerud J, Mokhtari D, Johansson M,
Christoffersson G, Lawler J, Welsh N and Carlsson PO:
Thrombospondin-1: An islet endothelial cell signal of importance
for β-cell function. Diabetes. 60:1946–1954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu X, Li Y, Alvero A, Li J, Wu Q, Xiao Q,
Peng Y, Hu Y, Li X, Yan W, et al: MicroRNA-222-3p/GNAI2/AKT axis
inhibits epithelial ovarian cancer cell growth and associates with
good overall survival. Oncotarget. 7:80633–80654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhang T, Zhou Y, Sun Y, Cao Y, Chang
X, Zhu Y and Han X: A presenilin/Notch1 pathway regulated by
miR-375, miR-30a, and miR-34a mediates glucotoxicity
induced-pancreatic beta cell apoptosis. Sci Rep. 6:361362016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Shi C, Wang C, Liu W, Chu Y, Xiang
Z, Hu K, Dong P and Han X: The role of miR-497-5p in myofibroblast
differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep.
7:409582017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin X, Guan H, Huang Z, Liu J, Li H, Wei
G, Cao X and Li Y: Downregulation of Bcl-2 expression by miR-34a
mediates palmitate-induced Min6 cells apoptosis. J Diabetes Res.
2014:2586952014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Xu X, Liang Y, Liu S, Xiao H, Li F,
Cheng H and Fu Z: miR-375 enhances palmitate-induced lipoapoptosis
in insulin-secreting NIT-1 cells by repression myotrophin (V1)
protein expression. Int J Clin Exp Pathol. 3:254–264.
2010.PubMed/NCBI
|
|
16
|
Lovis P, Roggli E, Laybutt DR, Gattesco S,
Yang JY, Widmann C, Abderrahmani A and Regazzi R: Alterations in
microRNA expression contribute to fatty acid-induced pancreatic
beta-cell dysfunction. Diabetes. 57:2728–2736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao J, Xu C, Fang Z, Li Y, Liu H, Wang Y,
Xu C and Sun Y: Androgen receptor regulated microRNA miR-182-5p
promotes prostate cancer progression by targeting the ARRDC3/ITGB4
pathway. Biochem Biophys Res Commun. 474:213–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang W, Liu G and Tang W: MicroRNA-182-5p
ameliorates liver ischemia-reperfusion injury by suppressing
Toll-like receptor 4. Transplant Proc. 48:2809–2814. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blume CJ, Hotz-Wagenblatt A, Hüllein J,
Sellner L, Jethwa A, Stolz T, Slabicki M, Lee K, Sharathchandra A,
Benner A, et al: p53-dependent non-coding RNA networks in chronic
lymphocytic leukemia. Leukemia. 29:2015–2023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
International Diabetes Federation. IDF
diabetes atlas. 7:(Brussels). International Diabetes Federation.
2015.
|
|
22
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 36((Suppl
1)): S67–S74. 2013.PubMed/NCBI
|
|
23
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37((Suppl
1)): S81–S90. 2014.PubMed/NCBI
|
|
24
|
Prentki M and Nolan CJ: Islet beta cell
failure in type 2 diabetes. J Clin Invest. 116:1802–1812. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hull RL, Kodama K, Utzschneider KM, Carr
DB, Prigeon RL and Kahn SE: Dietary-fat-induced obesity in mice
results in beta cell hyperplasia but not increased insulin release:
Evidence for specificity of impaired beta cell adaptation.
Diabetologia. 48:1350–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poitout V, Amyot J, Semache M, Zarrouki B,
Hagman D and Fontés G: Glucolipotoxicity of the pancreatic beta
cell. Biochim Biophys Acta. 1801:289–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue M, Jiang Y, Barnes RH II, Tokunaga
M, Martinez-Santibañez G, Geletka L, Lumeng CN, Buchner DA and Chun
TH: Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis
and insulin resistance in male mice. Endocrinology. 154:4548–4559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong P, Gonzalez-Quesada C, Li N, Cavalera
M, Lee DW and Frangogiannis NG: Thrombospondin-1 regulates
adiposity and metabolic dysfunction in diet-induced obesity
enhancing adipose inflammation and stimulating adipocyte
proliferation. Am J Physiol Endocrinol Metab. 305:E439–E450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terracciano D, Terreri S, de Nigris F,
Costa V, Celin GA and Cimmino A: The role of a new class of long
noncoding RNAs transcribed from ultraconserved regions in cancer.
Biochim Biophys Acta Rev Cancer. 1868:449–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin J, Ren W, Huang X, Li T and Yin Y:
Protein restriction and cancer. Biochim Biophys Acta Rev Cancer.
1869:256–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhi F, Shao N, Xue L, Xu Y, Kang X, Yang Y
and Xia Y: Characteristic microRNA expression induced by δ-opioid
receptor activation in the rat liver under prolonged hypoxia. Cell
Physiol Biochem. 44:2296–2309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cazanave SC, Mott JL, Elmi NA, Bronk SF,
Masuoka HC, Charlton MR and Gores GJ: A role for miR-296 in the
regulation of lipoapoptosis by targeting PUMA. J Lipid Res.
52:1517–1525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han YB, Wang MN, Li Q, Guo L, Yang YM, Li
PJ, Wang W and Zhang JC: MicroRNA-34a contributes to the protective
effects of glucagon-like peptide-1 against lipotoxicity in INS-1
cell. Chin Med J (Engl). 125:4202–4208. 2012.PubMed/NCBI
|