Introduction
Pregnancy-induced hypertension (PIH), also known as
gestational hypertension, is a pregnancy-specific condition
characterized by high blood pressure, edema, proteinuria and heart
and kidney failure (1). PIH is
defined as having a diastolic blood pressure (DBP) >90 mmHg and
systolic blood pressure (SBP) >140 mmHg (2). Furthermore, PIH can be classified as
mild, moderate and severe (3).
Severe PIH is called preeclampsia (PE), which typically occurs
after 20 weeks of gestation and may be accompanied by proteinuria,
twitch and coma (3). PE is diagnosed
why SBP ≥160 mmHg and DBP ≥110 mmHg (4). Previous studies have demonstrated that
PE is a common and severe health problem for fetuses and expectant
women, a leading cause of maternal and fetal mortality and
morbidity (5). PE is associated with
fetus maldevelopment and may lead to eclampsia and even miscarriage
(5–7). Worldwide, there are ~55,000 deaths
associated with PE reported annually (7), and the underlying cause of PE remains
unknown. There is currently no effective therapeutic or
preventative treatment available for PE.
Some previous studies have focused their
investigations on trophoblast and placental dysfunction, which
initiate pathogenesis (8).
Successful implantation and placentation induces a series of
cellular events associated with embryo attachment to the
endometrial wall, including embryonic trophoblast cell
proliferation and migration as well as invasion of the trophoblast
cells into the endometrium (9).
Furthermore, successful implantation is associated with remodeling
of the uterine spiral via extravillous trophoblast (EVT) cell
invasion (10). EVT cell invasion
results in enlarged vessel diameter and the perfusion of utero
placenta, which leads to increased blood flow volume and oxygen
transportation (11). As such,
abnormalities in artery remodeling may cause decreased blood flow
volume and placental hypoxia, resulting in trophoblast dysfunction
and possibly preeclampsia (12).
Studying this process may increase our understanding of the onset
and development of preeclampsia and potential novel treatment
methods (13). It has also been
reported that some signaling and genetic pathways involving
vascular endothelial growth factor (VEGF), placental growth factor
and transforming growth factor-β contribute to PE development
(7,14). Furthermore, PE affects the innate
immunity of the fetus due to increased levels of inflammatory
immune cells and cytokines and decreased the regulation of immune
cells and cytokines, including IL-6, certain chemokines (chemokine
ligand 5) and adhesion molecules (VCAM, ICAM and E-selectin)
(15).
MicroRNAs (miRNAs or miRs) are a family of small
non-coding RNAs that are able to regulate gene expression via
degrading mRNA at the post-translational level. Therefore,
dysregulation of miRNAs is associated with a number of diseases
(16). It has previously been
reported that C19MC, miR-371-3 cluster and C14MC encode
pregnancy-associated microRNAs that are responsible for the
subsequent onset of gestational hypertension (17,18).
It has been reported that miR-145 may be able to
regulate the expression of sex determining region Y-box 2 and
influence the proliferation and invasion ability of JAR and JEG-3
cells in the development of human choriocarcinomas (18,19).
Mucin (MUC1) is a type 1 transmembrane protein that serves a vital
role in trophoblast cell proliferation and migration (20). Due to its large size, MUC1 works as a
lubricant to and affects cell-cell and cell-substrate interactions,
reducing cell adhesion in tumor tissues (21). Furthermore, a previous study
demonstrated that MUC1 facilitates cell-cell adhesion by binding
intercellular adhesion molecule-1 (ICAM-1) and E-selectin (22). The aim of the present study was to
define the roles of miR-145 in trophoblast cell proliferation and
invasion. In addition, the impact of MUC1 on trophoblast cell
proliferation and invasion profile was explored.
Materials and methods
Patient tissues
Placenta villi tissues were obtained from 60
patients (age range, 25–35 years) who underwent caesarean section
at Huai'an First People's Hospital (Huai'an, China) between April
2014 and May 2016. The tissues included 30 normal placentas (NP) as
controls and 30 placentas from women with PE. The inclusion
criteria were as follows: A confirmed diagnosis of PE, systolic
blood pressure >140 mm Hg and diastolic blood pressure >90
mmHg. Patients were compared with 30 healthy controls which were
aged matched. All patients with complications of pregnancy,
including, twins, fetal gene abnormalities, maternal chronic
hypertension, aberrant liver enzyme levels, cardiovascular disease,
renal disease, diabetes or other infectious diseases were excluded
from the current study.
Fresh tissues were flash frozen in liquid nitrogen
(−196°C). The present study was approved by the Ethics Committee of
Huai'an First People's Hospital.
Cell lines
The HTR-8/SVneo cell line was purchased from Otwo
Biotech, Inc., (Shenzhen, China). and cultured in RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10%
fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) at
37°C in an atmosphere containing 5% CO2. The
subcultivation ratio was 1:3 to 1:8 and cells were sub-cultured 2
to 3 times a week. Cultured cells at passage 4–5 were subsequently
used for cell transfection, MTT, Transwell and flow cytometry
assays.
MiR-145 target gene predictions
TargetScan (http://www.targetscan.org/vert_71/), miRBase
(http://www.mirbase.org/) and miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/)
were used for miR-145 target gene prediction. MUC1 was identified
as a target gene of miR-145. Pearson's correlation analysis was
therefore performed for miR-145 and MUC1 in PE tissues.
Cell transfection
HTR-8/SVneo cells were seeded in 24 well-plates in
the density of 5×103 cells/well and divided into three
groups: The negative control group (NC), the miR-145 mimic group
transfected with miR-145 overexpression plasmids (miR-145 mimic)
and the miR-145 mimic control group transfected with empty plasmids
(mimic control). The constructed miR-145 mimic and mimic control
were purchased from Cyagen Biosciences, Inc. (Santa Clara, CA,
USA). A total of 500 ng pBR322 plasmid (cat. no. 15367014;
Invitrogen; Thermo Fisher Scientific, Inc.) and 2.5 µl
Lipofectamine® 2000 transfection reagent (11668027;
Invitrogen; Thermo Fisher Scientific, Inc.) were added to each
well. RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.) was added
to reach a total of 1 ml in each well. Following 6 h of incubation
at 37°C, the medium was replaced with fresh RPMI 1640 medium
containing 10% bovine serum albumin (cat. no. A8020; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Following 48 h transfection, the expression of miR-145 and MUC1
were verified using RT-qPCR and western blotting respectively, both
in the tissues and HTR-8/SVneo cells. This experiment was performed
in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) used for miR-145 mRNA
expression
RT-qPCR was employed to assess the relative
expression of miR-145 in NP tissue, PE tissue, the miR-145 mimic
and transfected HTR-8/SVneo cells. An RNeasy Mini Kit (Qiagen GmbH,
Hilden, Germany) was utilized to isolate RNA in tissues and cells
according to the manufacturer's protocol. RNA concentration was
determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA). The M-MLV 1st Strand kit
(C28025032; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was used to synthesize cDNA by RT according to the
manufacturer's protocol. A Power SYBR-Green Real-Time PCR Master
Mix kit (RP014A; Takara Bio, Inc., Otsu, Japan) was used for qPCR.
The temperature protocol for RT was as follows: 43°C for 30 min,
98°C for 5 min and 5°C for 5 min. The PCR conditions were as
follows: Pre-denaturation at 95°C for 6 min followed by 36 cycles
of initiation at 94°C for 30 sec, annealing at 60°C for 30 sec, and
elongation at 75°C for 90 sec. Samples were subsequently stored at
4°C. Endogenous U6 was used as a reference gene to normalize the
miR-145 expression. Human-miR-145 RT-qPCR Primer Set 200 rxn was
used as the miR-145 primers, forward, 5′-GAGAACTCCAGCTGGTCCTTA-3′,
and reverse, 5′-GGTGGGAAGGAGGCAAAT-3′ (AM30047; Applied biosystems;
Thermo Fisher Scientific, Inc.) and the RT-qPCR Primer Set 200 rxn
was used as U6 primers, forward, 5′-CGCTTCGGCAGCACATATACTAA-3′,
reverse, 5′-TATGGAACGCTTCACGAATTTGC-3′ (AM30303; Boyao
Biotechnology, Shanghai, China). This experiment was performed in
triplicate. The expression of miR-145 was determined using the
2−∆∆Cq method (23).
Western blotting for MUC1 protein
expression
Western blotting was performed to identify the
expression of MUC1 in PE tissues and HTR-8/SVneo cell transfected
with the miR-145 mimic. Tissues or Cells were lysed using radio
immunoprecipitation assay buffer (P0013B, Beyotime Institute of
Biotechnology, Haimen, China) and protein was quantified using a
bicinchoninic acid assay kit (23227; Beijing Biotides Biotechnology
Co., Ltd., Beijing, China). GAPDH was used as an internal
reference. Protein (30 µg) in tissues or cells was separated by 12%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Thermo Fisher Scientific, Inc.). Membranes were incubated with
antibodies against MUC1 (1:1,000; cat. no. PA525939; Thermo Fisher
Scientific, Inc.) and GAPDH (1:500; cat. no. PA1987; Thermo Fisher
Scientific, Inc.) for 1 h at 25°C. Membranes were blocked with 5%
bovine serum albumin (Beijing Solarbio Science & Technology
Co., Ltd.) for 1 h at 25°C. Membranes were subsequently incubated
for 45 min at 25°C with the goat anti-rabbit IgG (H+L) Highly
Cross-Adsorbed secondary antibody, Alexa Fluor® Plus 800
(1:20,000; cat. no. A32735, Thermo Fisher Scientific, Inc.). An
enhanced chemiluminescence (ECL) western blotting kit (32209;
Thermo Fisher Scientific, Inc.) was used to visualize the bands.
Gray values were obtained using ImageJ software version 1.51j8
(National Institutes of Health, Bethesda, MD, USA). This experiment
was performed in triplicate.
MTT assay for HTR-8/SVneo cell
proliferation
An MTT assay was performed to assess cell
proliferation. A total of 2,000 transfected HTR-8/SVneo cells were
seeded in 24-well plates and incubated at 37°C for 4 h in an
atmosphere containing 5% CO2. Subsequently, an MTT Cell
Viability Assay kit (KA1606; Abnova Corporation, Taipei, Taiwan)
was used according to the manufacturer's protocol. The optical
density at 570 nm wavelength was detected using microplate reader
at 24, 48 and 72 h following transfection. This experiment was
conducted in triplicate.
Matrigel assay for HTR-8/SVneo cell
invasion
Transwell culture inserts (8-mm pore size; Falcon;
BD Biosciences, Franklin Lakes, NJ, USA) were placed into the wells
of 24-well culture plates with separated the upper and lower
chambers. Matrigel (BD Biosciences) was pre-coated on the upper
side of the membrane and incubated at 37°C for 1 h for gel
formation. The membrane was hydrated in FBS for 2 h prior to use.
In the lower chamber, 600 µl RPMI 1640 (Thermo Fisher Scientific,
Inc.) containing 10% FBS was added and 1×105 cells/well
were seeded to the upper chamber. Cells in inserts were fixed using
4% paraformaldehyde at 4°C for 15 min and stained with 0.05%
crystal violet for 10 min at room temperature. Cells were counted
under a light microscope (magnification, ×200) following 72 h
incubation at 37°C.
Flow cytometry assay for HTR-8/SVneo
cell apoptosis
Following transfection, a flow cytometry assay was
performed to study the apoptosis of HTR-8/SVneo cells. Briefly, the
transfected cells (1×105 cells/well) were seeded in a
24-well plate and incubated at 37°C for 72 h. HTR-8/SVneo cells
were stained with propidium iodide and Annexin V-fluorescein
isothiocyanate (BD Biosciences) in the dark for 15 min at room
temperature. Stained cells were subsequently examined using a
FACScan laser flow cytometer (BD Biosciences) equipped with
CellQuest software version 3.1 (BD Biosciences).
Luciferase reporter gene analysis for
target verification
For the luciferase reporter assay, HTR-8/SVneo cells
(2,500 cells/well) were seeded in 96-well plates and incubated at
37°C in an atmosphere containing 5% CO2 for 24 h.
Subsequently, MUC1-3′UTR-wild type (WT) and MUC1-3′UTR-mutant (MUT)
plasmids (2.5 µg; Cyagen Biosciences, Inc.) were transfected into
the NC, miR-145 mimic and mimic control groups using
Lipofectamine® 2000. Then, a Luciferase Reporter Assay
kit (K801-200; Wuhan Amyjet Scientific Co., Ltd., Wuhan, China) was
used according to the manufacturer's instruction.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were analyzed using GraphPad Prism version 5.01
(GraphPad Software, La Jolla, CA, USA). Student's t test and
one-way analysis of variance followed a Dunnett's post hoc test
were employed for data comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Relative expression of miR-145 mRNA
and MUC1 protein in NP and PE tissues
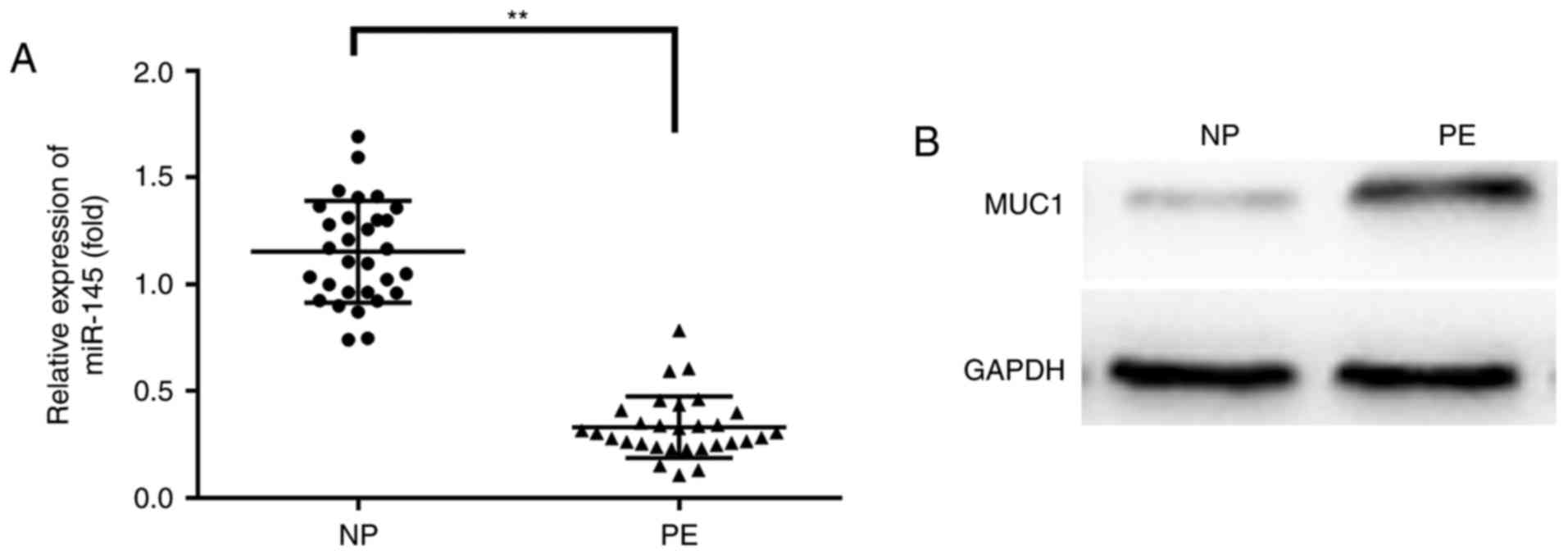
To investigate the expression profile of miR-145
mRNA and MUC1 protein in normal placental and the PE placental
tissues, RT-qPCR and western blotting were performed, respectively.
miR-145 was significantly downregulated and MUC1 was significantly
upregulated in PE compared with NP tissues (P<0.01; Fig. 1).
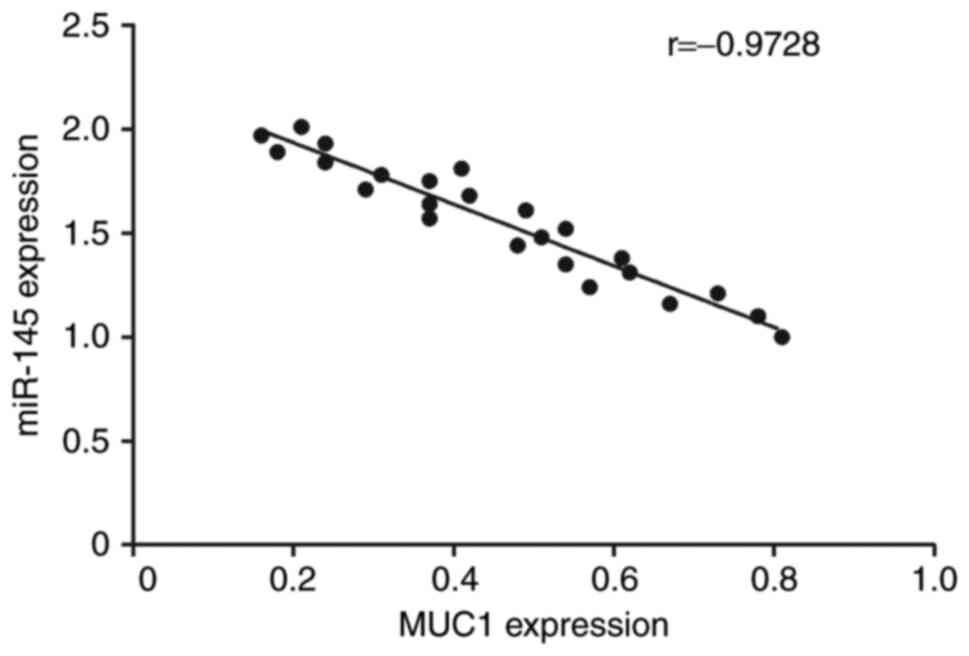
Correlation analysis between miR-145
and MUC1 expression
Correlation analysis was performed to investigate
the association between miR-145 and MUC1 expression level. The
results revealed that the expression of MUC1 was negatively
correlated with miR-145 expression (r=0.9728; Fig. 2).
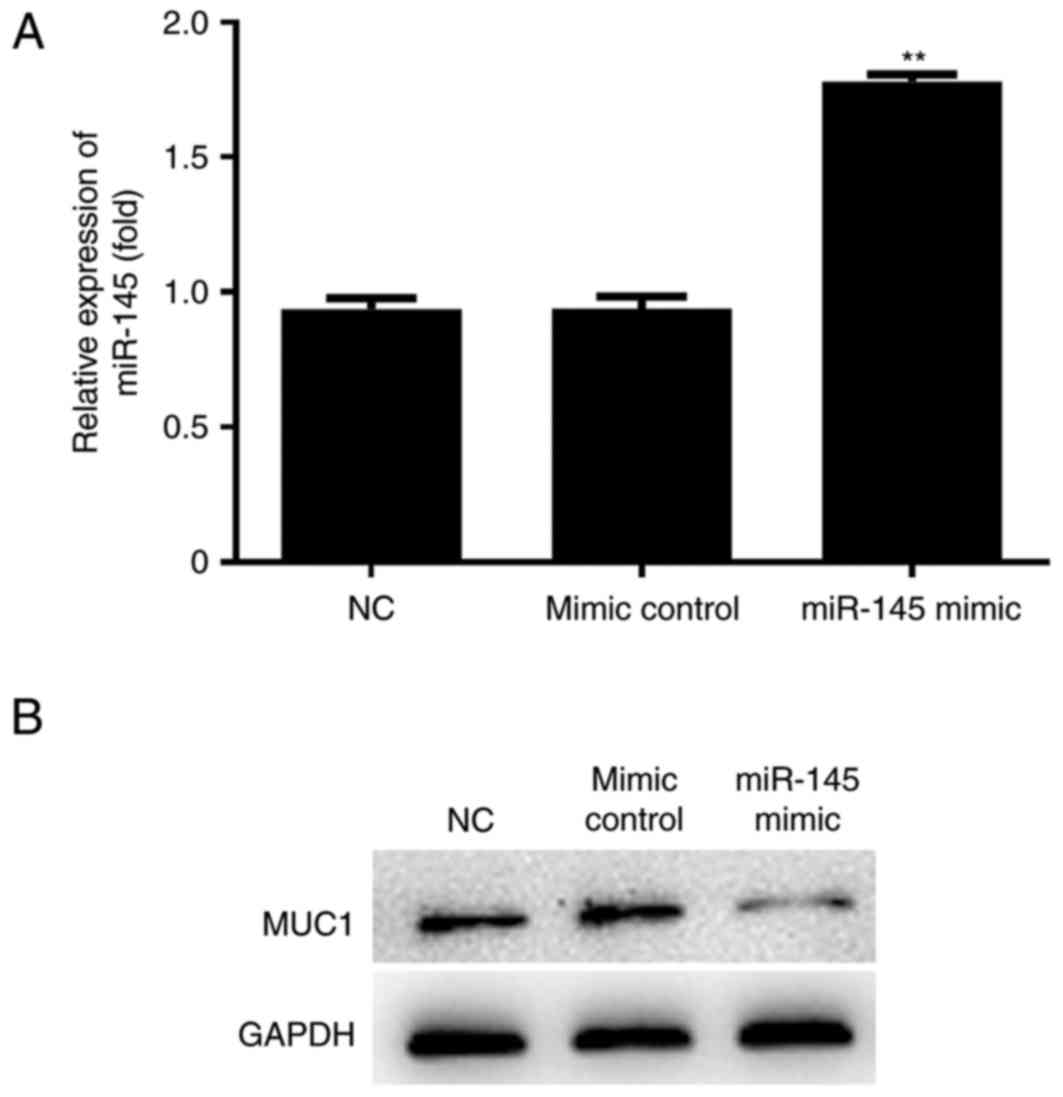
Relative expression of MUC1 in the
miR-145 mimic group
Following transfection of miR-145 mimic plasmids,
the expression of miR-145 and the MUC1 were determined by RT-qPCR
and western blotting respectively. miR-145 expression was
significantly higher (P<0.01) and MUC1 expression was markedly
downregulated in the miR-145 mimic group compared with the NC group
(Fig. 3).
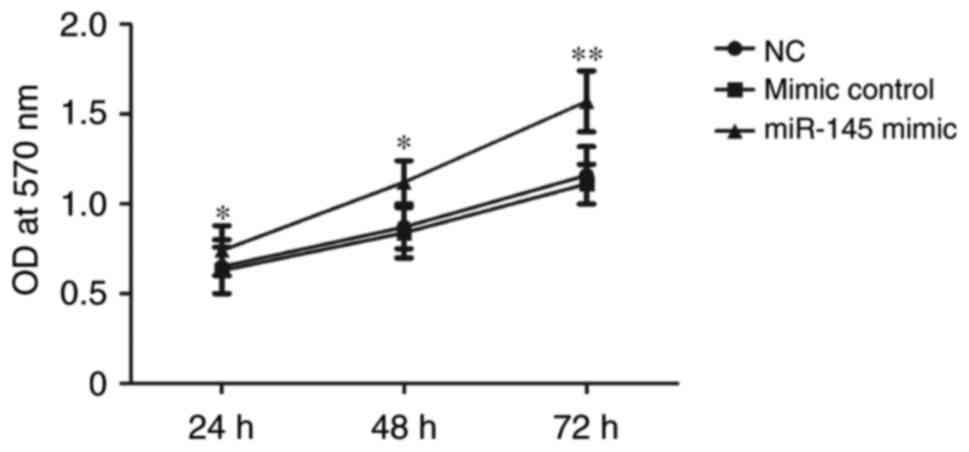
MTT assay for HTR-8/SVneo cell
proliferation determination
To explore the proliferation of HTR-8/SVneo cells,
and MTT assay was performed. HTR-8/SVneo cells were constructed
miR-145 mimic plasmids and mimic control plasmids. Results
demonstrated that the cell proliferation rate was significantly
increased in the miR-145 mimic group at 72 h following transfection
compared with the mimic control group (P<0.01; Fig. 4). This result suggested that miR-145
may therefore be able to promote cell proliferation.
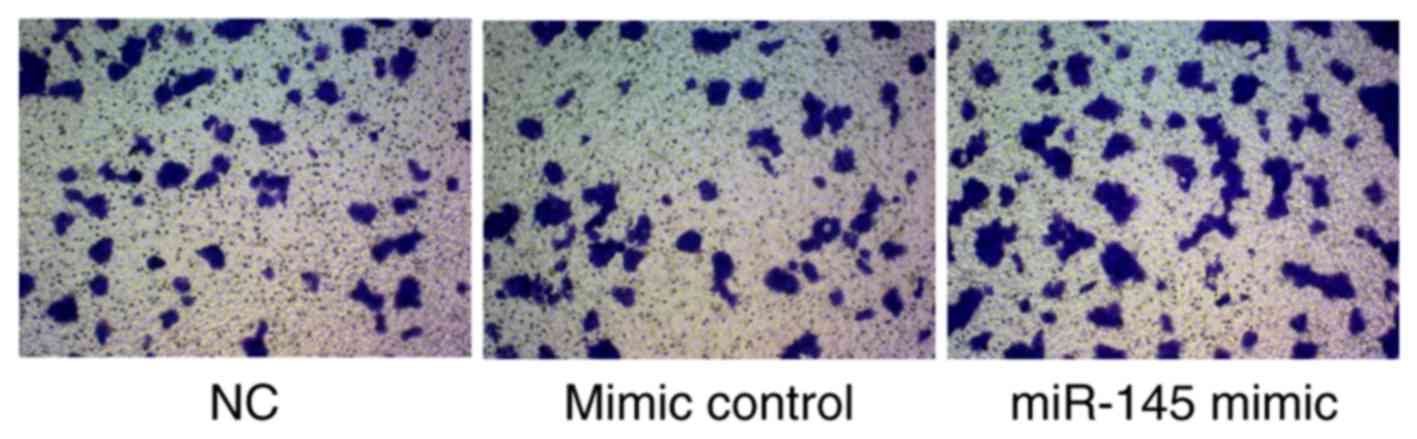
Transwell assay for HTR-8/SVneo cell
invasion determination
The effects of miR-145 on HTR-8/SVneo cell invasion
ability were assessed using a Transwell assay. Cell invasion was
markedly increased in the miR-145 mimic group compared with the
mimic control group (Fig. 5). Hence,
miR-145 may contribute to the invasion ability of HTR-8/SVneo
cells.
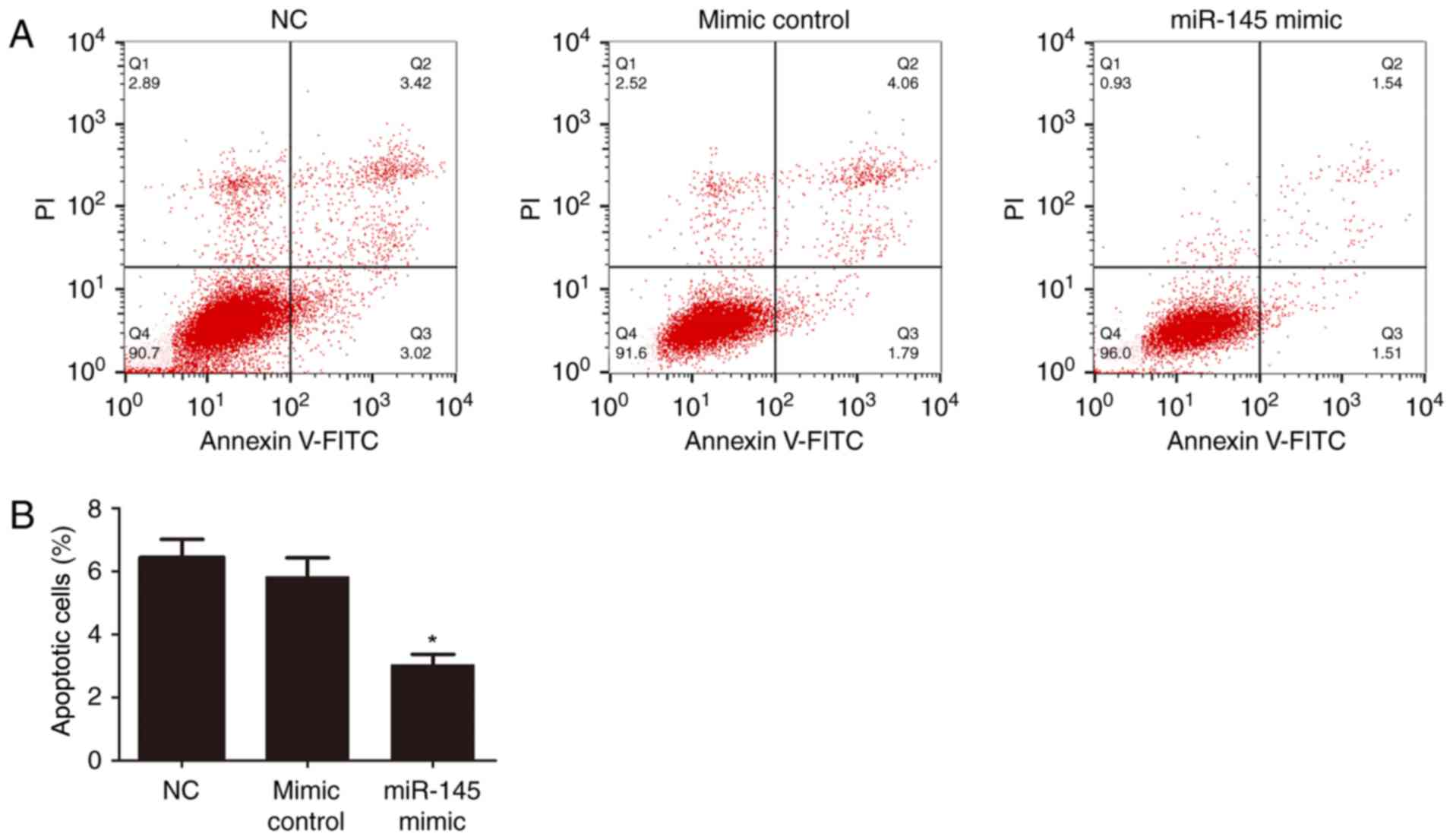
Flow cytometry assay for HTR-8/SVneo
cell apoptosis
Flow cytometry was performed at 72 h following
plasmid transfection. Subsequently, HTR-8/SVneo cells were stained
with propidium iodide and Annexin V-fluorescein isothiocyanate. The
results indicated that apoptosis was significantly decreased in the
miR-145 mimic group compared with the mimic control group and the
mimic control was not significantly different to the NC (P<0.05;
Fig. 6).
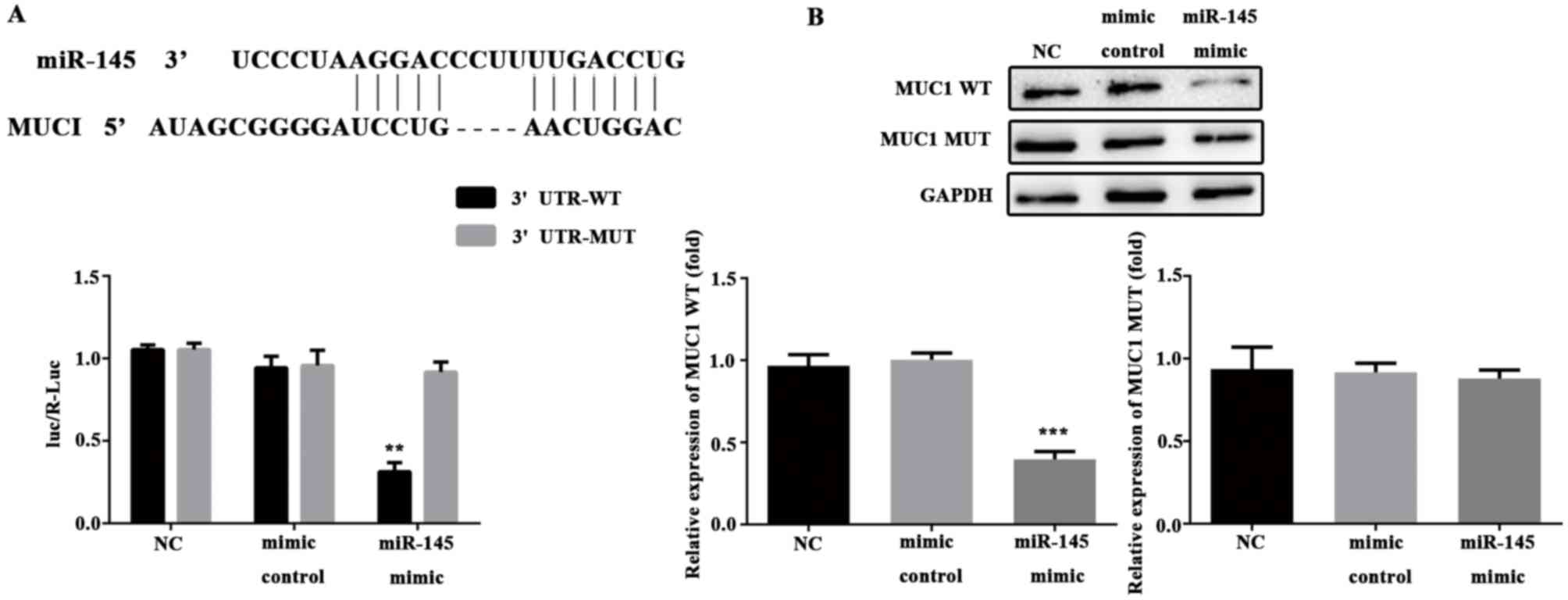
Luciferase reporter gene analysis for
target verification
The luciferase reporter system was used to verify
that MUC1 was a target gene of miR-145 in HTR-8/SVneo cells.
Firstly, the database was searched for target prediction. The
results revealed that MUC1 was a putative target gene of miR-145
(Fig. 7A). The constructed MUC1
mutant and wild type plasmids were co-transfected with miR-145
mimic and mimic control plasmids into HTR-8/SVneo cells. The
fluorescence intensity and results of western blotting were
significantly lower in the miR-145 mimic and MUC1-3′UTR-WT group
compared with the mimic control group (P<0.01, Fig. 7A; P<0.001, Fig. 7B). There were no significant
difference between mimic control and NC. Therefore, MUC1 confirmed
as a target gene of miR-145 in HTR-8/SVneo cells.
Discussion
It has previously been demonstrated that miRNA serve
vital roles in cell proliferation, migration, invasion and
apoptosis processes in various cancers (24). It is well known that miRNAs are able
to combine to the putative 3′-UTR of mRNAs to alter the function of
various genes at the post-translational level, and so it was
hypothesized that miRNAs may function as a regulator of some gene
that is associated with the development of placenta (25,26). In
addition, the underlying mechanisms of miRNAs in placenta have not
been fully explored (27). In the
present study, miR-145 was used as the target and the results
demonstrated that miR-145 was downregulated in PE tissues compared
with NP tissues. Furthermore, MUC1 was identified as a target gene
of miR-145 via gene prediction software and the luciferase reporter
assay. In addition, MTT, Transwell and flow cytometry assays was
performed in HTR-8/SVneo cells. Further investigation revealed that
miR-145 may promote HTR-8/SVneo cell proliferation and invasion
whilst suppressing cell apoptosis. Therefore, in the present study,
the roles of miR-145 in placenta development were identified and
its potential target MUC1 was explored.
It has previously been reported that miR-145
functions differently in various types of cancer (28). For example, miR-145 inhibits cell
growth in gallbladder cancer (29).
Suppressed cell migration and cancer invasion has also been
reported, which suggests that miR-145 may function as a tumor
suppressor (30). The results of the
present study are in accordance with those of a previous study that
indicated that miR-145 is a positive regulator of endogenously
regulated trophoblast expansion (19). Genes associated with trophoblast
invasion include heme oxygenase-1, epidermal growth factor (EGF),
VEGF and MUC1 (21,31,32). The
results of the present study and previous research indicate that
there are two pathways of MUC1 in cancer cell-cell interactions; in
tumor cells, MUC1 facilitates metastasis and inhibits cell-cell
adhesion by binding to ICAM-1 and E-selectin (21), whereas in the female reproductive
tract MUC1 overexpression reduced the invasion capacity of
HTR-8/SVneo cells (20). The primary
aim of the present study was to combine miR-145 and MUC1 to
investigate the effect of miR-145 in trophoblast cells and confirm
MUC1 as a target of miR-145.
The present study has several limitations due to the
experimental design. Although it was specified that MUC1 was a
target gene of miR-145 in HTR-8/SVneo cells, the underlying
mechanism remains to be elucidated. Future studies should be
performed to explore the whole signaling pathway associated with
miR-145 and MUC1 including the downstream genes associated with
cell proliferation, invasion and apoptosis. For instance, the
B-cell lymphoma 2/B-cell lymphoma 2-associated X protein signaling
pathway, which is a target of MUC1, is associated with cell
apoptosis (33). Furthermore,
proteins that serve roles in cell adhesion, including ICAM-1 and
E-selectin, may be used to assess cell migration and invasion
(21). Adrenomedullin 2 (ADM2) has
been reported to be associated with spontaneous abortion in humans
and inhibits the expression of MUCI (34). Serum ADM2 is downregulated in PE,
suggesting that decreased levels of ADM2 level may induce the
overexpression of MUC1 (34). In
future investigations, multiple factors that regulate the
expression of MUC1 should be explored.
In summary, miR-145 serves an important role in the
development of trophoblast cells. The results indicated that the
proliferation was promoted and invasion was inhibited by miR-145
targeting MUC1. Therefore, miR-145 may serve a role in the
development of PE via its effect on trophoblast cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and MZ conceived and designed the experiments and
analyzed the data. ZC performed the experiments and contributed to
the reagents/materials/analysis tools.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Huai'an First People's Hospital and written informed
consent was obtained from each patient prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mei Z, Huang B, Mo Y and Fan J: An
exploratory study into the role of miR-204-5p in pregnancy-induced
hypertension. Exp Ther Med. 13:1711–1718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
George EM and Granger JP: Endothelin: Key
mediator of hypertension in preeclampsia. Am J Hypertens.
24:964–969. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kintiraki E, Papakatsika S, Kotronis G,
Goulis DG and Kotsis V: Pregnancy-induced hypertension. Hormones
(Athens). 14:211–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obed S and Patience A: Birth weight and
ponderal index in pre-eclampsia: A comparative study. Ghana Med J.
40:8–13. 2006.PubMed/NCBI
|
|
5
|
Choudhury M and Friedman JE: Epigenetics
and microRNAs in preeclampsia. Clin Exp Hypertens. 34:334–341.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheikh AM, Small HY, Currie G and Delles
C: Systematic review of micro-RNA expression in pre-eclampsia
identifies a number of common pathways associated with the disease.
PLoS One. 11:e01608082016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gathiram P and Moodley J: Pre-eclampsia:
Its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 27:71–78.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conrad KP and Benyo DF: Placental
cytokines and the pathogenesis of preeclampsia. Am J Reprod
Immunol. 37:240–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Renaud SJ, Kubota K, Rumi MAK and Soares
MJ: The FOS transcription factor family differentially controls
trophoblast migration and invasion. J Biol Chem. 289:5025–5039.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wallace AE, Fraser R and Cartwright JE:
Extravillous trophoblast and decidual natural killer cells: A
remodelling partnership. Hum Reprod Update. 18:458–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hazan AD, Smith SD, Jones RL, Whittle W,
Lye SJ and Dunk CE: Vascular-leukocyte interactions : Mechanisms of
human decidual spiral artery remodeling in vitro. Am J Pathol.
177:1017–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burke SD and Karumanchi SA: Spiral artery
remodeling in preeclampsia revisited. Hypertension. 62:1013–1014.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anton L, Olarerin-George AO, Schwartz N,
Srinivas S, Bastek J, Hogenesch JB and Elovitz MA: miR-210 inhibits
trophoblast invasion and is a serum biomarker for preeclampsia. Am
J Pathol. 183:1437–1445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Craici IM, Wagner SJ, Weissgerber TL,
Grande JP and Garovic VD: Advances in the pathophysiology of
pre-eclampsia and related podocyte injury. Kidney Int. 86:275–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bounds KR, Newell-Rogers MK and Mitchell
BM: Four pathways involving innate immunity in the pathogenesis of
preeclampsia. Front Cardiovasc Med. 2:202015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong H, Liu CM, Liu DP and Liang CC: The
role of small RNAs in human diseases: Potential troublemaker and
therapeutic tool. Med Res Rev. 25:361–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hromadnikova I, Kotlabova K, Hympanova L,
Doucha J and Krofta L: First trimester screening of circulating
C19MC microRNAs can predict subsequent onset of gestational
hypertension. PLoS One. 9:e1137352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu F, Wang H, Zhang X, Liu T and Liu Z:
Cell proliferation and invasion ability of human choriocarcinoma
cells lessened due to inhibition of Sox2 expression by
microRNA-145. Exp Ther Med. 5:77–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farrokhnia F, Aplin JD, Westwood M and
Forbes K: MicroRNA regulation of mitogenic signaling networks in
the human placenta. J Biol Chem. 289:30404–30416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bojić-Trbojević Ž, Jovanović Krivokuća M,
Kolundžić N, Kadoya T, Radojčić L and Vićovac L: Interaction of
extravillous trophoblast galectin-1 and mucin(s)-Is there a
functional relevance? Cell Adh Migr. 10:179–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thirkill TL, Cao T, Stout M, Blankenship
TN, Barakat A and Douglas GC: MUC1 is involved in trophoblast
transendothelial migration. Biochim Biophys Acta. 1773:1007–1014.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geng Y, Yeh K, Takatani T and King MR:
Three to Tango: MUC1 as a ligand for both E-selectin and ICAM-1 in
the breast cancer metastatic cascade. Front Oncol. 2:762012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito M, Sferruzzi-Perri AN, Edwards CA,
Adalsteinsson BT, Allen SE, Loo TH, Kitazawa M, Kaneko-Ishino T,
Ishino F, Stewart CL and Ferguson-Smith AC: A trans-homologue
interaction between reciprocally imprinted miR-127 and Rtl1
regulates placenta development. Development. 142:2425–2430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paikari A, D Belair C and Blelloch R: The
eutheria-specific miR-290 cluster modulates placental growth and
maternal-fetal transport. Development. 144:3731–3743. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ouyang Y, Mouillet JF, Coyne CB and
Sadovsky Y: Review: Placenta-specific microRNAs in exosomes-good
things come in nano-packages. Placenta. 35 Suppl:S69–S73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iio A, Nakagawa Y, Hirata I, Naoe T and
Akao Y: Identification of non-coding RNAs embracing
microRNA-143/145 cluster. Mol Cancer. 9:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Letelier P, García P, Leal P, Álvarez H,
Ili C, López J, Castillo J, Brebi P and Roa JC: miR-1 and miR-145
act as tumor suppressor microRNAs in gallbladder cancer. Int J Clin
Exp Pathol. 7:1849–1867. 2014.PubMed/NCBI
|
|
30
|
Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei
Y, Li Y, Yang Q, Liu J, Wei JJ, et al: miR-145 inhibits tumor
growth and metastasis by targeting metadherin in high-grade serous
ovarian carcinoma. Oncotarget. 5:10816–10829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bilban M, Haslinger P, Prast J,
Klinglmüller F, Woelfel T, Haider S, Sachs A, Otterbein LE, Desoye
G, Hiden U, et al: Identification of novel trophoblast
invasion-related genes: Heme oxygenase-1 controls motility via
peroxisome proliferator-activated receptor gamma. Endocrinology.
150:1000–1013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knöfler M and Pollheimer J: IFPA award in
placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33 Suppl:S55–S62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nath S and Mukherjee P: Muc1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chauhan M, Balakrishnan M, Chan R and
Yallampalli C: Adrenomedullin 2 (ADM2) regulates Mucin 1 at the
maternal-fetal interface in human pregnancy. Biol Reprod.
93:1362015. View Article : Google Scholar : PubMed/NCBI
|