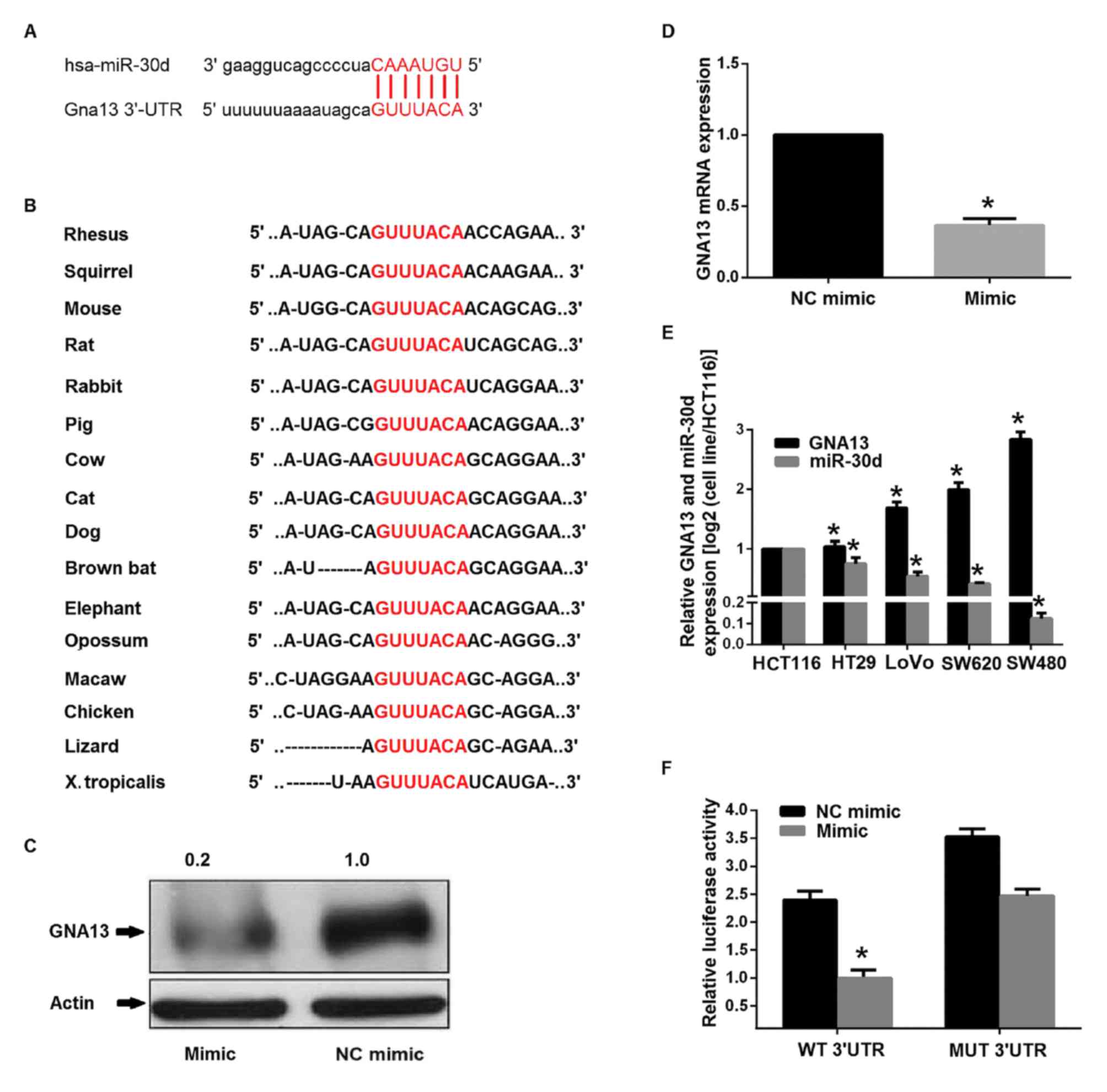
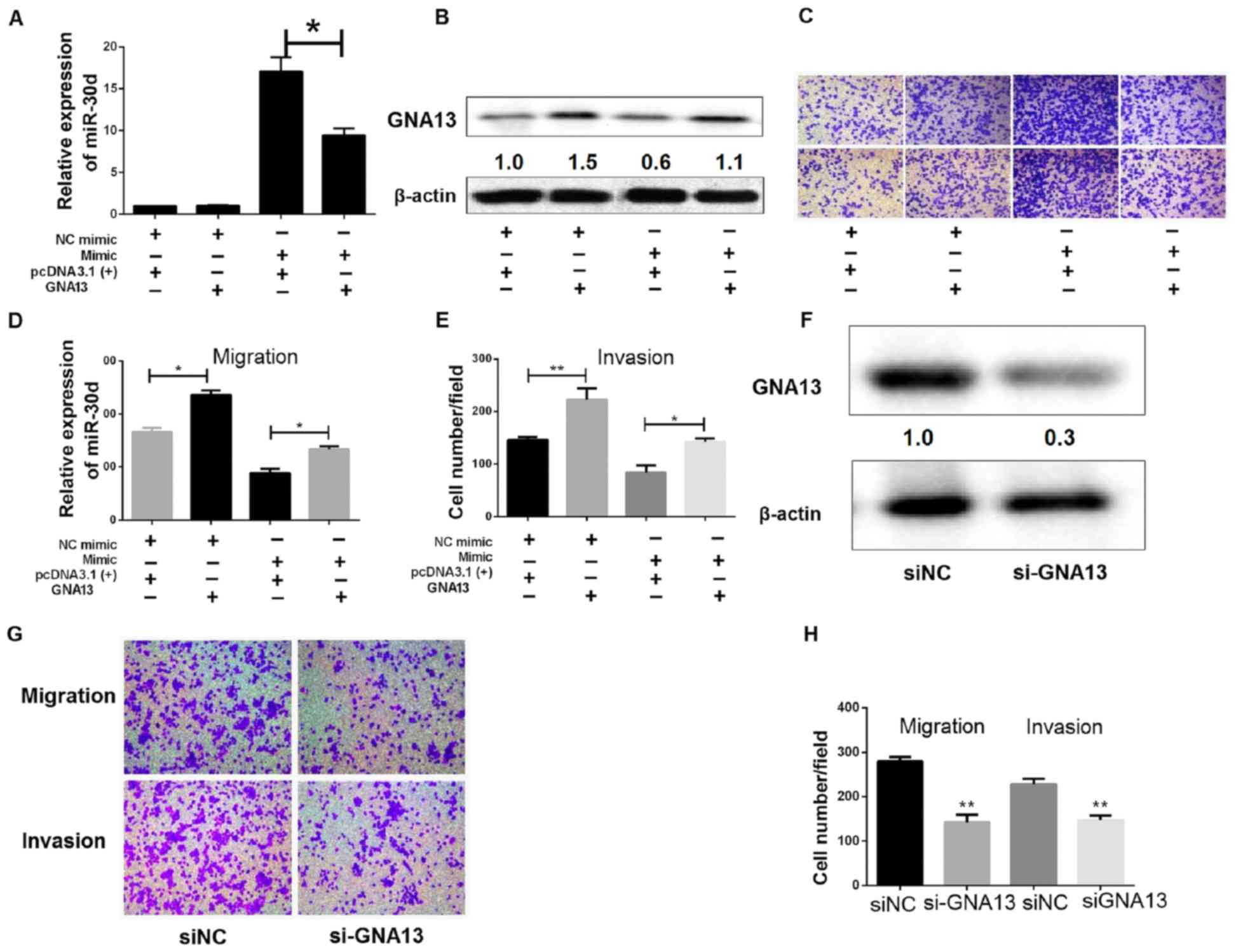
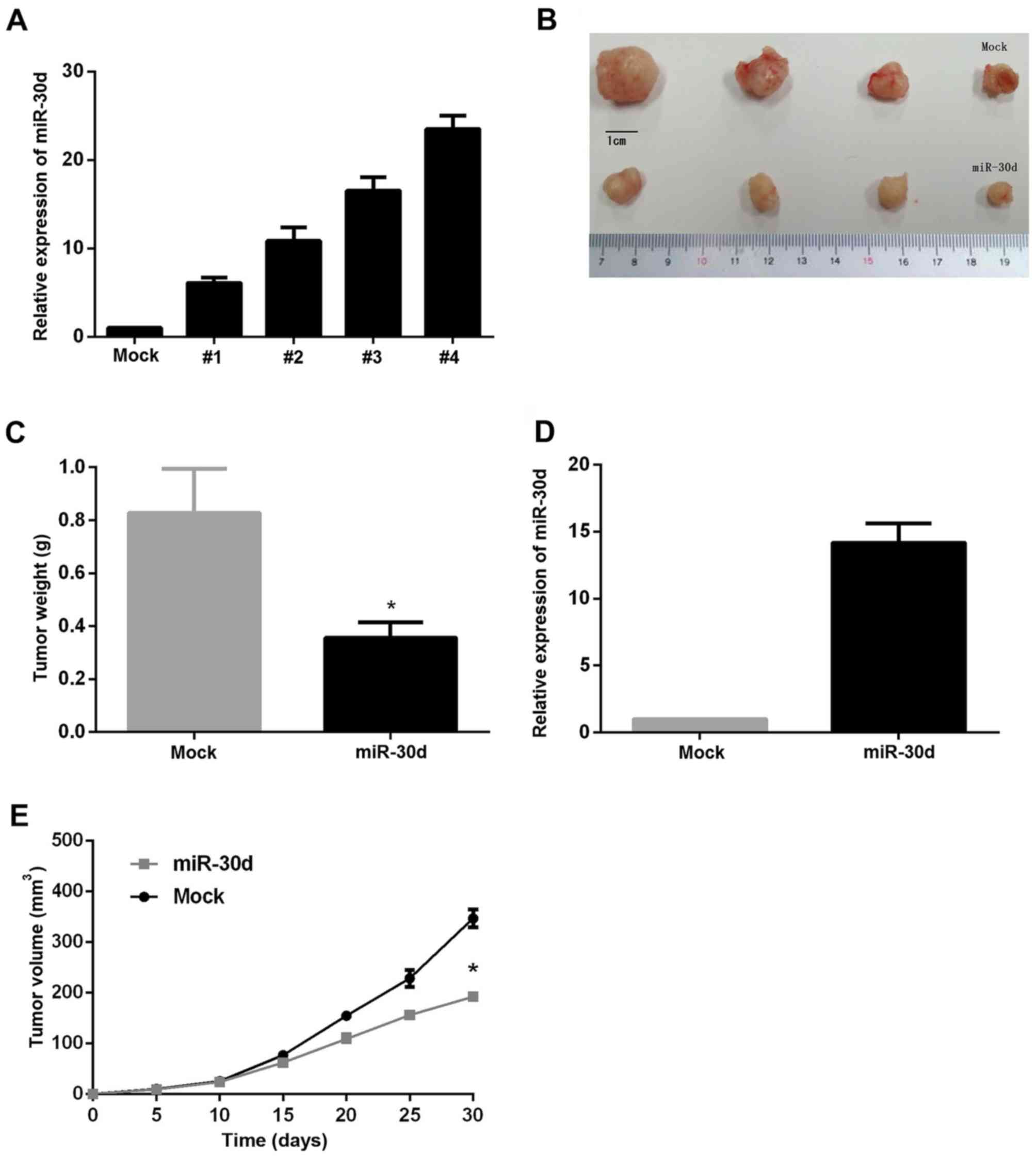
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiegering A, Isbert C, Dietz UA, Kunzmann
V, Ackermann S, Kerscher A, Maeder U, Flentje M, Schlegel N,
Reibetanz J, et al: Multimodal therapy in treatment of rectal
cancer is associated with improved survival and reduced local
recurrence-a retrospective analysis over two decades. BMC Cancer.
14:8162014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceelen WP: Progress in rectal cancer
treatment. ISRN Gastroenterol. 2012:6481832012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang TC, Yu D, Lee YS, Wentzel EA, Arking
DE, West KM, Dang CV, Thomas-Tikhonenko A and Mendell JT:
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet. 40:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng CW, Wang HW, Chang CW, Chu HW, Chen
CY, Yu JC, Chao JI, Liu HF, Ding SL and Shen CY: MicroRNA-30a
inhibits cell migration and invasion by downregulating vimentin
expression and is a potential prognostic marker in breast cancer.
Breast Cancer Res Treat. 134:1081–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Lin X, Wang F, Zhang B, Wang W, Shi
H, Zou B and Zhao J: Proliferation inhibition and the underlying
molecular mechanisms of microRNA-30d in renal carcinoma cells.
Oncol Lett. 7:799–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong K, Chen K, Han L and Li B:
MicroRNA-30b/c inhibits non-small cell lung cancer cell
proliferation by targeting Rab18. BMC Cancer. 14:7032014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugihara H, Ishimoto T, Watanabe M,
Sawayama H, Iwatsuki M, Baba Y, Komohara Y, Takeya M and Baba H:
Identification of miR-30e* regulation of Bmi1 expression mediated
by tumor-associated macrophages in gastrointestinal cancer. PLoS
One. 8:e818392013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of GalNAc
transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao J, Liang L, Huang S, Ding J, Tan N,
Zhao Y, Yan M, Ge C, Zhang Z, Chen T, et al: MicroRNA-30d promotes
tumor invasion and metastasis by targeting Galphai2 in
hepatocellular carcinoma. Hepatology. 51:846–856. 2010.PubMed/NCBI
|
|
20
|
Kwak SY, Kim BY, Ahn HJ, Yoo JO, Kim J,
Bae IH and Han YH: Ionizing radiation-inducible miR-30e promotes
glioma cell invasion through EGFR stabilization by directly
targeting CBL-B. FEBS J. 282:1512–1525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Yao X, Zhang J, Dong B, Chen Q,
Xue W, Liu D and Huang Y: Hypoxia-induced downregulation of miR-30c
promotes epithelial-mesenchymal transition in human renal cell
carcinoma. Cancer Sci. 104:1609–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li N, Kaur S, Greshock J, Lassus H, Zhong
X, Wang Y, Leminen A, Shao Z, Hu X, Liang S, et al: A combined
array-based comparative genomic hybridization and functional
library screening approach identifies mir-30d as an oncomir in
cancer. Cancer Res. 72:154–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu Y, Ryan SL, Elliott DJ, Bignell GR,
Futreal PA, Ellison DW, Bailey S and Clifford SC: Amplification and
overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at
8q24.22-q24.23 in medulloblastoma. PLoS One. 4:e61592009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye Z, Zhao L, Li J, Chen W and Li X:
miR-30d blocked transforming growth factor β1-induced
epithelial-mesenchymal transition by targeting Snail in ovarian
cancer cell. Int J Gynecol Cancer. 25:1574–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin ZY, Chen G, Zhang YQ, He HC, Liang YX,
Ye JH, Liang YK, Mo RJ, Lu JM, Zhuo YJ, et al: MicroRNA-30d
promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA
pathway and predicts aggressive outcome in prostate cancer. Mol
Cancer. 16:482017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang P, Garnett J, Creighton CJ, Al
Sannaa GA, Igram DR, Lazar A, Liu X, Liu C and Pollock RE:
EZH2-miR-30d-KPNB1 pathway regulates malignant peripheral nerve
sheath tumour cell survival and tumourigenesis. J Pathol.
232:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cervantes-Anaya N, Ponciano-Gómez A,
López-Álvarez GS, Gonzalez-Reyes C, Hernández-Garcia S,
Cabañas-Cortes MA, Garrido-Guerrero JE and Villa-Treviño S:
Downregulation of sorting nexin 10 is associated with
overexpression of miR-30d during liver cancer progression in rats.
Tumour Biol. 39:10104283176959322017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Hao Y, Li Y, Li R, Wu R, Wang S
and Fang Z: Amplification and up-regulation of MIR30D was
associated with disease progression of cervical squamous cell
carcinomas. BMC Cancer. 17:2302017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie R, Wu SN, Gao CC, Yang XZ, Wang HG,
Zhang JL, Yan W and Ma TH: MicroRNA-30d inhibits the migration and
invasion of human esophageal squamous cell carcinoma cells via the
post-transcriptional regulation of enhancer of zeste homolog 2.
Oncol Rep. 37:1682–1690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han YL, Cao XE, Wang JX, Dong CL and Chen
HT: Correlations of microRNA-124a and microRNA-30d with
clinicopathological features of breast cancer patients with type 2
diabetes mellitus. Springerplus. 5:21072016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar B, Khaleghzadegan S, Mears B, Hatano
K, Kudrolli TA, Chowdhury WH, Yeater DB, Ewing CM, Luo J, Isaacs
WB, et al: Identification of miR-30b-3p and miR-30d-5p as direct
regulators of androgen receptor signaling in prostate cancer by
complementary functional microRNA library screening. Oncotarget.
7:72593–72607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi N, Uemura H, Nagahama K, Okudela
K, Furuya M, Ino Y, Ito Y, Hirano H, Inayama Y, Aoki I, et al:
Identification of miR-30d as a novel prognostic maker of prostate
cancer. Oncotarget. 3:1455–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang
L, Liu L, Huang S, Zhao Y and He X: MicroRNA-30d-5p inhibits tumour
cell proliferation and motility by directly targeting CCNE2 in
non-small cell lung cancer. Cancer Lett. 362:208–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
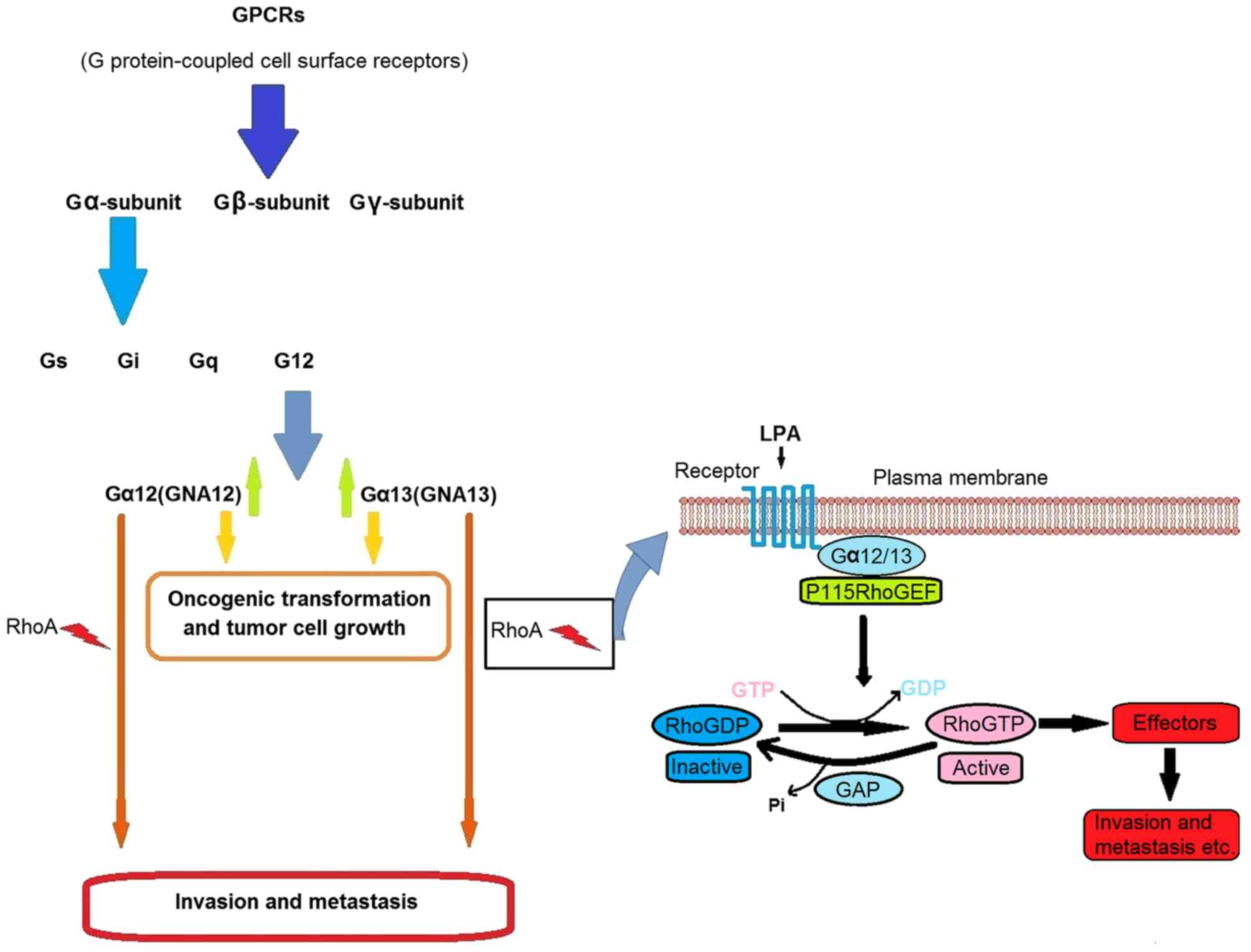
Dorsam RT and Gutkind JS:
G-protein-coupled receptors and cancer. Nat Rev Cancer. 7:79–94.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wettschureck N and Offermanns S: Mammalian
G proteins and their cell type specific functions. Physiol Rev.
85:1159–1204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YM, Lee S, Nam CW, Ha JH, Jayaraman
M, Dhanasekaran DN, Lee CH, Kwak MK and Kim SG: G(alpha)12/13
inhibition enhances the anticancer effect of bortezomib through
PSMB5 downregulation. Carcinogenesis. 31:1230–1237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan AM, Fleming TP, McGovern ES, Chedid
M, Miki T and Aaronson SA: Expression cDNA cloning of a
transforming gene encoding the wild-type G alpha 12 gene product.
Mol Cell Biol. 13:762–768. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu N, Bradley L, Ambdukar I and Gutkind
JS: A mutant alpha subunit of G12 potentiates the eicosanoid
pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad
Sci USA. 90:6741–6745. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kelly P, Casey PJ and Meigs TE: Biologic
functions of the G12 subfamily of heterotrimeric g proteins:
Growth, migration, and metastasis. Biochemistry. 46:6677–6687.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kelly P, Moeller BJ, Juneja J, Booden MA,
Der CJ, Daaka Y, Dewhirst MW, Fields TA and Casey PJ: The G12
family of heterotrimeric G proteins promotes breast cancer invasion
and metastasis. Proc Natl Acad Sci USA. 103:8173–8178. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kelly P, Stemmle LN, Madden JF, Fields TA,
Daaka Y and Casey PJ: A role for the G12 family of heterotrimeric G
proteins in prostate cancer invasion. J Biol Chem. 281:26483–26490.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheong SC, Chandramouli GV, Saleh A, Zain
RB, Lau SH, Sivakumaren S, Pathmanathan R, Prime SS, Teo SH, Patel
V and Gutkind JS: Gene expression in human oral squamous cell
carcinoma is influenced by risk factor exposure. Oral Oncol.
45:712–719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kozasa T, Hajicek N, Chow CR and Suzuki N:
Signalling mechanisms of RhoGTPase regulation by the heterotrimeric
G proteins G12 and G13. J Biochem. 150:357–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Guo L, Hadas J, Gutowski S, Sprang
SR and Sternweis PC: Activation of p115-RhoGEF requires direct
association of Gα13 and the Dbl homology domain. J Biol Chem.
287:25490–25500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Malchinkhuu E, Sato K, Maehama T, Mogi C,
Tomura H, Ishiuchi S, Yoshimoto Y, Kurose H and Okajima F: S1P(2)
receptors mediate inhibition of glioma cell migration through Rho
signaling pathways independent of PTEN. Biochem Biophys Res Commun.
366:963–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shumay E, Tao J, Wang HY and Malbon CC:
Lysophosphatidic acid regulates trafficking of beta2-adrenergic
receptors: The Galpha13/p115RhoGEF/JNK pathway stimulates receptor
internalization. J Biol Chem. 282:21529–21541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM, Zhou W, Ghosh S and Casey PJ: MicroRNA-31 controls G protein
alpha-13 (GNA13) expression and cell invasion in breast cancer
cells. Mol Cancer. 14:672015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM and Casey PJ: MicroRNA-182 and microRNA-200a control G-protein
subunit α-13 (GNA13) expression and cell invasion synergistically
in prostate cancer cells. J Biol Chem. 288:7986–7995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gardner JA, Ha JH, Jayaraman M and
Dhanasekaran DN: The gep proto-oncogene Gα13 mediates
lysophosphatidic acid-mediated migration of pancreatic cancer
cells. Pancreas. 42:819–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang JX, Yun M, Xu Y, Chen JW, Weng HW,
Zheng ZS, Chen C, Xie D and Ye S: GNA13 as a prognostic factor and
mediator of gastric cancer progression. Oncotarget. 7:4414–4427.
2016.PubMed/NCBI
|
|
51
|
Grzelinski M, Pinkenburg O, Büch T, Gold
M, Stohr S, Kalwa H, Gudermann T and Aigner A: Critical role of
G(alpha)12 and G(alpha)13 for human small cell lung cancer cell
proliferation in vitro and tumor growth in vivo. Clin Cancer Res.
16:1402–1415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Tang Q, Li M, Jiang S and Wang X:
MicroRNA-375 inhibits colorectal cancer growth by targeting PIK3CA.
Biochem Biophys Res Commun. 444:199–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H,
Wang W, Zhang L, Zhang X, Tang Q, et al: MicroRNA-25 functions as a
potential tumor suppressor in colon cancer by targeting Smad7.
Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vukobrat-Bijedic Z, Husic-Selimovic A,
Sofic A, Bijedic N, Bjelogrlic I, Gogov B and Mehmedovic A: Cancer
antigens (CEA and CA 19-9) as markers of advanced stage of
colorectal carcinoma. Med Arh. 67:397–401. 2013. View Article : Google Scholar
|
|
56
|
Tong J, Wang Y, Chang B, Zhang D and Wang
B: Associations between tumormarkers and the risk of colorectal
polyp recurrence in Chinese people. Int J Clin Exp Med.
8:6397–6405. 2015.PubMed/NCBI
|
|
57
|
Wang K, Liu F, Zhou LY, Ding SL, Long B,
Liu CY, Sun T, Fan YY, Sun L and Li PF: miR-874 regulates
myocardial necrosis by targeting caspase-8. Cell Death Dis.
4:e7092013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C and Shen H: Serum microRNA
signatures identified in a genome-wide serum microRNA expression
profiling predict survival of non-small-cell lung cancer. J Clin
Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|