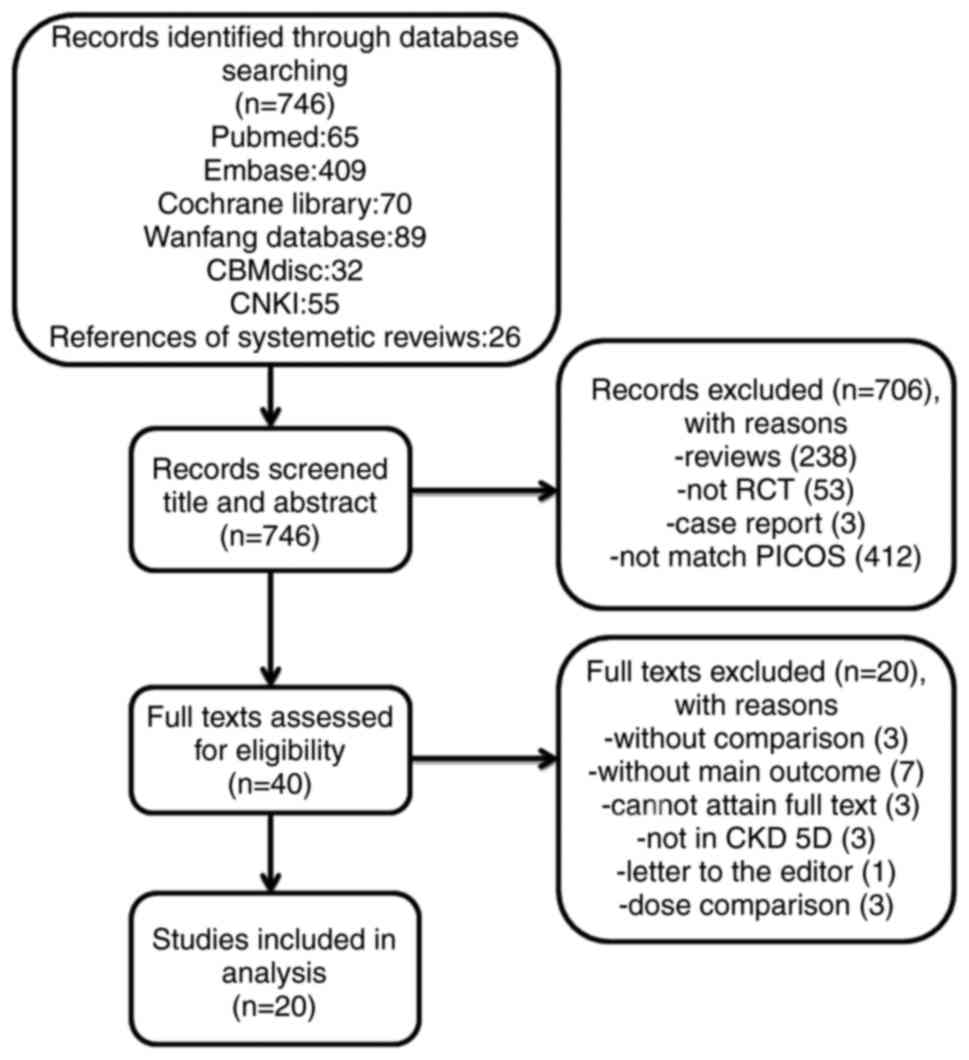
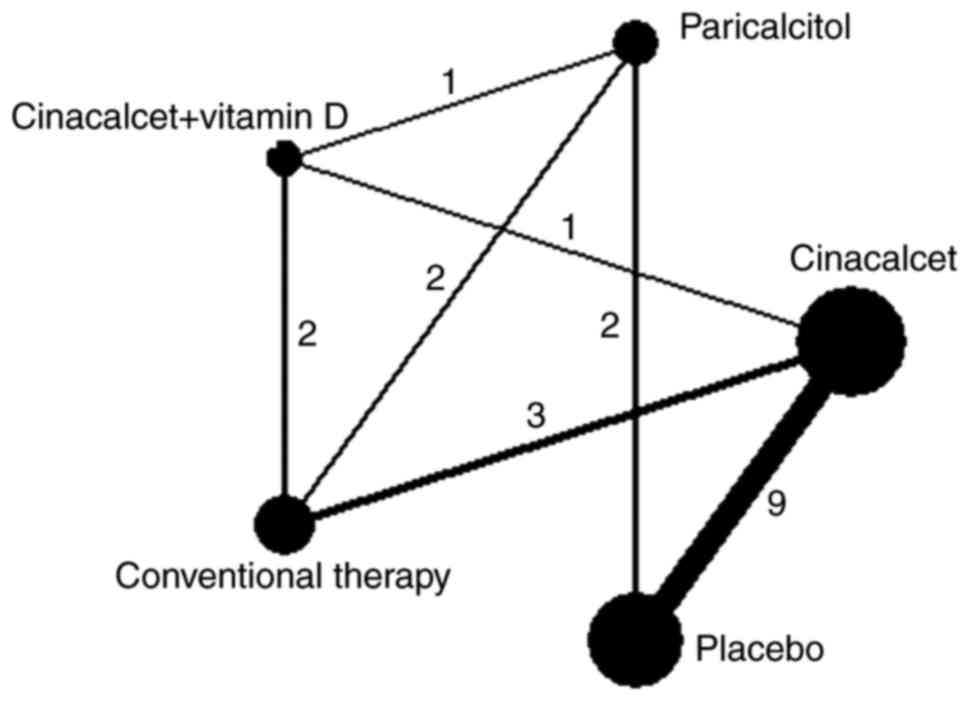
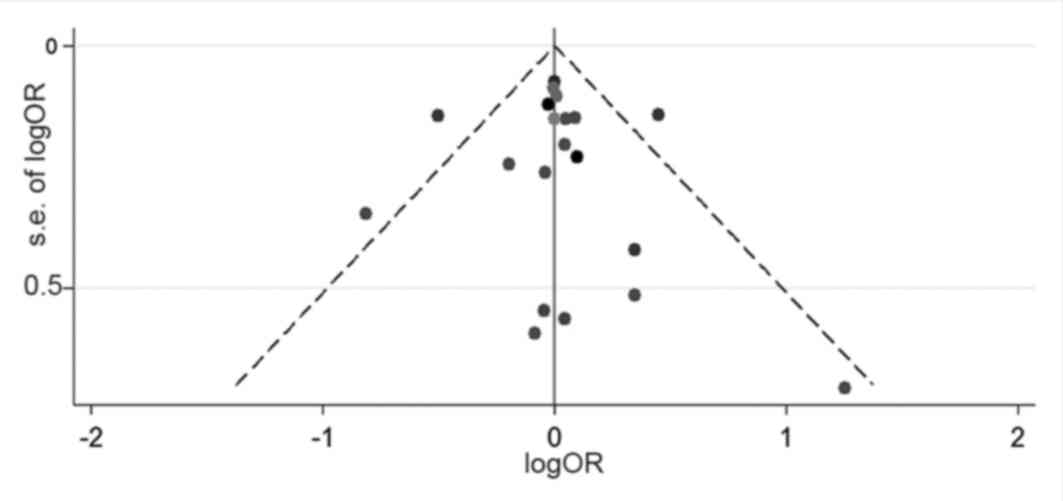
|
1
|
National Center for Chronic Disease
Prevention and Health Promotion. CS250738-A: National Chronic
Kidney Disease Fact Sheet. 2014, https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf
|
|
2
|
Zhang L, Wang F, Wang L, Wang W, Liu B,
Liu J, Chen M, He Q, Liao Y, Yu X, et al: Prevalence of chronic
kidney disease in China: A cross-sectional survey. Lancet.
379:815–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douthat WG, Castellano M, Berenguer L,
Guzmán MA, de Arteaga J, Chiurchiu CR, Massari PU, Garay G, Capra R
and de La Fuente JL: High prevalence of secondary
hyperparathyroidism in chronic kidney disease patients on dialysis
in argentina. Nefroloqia. 33:657–666. 2013.(In English,
Spanish).
|
|
4
|
Jeloka T, Mali M, Jhamnani A, Konde S and
Jadhav V: Are we overconcerned about secondary hyperparathyroidism
and underestimating the more common secondary hypoparathyroidism in
our dialysis patients? J Assoc Physicians India. 60:102–105.
2012.PubMed/NCBI
|
|
5
|
Block GA, Hulbert-Shearon TE, Levin NW and
Port FK: Association of serum phosphorus and calcium × phosphate
product with mortality risk in chronic hemodialysis patients: A
national study. Am J Kidney Dis. 31:607–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goodman WG, Goldin J, Kuizon BD, Yoon C,
Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al:
Coronary-artery calcification in young adults with end-stage renal
disease who are undergoing dialysis. N Engl J Med. 342:1478–1483.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzoni V, Trieste L and Turchetti G:
The cost-effectiveness of drug therapies to treat secondary
hyperparathyroidism in renal failure: A focus on evidence regarding
paricalcitol and cinacalcet. Expert Rev Pharmacoecon Outcomes Res.
15:622–624. 2015. View Article : Google Scholar
|
|
8
|
Moe SM, Chertow GM, Coburn JW, Quarles LD,
Goodman WG, Block GA, Drueke TB, Cunningham J, Sherrard DJ, McCary
LC, et al: Achieving NKF-K/DOQI bone metabolism and disease
treatment goals with cinacalcet HCl. Kidney Int. 67:760–771. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Li M, You L, Li H, Ni L, Gu Y,
Hao C and Chen J: Effects and safety of calcimimetics in end stage
renal disease patients with secondary hyperparathyroidism: A
meta-analysis. PLoS One. 7:e480702012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fishbane S, Shapiro WB, Corry DB, Vicks
SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S and
Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D
improves treatment of secondary hyperparathyroidism in dialysis
patients compared with vitamin D alone: The ACHIEVE study results.
Clin J Am Soc Nephrol. 3:1718–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urena-Torres P, Bridges I, Christiano C,
Cournoyer SH, Cooper K, Farouk M, Kopyt NP, Rodriguez M, Zehnder D
and Covic A: Efficacy of cinacalcet with low-dose vitamin D in
incident haemodialysis subjects with secondary hyperparathyroidism.
Nephrol Dial Transplant. 28:1241–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coyne D, Acharya M, Qiu P, Abboud H,
Batlle D, Rosansky S, Fadem S, Levine B, Williams L, Andress DL, et
al: Paricalcitol capsule for the treatment of secondary
hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis.
47:263–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin KJ, Gonzalez EA, Gellens M, Hamm
LL, Abboud H and Lindberg J: 19-Nor-1-alpha-25-dihydroxyvitamin D2
(Paricalcitol) safely and effectively reduces the levels of intact
parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol.
9:1427–1432. 1998.PubMed/NCBI
|
|
14
|
Alegre MM, Weyant MJ, Bennett DT, Yu JA,
Ramsden MK, Elnaggar A, Robison RA and O'Neill KL: Serum detection
of thymidine kinase 1 as a means of early detection of lung cancer.
Anticancer Res. 34:2145–2151. 2014.PubMed/NCBI
|
|
15
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valkenhoef G van, Tervonen T, Zwinkels T,
Brock B de and Hillege H: ADDIS: A decision support system for
evidence-based medicine. Decision Support Systems. 55:459–575.
2013. View Article : Google Scholar
|
|
18
|
Puhan MA, Schünemann HJ, Murad MH, Li T,
Brignardello-Petersen R, Singh JA, Kessels AG and Guyatt GH; GRADE
Working Group, . A GRADE working group approach for rating the
quality of treatment effect estimates from network meta-analysis.
BMJ. 349:g56302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thorlund K and Mills EJ: Sample size and
power considerations in network meta-analysis. Syst Rev. 1:412012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Block GA, Martin KJ, de Francisco AL,
Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa A,
Messa P, et al: Cinacalcet for secondary hyperparathyroidism in
patients receiving hemodialysis. New Engl J Med. 350:1516–1525.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Shafey EM, Alsahow AE, Alsaran K, Sabry
AA and Atia M: Cinacalcet hydrochloride therapy for secondary
hyperparathyroidism in hemodialysis patients. Ther Apher Dial.
15:547–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukagawa M, Yumita S, Akizawa T, Uchida E,
Tsukamoto Y, Iwasaki M and Koshikawa S; KRN1493 study group, .
Cinacalcet (KRN1493) effectively decreases the serum intact PTH
level with favorable control of the serum phosphorus and calcium
levels in Japanese dialysis patients. Nephrol Dial Transplant.
23:328–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansen D, Rasmussen K, Danielsen H,
Meyer-Hofmann H, Bacevicius E, Lauridsen TG, Madsen JK, Tougaard
BG, Marckmann P, Thye-Roenn P, et al: No difference between
alfacalcidol and paricalcitol in the treatment of secondary
hyperparathyroidism in hemodialysis patients: A randomized
crossover trial. Kidney Int. 80:841–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ketteler M, Martin KJ, Wolf M, Amdahl M,
Cozzolino M, Goldsmith D, Sharma A, Marx S and Khan S: Paricalcitol
versus cinacalcet plus low-dose vitamin D therapy for the treatment
of secondary hyperparathyroidism in patients receiving
haemodialysis: Results of the IMPACT SHPT study. Nephrol Dial
Transplant. 27:3270–3278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindberg JS, Moe SM, Goodman WG, Coburn
JW, Sprague SM, Liu W, Blaisdell PW, Brenner RM, Turner SA and
Martin KJ: The calcimimetic AMG 073 reduces parathyroid hormone and
calcium × phosphorus in secondary hyperparathyroidism. Kidney Int.
63:248–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindberg JS, Culleton B, Wong G, Borah MF,
Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, et
al: Cinacalcet HCl, an oral calcimimetic agent for the treatment of
secondary hyperparathyroidism in hemodialysis and peritoneal
dialysis: A randomized, double-blind, multicenter study. J Am Soc
Nephrol. 16:800–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin KJ, Jüppner H, Sherrard DJ, Goodman
WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary
LC, et al: First- and second-generation immunometric PTH assays
during treatment of hyperparathyroidism with cinacalcet HCl. Kidney
Int. 68:1236–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Messa P, Macário F, Yaqoob M, Bouman K,
Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, et
al: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara)
treatment algorithm for secondary hyperparathyroidism. Clin J Am
Soc Nephrol. 3:36–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quarles LD, Sherrard DJ, Adler S, Rosansky
SJ, McCary LC, Liu W, Turner SA and Bushinsky DA: The calcimimetic
AMG 073 as a potential treatment for secondary hyperparathyroidism
of end-stage renal disease. J Am Soc Nephrol. 14:575–583. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ross EA, Tian J, Abboud H, Hippensteel R,
Melnick JZ, Pradhan RS, Williams LA, Hamm LL and Sprague SM: Oral
paricalcitol for the treatment of secondary hyperparathyroidism in
patients on hemodialysis or peritoneal dialysis. Am J Nephrol.
28:97–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sprague SM, Llach F, Amdahl M, Taccetta C
and Batlle D: Paricalcitol versus calcitriol in the treatment of
secondary hyperparathyroidism. Kidney Int. 63:1483–1490. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sterrett JR, Strom J, Stummvoll HK, Bahner
U, Disney A, Soroka SD, Corpier C, Arruda JA, Schwanauer LE,
Klassen PS, et al: Cinacalcet HCI (Sensipar/Mimpara) is an
effective chronic therapy for hemodialysis patients with secondary
hyperparathyroidism. Clin Nephrol. 68:10–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wetmore JB, Gurevich K, Sprague S, Da Roza
G, Buerkert J, Reiner M, Goodman W and Cooper K: A randomized trial
of cinacalcet versus Vitamin D analogs as monotherapy in secondary
hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol.
10:1031–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mei C, Chen N, Ding X, Yu X, Wang L, Qian
J, Wang M, Jiang G, Li X, Hou F, et al: Efficacy and safety of
Cinacalcet on secondary hyperparathyroidism in Chinese chronic
kidney disease patients receiving hemodialysis. Hemodial Int.
20:589–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han YY, Wang T, Wenyu Z and Wenxiu C:
Clinical observation of calcitriol combined with cinacalcet in
hemodialysis patients with secondary hyperparathyroidism. Drug
Clinic. 30:1451–1454. 2015.
|
|
36
|
Cai P, Tang X, Qin W, Ji L and Li Z:
Comparison between paricalcitol and active non-selective vitamin D
receptor activator for secondary hyperparathyroidism in chronic
kidney disease: A systematic review and meta-analysis of randomized
controlled trials. Int Urol Nephrol. 48:571–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Shao L, Zhou H, Jiang W, Zhang W and
Xu Y: The efficacy of cinacalcet combined with conventional therapy
on bone and mineral metabolism in dialysis patients with secondary
hyperparathyroidism: A meta-analysis. Endocrine. 43:68–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cunningham J, Danese M, Olson K, Klassen P
and Chertow GM: Effects of the calcimimetic cinacalcet HCl on
cardiovascular disease, fracture, and health-related quality of
life in secondary hyperparathyroidism. Kidney Int. 68:1793–1800.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
EVOLVE Trial Investigators, . Chertow GM,
Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog
CA, Kubo Y, London GM, Mahaffey KW, et al: Effect of cinacalcet on
cardiovascular disease in patients undergoing dialysis. N Engl J
Med. 367:2482–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colloton M, Shatzen E, Miller G,
Stehman-Breen C, Wada M, Lacey D and Martin D: Cinacalcet HCl
attenuates parathyroid hyperplasia in a rat model of secondary
hyperparathyroidism. Kidney Int. 67:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamada S, Tokumoto M, Taniguchi M,
Toyonaga J, Suehiro T, Eriguchi R, Fujimi S, Ooboshi H, Kitazono T
and Tsuruya K: Two years of cinacalcet hydrochloride treatment
decreased parathyroid gland volume and serum parathyroid hormone
level in hemodialysis patients with advanced secondary
hyperparathyroidism. Ther Apher Dial. 19:367–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slatopolsky E, Finch J, Ritter C and
Takahashi F: Effects of 19-nor-1,25(OH)2D2, a new analogue of
calcitriol, on secondary hyperparathyroidism in uremic rats. Am J
Kidney Dis. 32 Suppl 2:S40–S47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schumock GT, Walton SM, Lee TA, Marx SE,
Audhya P and Andress DL: Comparative effectiveness of paricalcitol
versus cinacalcet for secondary hyperparathyroidism in patients
receiving hemodialysis. Nephron Clin Pract. 117:c151–c159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zittermann A and Koerfer R: Protective and
toxic effects of vitamin D on vascular calcification: Clinical
implications. Mol Aspects Med. 29:423–432. 2008. View Article : Google Scholar : PubMed/NCBI
|