Introduction
Congenital hydronephrosis is one of the common
childhood malformations in clinic which is usually caused by the
interaction of glomerular hemodynamics with the changes in renal
tubular function, with a morbidity of 1–2% in newborn infants
(1). Hydronephrosis can eventually
lead to renal failure in children, so it is very important to find
the early congenital hydronephrosis and treat it timely (2). Clinically, the neonatal hydronephrosis
is often diagnosed through imaging examination and a variety of
assisting biochemical indicators. However, the biochemical
indicators such as serum creatinine and blood urea nitrogen are
susceptible to various factors. Therefore, the diagnosis often lags
and cannot accurately reflect the extent of neonatal renal injury.
As a result, looking for indicators reflecting the degree of renal
injury of children with hydronephrosis at early stage is necessary
(3). Retinol binding protein (RBP)
is currently considered to be the most sensitive and stable
transporter that reflects the renal function changes in urine and
can be monitored at any time for the diagnosis of renal biopsy
morphology (4). Urinary
micro-albumin (ALB) is one of the early diagnosis markers for the
degree of glomerular injury (5).
Aquaporin-2 (AQP2) is a kind of aquaporin that widely exists in the
special channel for water transporting on the membrane and it is
the marker for the diagnosis of hydronephrosis (6). Monocyte chemoattractant protein 1
(MCP-1) is an inflammatory marker whose overexpression would result
in the shallow implantation of placenta in pregnant women and the
impaired endothelial function, thus bringing an adverse effect on
the normal growth of fetuses (7). In
the study, the expression levels of urinary RBP, ALB, AQP2 and
MCP-1 in the prenatal maternal blood were detected and analyzed to
provide the diagnostic proof for neonatal hydronephrosis.
Patients and methods
Patients
A total of 46 newborn infants with hydronephrosis
admitted to Hongqi Hospital Affiliated to Mudanjiang Medical
College (Mudanjiang, China) from December 2016 to November 2017
were selected as the observation group. Inclusion criteria: i)
child patients complying with the hydronephrosis diagnostic
criteria (8), ii) child patients who
were born within 28 days and iii) approval obtained from the Ethics
Committee in Hongqi Hospital Affiliated to Mudanjiang Medical
College and informed consent signed by the family members.
Exclusion criteria: i) patients with bilateral hydronephrosis and
ii) child patients with urinary tract infection and febrile
diseases. Compared with the 46 normal newborn infants at the same
period in the control group, there were no significant differences
in general materials of the child patients in the two groups
(P>0.05; Table I).
 | Table I.Baseline data of child patients in the
two groups. |
Table I.
Baseline data of child patients in the
two groups.
| Items | Control group
(n=46) | Observation group
(n=46) | t/χ2 | P-value |
|---|
| Sex
(male/female) | 27/19 | 25/21 | 0.044 | 0.833 |
| Time of birth
(days) | 6–21 | 6–23 |
|
|
| Average day-age
(days) | 13.58±5.43 | 13.83±5.52 | 0.219 | 0.827 |
| Body mass (kg) | 2.63±0.76 | 2.69±0.75 | 0.381 | 0.704 |
| Degree of
hydronephrosis (n, %) |
| Mild | 29 (63.04) | 30 (65.21) | 0.326 | 0.849 |
|
Moderate | 9 (19.57) | 7 (15.22) |
|
|
|
Severe | 8 (17.39) | 9 (19.57) |
|
|
Methods
The midstream urine samples were collected from two
groups of newborn infants in the morning (taken at 6:00 a.m.), and
were stored in a refrigerator at −80°C for detection. After removal
from the refrigerator during the detection, the urine samples were
thawed in water at 4°C and centrifuged at 8,100 × g for 5 min.
Neonatal urinary RBP and ALB were detected via the immunity
transmission turbidity method. The related kits were provided by
Shanghai Beijia Biochemical Reagent Co., Ltd. (Shanghai, China) and
the operation was conducted in strict accordance with the
instructions. Neonatal urinary AQP2 concentration was detected
through the enzyme-linked immunosorbent assay (ELISA). The related
kits were provided by R&D systems (Minneapolis, MN, USA) and
the determination was in strict accordance with the product
manual.
A total of 3 ml of peripheral blood was collected
from each pregnant woman in the two groups before the infants were
born, placed at 20°C for 30 min and centrifuged at 1,800 × g for 20
min at 4°C. The separated serum was poured into an Eppendorf (EP)
tube and stored in a refrigerator at −80°C. The serum MCP-1 level
was examined via ELISA. The operation was performed strictly
according to the protocol of the related kit (provided by Beyotime,
Shanghai, China), followed by sample adding, incubation, washing
with enzyme, incubation and washing and color development. After
the addition of terminative solution, the optical density (OD)
value in each well was measured sequentially through a microplate
reader (Bio-Rad, Hercules, CA, USA) at the wavelength of 450 nm
within 15 min, and the MCP-1 level was calculated.
Evaluation indexes
Judgement criteria for the hydronephrosis degree
(9): i) mild (renal pelvis and renal
calices were full and visualized during 10–30 min, but the cup
became shallow), ii) moderate (renal pelvis and renal calices
developed during 31–60 min, there were enlargement of renal pelvis
and renal calices as well as ureter expansion, but the calices cup
disappeared) and iii) severe (renal pelvis and renal calices
developed over 60 min and there was clumpy deformation of renal
pelvis and renal calices).
Neonatal urinary RBP and ALB
concentrations were detected through immunity transmission
turbidity method
Neonatal urinary AQP2 and MCP-1 concentrations in
peripheral blood of prenatal pregnant women were detected via
ELISA.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 (SPSS, Inc., Chicago, IL, USA) software was used for data
processing. Measurement data were presented as mean ± standard
deviation, and t-test was used for the examination. Measurement
data were shown as ratio, and χ2 test was used for the
examination. Diagnostic value was analyzed via receiver operating
characteristic (ROC) curve and the correlation was analyzed via
Pearsons correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
RBP, ALB and maternal MCP-1
levels
As for the conditions of RBP, ALB and maternal MCP-1
in the two groups of newborn infants, the levels of RBP and ALB in
the observation group were significantly higher than those in the
control group and the concentration of AQP2 in the observation
group was lower than that in the control group. The maternal MCP-1
level in the observation group was significantly higher than that
in the control group (P<0.05; Table
II).
 | Table II.Comparison of RBP, ALB AQP2 and
maternal MCP-1 levels in the two groups of newborn infants. |
Table II.
Comparison of RBP, ALB AQP2 and
maternal MCP-1 levels in the two groups of newborn infants.
| Groups | n | RBP (mg/l) | ALB (mg/ml) | AQP2 (ng/ml) | Maternal MCP-1
(ng/ml) |
|---|
| Observation
group | 46 | 7.64±2.63 | 1.59±0.78 | 7.63±1.24 | 208.63±16.43 |
| Control group | 46 | 1.96±0.75 | 0.37±0.15 | 12.79±2.35 | 141.22±13.28 |
| t value |
| 14.086 | 10.417 | 13.171 | 21.642 |
| P-value |
| <0.001 | <0.001 | <0.001 | <0.001 |
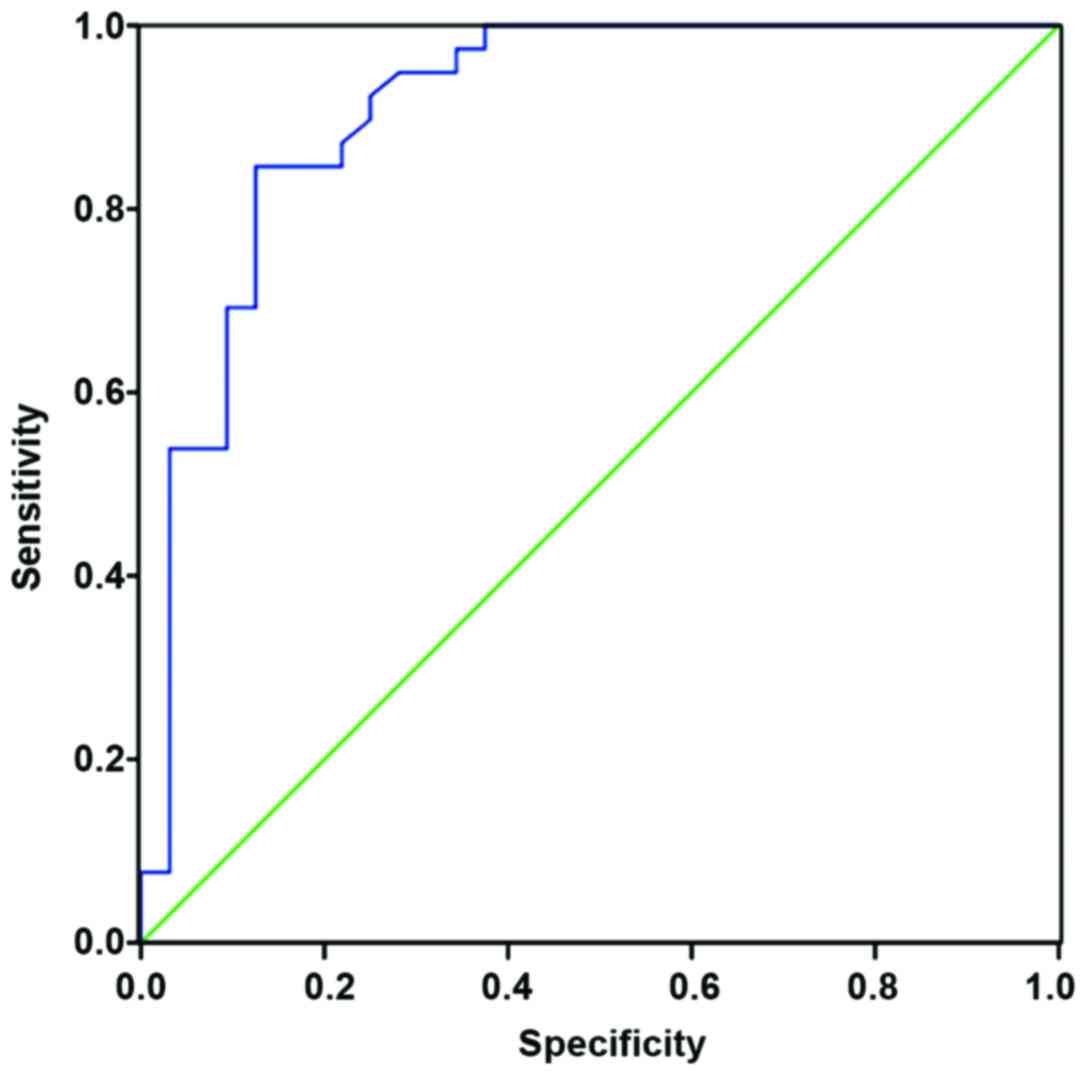
ROC curve for RBP, ALB and AQP2
ROC curve of the combined diagnosis of RBP, ALB and
AQP2 is shown in Fig. 1, with an
area under curve (AUC) of 0.913, sensitivity of 91.5% and
specificity of 89.8%.
Changes of RBP, ALB and AQP2 in child
patients before and after treatment
In terms of the changes of RBP, ALB and AQP2 in
child patients before and after treatment, the levels of RBP and
ALB in child patients after treatment were significantly lower than
those before treatment and AQP2 concentration was increased
compared with that before treatment (P<0.05; Table III).
 | Table III.RBP, ALB and AQP2 of child patients
before and after treatment. |
Table III.
RBP, ALB and AQP2 of child patients
before and after treatment.
| Time | n | RBP | ALB | AQP2 |
|---|
| Before treatment | 46 | 7.64±2.63 | 1.59±0.78 | 7.63±1.24 |
| After treatment | 46 | 2.56±1.25 | 0.68±0.15 | 11.89±1.35 |
| t value |
| 11.832 | 7.770 | 15.762 |
| P-value |
| <0.001 | <0.001 | <0.001 |
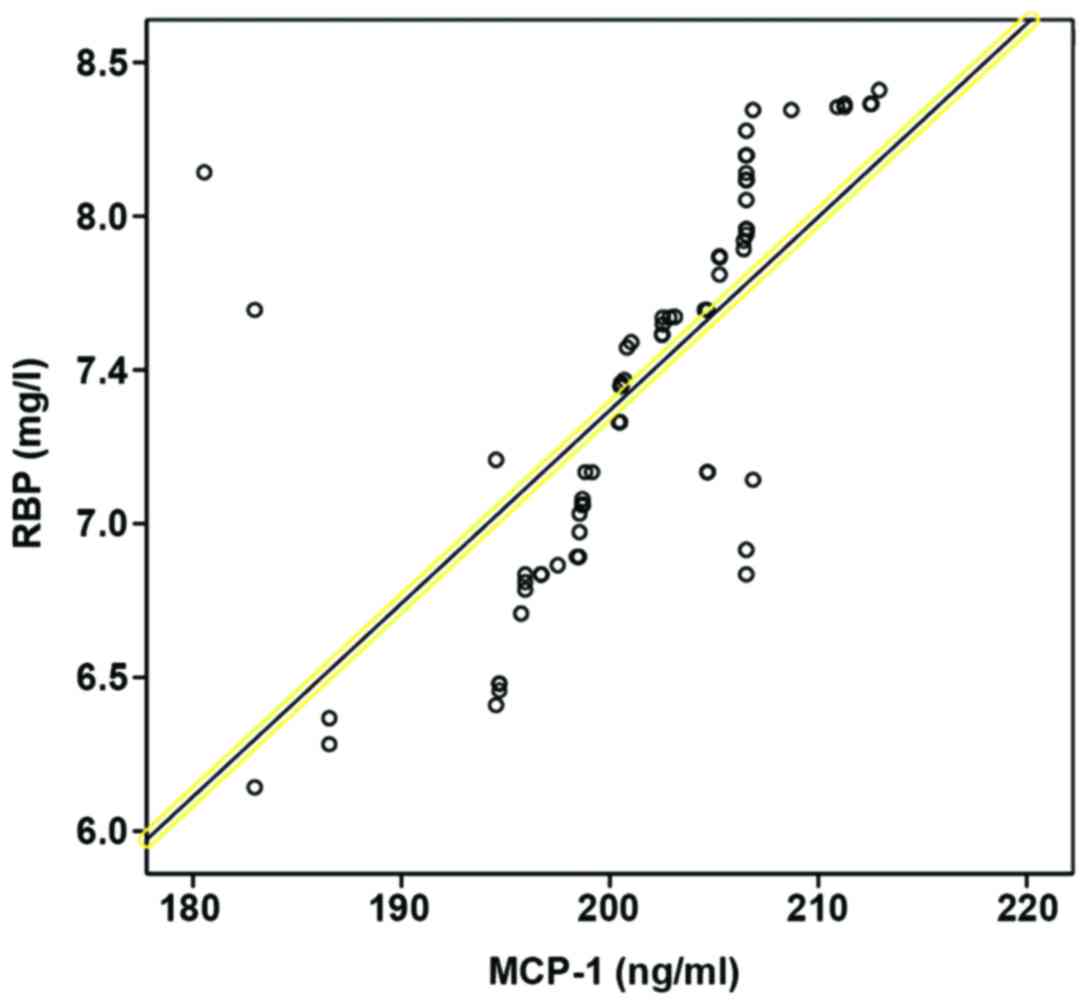
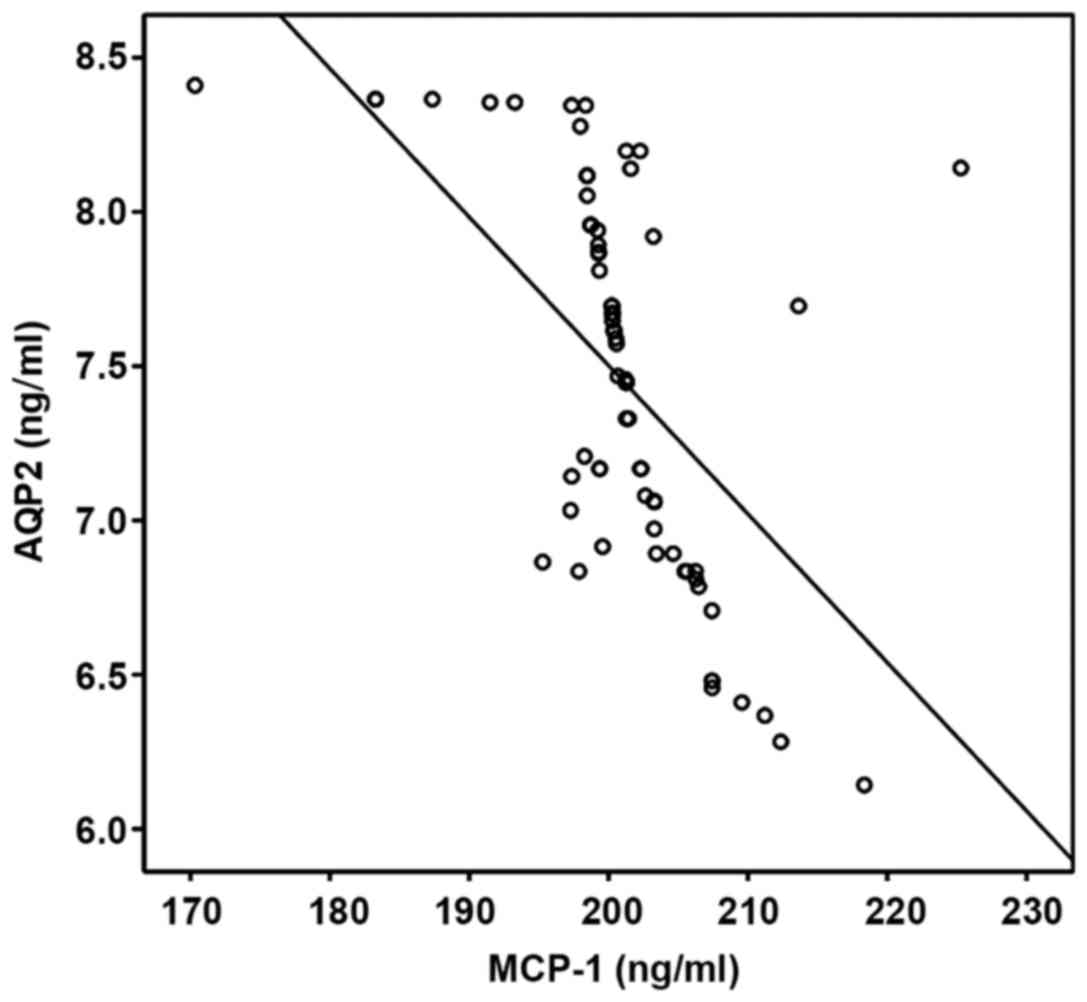
The correlation between RBP, ALB, AQP2
and prenatal MCP-1 level
The correlation between RBP, ALB, AQP2 and prenatal
MCP-1 level in pregnant women were analyzed via Pearsons
correlation coefficient. RBP and ALB were positively correlated
with MCP-1, but AQP2 and MCP-1 were negatively correlated
(P<0.05; Table IV, Figs. 2–4).
 | Table IV.Correlation analysis of RBP, ALB and
AQP2 with MCP-1. |
Table IV.
Correlation analysis of RBP, ALB and
AQP2 with MCP-1.
| Items | r | P-value |
|---|
| RBP | 0.568 | 0.003 |
| ALB | 0.501 | 0.014 |
| AQP2 | −0.445 | 0.017 |
Discussion
Neonatal hydronephrosis can account for >50% of
prenatal defects, which can be caused by the urinary catheter end
stenosis, vesicoureteral reflux, posterior urethral valve, ureteral
stenosis and ureterocele. Most of the neonatal hydronephrosis
results from the congenital ureteral renal pelvis junction
obstruction, accounting for approximately 85% (10). The urinary tract obstruction leads to
increasing pressure in the ureter and renal tubular and brings
direct or indirect damage to the renal tubular and renal
parenchyma. The manifestations are weakened reabsorption function
in the renal tubular, the damaged secretion function of hydrogen
ion and potassium ion, decreased urine concentration and abnormal
renal function in the distal segment of the kidney (11). With the progress of the disease, the
progressive decline of renal function eventually leads to renal
failure (12). At present, the
diagnosis of hydronephrosis mainly relies on imaging and technology
is very advanced. However, the routine detection such as serum
creatinine and blood urea nitrogen test for neonates, especially
for the early diagnosis of renal damage, is often laging. Surgery
should be performed when children's renal function begins to
deteriorate. Therefore, timely and dynamic diagnosis of changes in
renal function is very important.
RBP is a low molecular weight protein secreted by
hepatocytes and belongs to the retinol transporters. It is widely
present in body fluids such as blood, urine and cerebrospinal fluid
and plays an important role in the storage, transportation and
metabolism of vitamin A (13). In
clinic, RBP is often used as a marker of renal tubular injury. It
is simple to be detected, ready to be monitored and difficult to be
decomposed in urine. With strong stability, it is not affected by
the PH value of sphygmomanometer and other factors. Therefore, it
can provide effective supplement for the renal biopsy diagnosis
(14). Results of this study showed
that RBP level in children with hydronephrosis was significantly
higher than that in normal children, and it was significantly
decreased after treatment (P<0.05). This is because the
glomerular filtration in neonates with hydronephrosis decreases
while RBP continues to accumulate. When the renal tubular
reabsorption function is impaired, RBP cannot be reabsorbed and
degraded, thus it would be excreted with the urine. Then its
concentration in urine may increase. After effective treatment, the
renal function can recover effectively, RBP is absorbed and
degraded again, and therefore, the concentration is naturally
decreased in urine.
ALB is a highly sensitive middle-molecule protein
that is a marker of glomerular injury (15). Results of this study showed that the
level of ALB in children with hydronephrosis was significantly
higher than that in normal children, and the level of ALB was
significantly decreased after treatment (P<0.05). This is
because ALB is reabsorbed by proximal convoluted tubule in normal
people, while in neonates with hydronephrosis, the glomerular
filtration rate is decreased, so that the filtration of ALB far
exceeds the maximum absorption amount of renal tubular. As a
result, ALB enters into the urine and its content increases,
indicating that the early glomerular lesions occur where there is
some damage. Through the intervention treatment, ALB concentration
in the urine can be reduced.
AQP2 was first discovered in the early 1990s, it is
a member of the aquaporin family (16). AQP2 mainly exists in the cytoplasm of
principal cell of renal collecting duct and the cell membrane
towards the luminal side, which can increase the ability of water
reabsorption and urine concentration (17). It was indicated in the results of
this study that ALB level in children with hydronephrosis was
significantly lower than that in normal children and it was
significantly increased after treatment (P<0.05). This is due to
the fact that when hydronephrosis occurs in the neonates, the
concentration of AQP2 which carries on the trans-membrane
transportation of water is decreased, so that the water
permeability of collecting ductis also decreased. As a result, the
transmembrane transportation of water is hindered and the urine
concentration is decreased (18).
After effective treatment, the amount of AQP2 on the cell membrane
is increased, leading to an increase in water reabsorption.
MCP-1, a chemokine most closely associated with
pregnancy, plays a key role in maintaining mononuclear phagocyte
localization and the invasion of vascular endothelial trophoblasts
(19). Results of this study
revealed that the level of MCP-1 in maternal peripheral blood in
the observation group was significantly higher than that in the
control group (P<0.05). The reason may be that pregnant women
with overexpression of MCP-1 may have shallow implantation of
placenta, thus affecting the normal healthy growth of fetuses and
increasing the risk of hydronephrosis after birth of the fetuses.
In this study, Pearsons correlation coefficient analysis indicated
that VRBP and ALB had a positive correlation with MCP-1 while AQP2
and MCP-1 were negatively correlated (P<0.05). This may be
related to the fact that MCP-1 endows chemotaxis on a large number
of inflammatory cells and expand the inflammatory response. When
the level of MCP-1 content is changed, especially in the case of
its constantly increasing concentration, the trophoblast invasion
function is affected, and the microvessel density changes occur and
lead to placental atherosclerosis, thus causing a series of
pathological reactions such as elevated blood pressure (20). Substance exchange between mother and
baby is decreased and fetal hypoxia appears, which changes the
growth and development. This leads to kidney damage and
hydronephrosis, increased RBP and ALB levels and decreased AQP2
level, indicating that there is a positively and negatively
dependent relationship between the three indexes and MCP-1.
In conclusion, urinary RBP, ALB and AQP2 can be used
as diagnostic indicators for the degree of renal dysfunction, which
are closely related to the expression of MCP-1 in the peripheral
blood of pregnant women, and can be combined with imaging to guide
the diagnosis and treatment of neonatal hydronephrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ wrote the manuscript and collected the midstream
urine samples. YZ detected and analyzed neonatal urinary RBP and
ALB concentrations. LL performed ELISA. HW analyzed the changes of
RBP. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hongqi Hospital Affiliated to Mudanjiang Medical College
(Mudanjiang, China) and informed consents were signed by the
parents of the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masarwa I, Bahouth Z and Halachmi S: Giant
congenital hydronephrosis obstructing the gastrointestinal system
and the contralateral kidney in a new born. Urol Case Rep. 8:1–3.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyerholz DK and Hostetter SJ: Unilateral
perinephric pseudocyst secondary to hydronephrosis in a C57BL/6J
mouse. Vet Pathol. 42:496–498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Mashhadi A, Nevéus T, Stenberg A,
Karanikas B, Persson AE, Carlström M and Wåhlin N: Surgical
treatment reduces blood pressure in children with unilateral
congenital hydronephrosis. J Pediatr Urol. 11:91.e1–91.e6. 2015.
View Article : Google Scholar
|
|
4
|
Larson LM, Namaste SM, Williams AM,
Engle-Stone R, Addo OY, Suchdev PS, Wirth JP, Temple V, Serdula M
and Northrop-Clewes CA: Adjusting retinol-binding protein
concentrations for inflammation: Biomarkers Reflecting Inflammation
and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin
Nutr. 106 Suppl 1:390S–401S. 2017.PubMed/NCBI
|
|
5
|
Gong XZ, Zhou LF, Wang Q, Tang XC, Qian
YR, Wang YR, Lu L and Zhou JJ: Effect of Chuanhuang No. 1 recipe on
renal function and micro-inflammation in phase 3 chronic kidney
disease patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 35:137–141.
2015.(In Chinese).
|
|
6
|
Cui X, Zhang J, Li Y, Sun Y, Cao J, Zhao
M, Zhao Y, Zhao X, He Y and Han A: Effects of qili qiangxin capsule
on AQP2, V2R, and AT1R in rats with chronic heart failure. Evid
Based Complement Alternat Med. 2015:6394502015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satonaka H, Nagata D, Takahashi M, Kiyosue
A, Myojo M, Fujita D, Ishimitsu T, Nagano T, Nagai R and Hirata Y:
Involvement of P2Y12 receptor in vascular smooth muscle
inflammatory changes via MCP-1 upregulation and monocyte adhesion.
Am J Physiol Heart Circ Physiol. 308:H853–H861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santos AI, Violante L, Carmona S, Prata A,
Rodrigues Victor M, Santos JG, Araújo Sequeira J, Alves M, Papoila
AL and Piepsz A: Interobserver agreement on cortical tracer transit
in 99mTc-MAG3 renography applied to congenital hydronephrosis. Nucl
Med Commun. 38:124–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harding LJ, Malone PS and Wellesley DG:
Antenatal minimal hydronephrosis: Is its follow-up an unnecessary
cause of concern? Prenat Diagn. 19:701–705. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Q, Yang Y, Wang C, Hou Y and Chen H:
ATP5B and ETFB metabolic markers in children with congenital
hydronephrosis. Mol Med Rep. 14:5111–5115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vemulakonda VM, Wilcox DT, Torok MR, Hou
A, Campbell JB and Kempe A: Inter-rater reliability of postnatal
ultrasound interpretation in infants with congenital
hydronephrosis. Int Urol Nephrol. 47:1457–1461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie J, Zhou Y, Gao W, Li Z, Xu Z and Zhou
L: The relationship between amniotic fluid miRNAs and congenital
obstructive nephropathy. Am J Transl Res. 9:1754–1763.
2017.PubMed/NCBI
|
|
13
|
Zabetian-Targhi F, Mahmoudi MJ, Rezaei N
and Mahmoudi M: Retinol binding protein 4 in relation to diet,
inflammation, immunity, and cardiovascular diseases. Adv Nutr.
6:748–762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gursoy AY, Aynaoglu G, Caglar GS and
Soylemez F: Early second trimester retinol-binding protein-4 values
in cases with or without gestational diabetes mellitus risk
factors: A cross-sectional study. J Obstet Gynaecol Res. 41:55–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woo HD, Chiu WA, Jo S and Kim J: Benchmark
dose for urinary cadmium based on a marker of renal dysfunction: A
meta-analysis. PLoS One. 10:e01266802015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheema MU, Irsik DL, Wang Y, Miller-Little
W, Hyndman KA, Marks ES, Frøkiær J, Boesen EI and Norregaard R:
Estradiol regulates AQP2 expression in the collecting duct: A novel
inhibitory role for estrogen receptor α. Am J Physiol Renal
Physiol. 309:F305–F317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kristensen ML, Kierulf-Lassen C, Nielsen
PM, Krag S, Birn H, Nejsum LN and Nørregaard R: Remote ischemic
perconditioning attenuates ischemia/reperfusion-induced
downregulation of AQP2 in rat kidney. Physiol Rep. 4:42016.
View Article : Google Scholar
|
|
18
|
Arystarkhova E, Bouley R, Liu YB and
Sweadner KJ: Impaired AQP2 trafficking in Fxyd1 knockout mice: A
role for FXYD1 in regulated vesicular transport. PLoS One.
12:e01880062017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dekker Nitert M, Barrett HL, Denny KJ,
McIntyre HD and Callaway LK; BAMBINO group, . Exercise in pregnancy
does not alter gestational weight gain, MCP-1 or leptin in obese
women. Aust N Z J Obstet Gynaecol. 55:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams Waldorf KM, Singh N, Mohan AR, Young
RC, Ngo L, Das A, Tsai J, Bansal A, Paolella L, Herbert BR, et al:
Uterine overdistention induces preterm labor mediated by
inflammation: Observations in pregnant women and nonhuman primates.
Am J Obstet Gynecol. 213:830.e1–830.e19. 2015. View Article : Google Scholar
|