Introduction
Harlequin ichthyosis (HI) is a rare and severe
genetic skin disorder that occurs within the developing fetus, the
underlying mechanisms of which are not well understood (1). Infants born with HI typically exhibit
large, thick, plate-like scales covering the whole body associated
with severe ectropion, eclabium and flattened ears, that later
develop into a severe scaling erythroderma (2). The incidence of HI is relatively low,
occurring in ~1 in 200,000 births (3), however there is a lack of effective
treatment for the condition. The pathogenesis of HI was unknown
until a recent report by Akiyama et al (1), in which HI was found to be a lipid
metabolism disorder, caused by mutation in the adenosine
triphosphate (ATP)-binding cassette (ABC) transporter, ABCA12.
Historically, there has been a high early mortality rate in infants
with HI; however, improved neonatal management and the early
introduction of systemic retinoids may contribute to improved
prognosis. Mortality in these patients is most commonly caused by
sepsis, respiratory failure, or electrolyte imbalances (4). Early retinoid therapy and the
administration of antibiotics may improve the prognosis of HI.
Intrinsic factors may also be associated with the prognosis
(5). Homozygous mutations in ABCA12
resulting in truncation of the ABCA12 protein were previously
demonstrated to cause a severe HI phenotype, whereas heterozygous
ABCA12 missense mutations resulted in a less severe phenotype
(6). The current study suggests that
a multidisciplinary approach involving surgeons, ophthalmologists,
dermatologists, pediatricians, dieticians and psychologists is
required for improving treatment in the future. Although fetal HI
cases have been previously reported (3,4,7–9), two
fetuses presenting with HI from successive pregnancies in a single
woman are rare and have not previously been documented. The present
study reports a case of a woman who delivered two fetuses with HI
from two successive gestations at the ages of 35–36.
Case report
The present case is of a thirty-five year old woman
who became pregnant for the first time at the age of 35. The
patient had a regular period prior to the pregnancy. She was
admitted in Obstetrics Department of He Xian Memorial Hospital
(Guangdong, China) on 29th, July, 2007.
Treatment, including Progesterone 20 mg Q.D, human
chorionic gonadotropin (HCG) 2000 u Q.O.D, was offered during the
early stages of pregnancy (8 weeks) due to signs of threatened
miscarriage, such as vaginal bleeding and abdominal pain. A virus
test panel and toxoplasmosis test were conducted with negative
results, and no signs of hyperglycemia or hypertension were
observed during the pregnancy. The patient denied having a
consanguineous marriage or a history of contact with insecticide
and radioactive materials. As the disease is genetic, the
sister-in-law and mother of the patient were evaluated and had
unconfirmed ichthyotic-like lesions on their feet.
Sonography at twelve weeks of gestation found no
abnormalities in fetal development, though ultrasound performed at
the same time showed that the bipedal length of the fetus was
shorter than expected, compared with infants of the same age and
that the fetus had a relatively flattened nose. At thirty weeks of
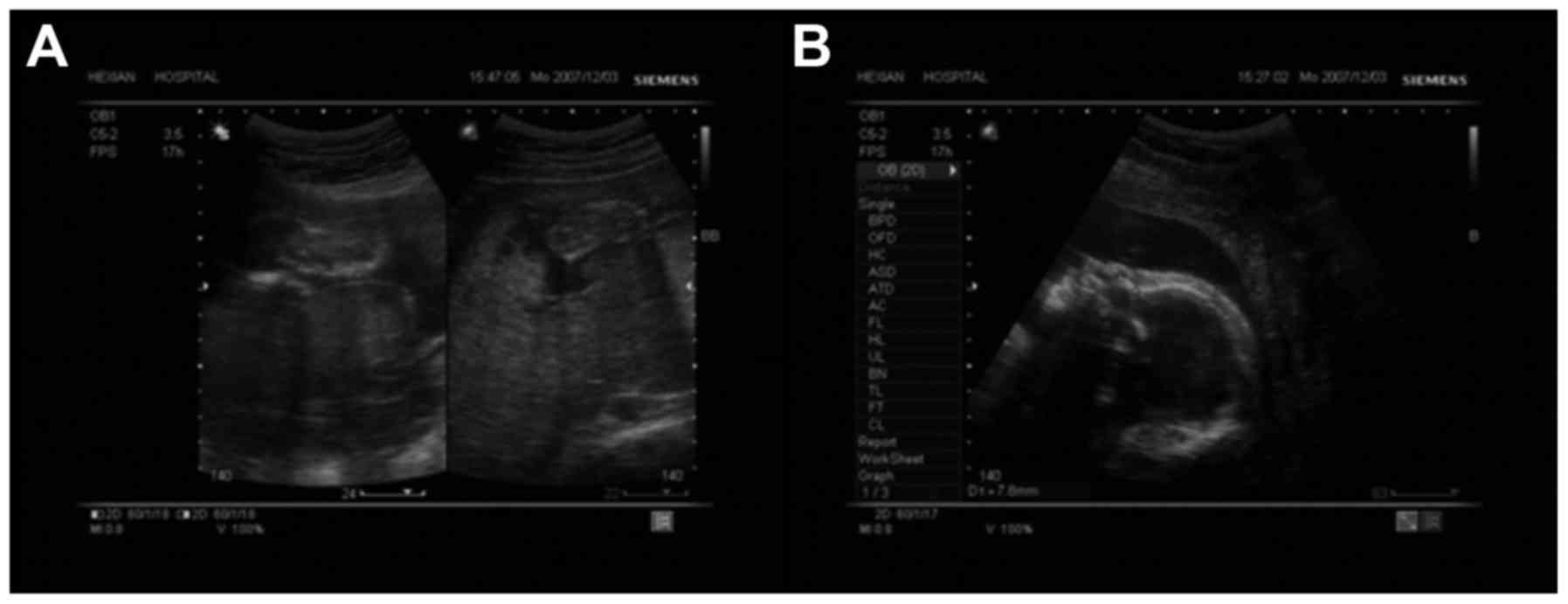
gestation, sonography identified thickened skin covering the body
of the fetus without obvious signs of edema (Fig. 1). In the thirty-first week of
gestation (16th January 2008), a live female infant was delivered
due to premature rupture of membranes. No abnormalities in the
amniotic fluid or placenta were observed during ultrasound
assessment. The neonate breathed in a normal and regular rhythm
(<60 breaths per minute) and had a normal heart rate of 145 bpm.
Scores of an Apgar test, performed as previously described
(10) (used to evaluate the health
status of neonates) were completed as follows: 9 at 1 min and 9 at
5 min, and the infant failed to suckle or bottle feed. The infant
had a body weight of 2.0 kg, a body length of 42 cm and a head
circumference of 31 cm, as a premature infant these measurements
are smaller than a normal full-term infant. The skin covering the
body exhibited hyperkeratosis and cracks that penetrated to
epidermis level were observed between thickened regions on the
head, neck, subaxillary, lower back rump areas and extremities.
Bilateral congenital upper eyelid eversion that appeared red in
colour was also observed. In addition, there was contraction and
deformation of the skin of the perioral area and lip eversion,
causing the mouth to be fixed in an O-shape. As a result, dentium
caverna was exposed. The infant had a flattened nose with no
obvious nasal bridge and the external ears appeared continuous with
the skin of the head. The infant also had external auditory canal
atresia with an accumulation of thick yellow substance. No hair had
grown and the middle and marginal head regions exhibited keratosis
and thickened skin with deep cracks. Furthermore, the infant had a
fixed flexion deformity of the extremities with a normal number of
fingers and toes. However, the fingers and toes were stubbed and
webbed with constriction of the skin. The infant had a normal vulvo
and anus. The infant succumbed two days after birth. The final
pathological diagnosis was severe fetus harlequin ichthyosis.
At the age of 36, the woman had a second pregnancy.
Similar to the first gestation, signs of threatened miscarriage
were observed during the early pregnancy stages, including vaginal
bleeding and abdominal pain. Furthermore, a urine culture performed
at week 10, was positive for E. coli. As a result, cystitis
was diagnosed and cefuroxime 3.5 g was administrated twice a day
for 5 days as an anti-bacterial treatment. The husband denied a
history of medication, sickness or contact with toxic substances in
the 3 months prior to the pregnancy. The woman underwent genetic
tests at sixteen weeks gestation for Down's syndrome, neural tube
defect and trisomy 18 syndrome, the results of which were all
negative. A karyotype analysis of the amniotic fluid at twenty
weeks of gestation exhibited no chromosomal abnormalities. However,
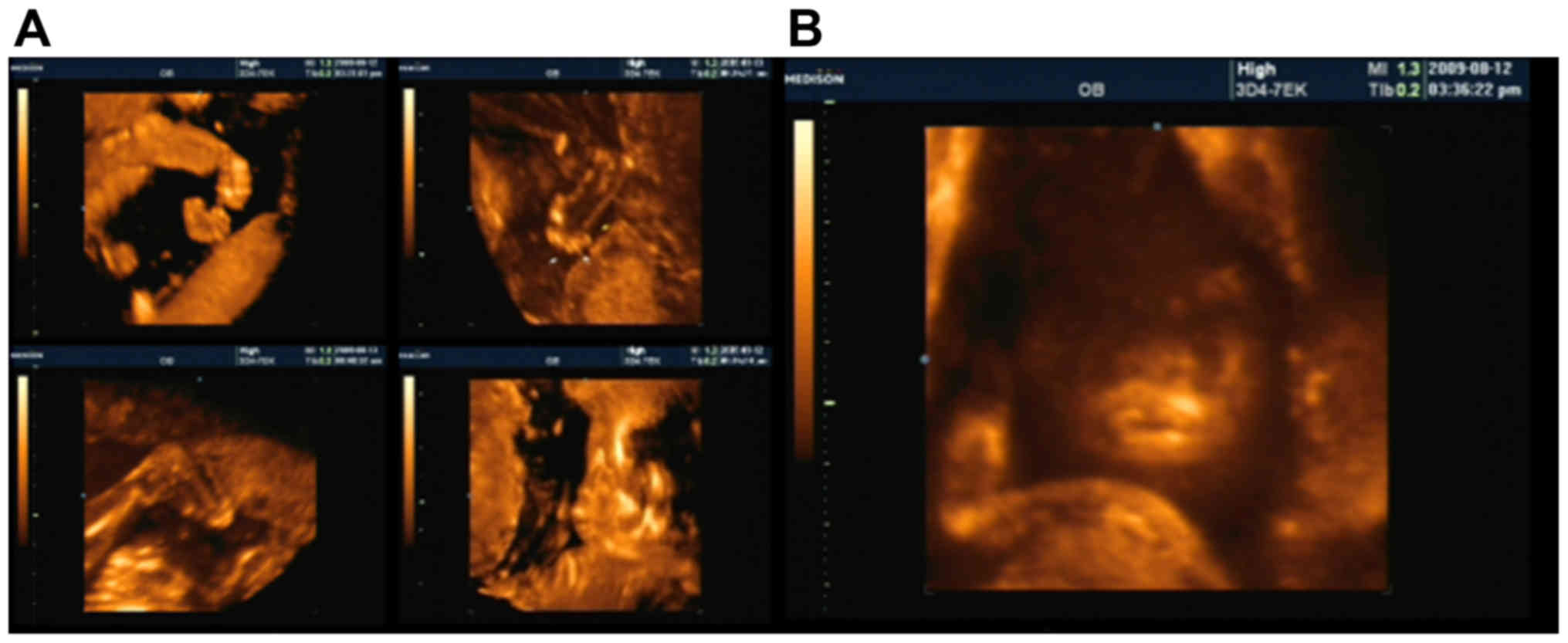
a three-dimensional sonography construction at twenty-four weeks of
gestation revealed bilaterally thickened soft tissue resonance in
the anterior region of the eyeballs, with the thickest region
measuring 3.6 mm. This is unusual, as the soft tissue resonance is
not identified in the anterior region of the eyeballs (11). The fetus also had fixed flexion
deformity of the extremities and thickened soft tissue in the lower
back region with deep cracks, which was measured by palpitation
(Fig. 2A). On the sagittal section,
the curve of the frontal bone and nasal tip appeared flattened and
dissipated. Two nostrils were observed, with a nasal wing width of
14 mm. The mouth was unable to fully close, leaving a gap of 30 mm
between the upper and lower lips, and the lips were markedly
thickened and everted (Fig. 2B). At
24 weeks, labor was induced and a stillborn infant was delivered.
In accordance with the sonography, the infant exhibited typical
characteristics of HI.
All subjects discussed in the present study provided
informed consent for the use of their data in the present
report.
Discussion
HI, otherwise known as keratosis diffusa
fetalis, is a distinct form of congenital ichthyosis, which
occurs due to mutation in the ABC transporters ABCA12, a cell
membrane transporter associated with lipid transportation (1,12).
Fetuses carrying this mutation have defective lipid secretion
within epidermal keratinocytes, leading to a loss of the skin lipid
barrier and development of harlequin-type ichthyosis (1). HI is a rare autosomal-recessive disease
with a high mortality rate for affected fetuses, although there is
limited epidemiological data currently (3). In the majority of cases, the fetus
succumbs during pregnancy, preventing the possibility of a lvive
birth (13). In a number of cases,
fetuses with HI have been born alive, though with severe
respiratory defects or feeding difficulties. Thus, the probability
of mortality for infants with HI is high, due to respiratory
failure, loss of fluid or skin infection (14). As such, prenatal diagnosis of fetal
HI is critical for appropriate perinatal and postnatal management
and to prepare parents for future pregnancies.
In the present study, two cases of successive fetal
HI were reported, a phenomenon that has not previously been
documented. In particular, the prenatal sonography images of each
HI fetus was analyzed, in which a discontinuity of the ultrasound
signal indicated a thickening of the skin across the body.
Furthermore, the fetuses exhibited thickened and convex lips. Fixed
flexion of the extremities, short digits and an open mouth were
also observed by two-dimensional sonography. Such characteristics
are typical of fetal HI (15).
Sonography is an important method of diagnosing of
HI, although it is unable to conclusively differentiate fetal HI
from other fetal diseases, including fetal macroglossia and
congenital tumor-like fetus angeioma (15). The development of the latter
conditions is detected by sonography as a marked enlargement of the
tongue, with extension of the tongue from the mouth, causing
opening of the mouth itself. Such features appear similar to those
of fetal HI; however, macroglossia is invariably associated with
genetic disorders, including trisomy 21 syndrome (Down's syndrome)
and Beckwith-Wiedemann syndrome. Therefore, genetic testing is a
critical method to differentiate HI from other macroglossia genetic
diseases (16). Another disease
requiring a differentiating diagnostic method is congenital
hemangioma. The thickened tongue in fetuses with congenital
hemangioma typically exhibits blood flow, as observed by color
Doppler imaging (17), therefore
differentiation is less challenging. However, HI also has distinct
sonographic characteristics that aid in its diagnosis, as the
discontinuous reflecting signal, indicating thickened skin, and
fixed flexion deformity of the extremities observed in sonography
of HI are absent in congenital hemangioma and fetal macroglossia
(17).
As HI is a severe congenital abnormality, fetuses
commonly experience respiratory defects and/or severe clinical
complications, including skin infections and sepsis (12). This leads to a high probability of
mortality for the few cases of live born infants. Therefore,
prenatal diagnosis of HI principally via sonographic techniques is
critical in managing the condition. Due to the recent establishment
of a genetic basis for HI, genetic screening for candidate gene
mutations associated with HI, such as ABCA12, may aid in prenatal
diagnosis. In turn, early diagnosis by genetic screening may reduce
the physical and mental impacts of fetal HI for parents and
relatives.
References
|
1
|
Akiyama M, Sugiyama-Nakagiri Y, Sakai K,
McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K,
Sasaki R, et al: Mutations in lipid transporter ABCA12 in harlequin
ichthyosis and functional recovery by corrective gene transfer. J
Clin Invest. 115:1777–1784. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Layton J: A review of harlequin
ichthyosis. Neonatal Netw. 24:17–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jilumudi UB: Harlequin ichthyosis: A
medico legal case report & review of literature with peculiar
findings in autopsy. J Forensic Leg Med. 19:352–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parikh K, Brar K, Glick JB, Flamm A and
Glick SA: A case report of fatal harlequin ichthyosis: Insights
into infectious and respiratory complications. JAAD Case Rep.
2:301–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Washio K, Sumi M, Nakata K, Fukunaga A,
Yamana K, Koda T, Morioka I, Nishigori C and Yamanishi K: Case of
harlequin ichthyosis with a favorable outcome: Early treatment and
novel, differentially expressed, alternatively spliced transcripts
of the ATP-binding cassette subfamily A member 12 gene. J Dermatol.
Mar 11–2017.(Epub ahead of print). View Article : Google Scholar
|
|
6
|
Akiyama M: ABCA12 mutations and autosomal
recessive congenital ichthyosis: A review of genotype/phenotype
correlations and of pathogenetic concepts. Hum Mutat. 31:1090–1096.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moreau S, Salame E, Goullet de Rugy M and
Delmas P: Harlequin fetus: A case report. Surg Radiol Anat.
21:215–216. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai SW, Chen YC, Tsai FJ, Wu MT, Peng CT,
Lin CC and Tsai CH: Harlequin ichthyosis: Report of one case.
Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 39:412–414.
1998.PubMed/NCBI
|
|
9
|
Singalavanija S, Sangtawesin V, Horpoapan
S and Ratrisawadi V: Harlequin baby: A case report. J Med Assoc
Thai. 81:365–370. 1998.PubMed/NCBI
|
|
10
|
Ahmed H and O'Toole EA: Recent advances in
the genetics and management of harlequin ichthyosis. Pediatr
Dermatol. 31:539–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernández-Martin A and González-Sarmiento
R: Recent advances in congenital ichthyoses. Curr Opin Pediatr.
27:473–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai S, Flatley C and Kumar S: Perinatal
risk factors for low and moderate five-minute Apgar scores at term.
Eur J Obstet Gynecol Reprod Biol. 210:251–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bongain A, Benoit B, Ejnes L, Lambert JC
and Gillet JY: Harlequin fetus: Three-dimensional sonographic
findings and new diagnostic approach. Ultrasound Obstet Gynecol.
20:82–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmuth M, Gruber R, Elias PM and Williams
ML: Ichthyosis update: Towards a function-driven model of
pathogenesis of the disorders of cornification and the role of
corneocyte proteins in these disorders. Adv Dermatol. 23:231–256.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wortsman X, Aranibar L and Morales C:
Postnatal 2- and 3-dimensional sonography of the skin and nail in
congenital autosomal recessive ichthyosis correlated with cutaneous
histologic findings. J Ultrasound Med. 30:1437–1439. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soejima H and Higashimoto K: Epigenetic
and genetic alterations of the imprinting disorder
Beckwith-Wiedemann syndrome and related disorders. J Hum Genet.
58:402–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vohra N, Rochelson B and Smith-Levitin M:
Three-dimensional sonographic findings in congenital (harlequin)
ichthyosis. J Ultrasound Med. 22:737–739. 2003. View Article : Google Scholar : PubMed/NCBI
|