Introduction
In recent years, the number of cases of diabetes
mellitus (DM) in Thailand has been rapidly increasing and the
prevalence of DM per 100,000 individuals increased from 1,032.50 in
2014 to 1,292.79 in 2016 (1).
Diabetes causes numerous severe complications, including pain and
sensory and movement disorders. These complications negatively
affect the quality of life of individuals and impose a
socio-economic burden on the society (2). Nerve compression syndrome, also known
as entrapment neuropathy, is a type of diabetic neuropathy commonly
identified among patients with diabetes (3). Pain and inflammation associated with
the nerve compression syndrome may be alleviated by local steroid
injection; however, this injection can cause adverse side effects
predominantly for bones and skin, including skin depigmentation,
skin atrophy and a decrease in bone mineral density (4,5). Surgery
to remove the compression is a more permanent treatment option;
nevertheless, full decompression and complete functional recovery
are not always attainable (6).
Therefore, alternative treatment methods, including administration
of medicinal herbs, may benefit both patients and the society.
DM frequently leads to long-term hyperglycemia,
which alters the neurochemical pathways in the nerves and increases
the susceptibility to compression (7). Numerous possible explanations for this
increased susceptibility to nerve compression have been proposed.
It has been hypothesized that excessive glucose is reduced to
sorbitol in the polyol pathway, and subsequently, sorbitol promotes
nerve swelling due to its low plasma permeability, and may promote
osmosis (8). The second possible
explanation is that hyperglycemia induces the production of
advanced glycation end-products (AGEs) (9). AGEs may impair the vascular supply to
the nerve tissues, including nerve fibers, Schwann cells and
endothelial cells of vasa nervosum, and consequently, promote the
destruction of the nerve fibers (10,11). The
third possible explanation is that glucose overload may induce an
increased activity of electron transport chain due to nerve axons
being rich in mitochondria (12).
The excessive of production free radicals may subsequently cause
oxidative damage to DNA, proteins and lipids (13). It has been previously demonstrated
that antioxidants serve an important role in the prevention of cell
damage against reactive oxygen species (14). Therefore, the identification of a
plant exhibiting both antioxidative and hypoglycemic properties
would be beneficial for the treatment of patients with diabetes.
Numerous studies have reported that plants containing antioxidants
could promote nerve recovery following a crush injury (15–17).
Azadirachta indica A. Juss. var.
siamensis Valeton (A. indica), also known as Sadao in
Thailand, is a member of the Meliaceae family (18). The edible parts of A. indica
are young leaves and young flowers (19). A. indica is considered a safe
medicinal plant and previous studies indicated that it exhibits
antioxidative (20),
anti-inflammatory (21),
antiulcerative (22), and anticancer
effects (23). Furthermore, A.
indica has been demonstrated to exhibit antidiabetic properties
(24). Specifically, the current
study hypothesizes that A. indica has the potential to
reduce oxidative stress in a diabetic condition and potentially to
promote nerve recovery. However, the effect of A. indica
flower extract on the functional recovery of sciatic nerve crush
injury in diabetic rats remains to be elucidated.
Materials and methods
Plant material and extract
preparation
A. indica flowers were collected from
Maetumboonyong, Mueang, Phayao, Thailand during December 2015 to
January 2016. The voucher specimen (no. 003805) was deposited in
the herbarium of the Faculty of Biology, Naresuan University,
Mueang, Phitsanulok, Thailand. The sample was authenticated by Dr
Pranee Nangngam, the Faculty of Biology, Naresuan University,
Mueang, Phitsanulok, Thailand. The flowers were washed and blended
with distilled water (plant to water ratio, 1:3). A powdered
extract was obtained after this solution was filtered and
freeze-dried. The powder extract was stored at −20°C until further
use.
Animals
A total of 52 eight-week old male Wistar rats
(weight, 200–220 g) were obtained from the Nomura Siam
International Co., Ltd. (Bangkok, Thailand). During the
experimental procedure, 3 rats were housed per cage and maintained
under a 12-h light/dark cycle with food and water ad
libitum. The rats were randomly divided into seven groups
including: i) Group 1, untreated rats not subjected to surgery
(n=8); ii) group 2, rat models of DM with sham surgery administered
distilled water (n=5); iii) group 3, rat models of DM with sciatic
nerve crush surgery administered distilled water (n=8); iv) group
4, rat models of DM with sciatic nerve crush surgery administered
A. indica extract at a dose of 250 mg/kg animal body weight
(BW; n=8); v) group 5, rat models of DM with sciatic nerve crush
surgery administered A. indica extract at a dose of 500
mg/kg (n=8); vi) group 6, rat models of DM with sciatic nerve crush
surgery administered A. indica extract at a dose of 750
mg/kg (n=8); and vii) group 7, rat models of DM with sciatic nerve
crush surgery administered vitamin C (Chem-Supply, Gillman, South
Australia, Australia) at a dose of 100 mg/kg BW (n=7). The rats in
groups 2–7 were administered either vehicle (distilled water),
A. indica flower extract or vitamin C once daily for 21
consecutive days following the sham operation or sciatic nerve
crush surgery. At the end of the experiment, rats were euthanized
with an overdose of sodium pentobarbital (intraperitoneal injection
at dose of 100 mg/kg animal BW). Subsequently, left sciatic nerves
were collected and half of each nerve was used to determine the
malondialdehyde (MDA) levels and activity of superoxide dismutase
(SOD). The other half of each nerve was used for histological
analysis (axon density).
DM induction and sciatic nerve crush injury. DM was
induced by a single intraperitoneal injection of streptozotocin
(STZ) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at dose of 55
mg/kg animal BW. The fasting blood glucose level was assessed 72 h
after the injection. The rats with plasma glucose levels of ≥250
mg/dl were regarded as diabetic rats and were enrolled for further
surgery. A sciatic nerve crush surgery was performed following
anesthesia using intraperitoneal injection of sodium pentobarbital
(50 mg/kg animal BW). An incision was made on the posterior left
thigh and the sciatic nerve was carefully exposed at a point
immediately distal to the gluteus maximus muscle. The nerve was
crushed at a distance of 5 mm from the sciatic notch (midthigh) for
30 sec using hemostatic forceps. The sham rats received the same
surgery without a nerve crushing injury. To minimize the
variability in the experimental process, each surgery was performed
by the same person.
Walking track analysis
As previously described by Bain et al
(25), the current study analyzed
the sciatic function index (SFI) from the walking track analysis of
the rats. Prior to the surgery, the rats were given five trials of
walking on conventional paper. Recovery was assessed on day 3, 6,
9, 12, 15, 18 and 21 after surgery using one walking trial.
Briefly, the rats were held by the chest and their hind feet were
pressed down onto a stamp pad soaked with water-soluble black ink.
The rats subsequently walked down a confined walkway (width, 21 cm;
length, 120 cm) with a dark shelter at the end of the corridor to
encourage movement such that inked footprints were left on the
paper. The following measurements were subsequently taken: i) The
distance from the heel to the third toe defined as the print length
(PL); ii) the distance from the first to fifth toe defined as toe
spread (TS); and iii) the distance from the second to the fourth
toe defined as the intermediate toe spread (ITS). The SFI value of
each group was calculated using the Bain formula (25): SFI = (−38.3 × PLF) + (109.5 × TSF) +
(13.3 × ITF) - 8.8, where PLF (print length factor) = (experimental
PL-normal PL)/normal PL; TSF (toe spread factor) = (experimental
TS-normal TS)/normal TS; ITF (intermediate toe spread factor) =
(experimental ITS - normal ITS)/normal ITS. An SFI value close to 0
indicates normal function. In the present study however, SFI values
between (−10)-(10) were considered
to be within the normal range (16).
Foot withdrawal reflex
A modified version of the hot plate test was used to
determine the functional sensory recovery of the rats on day 3, 6,
9, 12, 15, 18 and 21 after surgery (26). Prior to each testing session, the
rats were acclimatized to the non-heated plate for 10 min.
Subsequently, the time it took the rat to remove its paw from a hot
plate [50°C, 5°C lower than in the original study by Menéndez et
al (26)] was recorded and
defined as the paw withdrawal latency (PWL). No rat was allowed to
remain in contact with the heated plate for >20 sec. PWL was
measured with the left paw three times and the mean was
subsequently calculated. The time interval between sessions was 5
min.
Rotarod test. A rotarod apparatus model Acceler
Rota-Rod (Jones & Roberts) for rats 7750 (Comerio, VA, Italy)
was used to determine sensorimotor coordination of the rats. The
apparatus was provided by the Division of Neuroanatomy, Department
of Anatomy Histology and Embryology, Medical University Innsbruck,
Innsbruck, Austria. All rats underwent a 7-day training program.
Rats were trained to walk against the motion of the rotating drum
at a constant speed of 10 rpm for a maximum of 1 min. If a rat fell
off the rod during a training trial, it was put back on the
rotating drum. Each rat received four training trials per day with
an interval trial time of 10 min. Although the accelerating mode
was more suitable to evaluate the sensorimotor coordination of rats
(15,27), the poor motor function of the rats of
the present study following surgery prevented the use of that
specific mode. A previous study by Abada et al (28) demonstrated that the best rotarod
performance of rats is achieved when the speed used on the test day
is faster than that on the training days. This is due to rats
having already learnt how to climb using a constant speed (10 rpm).
Therefore, the speed of the rotarod was set to a constant speed of
12 rpm for the test. The amount of time that the rat remained on
the rotating drum was recorded for three trials and the values were
averaged to obtain a falling latency. The maximum allowed duration
was 30 sec. The apparatus was sprayed with a 70% ethanol solution
between sessions and wiped with tissue paper.
Homogenate preparation. The left sciatic nerve was
isolated from each rat at the end of the experiment. Homogenate was
prepared in 1 ml of 0.1 M phosphate buffer (pH 7.4). The obtained
nerve homogenate was adjusted to 10% w/v and centrifuged at 10,000
× g and 4°C for 1 h using a microcentrifuge (1-15PK; Sigma
Laborzentrifugen GmbH; Osterode am Harz, Germany). The supernatant
was harvested and processed for the estimation of biochemical
parameters.
Determination of MDA levels. As previously described
by Ohkawa et al (29), the
level of MDA was assessed using thiobarbituric acid reactive
substances. Briefly, 100 µl of nerve homogenate solution, 100 µl of
8.1% (w/v) sodium dodecyl sulfate, 750 µl of 20% (v/v) acetic acid
(pH 3.5) and 750 µl of 0.8% (w/v) thiobarbituric acid (all from
Sigma-Aldrich; Merck KGaA) were mixed thoroughly and boiled in a
water bath at 95°C for 1 h then cooled immediately under running
tap water. After cooling, 500 µl of chilled water and 2.5 ml of a
mixture of butanol and pyridine (15:1 v/v; each Sigma-Aldrich;
Merck KGaA) were added into each sample and mixed well. The
solution was subsequently centrifuged at 800 × g and 4°C for 20
min. The upper pink layer was harvested and the absorbance was
measured at a wavelength of 532 nm using a spectrophotometer
(GENESYS™ 20; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
1,1,3,3-Tetramethoxypropane (Sigma-Aldrich; Merck KGaA) was used as
a standard MDA. The level of MDA was expressed in nmol/mg
protein.
Determination of SOD activity. SOD activity was
determined via a nitroblue tetrazolium reduction assay, as
previously described (30). The
xanthine-xanthine oxidase system was used as a superoxide
generator. Briefly, a reaction mixture containing 20 µl of nerve
homogenate solution and 200 µl of reaction mixture consisting of 57
mM phosphate buffer solution (KH2PO4), 0.1 mM EDTA, 10 mM
cytochrome C solution, 50 µM of xanthine solution and 20 µl of
xanthine oxidase solution (0.90 mU/ml) was prepared at 25°C for 5
min. All aforementioned reagents were from Sigma-Aldrich; Merck
KGaA. The absorbance was measured at a wavelength of 415 nm with an
ultraviolet-spectrophotometer (Biochrom 4060; Pharmacia Biotech,
Uppsala, Sweden). A system devoid of xanthine oxidase served as the
control and the three parallel experiments were conducted. SOD
activity was expressed in U/mg protein.
Histological assessment. The left sciatic nerve was
collected from each rat at the end of the experiment. Half of each
nerve was fixed in 4% paraformaldehyde at 4°C for 48 h and
processed for paraffin embedding. Sections (5-µm-thick) were cut
using a microtome and stained with hematoxylin for 8 min and eosin
for 1 min (H&E) at room temperature. A light microscope was
subsequently used to examine the morphology of the axon for each
nerve. The axon density was determined using the ImageJ software
version 1.51 (National Institutes of Health, Bethesda, MD, USA) at
a ×20 magnification.
Statistical analysis. All data are presented as the
mean ± standard error of the mean. Statistical analysis was
performed using one-way analysis of variance followed by LSD post
hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of A. indica flower extract on
motor functional recovery
The effect of A. indica flower extract on SFI
is presented in Table I. According
to a previous study, normal walking pattern SFI can range between
−10 and 10 (16). Before the crush
injury, rats in all groups exhibited normal SFI. After the crush
injury, rats underwent functional testing every 3 days. The rats
with DM subjected to a sciatic nerve crush injury had a
significantly lower SFI compared with rats in the control group
(P<0.001) and rats with DM subjected to sham surgery
(P<0.001). These differences indicated abnormal walking patterns
among rats with nerve crush injury. Compared with the
vehicle-treated group subjected to crush injury, administration of
A. indica flower extract at a high dose (750 mg/kg animal
BW) significantly increased SFI on postoperative day 18 and 21
(P<0.05 and P<0.00l, respectively). Administration of A.
indica flower extract at the medium dose (500 mg/kg animal BW)
significantly increased SFI on day 21 compared with the rats
subjected to crush injury and treated with vehicle (P<0.01).
Furthermore, administration of vitamin C significantly increased
SFI on days 18 and 21 compared with the rats with crush injury
treated with vehicle (P<0.05 and P<0.0l, respectively).
However, the SFI values on day 21 were between −20 and −40, and,
therefore, the SFI did not return to normal following treatment
with A. indica flower extract or vitamin C.
 | Table I.Effect of A. indica flower
extract on SFI. |
Table I.
Effect of A. indica flower
extract on SFI.
|
| SFI |
|---|
|
|
|
|---|
| Group | Baseline | Day 3 | Day 18 | Day 21 |
|---|
| Control | −4.24±2.67 | −8.68±1.44 | −8.75±1.25 | −8.30±1.21 |
| DM + sham +
vehicle | −8.00±1.09 | −11.45±1.64 | −3.69±3.82 | −5.00±1.75 |
| DM + crush +
vehicle | −4.98±1.70 |
−69.42±1.57c,d |
−55.40±4.86c,d |
−43.70±2.41c |
| DM + crush + A.
indica 250 | −7.90±1.59 |
−68.07±2.94c,d |
−46.69±2.32c,d |
−39.02±1.88c |
| DM + crush + A.
indica 500 | −4.15±1.75 |
−69.46±2.43c,d |
−40.93±7.03b,d |
−30.40±5.20c,d,f |
| DM + crush + A.
indica 750 | −4.26±1.33 |
−66.66±0.98c,d |
−37.33±7.47b,d,e |
−22.44±2.32b,d,g |
| DM + crush + Vit
C | −4.88±1.87 |
−68.33±2.25c,d |
−31.47±11.86a,d,e |
−28.97±4.09c,d,f |
Effect of A. indica flower extract on
sensory functional recovery
A hot plate test was used to assess the sensory
function of rats and analyze PWL. The longer PWL indicates poor
sensory function (31). The results
of functional recovery, as determined by walking track analysis,
foot withdrawal reflex and rotarod tests, were recorded on days 3,
18 and 21 following sciatic nerve surgery (sham or crush; Tables I–III) as these results revealed significant
changes. The effect of A. indica flower extract on PWL is
presented in Table II. The results
revealed that on day 3, rats with sciatic nerve crush injury
treated with vehicle exhibited a significantly longer PWL compared
with the control group (P<0.001) and the vehicle group with sham
operation (P<0.001). Moreover, the sciatic nerve crush injury
groups exhibited a significantly longer PWL, as compared with the
control group (P<0.001) and vehicle group with sham operation on
day 3 (P<0.001). This indicated that a sciatic nerve crush
significantly impaired sensory function.
 | Table III.Effect of A. indica flower
extract on falling latency. |
Table III.
Effect of A. indica flower
extract on falling latency.
|
| Falling latency,
sec |
|---|
|
|
|
|---|
| Group | Baseline | Day 3 | Day 18 | Day 21 |
|---|
| Control | 16.00±1.75 | 18.14±1.38 | 24.00±2.54 | 24.62±1.60 |
| DM + sham +
vehicle | 17.22±1.57 | 16.89±1.47 | 22.28±3.08 | 23.72±4.27 |
| DM + crush +
vehicle | 16.58±1.98 |
10.92±0.42c,f | 17.29±1.79 |
17.75±1.34a |
| DM + crush + A.
indica 250 | 16.46±1.54 |
12.54±0.87c,e | 21.75±3.78 | 22.58±2.09 |
| DM + crush + A.
indica 500 | 17.29±1.54 |
12.10±0.80c,e | 22.19±2.08 | 23.05±2.43 |
| DM + crush + A.
indica 750 | 17.17±2.44 |
12.96±1.31b,d | 22.67±1.59 | 23.67±2.34 |
| DM + crush + Vit
C | 16.19±0.71 |
12.97±1.02b,d | 20.67±3.75 | 21.62±2.25 |
 | Table II.Effect of A. indica flower
extract on paw withdrawal latency. |
Table II.
Effect of A. indica flower
extract on paw withdrawal latency.
|
| Paw withdrawal
latency, sec |
|---|
|
|
|
|---|
| Group | Baseline | Day 3 | Day 18 | Day 21 |
|---|
| Control | 4.19±0.32 | 4.38±0.20 | 4.10±0.23 | 4.48±0.27 |
| DM + sham +
vehicle | 3.94±0.22 | 4.61±0.23 | 4.44±0.40 | 4.11±0.41 |
| DM + crush +
vehicle | 3.96±0.24 |
15.54±0.98b,d |
10.21±1.27a,c |
9.83±1.39a,c |
| DM + crush + A.
indica 250 | 4.04±0.23 |
15.75±0.66b,d |
10.00±1.92a,c |
9.50±2.06a,c |
| DM + crush + A.
indica 500 | 4.14±0.53 |
15.29±0.99b,d | 7.43±2.12 | 7.24±1.59 |
| DM + crush + A.
indica 750 | 4.00±0.24 |
14.92±1.29b,d |
6.58±0.66e |
5.75±0.56e |
| DM + crush + Vit
C | 4.14±0.26 |
15.10±1.40b,d | 7.38±1.00 |
6.19±0.62e |
Compared with the vehicle group with
crush injury, the A. indica
flower extract administered at a high dose (750
mg/kg animal BW) resulted in a significantly shorter PWL on
postoperative day 18 and 21 (both P<0.05) while the vitamin C
group exhibited a significantly shorter PWL only on postoperative
day 21 (P<0.05).
Effect of A. indica flower extract on
sensorimotor coordination. The effect of A. indica flower
extract on the mean falling latency is presented in Table III. Prior to the crush injury, rats
were subjected to rotarod testing to determine the baseline falling
latency. There was no significant difference in falling latency
between the groups at baseline. The group of diabetic rats that
received vehicle treatment and sham operation exhibited a decreased
mean falling latency compared with the control group on day 3
following the crush injury; however, the difference was not
statistically significant. Furthermore, the sciatic nerve crush
injury groups exhibited a significantly shorter falling latency
compared with the control group and vehicle group with sham
operation on day 3 (all P<0.05). This suggests that a sciatic
nerve crush significantly impaired sensorimotor coordination. In
addition, compared with the vehicle group, the groups administered
with A. indica flower extract at all doses exhibited an
increase in the average falling latency. Nonetheless, there was no
significant difference among the groups.
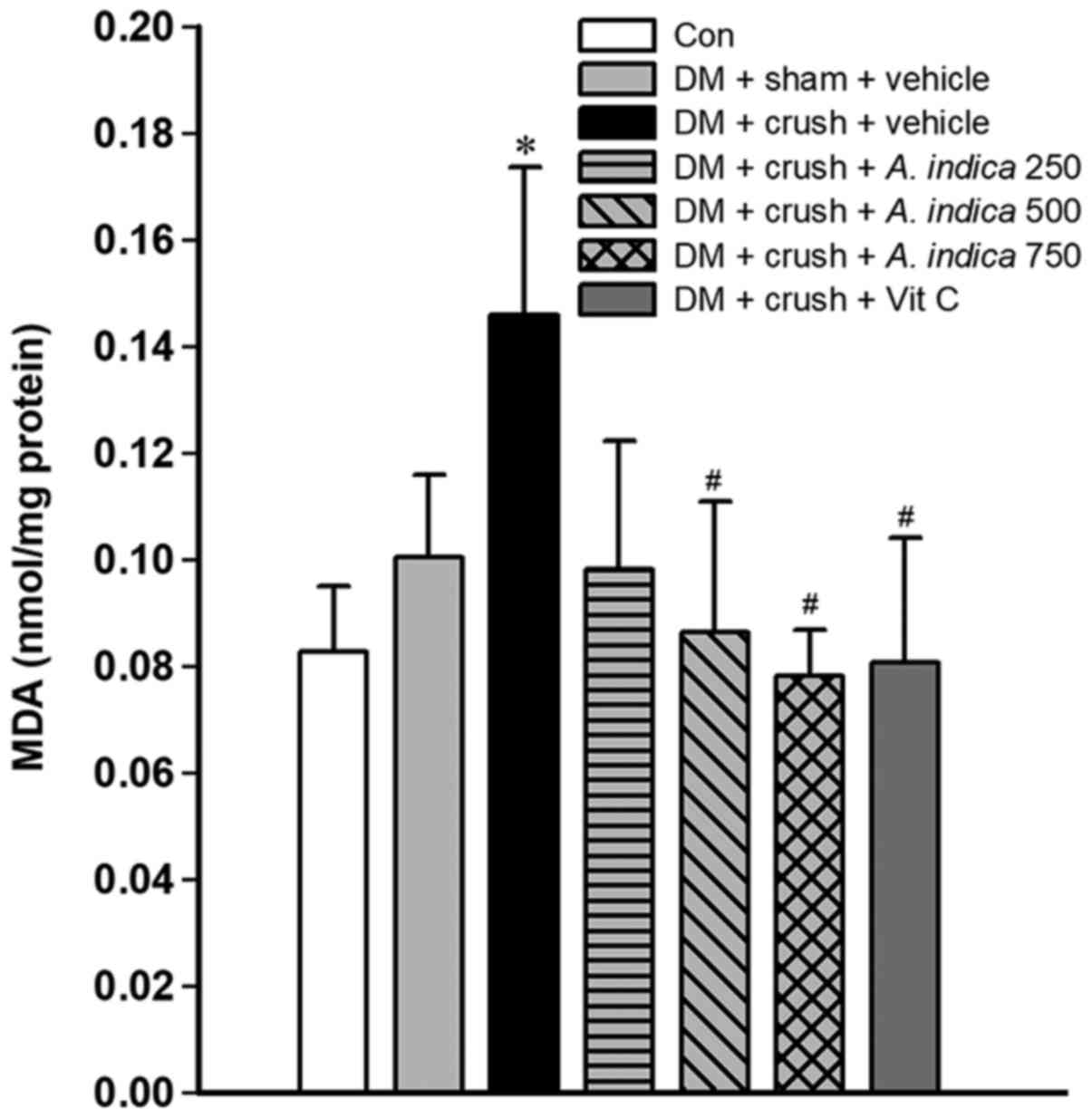
Effect of A. indica flower extract on MDA
levels. Oxidative stress is one of the most important mechanisms
promoting nerve injury and delaying nerve recovery (32). The present study investigated the
effect of the A. indica flower extract on MDA levels, an
indicator of lipid peroxidation in injured sciatic nerve tissue
(33) (Fig. 1). The diabetic rats in the sham
surgery group exhibited increased MDA levels compared with the rats
in the control group, however this difference was not statistically
significant. Compared with the control group, sciatic nerve crush
injury increased the expression of MDA in the group with vehicle
treatment and crush injury (P<0.05). Compared with the vehicle
group with crush injury, the levels of MDA were significantly lower
in rats treated with medium and high doses (500 and 750 mg/kg
animal BW) of A. indica flower extract and group treated
with vitamin C (all P<0.05).
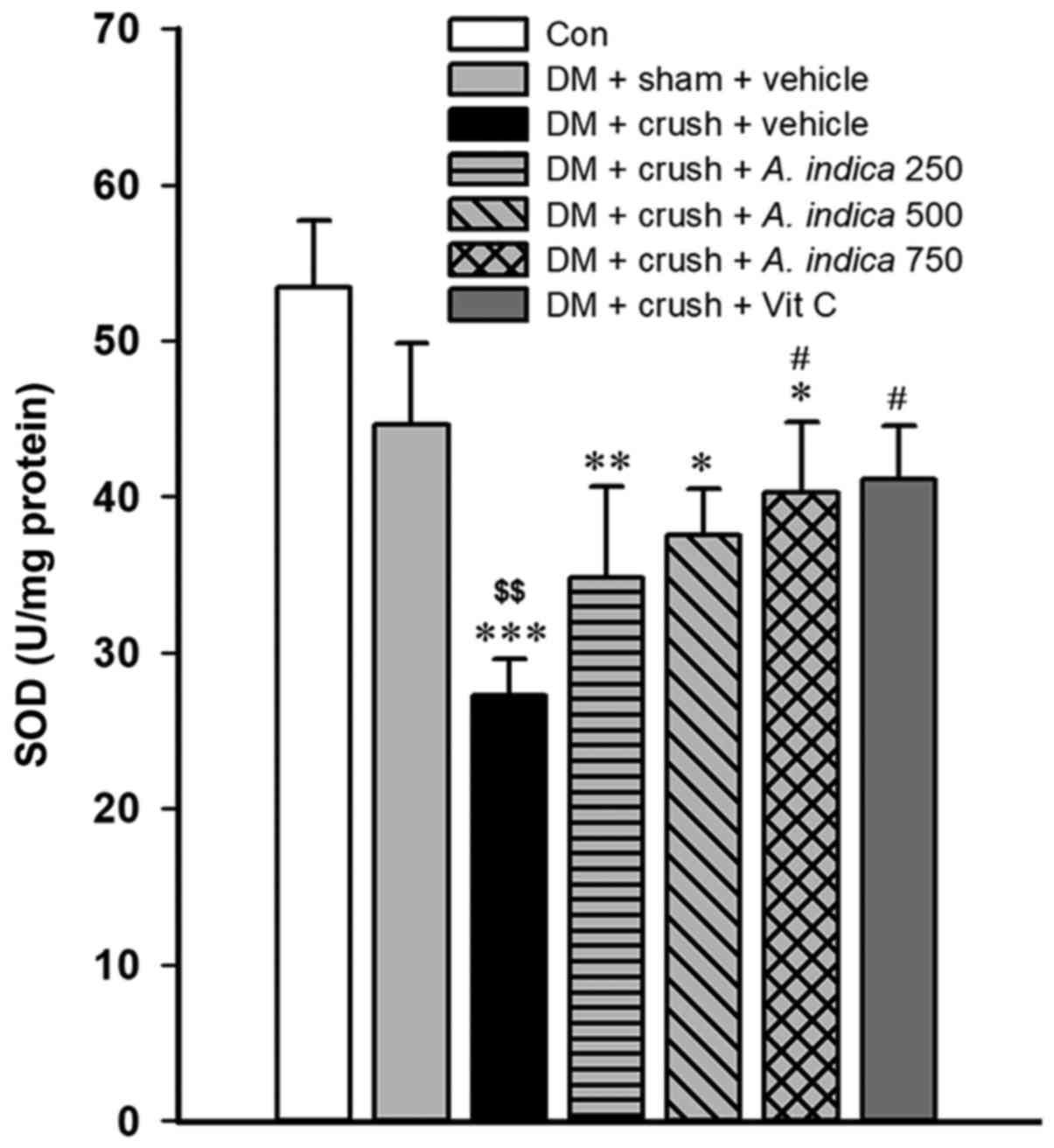
Effect of A. indica flower extract on SOD
activity. SOD serves an important role in the free radical
scavenging enzyme system (34).
Therefore, the activity of SOD in sciatic nerve homogenate was
determined. The results revealed that the rat models of DM treated
with vehicle and subjected to the sciatic nerve crush injury
demonstrated a significantly lower activity of SOD compared with
both the control group and vehicle group subjected to sham
operation (P<0.001 and P<0.01, respectively; Fig. 2). Rats with DM subjected to sham
surgery and treated with vehicle exhibited a lower SOD activity
compared with the rats in the control group, however, this
difference was not statistically significant. Compared with the
vehicle group with crush injury, the rats treated with a high dose
(750 mg/kg animal BW) of A. indica flower extract and rats
treated with vitamin C exhibited a significantly increased SOD
activity (both P<0.05).
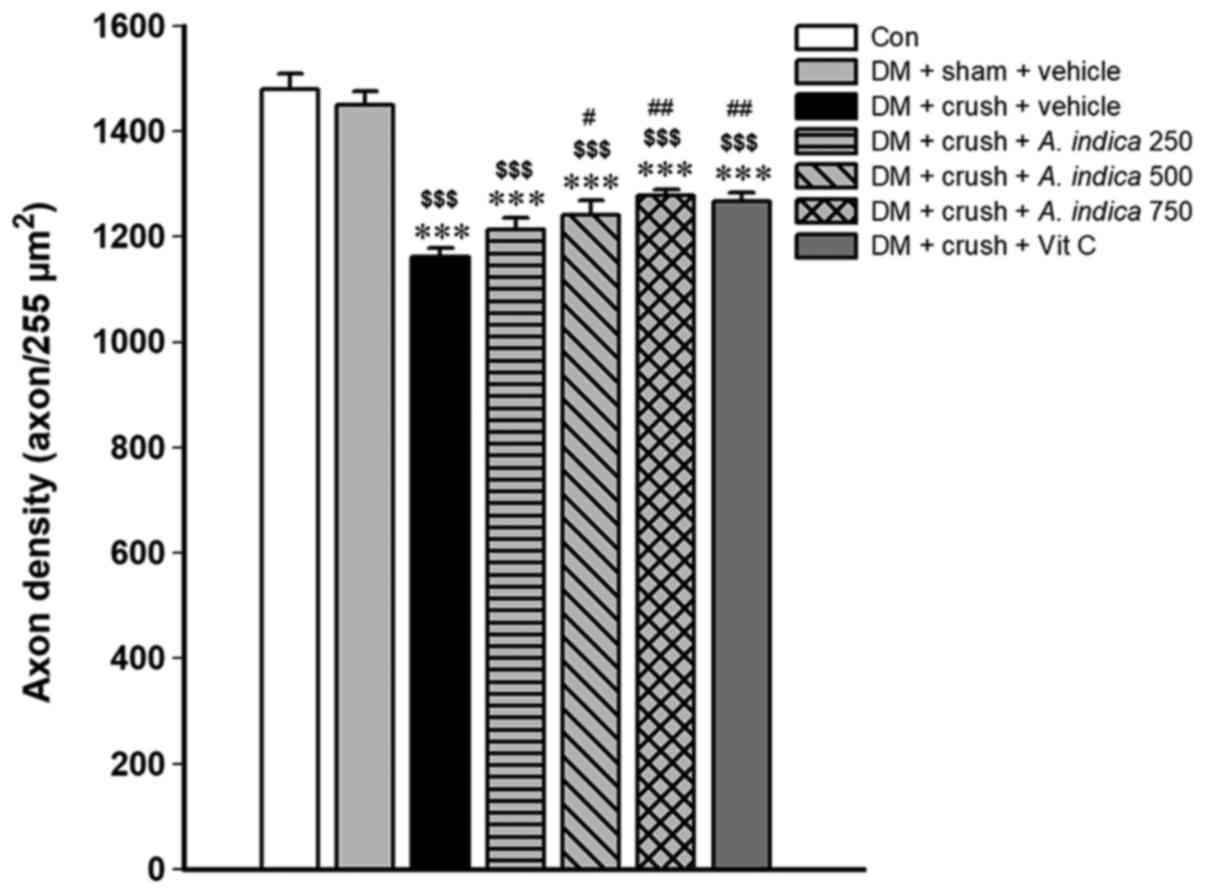
Effect of A. indica flower extract on axon
density. The functional recovery following a nerve injury is
associated with the morphological change of the nerve. Therefore,
the axon density of the nerve was evaluated to assess nerve
regeneration. The effect of A. indica flower extract on axon
density is presented in Fig. 3. The
results indicated that the axon density of rat models of DM
subjected to sham surgery was not significantly different compared
with the control group. Compared with both the control group and
rat models of DM subjected to sham surgery, the rats in the group
subjected to a sciatic nerve crush injury exhibited a significantly
lower axon density (P<0.001 and P<0.01, respectively).
Treatments with A. indica flower extract at medium and high
doses (500 and 750 mg/kg animal BW) and vitamin C resulted in
increased axon density compared with the vehicle group with crush
injury (P<0.05, P<0.01 and P<0.01, respectively).
Discussion
The present study demonstrated that treatment with
A. indica flower extract for 21 days enhanced the functional
recovery of a sciatic nerve crush injury in rats with STZ-induced
DM. Additionally, the improvement of both sensory and motor
functions was observed on day 18 after the crush injury. However,
the full recovery of both motor and sensory function was not
observed within 21 days.
Walking track analysis was used to determine the
motor function of the rats. All rats exhibited normal motor
function as manifested by normal SFI values (between −10 to 10)
prior to the nerve injury. Compared with the control group, all
groups of rats subjected to the crush injury exhibited lower SFI
values indicating poor motor function. These values gradually
improved over the 21-day period; however, recovery to pre-crush
values was not observed in any group. The treatment with A.
indica flower extract at a dose of 750 mg/kg animal BW improved
SFI value at day 18 and 21 after the crush injury. However, the
recovery was not complete as manifested by SFI values outside the
normal range. In normal untreated rats, the full functional
recovery of the sciatic nerve was observed at ~14–21 days after the
crush injury (35,36). Hadlock et al (37) reported that on postoperative day 34,
the recovery in normal untreated rats reached a plateau level and
the pre-crush level was not reached. However, another study
demonstrated that on day 21 after the crush injury, complete
functional motor recovery was not observed in crocin treated rats
(17). It has been suggested that
nerve recovery of rats with DM was slower compared with normal
healthy rats due to the pathophysiology of DM (38). A previous study demonstrated that
hyperglycemia significantly lowered the rate of nerve recovery in
rats with DM induced by STZ (6).
Furthermore, one study indicated that in rats with DM induced by
STZ, the motor functional recovery was not observed on
postoperative day 21 (39), and
these results were supported by the present study. However,
different methods used to induce the nerve crush injury in previous
studies (17,35–37,39),
including the use of hemostatic forceps and Jewelers forceps, may
have resulted in different magnitudes of the injury. Mazzer et
al (40) further suggested that
the different crushing loads can produce a different degree of
axonotmesis injury. Therefore, the difference in motor functional
recovery may be associated with the experimental conditions and the
pathophysiological response of peripheral nerves to the level of
different crushing loads and different methods to induce nerve
injury.
Entrapment neuropathies are highly prevalent among
patients with DM. Muscle weakness and poor sensation lead to the
impairment of motor and sensory functions. The present study used a
crush injury to induce sciatic nerve compression as this method
induced mechanical pressure and ischemic injury to the nerve
(41). The results of the present
study support the conclusions of George et al (42). Specifically, the present study
demonstrated that the crush injury significantly decreased the
thermal sensitivity and induced hypoalgesia of the operated hind
paw of rats with DM on day 3 after the crush injury (42). All groups of rats subjected to the
crush injury exhibited hypoalgesia to the heat stimulus; however,
this impairment improved over time. The recovery was not complete
compared with normal rats; however, the rats with DM subjected to
the crush injury that were administered a high dose (750 mg/kg
animal BW) of A. indica flower extract exhibited a
significant improvement in recovery from nociception deficit on
postoperative day 18 and 21. Pavić et al (43) reported similar results with regard to
sensory function 4 weeks following the crush. Furthermore, rats
with DM subjected to sham operation presented the same response to
the heat stimulus as observed among normal rats; however rats with
DM and sciatic nerve crush injury presented hypoalgesia. This
observation indicates that injection with STZ cannot induce sensory
neuropathy in rats within 3 weeks following the injection. By
contrast, a previous study demonstrated that diabetic ddY mice
exhibited hypoalgesia when exposed to a mechanical nociceptor
stimulus 5 weeks following induction of DM (44). In addition, mechanical
hyposensitivity and the decreased motor nerve conduction velocity
were developed at 6 and 8 weeks following induction of diabetes in
another study (45). Prior research
has demonstrated different responses to mechanical and thermal
stimuli in rats with DM (46). The
present study evaluated the response to the heat stimulus; however,
the response to the mechanical stimulus remains to be elucidated.
Therefore, future studies should investigate the response of rats
with DM and sciatic nerve crush injury to different mechanical
stimuli.
A rotarod test has been commonly used to test
neurological deficits in experimental rodents, especially motor
coordination and balance (15,47). For
example, a study by Mucimapura et al (15) used a rotarod test to determine
sensorimotor coordination. The results of the present study
indicated that rats with DM and sciatic nerve injury demonstrated a
shorter falling latency compared with the normal rats.
Administration with A. indica flower extract failed to
improve sensorimotor coordination. Therefore, the functional
recovery of sensory and motor functions demonstrated by the results
of the walking track analysis and the heat withdrawal reflex test
did not correspond with the results of the rotarod test. There may
be other factors, including cardiopulmonary endurance, that
influence the sensorimotor coordination of the rats. Jolivalt et
al (48) suggested that the
performance in the accelerating rotarod test was not affected by
thermal hypoalgesia. However, the present study did not use the
accelerating mode of the rotarod, as rats exhibited poor leg
strength following crush injury. Furthermore, the large size of the
rats meant that they fitted tightly in the rotarod compartment and
previous studies have demonstrated a significant correlation
between high body weight and low rotarod performance (49,50). In
addition, the low height of the rod from the base was also reported
to influence the mice to jump off the apparatus (51). Therefore, these factors may have
influenced the results.
Several studies demonstrated that individuals with
DM tend to exhibit increased levels of reactive oxygen species and
oxidative stress markers, with an accompanying decrease in
antioxidant levels (52–54). In addition, previous studies
indicated that oxidative stress serves a role in nerve destruction
following injury (17,55). Furthermore, it has been demonstrated
that antioxidants serve important roles in nerve recovery (56,57). MDA
is an oxidative stress marker (58),
while SOD is an antioxidative enzyme that detoxifies superoxide
anions (59). Reduced activity of
SOD increases the generation of reactive oxygen species leading to
the damage of cellular organelles and increased lipid peroxidation
(13). These consequences of
oxidative stress can further aggravate the complications associated
with DM (60). A previous study
indicated that SOD was inactivated by glycation (61). Another study reported that SOD
activity was decreased in STZ-induced rat models of DM (62). An injection with STZ was reported to
increase MDA levels and decrease the activity of SOD in rats with
DM (63,64). The results of the present study
indicated that the rats with DM and sham surgery exhibited
increased MDA levels and decreased SOD activity compared with rats
in the control group. However, this difference was not
statistically significant. This result indicates that the STZ
injection may not be the only factor affecting the oxidative stress
in the sciatic nerve tissue of rats with DM. Previous studies
demonstrated that crush injury induced an increase in the oxidative
stress of rats with STZ-induced DM (65,66). The
present results demonstrated that rats with DM subjected to sciatic
nerve crush injury and treated with vehicle demonstrated elevated
levels of MDA. This result indicated that sciatic nerve crush
injury, used as a model of nerve compression syndrome, induced
oxidative stress in rats with DM. Administration of the A.
indica flower extract significantly decreased MDA levels and
increased the activity of SOD in the sciatic nerve homogenate. The
same observations were made among rats treated with vitamin C.
Therefore, these results suggested that A. indica flower
extract may improve functional recovery by decreasing the oxidative
stress and increasing antioxidative enzyme activity. However, the
alteration of other parameters involved in the oxidative stress
pathway should be further investigated.
Crush injury has previously been used to model
axonotmesis injury (67). This model
is widely used to evaluate peripheral nerve regeneration (34,68).
Crush injury damages the axons covered with the supporting
structures, including endoneurium, perineurium and epineurium,
resulting in secondary Wallerian degeneration distal to the site of
injury (69). A previous study
demonstrated that crushing load significantly reduced the density
of nerve fibers (39). The results
of the present study indicated that there was no statistically
significant difference in myelinated axon density between rats with
DM subjected to sham operation and rats in the control group. By
contrast, 21 days following the crush injury, the crush group
exhibited significantly lower myelinated axon density compared with
the vehicle group with sham injury. Administration of A.
indica flower extract at dose of 500 and 750 mg/kg animal BW
induced a significant increase in axon density compared with the
vehicle treatment. Tong et al (70) reported that functional recovery
following sciatic nerve crush injury was associated with
remyelination rather than axon regeneration. Therefore, the A.
indica flower extract may accelerate the remyelination process
leading to an increased density of nerve fibers. Nevertheless,
electrophysiological, morphological and histological studies should
be performed to assess peripheral nerve recovery fully. However,
due to the limited availability of laboratory equipment, the
present study only observed histological alterations using H&E
staining. Therefore, future studies with the aforementioned
additional techniques should examine nerve recovery in further
detail.
The results of the present study indicated that high
and medium doses of flower extract were more effective compared
with low doses in certain experiments. Treatment with the A.
indica flower extract at medium and high doses (500 and 750
mg/kg animal BW, respectively) significantly improved motor and
sensory functional recovery. This may be due to the higher
concentration of active ingredients in the higher doses of the
extract. The primary constituent identified in the flower extract
was quercetin, as reported previously by Duangjai et al
(71). This result was congruent
with a previous study, which indicated that rutin and quercetin
flavonoids were the primary antioxidative components in A.
indica flowers (19). Quercetin
was reported to significantly improve the functional recovery of
the sciatic nerve following crush injury in C57BL/6J mice (57) and STZ-induced rat models of DM
(39). Quercetin facilitates nerve
recovery by increasing the density of myelinated axons (72). Therefore, quercetin may be the active
component which promoted the functional recovery of the sciatic
nerve following the crush injury induced in the present study.
In conclusion, the results of the present study
indicated that A. indica flower extract accelerated the
functional recovery of sciatic nerve injury in rat models of DM. It
may be hypothesized that the recovery was associated with the
antioxidative effects induced by the flower extract. These results
may be beneficial for the application of alternative treatments for
patients with DM and nerve injury. Nevertheless, additional studies
are required to specify the time required to attain complete
functional recovery and clarify the underlying mechanisms of
treatment with the flower extract.
Acknowledgements
The authors would like to thank Dr Pennapa
Chonpathompikunlert from the Expert Centre of Innovative Health
Food, Thailand Institute of Scientific and Technological Research,
Khlong Luang, Pathum Thani for additional chemical support. Thanks
are also given to Mr. Theera Chanmanee and Mr. Panumat Deiam from
the Division of Anatomy, School of Medical Sciences, University of
Phayao for histological assessment. Finally, the authors wish to
commend the Division of Research Administration and Educational
Quality Assurance, University of Phayao for managing the grant and
the University of Phayao for providing research facilities.
Funding
The present study was financially supported by the
University of Phayao, Mueang, Phayao, Thailand (grant no.
RD59052)
Availability of data and materials
Datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NS conceived and designed the present study and
analyzed the data. NS, RK, AD, TH and ST performed the experiments.
NS and TH prepared the manuscript. All authors read and approved
the final manuscript prior to submission.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of the Laboratory Animal Research Center, University of
Phayao (approval no. 58 01 04 0035).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DM
|
diabetes mellitus
|
|
STZ
|
streptozotocin
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
SFI
|
sciatic function index
|
|
PWL
|
paw withdrawal latency
|
References
|
1
|
Strategy and Planning Division, Ministry
of Public Health, Thailand: Summary report of illness.
2016.http://bps.moph.go.th/new_bps/sites/default/files/ill_2559_full_edit.pdfDecember
25–2017
|
|
2
|
Schofield D, Cunich MM, Shrestha RN,
Passey ME, Veerman L, Callander EJ, Kelly SJ and Tanton R: The
economic impact of diabetes through lost labour force participation
on individuals and government: Evidence from a microsimulation
model. BMC Public Health. 14:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinik A, Mehrabyan A, Colen L and Boulton
A: Focal entrapment neuropathies in diabetes. Diabetes Care.
27:1783–1788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HJ and Park SH: Median nerve injuries
caused by carpal tunnel injections. Korean J Pain. 27:112–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kerezoudis P, Rinaldo L, Alvi MA, Hunt CL,
Qu W, Maus TP and Bydon M: The effect of epidural steroid
injections on bone mineral density and vertebral fracture risk: A
systematic review and critical appraisal of current literature.
Pain Med. 19:569–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang PH, Yang CC, Su WR, Wu PT, Cheng SC
and Jou IM: Effects of decompression on behavioral,
electrophysiologic, and histomorphologic recovery in a chronic
sciatic nerve compression model of streptozotocin-induced diabetic
rats. J Pain Res. 10:643–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sessions J and Nickerson DS: Biologic
basis of nerve decompression surgery for focal entrapments in
diabetic peripheral neuropathy. J Diabetes Sci Technol. 8:412–418.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomlinson DR and Gardiner NJ: Glucose
neurotoxicity. Nat Rev Neurosci. 9:36–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Negre-Salvayre A, Salvayre R, Augé N,
Pamplona R and Portero-Otín M: Hyperglycemia and glycation in
diabetic complications. Antioxid Redox Signal. 11:3071–3109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen RJ, Lin CC and Ju MS: In situ
biomechanical properties of normal and diabetic nerves: An
efficient quasi-linear viscoelastic approach. J Biomech.
43:1118–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu F, Sano Y, Haruki H and Kanda T:
Advanced glycation end-products induce basement membrane
hypertrophy in endoneurial microvessels and disrupt the blood-nerve
barrier by stimulating the release of TGF-β and vascular
endothelial growth factor (VEGF) by pericytes. Diabetologia.
54:1517–1526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S,
Avishai A, Komuro H and Trapp BD: Myelination and axonal electrical
activity modulate the distribution and motility of mitochondria at
CNS nodes of Ranvier. J Neurosci. 31:7249–7258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rains JL and Jain SK: Oxidative stress,
insulin signaling, and diabetes. Free Radic Biol Med. 50:567–575.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mucimapura S, Wattanathorn J, Thongrong S,
Chaisiwamongkol K and Sripanidkulchai B: Morus alba enhanced
functional recovery after sciatic nerve crush injury. Am J Agric
Biol Sci. 5:294–300. 2010. View Article : Google Scholar
|
|
16
|
Thipkaew C, Wattanathorn J and Muchimapura
S: The beneficial effect of asiaticoside on experimental neuropathy
in diabetic rats. Am J Appl Sci. 9:1782–1788. 2012. View Article : Google Scholar
|
|
17
|
Tamaddonfard E, Farshid AA, Ahmadian E and
Hamidhoseyni A: Crocin enhanced functional recovery after sciatic
nerve crush injury in rats. Iran J Basic Med Sci. 16:83–90.
2013.PubMed/NCBI
|
|
18
|
Subapriya R and Nagini S: Medicinal
properties of neem leaves: A review. Curr Med Chem Anticancer
Agents. 5:149–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaisawangwong W and Gritsanapan W:
Quality assessment and scavenging activity of Siamese neem flower
extract. Nat Prod Res. 27:394–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sultana B, Anwar F and Przybylski R:
Antioxidant activity of phenolic components present in barks of
Azadirachta indica, Terminalia arjuna, Acacia nilotica, and
Eugenia jambolana Lam. trees. Food Chem. 104:1106–1114.
2007. View Article : Google Scholar
|
|
21
|
Jagadeesh K, Srinivas K and Revankar SP:
Anti inflammatory effect of Azadirachta Indica (Neem) in
albino rats: An experimental study. IOSR J Pharm. 4:34–38.
2014.
|
|
22
|
Bhajoni PS, Meshram GG and Lahkar M:
Evaluation of the antiulcer activity of the leaves of
Azadirachta indica: An experimental study. Integr Med Int.
3:10–16. 2016. View Article : Google Scholar
|
|
23
|
Hao F, Kumar S, Yadav N and Chandra D:
Neem components as potential agents for cancer prevention and
treatment. Biochim Biophys Acta. 1846:247–257. 2014.PubMed/NCBI
|
|
24
|
Bhat M, Kothiwale SK, Tirmale AR, Bhargava
SY and Joshi BN: Antidiabetic properties of Azadirachta
indica and Bougainvillea spectabilis: In vivo studies in
murine diabetes model. Evid Based Complement Alternat Med.
2011:5616252011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bain JR, Mackinnon SE and Hunter DA:
Functional evaluation of complete sciatic, peroneal, and posterior
tibial nerve lesions in the rat. Plast Reconstr Surg. 83:129–138.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menéndez L, Lastra A, Hidalgo A and
Baamonde A: Unilateral hot plate test: A simple and sensitive
method for detecting central and peripheral hyperalgesia in mice. J
Neurosci Methods. 113:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bohlen M, Cameron A, Metten P, Crabbe JC
and Wahlsten D: Calibration of rotational acceleration for the
rotarod test of rodent motor coordination. J Neurosci Methods.
178:10–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abada YS, Nguyen HP, Schreiber R and
Ellenbroek B: Assessment of motor function, sensory motor gating
and recognition memory in a novel BACHD transgenic rat model for
huntington disease. PLoS One. 8:e685842013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wattanathorn J, Thiraphatthanavong P,
Muchimapura S, Thukhammee W, Lertrat K and Suriharn B: The combined
extract of Zingiber officinale and Zea mays (Purple
Color) improves neuropathy, oxidative stress, and axon density in
streptozotocin induced diabetic rats. Evid Based Complement
Alternat Med. 2015:3010292015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lennertz RC, Medler KA, Bain JL, Wright DE
and Stucky CL: Impaired sensory nerve function and axon morphology
in mice with diabetic neuropathy. J Neurophysiol. 106:905–914.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vincent AM, Russell JW, Low P and Feldman
EL: Oxidative stress in the pathogenesis of diabetic neuropathy.
Endocr Rev. 25:612–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aziza SAH, El-Haggar M, Abo-Zaid OA,
Hassanien MR and El-Shawarby R: Biomarkers of oxidative stress of
sciatic nerve tissues in experimental diabetic neuropathy. J Med
Sci. 14:12–20. 2014. View Article : Google Scholar
|
|
34
|
Senoglu M, Nacitarhan V, Kurutas EB,
Senoglu N, Altun I, Atli Y and Ozbag D: Intraperitoneal
Alpha-Lipoic Acid to prevent neural damage after crush injury to
the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj.
4:222009.PubMed/NCBI
|
|
35
|
Ramli D, Aziz I, Mohamad M, Abdulahi D and
Sanusi J: The changes in rats with sciatic nerve crush injury
supplemented with evening primrose oil: Behavioural, Morphologic,
and Morphometric Analysis. Evid Based Complement Alternat Med.
2017:34764072017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xavier AM, Serafim KG, Higashi DT, Vanat
N, Flaiban KK, Siqueira CP, Venâncio EJ and Ramos SP: Simvastatin
improves morphological and functional recovery of sciatic nerve
injury in Wistar rats. Injury. 43:284–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hadlock TA, Heaton J, Cheney M and
Mackinnon SE: Functional recovery after facial and sciatic nerve
crush injury in the rat. Arch Facial Plast Surg. 7:17–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kennedy JM and Zochodne DW: Impaired
peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv
Syst. 10:144–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thipkaew C, Wattanathorn J and Muchimapura
S: Electrospun nanofibers loaded with quercetin promote the
recovery of focal entrapment neuropathy in a rat model of
streptozotocin-induced diabetes. BioMed Res Int. 2017:20174932017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mazzer PY, Barbieri CH, Mazzer N and Fazan
VP: Morphologic and morphometric evaluation of experimental acute
crush injuries of the sciatic nerve of rats. J Neurosci Methods.
173:249–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao Y, Weng C and Wang X: Changes in nerve
microcirculation following peripheral nerve compression. Neural
Regen Res. 8:1041–1047. 2013.PubMed/NCBI
|
|
42
|
George A, Buehl A and Sommer C: Wallerian
degeneration after crush injury of rat sciatic nerve increases
endo- and epineurial tumor necrosis factor-alpha protein. Neurosci
Lett. 372:215–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pavić R, Pavić ML, Tvrdeić A, Tot OK and
Heffer M: Rat sciatic nerve crush injury and recovery tracked by
plantar test and immunohistochemistry analysis. Coll Antropol. 35
Suppl 1:93–100. 2011.
|
|
44
|
Murakami T, Iwanaga T, Ogawa Y, Fujita Y,
Sato E, Yoshitomi H, Sunada Y and Nakamura A: Development of
sensory neuropathy in streptozotocin-induced diabetic mice. Brain
Behav. 3:35–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kambiz S, van Neck JW, Cosgun SG, van
Velzen MH, Janssen JA, Avazverdi N, Hovius SE and Walbeehm ET: An
early diagnostic tool for diabetic peripheral neuropathy in rats.
PLoS One. 10:e01268922015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brussee V, Guo G, Dong Y, Cheng C,
Martinez JA, Smith D, Glazner GW, Fernyhough P and Zochodne DW:
Distal degenerative sensory neuropathy in a long-term type 2
diabetes rat model. Diabetes. 57:1664–1673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carter RJ, Morton J and Dunnett SB: Motor
coordination and balance in rodents. Curr Protoc Neurosci. Aug
1–2001.(Epub ahead of print). doi: 10.1002/0471142301.ns0812s15.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jolivalt CG, Rodriguez M, Wahren J and
Calcutt NA: Efficacy of a long-acting C-peptide analogue against
peripheral neuropathy in streptozotocin-diabetic mice. Diabetes
Obes Metab. 17:781–788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McFadyen MP, Kusek G, Bolivar VJ and
Flaherty L: Differences among eight inbred strains of mice in motor
ability and motor learning on a rotorod. Genes Brain Behav.
2:214–219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cook MN, Bolivar VJ, McFadyen MP and
Flaherty L: Behavioral differences among 129 substrains:
Implications for knockout and transgenic mice. Behav Neurosci.
116:600–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deacon RM: Measuring motor coordination in
mice. J Vis Exp. 75:e26092013.
|
|
52
|
Sharma R, Buras E, Terashima T, Serrano F,
Massaad CA, Hu L, Bitner B, Inoue T, Chan L and Pautler RG:
Hyperglycemia induces oxidative stress and impairs axonal transport
rates in mice. PLoS One. 5:e134632010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Murali R, Karthikeyan A and Saravanan R:
Protective effects of D-limonene on lipid peroxidation and
antioxidant enzymes in streptozotocin-induced diabetic rats. Basic
Clin Pharmacol Toxicol. 112:175–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Babizhayev MA, Strokov IA, Nosikov VV,
Savel'yeva EL, Sitnikov VF, Yegorov YE and Lankin VZ: The role of
oxidative stress in diabetic neuropathy: Generation of free radical
species in the glycation reaction and gene polymorphisms encoding
antioxidant enzymes to genetic susceptibility to diabetic
neuropathy in population of type I diabetic patients. Cell Biochem
Biophys. 71:1425–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Serarslan Y, Bal R, Altug ME, Kontaş T,
Keleş ON, Unal D and Unal B: Effects of trimetazidine on crush
injury of the sciatic nerve in rats: A biochemical and
stereological study. Brain Res. 1247:11–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lanza C, Raimondo S, Vergani L, Catena N,
Sénès F, Tos P and Geuna S: Expression of antioxidant molecules
after peripheral nerve injury and regeneration. J Neurosci Res.
90:842–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen MM, Qin J, Chen SJ, Yao LM, Zhang LY,
Yin ZQ and Liao H: Quercetin promotes motor and sensory function
recovery following sciatic nerve-crush injury in C57BL/6J mice. J
Nutr Biochem. 46:57–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yoshida Y, Umeno A and Shichiri M: Lipid
peroxidation biomarkers for evaluating oxidative stress and
assessing antioxidant capacity in vivo. J Clin Biochem Nutr.
52:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fridovich I: Superoxide radical and
superoxide dismutases. Annu Rev Biochem. 64:97–112. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jabeen R, Saleemuddin M, Petersen J and
Mohammad A: Inactivation and modification of superoxide dismutase
by glyoxal: Prevention by antibodies. Biochimie. 89:311–318. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sadri H, Goodarzi MT, Salemi Z and Seifi
M: Antioxidant effects of Biochanin A in streptozotocin induced
diabetic rats. Braz Arch Biol Technol. 60:e17160742017. View Article : Google Scholar
|
|
63
|
Aizzat O, Yap SW, Sopiah H, Madiha MM,
Hazreen M, Shailah A, Wan JW, Nur SA, Srijit D, Musalmah M, et al:
Modulation of oxidative stress by Chlorella vulgaris in
streptozotocin (STZ) induced diabetic Sprague-Dawley rats. Adv Med
Sci. 55:281–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sellamuthu PS, Arulselvan P, Kamalraj S,
Fakurazi S and Kandasamy M: Protective nature of mangiferin on
oxidative stress and antioxidant status in tissues of
streptozotocin-induced diabetic rats. ISRN Pharmacol.
2013:7501092013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Farshid AA and Tamaddonfard E:
Histopathological and behavioral evaluations of the effects of
crocin, safranal and insulin on diabetic peripheral neuropathy in
rats. Avicenna J Phytomed. 5:469–478. 2015.PubMed/NCBI
|
|
66
|
Nasiry D, Khalatbary AR, Ahmadvand H,
Amiri Talebpour F and Akbari E: Protective effects of methanolic
extract of Juglans regia L. leaf on streptozotocin-induced
diabetic peripheral neuropathy in rats. BMC Complement Altern Med.
17:4762017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Magill CK, Tong A, Kawamura D, Hayashi A,
Hunter DA, Parsadanian A, Mackinnon SE and Myckatyn TM:
Reinnervation of the tibialis anterior following sciatic nerve
crush injury: A confocal microscopic study in transgenic mice. Exp
Neurol. 207:64–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gao S, Fei M, Cheng C, Yu X, Chen M, Shi
S, Qin J, Guo Z and Shen A: Spatiotemporal expression of PSD-95 and
nNOS after rat sciatic nerve injury. Neurochem Res. 33:1090–1100.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sunderland S: A classification of
peripheral nerve injuries producing loss of function. Brain.
74:491–516. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tong LL, Ding YQ, Jing HB, Li XY and Qi
JG: Differential motor and sensory functional recovery in male but
not female adult rats is associated with remyelination rather than
axon regeneration after sciatic nerve crush. Neuroreport.
26:429–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Duangjai A, Nuengchamnong N, Lee LH, Goh
BH, Saokaew S and Suphrom N: Characterisation of an extract and
fractions of Azadirachta indica flower on cholesterol
lowering property and intestinal motility. Nat Prod Res. 19:1–4.
2017. View Article : Google Scholar
|
|
72
|
Wang W, Huang CY, Tsai FJ, Tsai CC, Yao CH
and Chen YS: Growth-promoting effects of quercetin on peripheral
nerves in rats. Int J Artif Organs. 34:1095–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|