Introduction
Olfactory ensheathing cells (OECs) have an important
role as seed cells in central nervous system (CNS) repair (1–3). OECs
secrete a variety of neurotrophic factors, including nerve growth
factors, as well as extracellular matrix molecules, to improve the
microenvironment after nerve injury, reduce glial scarring and
prevent neuronal apoptosis (4).
Previous studies using animal models have also reported that OEC
transplantation significantly promoted nerve fiber regeneration and
partial functional recovery (5).
However, at present, the efficacy of cell
transplants in repairing the CNS is not ideal as inflammatory
substances and glial scar formation has been demonstrated to
inhibit the secretion of extracellular matrix components and cell
scaffolds (6). In recent years,
promising tissue-engineered biomaterial scaffolds have been
demonstrated to have the capacity to improve the repair of CNS
injury. The ideal biomaterials should have biodegradability,
biocompatibility, excellent mechanical properties and flexibility
(7,8). Natural biodegradable materials include
chitosan, polypeptide hydrogel, poly L-lactic acid (PLL),
chitosan/polyethylene stents and non-cellular scaffolds, which have
been considered for the repair of nerve damage (9–11). Our
group has been working on natural Tussah silk fibroin (TSF)
biomedical materials for numerous years (12). TSF is a natural protein with a
polymer structure. Its amino acid composition is characterized by a
large number of arginine-glycine-aspartic acid (RGD) tripeptide
sequences. This RGD tripeptide sequence structure is known to
facilitate cell adhesion. TSF also has good biocompatibility, with
no toxic effects on cells and organisms, and a low likelihood of
inflammatory reaction or immune rejection (13–16).
In recent years, the use of this electrospinning
technology has led to great progress in the preparation of
tissue-engineered materials. The diameter, structure distribution,
molecular conformation and crystallinity of TSF may be controlled
using electrospinning technology (17). When the fiber diameter is decreased
from microns to nanometers, the material properties are improved
accordingly.
A previous study by our group indicated that TSF
scaffolds prepared by electrostatic spinning have good
biocompatibility with OECs and may support their growth and
migration (12). However, the study
also suggested that the diameter of nanoscale fibers affects the
biological effects of OECs. Previous studies have demonstrated that
the diameter of electrospun nanofibers has a significant effect on
cell behavior (18–20). In one study, fibers with a diameter
of 400 and 1,200 nm were prepared for evaluating their effect on
cell behavior (21), and the results
suggested that the diameter of the scaffold had a marked impact on
neural cell behavior, with a significant increase in the
cell-spreading area observed on 400-nm silk fibroin (SF). A
significantly enhanced migration efficiency of astrocytes grown on
SF scaffolds was demonstrated, which highlighted the effects of SF
nanofibers to enhance cell migration. As it was indicated that the
diameter of SF nanofibers may be an important factor in the
construction of biomimetic microenvironments suitable for cell
growth, the present study aimed to assess the ideal diameter of TSF
as a tissue engineering scaffold material.
Materials and methods
Materials and sample preparation
TSF nanofibers were prepared as previously reported
(22). In brief, TSF fibers were
boiled in 0.5% (w/w) Na2CO3 aqueous solution
for 30 min twice for degumming, followed by thorough rinsing with
sufficient deionized water to remove the glue-like sericin. The
degummed TSF fibers were dissolved in 16 M lithium thiocyanate
solution and the mixture was heated with hotplate stirrers at 50°C
for 1 h, followed by dialysis with cellulose tubular membranes in
distilled water for 3 days (molecular weight cut-off, 8,000–14,000
kDa). A TSF film was prepared by spreading the TSF solution onto a
polyethylene plastic board and leaving it to dry at room
temperature. The electrospinning solution was prepared by
dissolving the TSF film in hexafluoroisopropanol with hotplate
stirrers for 1 week at 25°C. The spin solution was transferred to a
10-ml syringe with an 18 G needle. The flow rate was 0.5 ml/h, the
voltage was gradually increased to 16 kV and the collection
distance was 12 cm. TSF nanofibers with diameters of 400 and 1,200
nm were generated using spin solutions with concentrations of 8 and
16wt%, respectively. The as-spun TSF nanofibers were immersed in
75% (v/v) ethanol/water for 30 min to induce a structural change,
and then dried at room temperature for 24 h. Finally, the TSF
nanofibers were X-rayed prior to use. X-ray diffraction experiments
were measured on X'Pert Pro MPD (PANalytical B.V., Almelo,
Netherlands) in transmittance mode to investigate the crystalline
structure of samples at a wavelength of 0.154 nm. Lastly, the
intensity of the incident beam, the sample absorption and the
background were corrected for changes (23).
Material characterization
The samples were cut into squares of 1
mm2 and fixed on copper chips. After spraying with gold,
they were observed using a scanning electron microscope (SEM)
(23,24). In order to calculate the diameter of
the nanofibers, SEM images of >100 independent fibers were
analyzed by ImageJ software (version 1.47; National Institutes of
Health, Bethesda, MD, USA). Fourier Transform Infrared Spectroscopy
(FTIR) is a powerful and commonly used tool for secondary structure
analysis; the conformational changes and secondary structure of TSF
nanofibers after ethanol treatment were determined by FTIR
(24,25).
In vitro primary cultured OECs on TSF
scaffolds
All animal experiments of the present study were
approved by the Ethics Committee of the Second Affiliated Hospital
of Soochow University (Suzhou, China) and performed in accordance
with the Guidelines for the Welfare of Animals of Soochow
University (Suzhou, China) (12) as
well as the National Institute of Health's Guide for the Care and
Use of Laboratory Animals (26).
Primary cultures of OECs were prepared from 30 male Sprague Dawley
rats (weight, 100–150 g; age, 4–5 weeks; Experimental Animal Center
of Soochow University) as reported previously (12). The culture medium for OECs consisted
of Dulbecco's modified Eagle's medium (DMEM)/F-12 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1% glutamine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 2%
penicillin-streptomycin (Harbin Pharmaceutical Group Co., Ltd.,
Harbin, China). The culturing condition was as follows: 37°C in a
humidified atmosphere containing 5% CO2. After 10 days
in culture, OECs were used for a biocompatibility evaluation with
TSF nanofibers. The OECs were detached with 0.1% trypsin
(Sigma-Aldrich; Merck KGaA) at 37°C for 10 min, centrifuged at 157
× g for 5 min and then resuspended. Subsequently, the OECs
(1.0×105) were seeded on coverslips coated with 400-nm
or 1,200-nm TSF fibers or PLL (Sigma-Aldrich; Merck KGaA) that had
been pre-wetted with culture medium in 35-mm Petri dishes (Corning
Inc., Corning, NY, USA), the dimensions of TSF fibers were 10×10×1
mm, the thickness was 1 mm; PLL served as a positive control. The
nanofibers were incubated in 1 ml complete culture medium at 37°C
for 30 min prior to seeding. Cell suspension (1 ml) was placed on
the fibers in each dish, and 1 ml culture medium was added after 4
h. The culture medium was changed every 3 days. After culture for 4
days, the samples were observed with an inverted phase-contrast
microscope and SEM, using the same method of SEM as described
above.
Immunofluorescence staining and
quantitative analysis for OECs on TSF scaffolds
OECs were seeded on the TSF nanofibers or PLL-coated
coverslips as described above, and their identity was confirmed by
immunostaining. Cells on the fibers and PLL were fixed for 30 min
at room temperature with 4% paraformaldehyde (Zhongde Chemical
Products Trade Co., Ltd., Tianjin, China) and then blocked for 30
min in PBS containing 3% bovine serum albumin (BSA), 0.2% Triton
X-100 and 0.02% NaN3 (v/v) (all from Sigma-Aldrich;
Merck KGaA). The cells were incubated with rabbit polyclonal
anti-nerve growth factor receptor (NGFR) p75 (cat. no. BA0514-2;
Wuhan Boster Biological Technology, Ltd., Wuhan, China), diluted to
1:200 in PBS, at 4°C overnight. Following three washes with PBS,
cells were incubated with fluorescein isothiocyanate-conjugated
goat anti-rabbit secondary antibodies (cat. no. BA1105; Wuhan
Boster Biological Technology, Ltd.) diluted 1:70 in PBS for 1 h at
room temperature. Following three washes with PBS, cell nuclei were
stained with Hoechst 33258 diluted 1:100 in PBS at room temperature
for 15 min. Cover slips were washed with PBS, mounted with 50%
glycerin in PBS and imaged with an AF6000 fluorescence microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
To evaluate the growth of OECs on TSF nanofibers,
the spreading area and the longest cellular process of OECs on TSF
nanofibers were quantified at 1, 4 and 7 days. In brief, at least
10 individual cells from three randomly selected fields were
measured with ImageJ 1.47 software on the basis of NGFR p75
immunostaining. At least five coverslips were included for each
experimental group. Experiments were repeated three times.
Crystal violet staining for cell
adhesion assays
The attachment test for OECs on TSF nanofibers was
performed as previously described (27). In brief, 500 µl OEC suspension
(1×105 cells/ml) was seeded into TSF nanofibers and a
24-well PLL-coated plate. Plates were coated with PLL by adding 1
ml 0.1% mg/ml PLL solution to each well at room temperature
overnight. The PLL solution was aspirated and the plates were left
to dry on a clean bench. OECs were incubated for 1 or 3 h in an
atmosphere of 5% CO2 at 37°C. The loose and unadhered
cells were removed. The wells were gently washed twice with PBS.
The adherent cells were fixed with 15% formalin in PBS for 15 min.
Once the fixation was completed, the wells were washed twice with
PBS and stained with 0.05 g/ml crystal violet at 37°C for 15 min.
In each well, the number of cells was counted in five microscopic
fields (magnification, ×200) and images were captured under an
Olympus microscope (Olympus Corp., Tokyo, Japan).
MTT cell proliferation assay
The proliferation of OECs on TSF and PLL was
examined at 1, 4, 7 and 10 days. At each time-point, 20 µl MTT
(Sigma-Aldrich; Merck KGaA) dissolved in PBS at 5 mg/ml was added
to each well, followed by incubation at 37°C for 4 h. Then, the
medium was discarded and 150 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA) was added to each well to dissolve the dark blue
crystals with agitation for 10 min. The absorbance was measured at
490 nm with a microplate reader (iMark™ Microplate
Absorbance Reader-168-1130; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Live/Dead Kit cell viability
analysis
The Live/Dead viability/cytotoxicity kit (cat. no.
L3224; Invitrogen; Thermo Fisher Scientific, Inc.), including 0.5
mM calcein AM and 0.5 mM ethidium homodimer-1 (Ethd-1) dissolved in
1 ml DMEM/F-12, was used for quantitative cell viability analysis
after 6 days of culture on PLL or TSF scaffolds in a 6-well plate.
In live cells, calcein displays bright green fluorescence, while in
dead cells, Ethd-1 binding to nucleic acids results in red
fluorescence. Live/dead reagent was added to cells, followed by
incubation at 37°C for 30 min and subsequent washing with PBS twice
and observation under a fluorescence microscope. In each well,
~3,000 cells were counted from randomly selected fields.
Live-cell imaging for migration
assay
The migration of OECs on TSF nanofibers was assayed
by timelapse video microscopy. An environmental chamber was used to
maintain optimal growth conditions for OECs at 37°C. In brief,
coverslips with cells were loaded and observed every 5 min using a
10X objective under a Leica DMI 6000 B microscope for a period of
400 min. The forward migration index (FMI) was calculated as the
ratio of forward progress (the net distance a cell progressed) to
the total path length (total distance a cell traveled through the
field).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis was performed using analysis of
variance followed by a Dunn-Bonferroni post-hoc test for multiple
group comparisons using GraphPad Prism 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
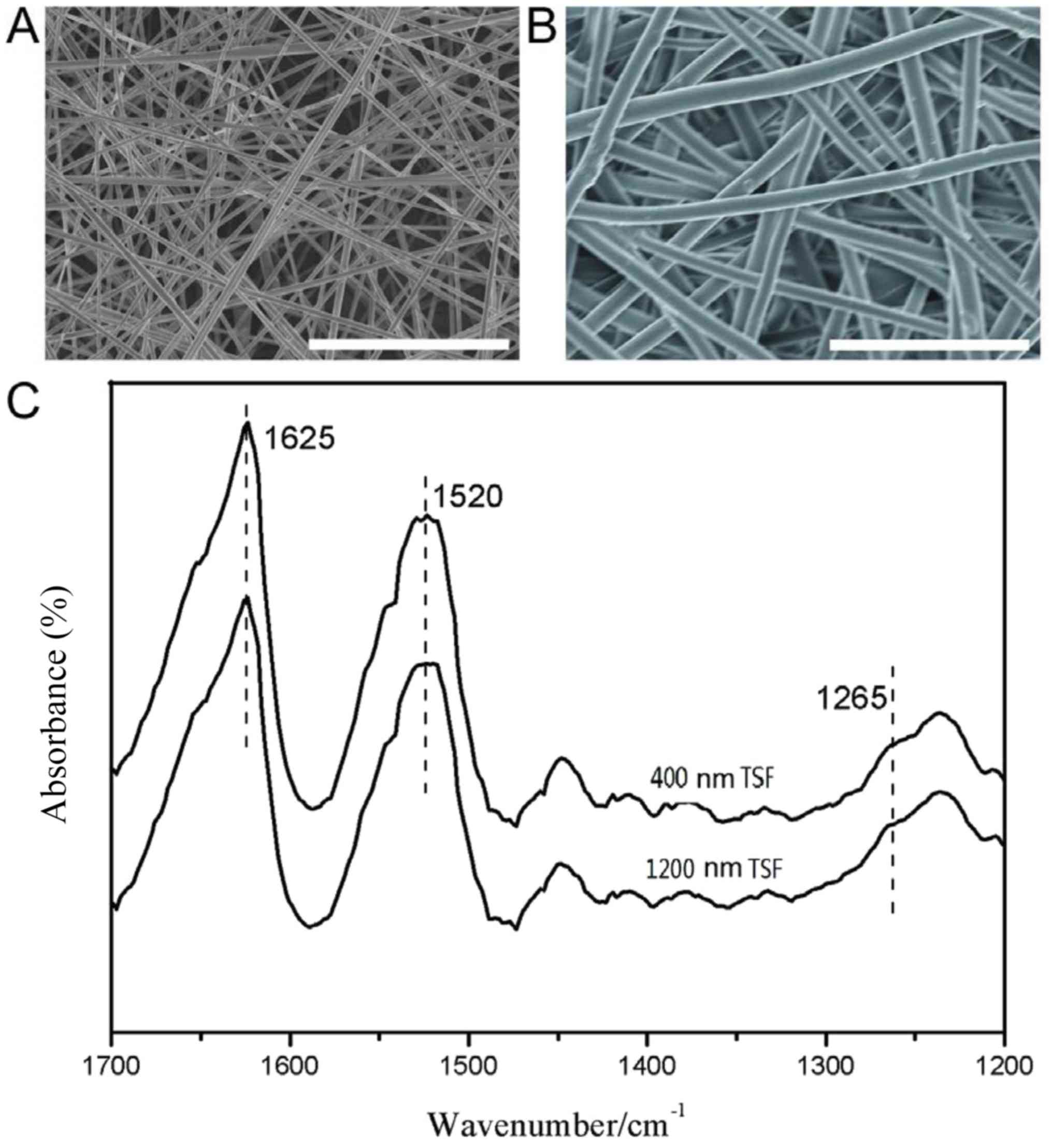
Morphology and structure of TSF
In order to assess the role of the nanofiber
diameter in regulating cell behavior, TSF nanofibers of different
diameter were prepared. As presented in Fig. 1A and B, the diameters of the two
types of TSF nanofiber were 400±44 and 1,200±95 nm, respectively;
the nanofibers exhibited a smooth surface with micro-sized pores to
allow for cell proliferation and penetration. Although native silk
fibroin is stable in water, the regenerated silk material is
usually post-treated with a solvent to achieve stability. In the
present study, 75% ethanol was employed to treat as-spun TSF
nanofibers and induce this structural change.
As presented in Fig.
1C, the FTIR spectra of TSF nanofibers exhibited absorption
bands at 1,625 cm−1 (amide I), 1,520 cm−1
(amide II) and 1,265 cm−1 (amide III), attributed to a
β-sheet structure. The crystalline β-sheet structure is the basis
for the water stability and strength of the TSF nanofibers. Thus,
water-stable TSF nanofibers with different diameters were
successfully prepared.
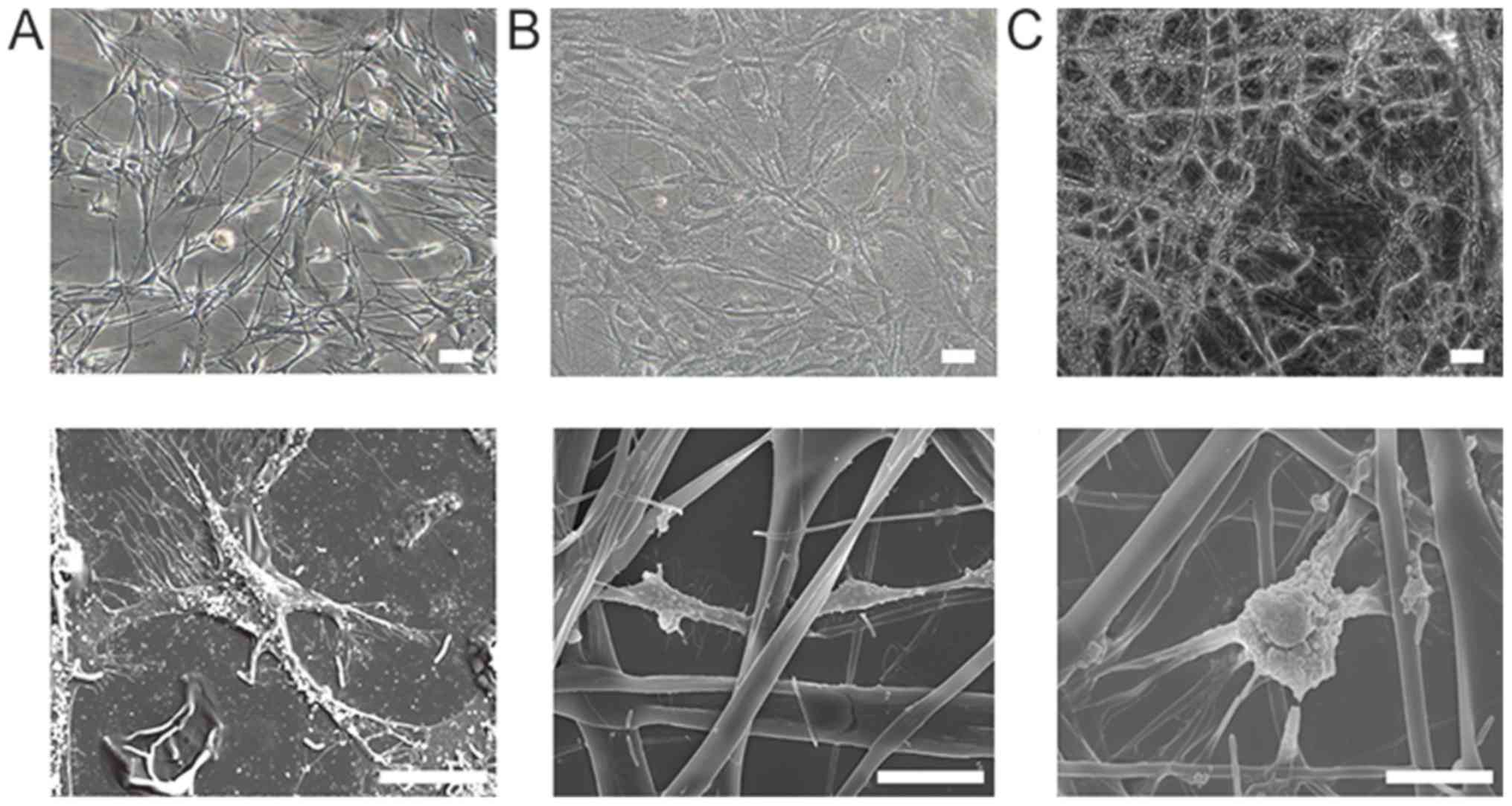
Morphology and structure of OECs on
TSF
An inverted-phase contrast microscope was used to
observe the OECs cultured on PLL and TSF (400 and 1,200 nm) after 4
days (Fig. 2A-C). Most OECs
presented with a bipolar or tripolar morphology, with excellent
refraction. The cells on PLL were connected through cell processes.
The OECs on TSF followed the fiber direction, and adjacent cells
formed connections through neurites. As presented in Fig. 2D and E, SEM revealed that OECs grew
on the surface and pores of the TSF microfibers after 4 days of
culture. The surfaces of the cells produced different cellular
processes, crossing the microporous surface to form intercellular
connections. In addition, extracellular matrix production was
observed in gaps in the material. Most of the OECs on the 400-nm
TSF displayed flat cell bodies and elongated protuberances, with
the cells tightly linked. The cellular connections of OECs on
1,200-nm TSF were fewer than those on 400-nm TSF.
Immunostaining and growth parameters
of OECs on TSF
NGFR p75 staining indicated that OECs on TSF
retained their phenotype. Hoechst 33258 staining revealed that the
nuclei of the OECs were round or oval; no chromatin condensation or
apoptotic body formation was observed and the staining was uniform,
indicating that there were no obvious apoptotic phenomena of OECs
grown on TSF nanofibers of either diameter.
As presented in Fig.
3, the difference in the area of OEC spreading on the two types
of TSF nanofiber after 1 day was relatively small
(0.9–1.5×103 µm2). After 4 days, the area of
cell spreading on 400 nm TSF fibers reached 3.8×103
µm2, which was significantly higher than that on 1,200
nm TSF (3×103 µm2; P<0.05). Significant
differences in the spreading area between the OECs grown on PLL or
400 nm TSF fibers and the 1,200 nm TSF fibers were obtained at 7
days (P<0.05). Quantitative analysis also indicated that the
maximum process length of OECs on 400 nm TSF fibers was
significantly longer than that of OECs on 1,200 nm fibers at 4 and
7 days (P<0.05).
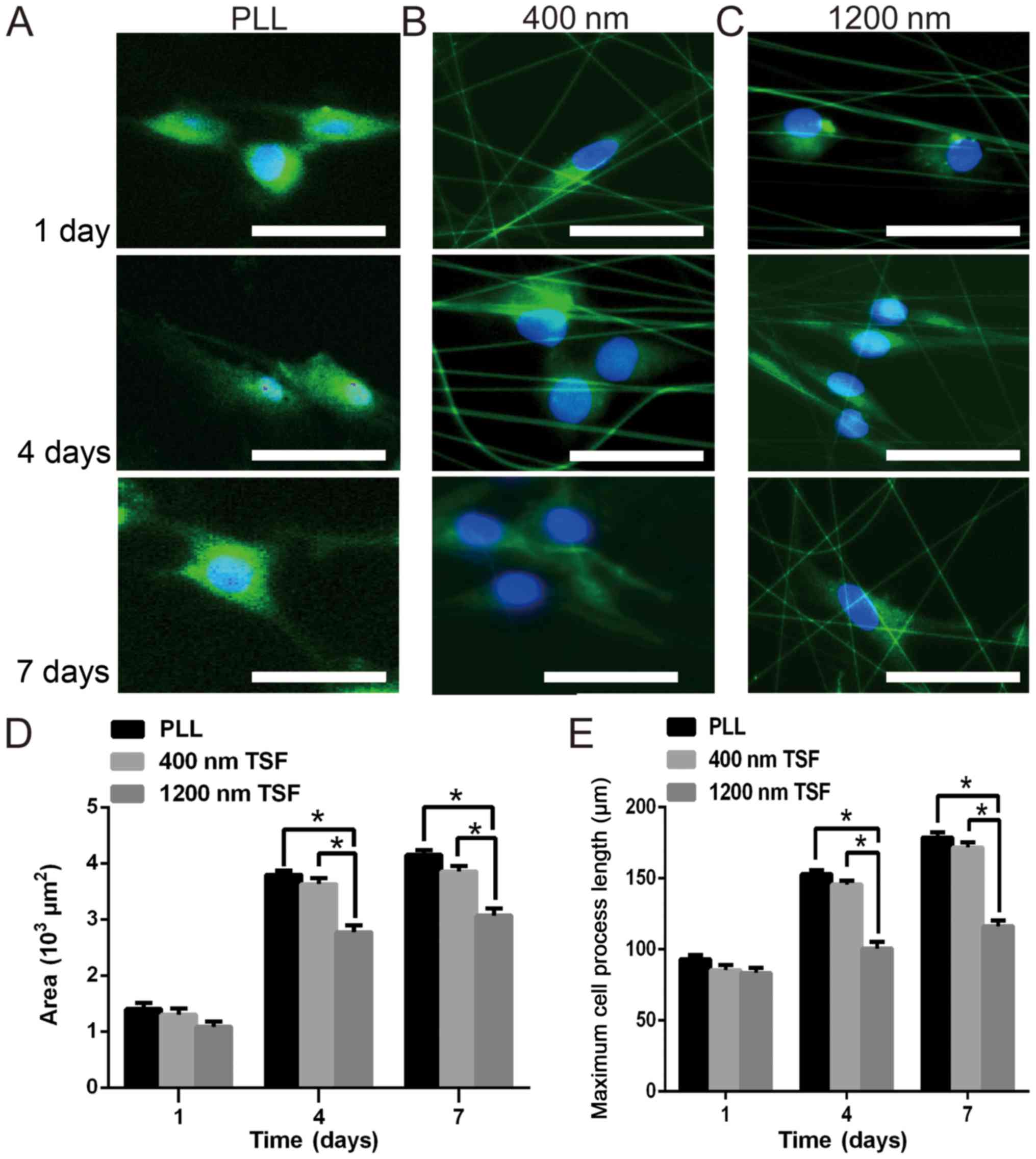 | Figure 3.Effect of the TSF scaffold diameter
on the growth and spread of OECs. Immunocytochemistry staining of
OECs with anti-NGFR p75 antibodies (green) and Hoechst 33258
(blue). OECs were cultured on (A) PLL and (B and C) TSF nanofibers
with a diameter of (B) 400 nm and (C) 1,200 nm, and were observed
at 1, 4 and 7 days (scale bar, 25 µm) using a fluorescence
microscope. (D) The spreading area and (E) the longest process of
OECS was quantified at 1, 4 and 7 days. The area of OECs on the
400-nm TSF fibers reached 3.8×103 µm2, which
was significantly higher than on 1,200-nm TSF, which reached
3.0×103 µm2. There were also significant
differences in the spread area of cells between PLL fibers and
1,200 nm TSF fibers at 7 days. In addition, the longest process of
OECs on 400-nm TSF was significantly longer than that of OECs on
1,200-nm TSF on days 4 and 7. *P<0.05. TSF, Tussah silk fibroin;
OECs, olfactory ensheathing cells; PLL, poly-L-lysine. |
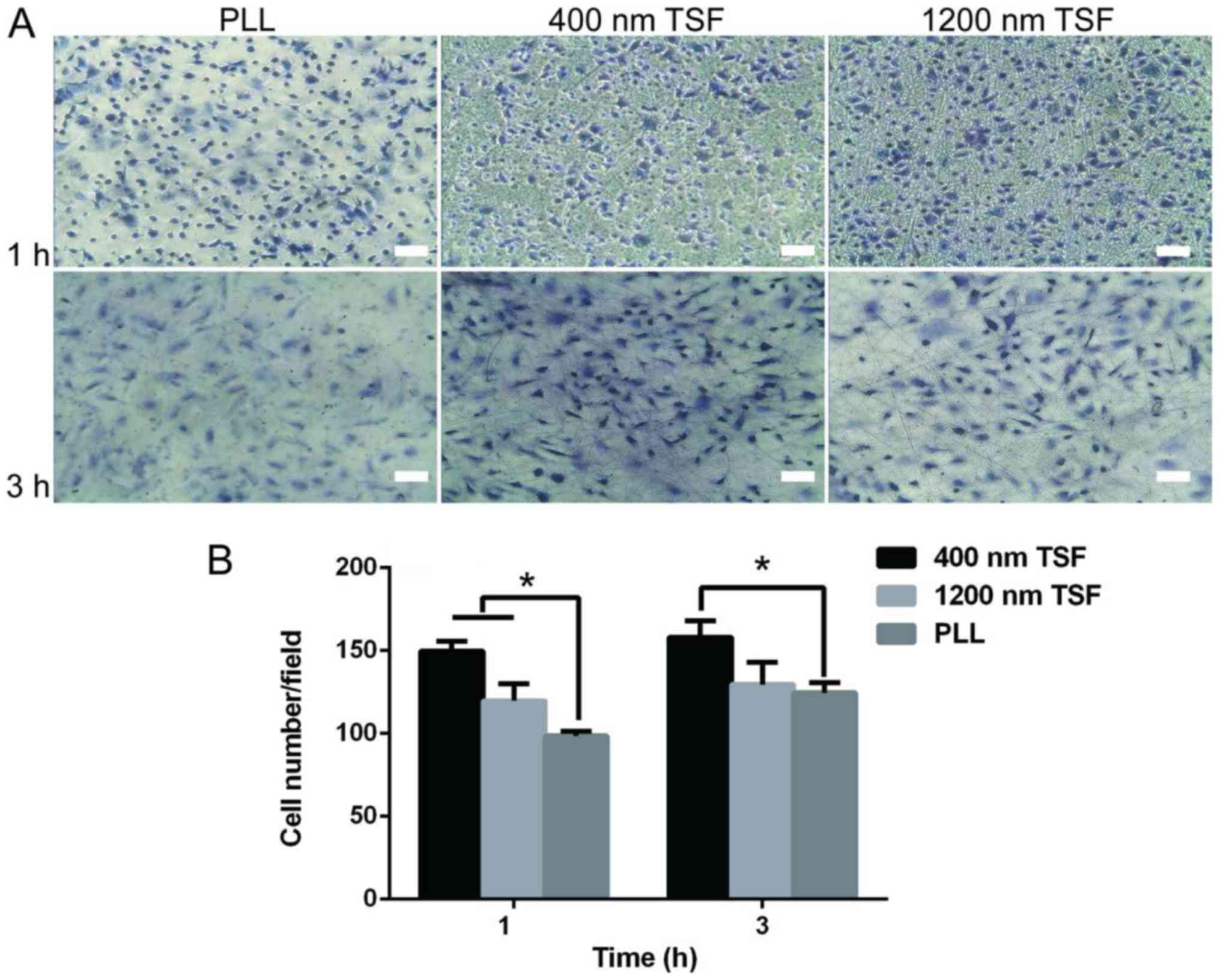
Cell adhesion of OECs on TSF
The cell adhesion on 400 nm TSF was greater than
that on PLL or 1,200 nm TSF (P<0.05). The cell adhesion on PLL
was the lowest (Fig. 4). This may be
due to TSF containing more RGD sequences.
Proliferation and viability of OECs on
TSF
As presented in Fig.
5A-C, the morphology of OECs on TSF was normal. The majority of
the cells were distributed along the fiber and evenly distributed.
According to an MTT assay (Fig. 5D),
the absorbance value in the 1,200 nm TSF group was significantly
less than that in the 400 nm TSF and PLL groups at 4 days (both
P<0.05), and the absorbance value of the 1,200 nm TSF group was
significantly less than that of the PLL group at 7 days
(P<0.05). However, there were no differences between the groups
at 1 and 10 days. These results indicate that, compared with PLL,
TSF has good biocompatibility and did not induce any marked
cytotoxicity on OECs.
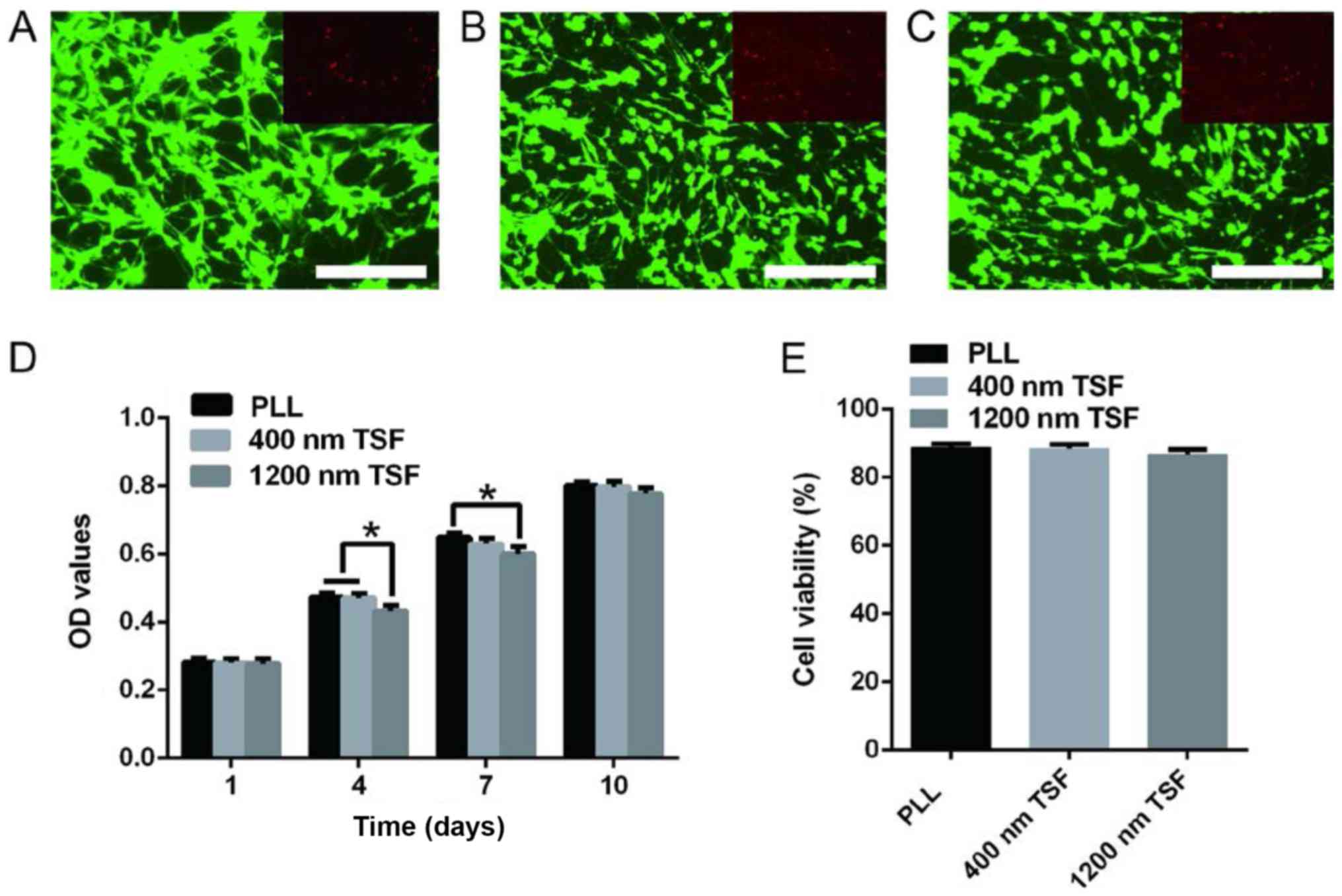 | Figure 5.Effects of (A) PLL, (B) 400-nm TSF
and (C) 1,200-nm TSF on OEC proliferation and viability. (D) After
4 days of culture, the absorbance value in the 1,200-nm TSF group
was significantly lower than that in the 400-nm TSF or the PLL
group, as well as the PLL group at 7 days. Scale bar, 250 µm. (E)
Live/dead cell staining revealed no significant differences between
the groups. *P<0.05. TSF, Tussah silk fibroin; OECs, olfactory
ensheathing cells; PLL, poly-L-lysine; OD, optical density. |
The cell viability and death rates were not
significantly different in the TSF groups (Fig. 5E). These results indicated that TSF
nanofibers successfully supported the survival, growth and
proliferation of OECs.
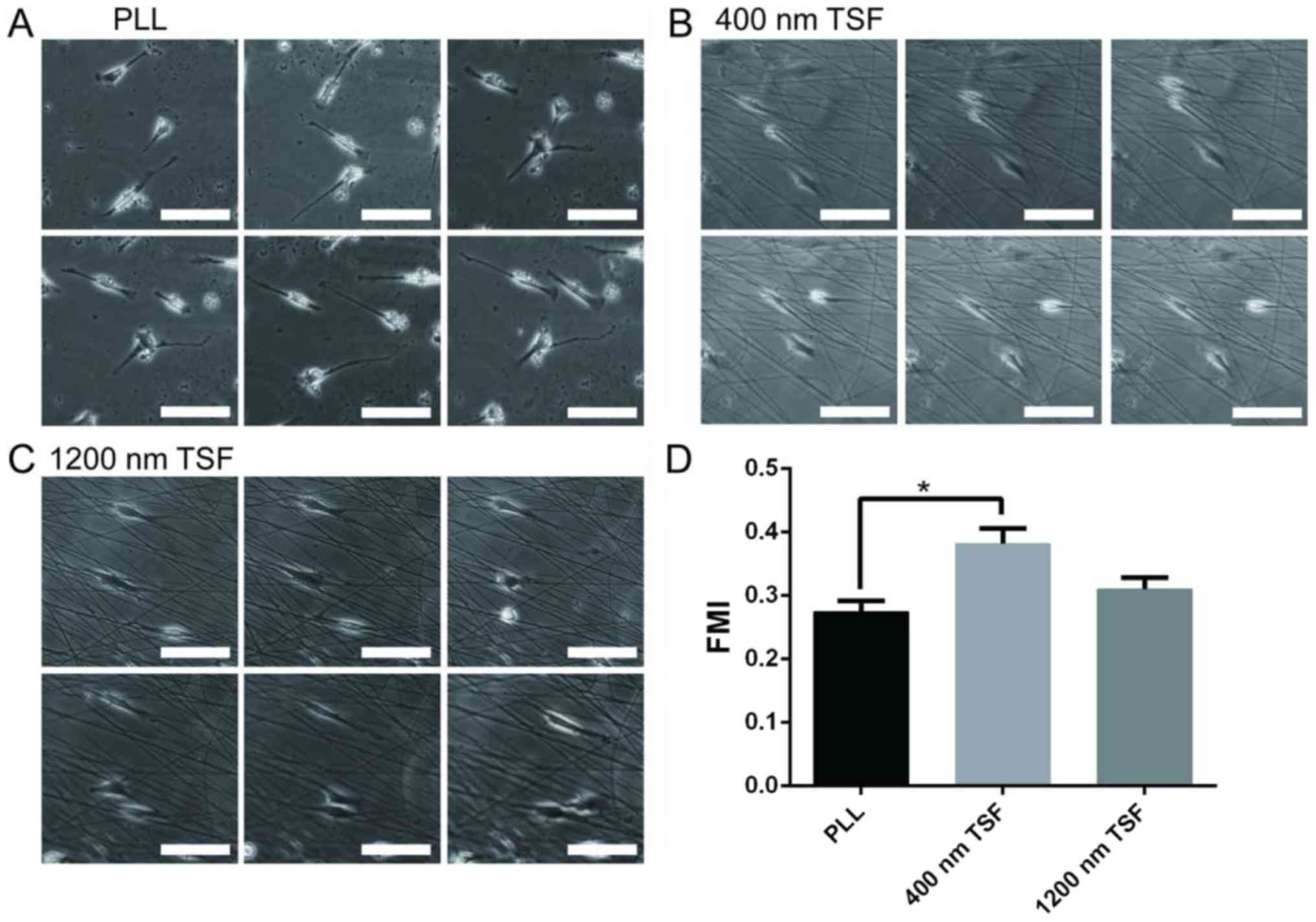
Cell migration of OECs on TSF
Cell migration was analyzed by single-cell
trajectories. The starting point of each cell migration was
standardized to the intersection point of the X- and Y-axes. OECs
on PLL migrated in random directions, whereas those on 400- and
1,200-nm TSF moved along the fiber. In order to measure cell
motility, the FMI was calculated and analyzed (Fig. 6). Quantitative analysis suggested
that the FMI of OECs in the 400-nm TSF group was significantly
higher than that in the PLL group (P<0.05).
Discussion
It has been verified that TSF has good
biocompatibility, permeability and biodegradability, with a low
inflammatory response and other favorable qualities (28,29). TSF
supports cell growth, proliferation and differentiation; in
vivo studies have also reported that it facilitates tissue
repair (30,31). A further advantage of TSF is that it
promotes cell adhesion and migration through the RGD tripeptide
sequence, a biometric signal (32).
It has been reported that TSF was beneficial for the adhesion and
growth of fibroblasts and bone marrow mesenchymal stem cells in a
mouse model (33,34).
The present study indicates that the diameter of
nanofibers has an important role in the arrangement and specific
biological behavior of cells. OECs were observed to be well-aligned
on 400-nm TSF, while cells were randomly arranged on 1,200-nm TSF.
This result suggests that a smaller diameter (400 nm) of TSF
nanofibers may promote cell alignment when compared with a larger
diameter (1,200 nm). This result is similar that of a previous
study by our group on OECs cultured on fabricated SF scaffolds of
different diameters (35). The RGD
tri-peptide sequence structure of TSF has been demonstrated to
facilitate cell adhesion (13). TSF
has also been shown to possess good biocompatibility with no toxic
effects on cells and organisms, and a low likelihood of
inflammatory reaction or immune rejection (15). Through the current study, it was
speculated that a smaller diameter of TSF nanofibers is more
similar to the microenvironment created by the natural
extracellular matrix, which is suitable for cell survival and
proliferation. However, the specific biochemical effects and
directional guidance of TSF nanofibers, as well as the underlying
mechanisms, still require further study.
Based on the above results, it may be speculated
that the precise control of the direction of nanofibers may control
the arrangement and directional migration of cells to promote the
regeneration of axons and the repair of central nerve injury. This
may be facilitated by the application of physical or chemical
stimuli, including electrical stimulation or growth factor release
(36).
In conclusion, through electrospinning, a
three-dimensional TSF scaffold material with a controllable
diameter, smooth surface and uniform pore spacing was prepared.
These TSF nanofibers do not affect the phenotype of OECs, and
support their adhesion, migration, growth and proliferation. The
performance of the 400-nm TSF fibers, including OEC adhesion,
proliferation and migration, was improved relative to that of the
1,200-nm TSF fibers. This indicates that TSF nanofibers with a
diameter of 400-nm may be a superior scaffold material for
repairing nerve injury. However, the mechanisms of this effect
require further study.
Acknowledgements
The authors would like to thank Professor Huanxiang
Zhang (Department of Cell Biology, Jiangsu Key Laboratory of Stem
Cell Research, Medical College of Soochow University, Suzhou,
China) for the guidance provided during the experiment.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81271723) and the Applied
Basic Research Project of Suzhou (grant no. SYS201622).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YS conceived and designed the experiments. PW, HZ
and PZ performed the experiments and wrote the paper. XW collected
data. XD and JC analyzed the data. BZ interpreted the results and
revised the manuscript. All authors discussed and approved the
final manuscript.
Ethical approval and consent to
participate
All animal experiments of the present study were
approved by the Ethics Committee of the Second Affiliated Hospital
of Soochow University (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests regarding this study.
References
|
1
|
Voronova АD, Stepanova OV, Valikhov MP,
Chadin AV, Dvornikov АS, Reshetov IV and Chekhonin VP: Preparation
of human olfactory ensheathing cells for the therapy of spinal cord
injuries. Bull Exp Biol Med. 164:523–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Straley KS, Foo CW and Heilshorn SC:
Biomaterial design strategies for the treatment of spinal cord
injuries. J Neurotrauma. 27:1–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu M, Gao Z, Li X, Zhao F, Guo L, Liu J
and He X: Feasibility of diffusion tensor imaging for assessing
functional recovery in rats with olfactory ensheathing cell
transplantation after contusive spinal cord injury (SCI). Med Sci
Monit. 23:2961–2971. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu M, Gao Z, Li X, Guo L, Lu T, Li Y and
He X: Conditioned medium of olfactory ensheathing cells promotes
the functional recovery and axonal regeneration after contusive
spinal cord injury. Brain Res. 1654:43–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Au E and Roskams AJ: Olfactory ensheathing
cells of the lamina propria in vivo and in vitro. Glia. 41:224–236.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khankan RR, Griffis KG, Haggerty-Skeans
JR, Zhong H, Roy RR, Edgerton VR and Phelps PE: Olfactory
ensheathing cell transplantation after a complete spinal cord
transection mediates neuroprotective and immunomodulatory
mechanisms to facilitate regeneration. J Neurosci. 36:6269–6286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Führmann T, Anandakumaran PN and Shoichet
MS: Combinatorial therapies after spinal cord injury: How can
biomaterials help? Adv Healthc Mater. 6:2017.(doi:
10.1002/adhm.201601130). View Article : Google Scholar
|
|
8
|
Zhang Q, Yan S, You R, Kaplan DL, Liu Y,
Qu J, Li X, Li M and Wang X: Multichannel silk protein/laminin
grafts for spinal cord injury repair. J Biomed Mater Res A.
104:3045–3057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Ge J, Wang Y, Qi F, Ma T, Wang M,
Yang Y, Liu Z, Huang J and Luo Z: A synthetic oxygen
carrier-olfactory ensheathing cell composition system for the
promotion of sciatic nerve regeneration. Biomaterials.
35:1450–1461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabiri M, Oraee-Yazdani S, Dodel M,
Hanaee-Ahvaz H, Soudi S, Seyedjafari E, Salehi M and Soleimani M:
Cytocompatibility of a conductive nanofibrous carbon nanotube/poly
(L-Lactic acid) composite scaffold intended for nerve tissue
engineering. EXCLI J. 14:851–860. 2015.PubMed/NCBI
|
|
11
|
Zhou M, Qiao W, Liu Z, Shang T, Qiao T,
Mao C and Liu C: Development and in vivo evaluation of
small-diameter vascular grafts engineered by outgrowth endothelial
cells and electrospun chitosan/poly(ε-caprolactone) nanofibrous
scaffolds. Tissue Eng Part A. 20:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Z, Shen Y, Zhang F, Zuo B, Lu Q, Wu P,
Xie Z, Dong Q and Zhang H: Control of olfactory ensheathing cell
behaviors by electrospun silk fibroin fibers. Cell Transplant. 22
Suppl 1:S39–S50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sofia S, McCarthy MB, Gronowicz G and
Kaplan DL: Functionalized silk-based biomaterials for bone
formation. J Biomed Mater Res. 54:139–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pavoni E, Tozzi S, Tsukada M and Taddei P:
Structural study on methacrylamide-grafted Tussah silk fibroin
fibres. Int J Biol Macromol. 88:196–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Shao W, Qian W, He J, Zhou Y, Qi K,
Wang L, Cui S and Wang R: Biomineralized poly (l-lactic-co-glycolic
acid)-tussah silk fibroin nanofiber fabric with hierarchical
architecture as a scaffold for bone tissue engineering. Mater Sci
Eng C Mater Biol Appl. 84:195–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asakura T, Nishi H, Nagano A, Yoshida A,
Nakazawa Y, Kamiya M and Demura M: NMR analysis of the fibronectin
cell-adhesive sequence, Arg-Gly-Asp, in a recombinant silk-like
protein and a model peptide. Biomacromolecules. 12:3910–3916. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min BM, Lee G, Kim SH, Nam YS, Lee TS and
Park WH: Electrospinning of silk fibroin nanofibers and its effect
on the adhesion and spreading of normal human keratinocytes and
fibroblasts in vitro. Biomaterials. 25:1289–1297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang F, Murugan R, Wang S and Ramakrishna
S: Electrospinning of nano/micro scale poly(L-lactic acid) aligned
fibers and their potential in neuronal tissue engineering.
Biomaterials. 26:2603–2610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smeal RM, Rabbitt R, Biran R and Tresco
PA: Substrate curvature influences the direction of nerve
outgrowth. Ann Biomed Eng. 33:376–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smeal RM and Tresco PA: The influence of
substrate curvature on neurite outgrowth is cell type dependent.
Exp Neurol. 213:281–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu J, Wang D, Wang H, Dong Y, Zhang F, Zuo
B and Zhang H: Electrospun silk fibroin nanofibers in different
diameters support neurite outgrowth and promote astrocyte
migration. J Biomed Mater Res A. 101:2667–2678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Ye R, Wei Y, Wang H, Xu X, Zhang
F, Qu J, Zuo B and Zhang H: The effects of electrospun TSF
nanofiber diameter and alignment on neuronal differentiation of
human embryonic stem cells. J Biomed Mater Res A. 100:632–645.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panda N, Bissoyi A, Pramanik K and Biswas
A: Development of novel electrospun nanofibrous scaffold from P.
Ricini and A. Mylitta silk fibroin blend with improved surface and
biological properties. Mater Sci Eng C Mater Biol Appl. 48:521–532.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khamhaengpol A and Siri S: Composite
electrospun scaffold derived from recombinant fibroin of weaver ant
(Oecophylla smaragdina) as cell-substratum. Appl Biochem
Biotechnol. 183:110–125. 2010. View Article : Google Scholar
|
|
25
|
Yang R, Wu P, Wang X, Liu Z, Zhang C, Shi
Y, Zhang F and Zuo B: A novel method to prepare tussah/Bombyx mori
silk fibroin-based films. RSC Adv. 8:22069–22077. 2018. View Article : Google Scholar
|
|
26
|
Parker J: The protection of laboratory
animals: A response to Stephenson. J Med Philos. 19:389–394. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Min BM, Jeong L, Nam YS, Kim JM, Kim JY
and Park WH: Formation of silk fibroin matrices with different
texture and its cellular response to normal human keratinocytes.
Int J Biol Macromol. 34:281–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang Y, Zhang Q, Feng J, Wang N, Xu W
and Yang H: The effect of native silk fibroin powder on the
physical properties and biocompatibility of biomedical polyurethane
membrane. Proc Inst Mech Eng H. 231:337–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi Y, Wang H, Wei K, Yang Y, Zheng RY, Kim
IS and Zhang KQ: A review of structure construction of silk fibroin
biomaterials from single structures to multi-level structures. Int
J Mol Sci. 18:pii: E237. 2017. View Article : Google Scholar
|
|
30
|
Zhang W, Chen L, Chen J, Wang L, Gui X,
Ran J, Xu G, Zhao H, Zeng M, Ji J, et al: Silk fibroin biomaterial
shows safe and effective wound healing in animal models and a
randomized controlled clinical trial. Adv Healthc Mater.
6:2017.(doi: 10.1002/adhm.201700121). View Article : Google Scholar
|
|
31
|
Fernández-García L, Marí-Buyé N, Barios
JA, Madurga R, Elices M, Pérez-Rigueiro J, Ramos M, Guinea GV and
González-Nieto D: Safety and tolerability of silk fibroin hydrogels
implanted into the mouse brain. Acta Biomater. 45:262–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Yoo R, Wells M, Beebe TP Jr,
Biran R and Tresco P: Neurite outgrowth on well-characterized
surfaces: Preparation and characterization of chemically and
spatially controlled fibronectin and RGD substrates with good
bioactivity. Biomaterials. 26:47–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luan XY, Wang Y, Duan X, Duan QY, Li MZ,
Lu SZ, Zhang HX and Zhang XG: Attachment and growth of human bone
marrow derived mesenchymal stem cells on regenerated antheraea
pernyi silk fibroin films. Biomed Mater. 1:181–187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Minoura N, Aiba S, Gotoh Y, Tsukada M and
Imai Y: Attachment and growth of cultured fibroblast cells on silk
protein matrices. J Biomed Mater Res. 29:1215–1221. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen Y, Qian Y, Zhang H, Zuo B, Lu Z, Fan
Z, Zhang P, Zhang F and Zhou C: Guidance of olfactory ensheathing
cell growth and migration on electrospun silk fibroin scaffolds.
Cell Transplant. 19:147–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aznar-Cervantes S, Pagán A, Martínez JG,
Bernabeu-Esclapez A, Otero TF, Meseguer-Olmo L, Paredes JI and
Cenis JL: Electrospun silk fibroin scaffolds coated with reduced
graphene promote neurite outgrowth of PC-12 cells under electrical
stimulation. Mater Sci Eng C Mater Biol Appl. 79:315–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|