Introduction
Lung cancer is a common type of thoracic malignant
tumor and the leading cause of cancer-associated mortality
worldwide (1). Radiotherapy is one
of the major therapies for the treatment of thoracic malignant
tumors, particularly for inoperable tumor patients (2). An increase of the radiotherapy dose
delivered to the tumor during the process of radiotherapy may
increase the local control rate of the tumor. However, radiotherapy
of pulmonary tumors causes damage to the corresponding peripheral
tissues and organs, including the lung, heart, esophagus and spinal
cord. While radiotherapy is efficient for treating thoracic
malignant tumors, it induces certain radiotherapy-associated
adverse reactions, including radiation pneumonitis (RP) and
radiation esophagitis. RP is one of the most common dose-limiting
toxicity syndromes after radiotherapy or concurrent
chemoradiotherapy for patients with thoracic malignant tumors
(3). Due to RP, the clinical use of
higher and more effective radiation doses is not possible, and
together with other methods of tumor treatment, it affects the
quality of life of patients; severe RP may even endanger the life
of affected patients (4,5).
Stereotactic body radiotherapy (SBRT), which has
been implemented in the clinic in the late 1950s, is a
radio-surgical technology for the treatment of extracranial lesions
(6). SBRT features a high
repeatability of posture fixation and high conformity of dose
distribution, and is able to individually measure tumors during
initial image acquisition, treatment and exposure to irradiation;
it may be used to formulate and implement targeted plans, and to
precisely administer irradiation under the guidance of online and
offline images (7). The precise
positioning system of SBRT greatly reduces the radiation dose
reaching surrounding normal tissues and organs, while maximising
the dose in tumor areas. SBRT has a high efficacy for inoperable
patients with early-stage non-small cell lung cancer (NSCLC)
(8). RP is one of the most common
complications of early-stage NSCLC after the treatment with SBRT. A
phase III randomised controlled trial on radiotherapy and
chemotherapy of stage-III NSCLC reported that a majority of
patients suffered from RP after treatment with SBRT (9).
A previous study indicated that the development of
RP is a repair process for injury that involves multiple factors
and biomolecular complexes (10).
Clinical research showed that some blood cytokines, including
interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α,
transforming growth factor-β and platelet-derived growth factor,
were associated with the occurrence of RP (11), but their clinical value in treatment
is still unclear. At present, no clear biomarker may be used to
predict RP. In the course of radiotherapy for lung and breast
cancer, healthy lung tissue is also subject to radiation. When lung
cancer is treated with radiation, the safe radiation dose is
significantly associated with the risk of RP in the surrounding
normal lung tissue. This risk is dose-dependent and is commonly
predicted by using metrics, including the percentage of volume that
received ≥20 Gy (V20), which are usually formulated under the
assumption of homogeneous pulmonary function. Because of the uneven
distribution of function throughout the lung, the risk of RP may be
reduced if high-functioning lung areas are identified in advance
and avoided preferentially during treatment (12,13). It
is indicated that radiation-induced lung injury can be predicted
and avoided to a certain extent (12,13).
Therefore, finding a suitable predictor is significant for avoiding
the occurrence of RP.
Patients and methods
Patients
NSCLC patients admitted to Henan Province
Anti-Cancer Hospital (Zhengzhou, China) between October 2014 and
June 2016 were selected for the present study. All patients were
diagnosed as stage I NSCLC according to the criteria in the 7th
edition of the guidelines of the American Joint Committee on Cancer
(14) and TI-4N0M0 by
histopathological detection. If lymph nodes with a size of <1 cm
in the hilus pulmonis and mediastinum or no abnormal mediastinal
lymph nodes on positron emission tomography-computed tomography
(PET/CT) were present, the nodal status was considered as N0. The
pathological types of the primary lesions were squamous-cell
carcinoma, adenocarcinoma, large cell carcinoma, large cell
neuroendocrine carcinoma and other unclassified NSCLC. The present
study obtained approval from the institutional review board of
Henan Province Anti-Cancer Hospital (Zhengzhou, China). All
patients provided written informed consent.
The exclusion criteria were as follows: i) Local or
distant metastasis confirmed by PET inspection or post-operative
pathological staging, or primary cancer or pre-cancerous lesions in
the past three years (except disease-free survival and survival
time after eradication therapy of aggressive tumors of >3 years,
carcinoma in situ and early-stage skin cancer through
eradication treatment); ii) primary tumor diameter >5 cm; iii)
history of chemotherapy, pulmonary lobectomy or pneumonectomy; iv)
the same radiation field with that of the present study and
previous radiotherapy; v) pure bronchioloalveolar carcinoma; vi)
active systemic infection or pulmonary and pericardial infection;
vii) women attempting to conceive, during pregnancy or breast
feeding, or sexually active, heterosexual men unable or unwilling
to use contraception in accordance with the acceptable medical
methods (note: This item is required, as the therapeutic methods
applied in the present study may lead to sperm cell malformation);
viii) unintended weight loss of >10% of body weight over the
previous three months; ix) patients with chronic obstructive
pulmonary disease and/or heart disease who were inoperable as
determined by an experienced thoracic cancer surgeon, patients who
refused surgery and those with a performance status score of
≤2.
SBRT schedules
For central lung cancer, a total SBRT dose of 55 Gy
was delivered by administration of 5 times within 5–7 days, with a
treatment interval of 1–3 days (15). Peripheral lung cancer was treated
with a total SBRT dose of 48 Gy administered over 4 times within
5–7 days, with a treatment interval of 1–3 days. These treatments
were required to be completed within ten days.
For all patients, the radiation dose was the
marginal dose of the planning target volume (PTV). During the
calculation of the dose, the heterogeneity of tissues was
considered. A requirement during the establishment of the
radiotherapy schedule was that the target volume was enclosed by
the isodose curve of 95%.
Establishment of radiotherapy schedules
and radiation dose
General principles
The treatment aimed to deliver a high dose to the
target volume and reduce the dose reaching the surrounding normal
tissues. The calculation of radiation dose and the measurement with
monitoring device (RadHalo™ RDP and FM Spectroscopic Area Monitors;
each, Thermo Fisher, Scientific, Inc., Waltham, MA, USA) was based
on the heterogeneity of tissues. Tumors with a shallow depth of
<2 cm were treated with gamma rays of 6 MV or below. High-energy
gamma ray (16–18 MV) mayoptimize the dose-response curves on the
target volume. The calculation of radiation dose excluded the
reconstructed images after a breath by using 3-dimensional (3D)-CT.
Hot spots in the internal target volume (ITV) were allowed, with
the maximum dose being ≤140% of the standard dose. The lung is one
of the major dose volume-limiting organs for thoracic radiotherapy.
Numerous dosimetric parameters, including the V5, V20, V30 and the
mean lung dose, have been reported to be associated with radiation
toxicity to the lung. Although no standardization has been
performed for delineating the normal lung for dose computation and
the dosimetric cut-off is controversial, clinical trials and the
National Comprehensive Cancer Network practice guidelines have set
the V20 and the mean lung dose as limits on lung dosimetry
(16,17).
Criteria of successful treatment
plans
i) Normalization: The dose of the treatment plan is
normalized to a 100% dose point at the center of the PTV. This
point usually coincides with the isocenter (but this is not a
requirement). ii) Coverage of isodose curves on the target volume:
The dose delivered to 95% of the PTV, 99% of the ITV and 100% of
the gross tumor volume is identical to the prescribed dose,
respectively. iii) Dose heterogeneity on the target volume: The
dose at the isodose point on the body surface must be >60 and
<90% of the dose at the center of PTV (the PTV is equal to that
mentioned in the above point i). iv) Maximum dose: Patients are
treated with the absolute corresponding maximum dose in the
treatment plan, and the dose point must be within the PTV. v)
Prescribed isodose: The dose delivered to the prescribed isodose
surface must be at least 60% and no more than 90% of the maximum
dose. vi) Coverage of prescribed isodose surface: The coverage of
the prescribed isodose surface covers 95% of the PTV, or at least
99% of the PTV is treated with 90% of the prescribed dose. vii)
High-dose leakage: The total volume of all soft tissues outside the
PTV with >105% of the dose <15% of the volume of the PTV.
viii) Application of 3D coplanar or non-coplanar beams provides
each patient with a highly conformal prescribed dose distribution.
In general, when the number of radiation beams is >10, they are
applied approximately to the same radiation weight. A larger number
of beams is generally used for larger lesions. When static beams
are used, application of at least 7 non-penetrated beams is
required. When the arc spinning technique is applied, the
accumulative angle of all beams is 340 degrees at least. In order
to gain an acceptable scope, the size and shape of the aperture on
radiation fields is almost the same as the projection on the PTV.
The only exception is that when the observed minimum diameter of
the radiation fields is 3.5 cm during the treatment of smaller
lesions, 60–90% of the PTV is usually covered (maximum dose, 100%).
However, higher isodoses (hot spots) must be applied within the
target volume, not to the surrounding normal tissues. The isocenter
of the treatment or the point setting in stereotactic coordinates
depends on systematic data-points, which may be adjusted by
location images prior to treatment.
Single-photon emission computed
tomography (SPECT) pulmonary perfusion imaging
SPECT (18) and PET
(19) are two types of CT technology
applied in nuclear medicine. SPECT, which detects photons and PET,
which detects the positrons emitted form images that are
collectively referred to as ECT. Pulmonary perfusion imaging is
able to reflect lung regions with different functional activity,
and radiotherapy plans established on the basis of pulmonary
perfusion images exhibit high biological conformality, which allows
for sparing the most vulnerable non-cancerous tissues from
radiation treatment. In addition, the presence of defects or fluid
displayed on pulmonary perfusion images was consistent with the
pulmonary function, based on which it was possible to estimate the
occurrence of lung injury.
Pulmonary perfusion imaging was performed using a
dual-head SPECT-CT (Philips, Eindhoven, The Netherlands) at the
Affiliated Cancer Hospital of Zhengzhou University/Henan Cancer
Hospital (Zhengzhou, China). 99mTc macroaggregated
albumin (MAA) was used as a marker. The patient was made to lay
flat on the inspection parallel board in the supine position with
their elbows held in front of their forehead. The position and
placement of the patients was in accordance with that during
radiotherapy. A total of 185 MBq 99mTc-MAA was slowly
injected through the brachium vein of the patient. Lung static
images were immediately captured from the front, back, left
anterior, left posterior, right anterior, right posterior, left
lateral and right lateral views. The required acquisition matrix
was 128×128 and the acquisition counter in each position was set at
5×105/frame. Cross-sectional images of coronal, cross
and sagittal sections were collected for each patient, with one
frame for 20 sec every 60 degrees, at double magnification and each
probe was rotated 180 degrees.
Cytokine detection
In the morning prior to SBRT, 2 ml fasting blood was
collected from each patient in EDTA anti-coagulative tubes. Within
the next hour, the blood was centrifuged at 1,000 × g for 10 min at
4°C. The separated plasma was stored at −20°C. The cytokines were
measured within 2 h after the separated plasma was defrosted at
room temperature. The serum levels of apurinic/apyrimidinic
endonuclease-1 (Ape-1; cat. no. 65920), intercellular adhesion
molecule (ICAM)-1 (cat. no. 70220) and IL-17A (cat. no. 4176AF-50)
were detected using ELISA kits (NeoBioscience, Shenzhen, China)
according to the manufacturer's protocol. A standard curve was
established, from which the concentration of the antigen in the
samples was determined. Titer was considered to be present at an
optical density greater than that of the blank control well.
Diagnosis and evaluation of RP
All patients were examined at least once a week
during the treatment. For follow-up, a routine chest CT scan was
performed at 4–6 weeks after radiotherapy and every three months
thereafter. If any suspicious RP symptoms (e.g., severe coughing,
elevated temperature and choking sensation in the chest) occurred
at any time in the process of or after radiotherapy, chest CT
examination for confirmation was promptly performed. RP was
diagnosed according to patients' clinical symptoms (coughing or
dyspnea) and imaging abnormalities, including new ground-glass
opacity changes, irregular enhancement or consolidation changes in
the radiation field. For the diagnosis of RP, it was required to
exclude intrapulmonary infection and the development of pulmonary
lesions, and the stage of RP was determined based on the severity
of the symptoms.
RP was divided into the following stages according
to the standards of acute RP established by the Radiation Therapy
Oncology Group (20): 0, no obvious
change in symptoms and signs after treatment compared with those
prior to treatment; I, mild cough or a cough reflex in response to
forceful expiration; II, persistent cough that requires treatment
with narcotic antitussive or dyspnea in response to light exercise
but no dyspnea in the resting state; III, severe cough for which
narcotic antitussive ineffective, dyspnea in the resting state or
acute pneumonia confirmed by radiological images, which may be
treated with intermittent oxygen inhalation or cortical hormones;
IV, severe respiratory insufficiency which requires treatment with
continuous oxygen inhalation or assisted ventilation; V, death from
RP.
Statistical analysis
SPSS 11.5 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for data processing. The Chi-squared test was
used to assess differences between groups for categorical/numerical
variables. Data of two groups were subjected to normal
distribution-homogeneity of variance (homogeneity test of variance)
via use of a Student's t-test. If the above conditions were not
met, then a rank sum test was performed. Measurement data were
expressed as the mean ± standard deviation, and the P-value was
used for assessment. P<0.05 was considered to indicate a
significant difference.
Results
Basic information
In the present study, patients with high levels of
Ape-1, ICAM-1 and IL-17A prior to SBRT were divided into group A,
while patients without high levels of Ape-1, ICAM-1 and IL-17A were
assigned to group B. The median cut-off values for Ape-1, ICAM-1
and IL-17A were 4.2, 3.0 and 5.1 µg/l. There was no significant
difference in age, sex, tumor type and tumor stage distribution
between the two groups (P>0.05; Table
I).
 | Table I.Basic data of patients in the two
groups. |
Table I.
Basic data of patients in the two
groups.
| Variable | Group A (n=23) | Group B (n=23) | P-value |
|---|
| Age | 66.1±11.23 | 64.2±10.61 | 0.18 |
| Males/females | 17/6 | 16/7 | 0.74 |
| Cancer stage
(12) |
| IA | 4 (17.4%) | 3 (13.0%) | 0.85 |
| IB | 3 (13.0%) | 4 (17.4%) |
|
| IIA | 5 (21.7%) | 6 (26.1%) |
|
| IIB | 6 (26.1%) | 4 (17.4%) |
|
| IIIA | 2 (8,7%) | 4 (17.4%) |
|
|
IIIB | 3 (13.0%) | 2 (8.7%) |
|
| Histological
type |
|
Squamous cell carcinoma | 14 (60.1%) | 13 (56.5%) | 0.81 |
|
Adenocarcinoma | 7 (30.4%) | 8 (34.8%) |
|
| Large
cell carcinoma | 2 (8.7%) | 2 (8.7%) |
|
| Lung radiation
dose |
| V5 | 26.8±0.84 | 27.4±0.98 | 0.90 |
|
V20 | 40.6±1.32 | 48.3±0.93 | 0.25 |
|
V30 | 48.4±1.09 | 55.4±1.11 | 0.46 |
| Smoking
history | 42.5±2.11 | 48.7±1.98 | 0.45 |
| MLD | 48.4±2.17 | 55.4±1.96 | 0.46 |
SPECT imaging
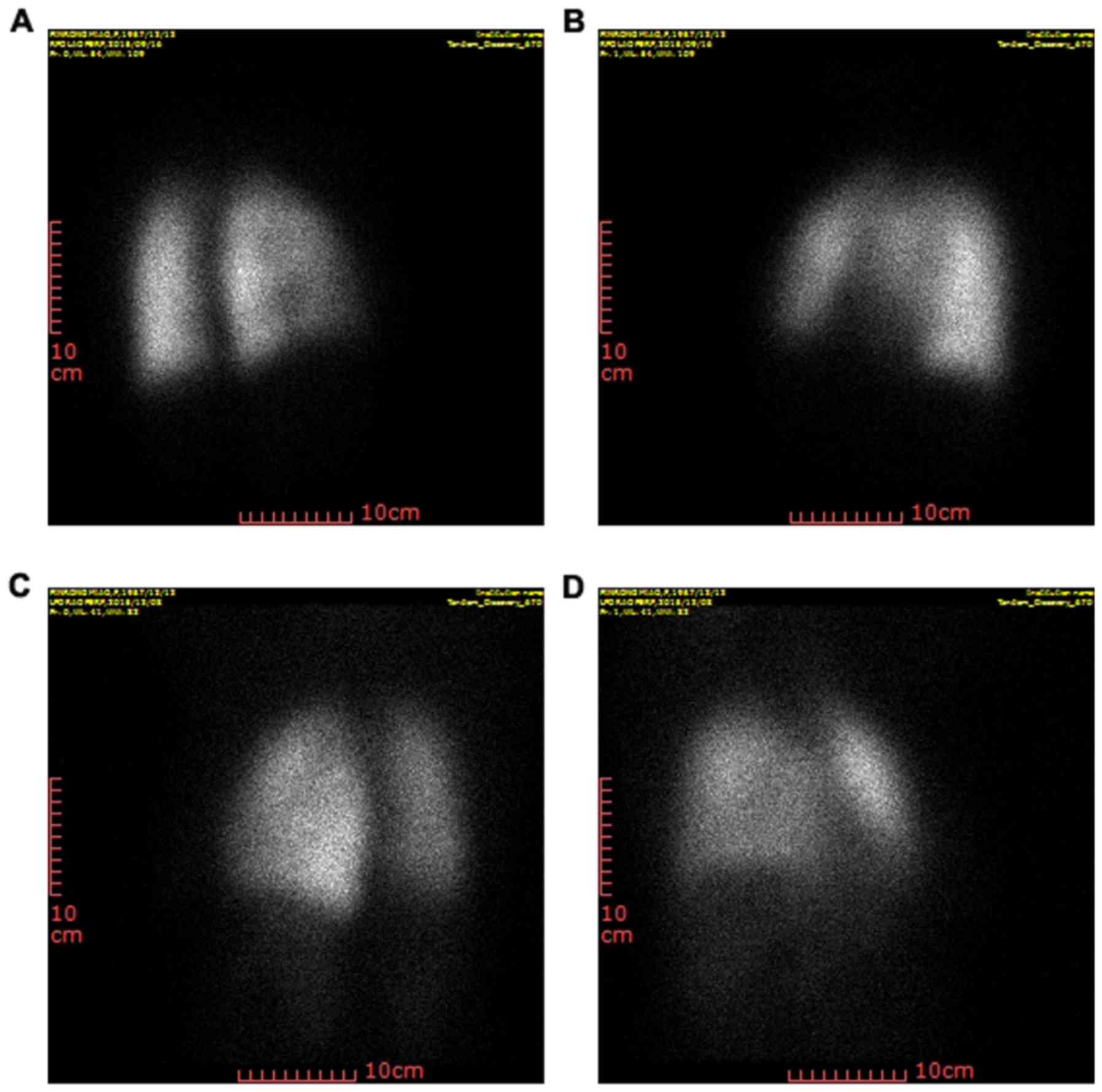
Prior to SBRT, patients were subjected to SPECT,
provided a comprehensive information on the condition and function
of the lung to establish ideal radiotherapy plans. The images in
Fig. 1A and B were captured prior to
SBRT and those in Fig. 1C and D were
obtained after radiotherapy. No RP occurred in any of the patients
with NSCLC prior to SBRT. However, after SBRT, ground-glass opacity
changes and irregular enhancement were observed, which demonstrated
that the occurrence of RP (21) was
~95.6%. Furthermore, the occurrence of stage II–III RP was
~47.8%.
Basal Ape-1, ICAM-1 and IL-17A levels
are associated with the incidence of RP
Prior to SBRT, the Ape-1, ICAM-1 and IL-17A levels
in group A were significantly higher than those in group B
(P<0.05). There was no significant difference between the two
groups in Ape-1, ICAM-1 or IL-17A levels, age, sex, tumor stage or
tumor type. After SBRT, the levels of Ape-1, ICAM-1 and IL-17A in
group A were still higher than those in group B (Table II).
 | Table II.Serum levels of Ape-1, ICAM-1 and
IL-17A in the patients of the two groups (µg/l). |
Table II.
Serum levels of Ape-1, ICAM-1 and
IL-17A in the patients of the two groups (µg/l).
|
Group/time-point | Ape-1 | ICAM-1 | IL-17A |
|---|
| Group A |
|
Pre-SBRT | 16±1.21 | 19±0.92 | 14±0.69 |
|
Post-SBRT | 21 | 18±0.48 | 19±1.09 |
|
P-value | 0.02 | 0.01 | 0.02 |
| Group B |
|
Pre-SBRT | <4.2±0.17 | <3.0±0.32 | <5.1±0.25 |
|
Post-SBRT | <4.1±0.76 | <2.1±0.46 | <4.2±0.86 |
|
P-value | 0.06 | 0.05 | 0.07 |
After SBRT, 22 patients in group A suffered from RP,
while in group B, 17 were without RP. The difference in the
incidence of RP between the two groups was significant
(P=1.66×10−12; Table
III). Higher Ape-1, ICAM-1 and IL-17A levels were associated
with a higher risk of RP. A further analysis should be performed to
verify whether these factors have significant prognostic value.
 | Table III.Incidence of RP after SBRT in groups
A and B (n=23 each). |
Table III.
Incidence of RP after SBRT in groups
A and B (n=23 each).
|
|
|
| Stage of RP |
|
|---|
|
|
|
|
|
|
|---|
| Group | No RP % | RP % | I % | II % | III % | P-value |
|---|
| A | 1 (4.3) | 22 (95.6) | 11 (47.8) | 7 (30.4) | 4 (17.4) |
1.66×10−12 |
| B | 17 (73.9) | 6 (26.1) | 3 (13.0) | 2 (8.7) | 1 (4.3) |
|
Discussion
RP is a serious adverse effect of SBRT in lung
cancer patients. The factors associated with the occurrence of RP
have been thoroughly discussed (22). Studies on RP have provided tools for
developing improved radiotherapy plans for lung cancer patients,
but to the best of our knowledge, no effective factor for the
prediction of RP has been provided (23–25). The
prognostic value of the cytokines Ape-1, ICAM-1 and IL-17A used in
combination should be assessed via multivariate logistic regression
analysis.
Ape-1 is a perfect paradigm of the functional
complexity of a biological macromolecule (26). It has a crucial role in controlling
cellular processes, including apoptosis, proliferation and
differentiation. It also inhibits oxidative stress by inhibiting
reactive oxygen species generation via the cytoplasmic small
guanosine triphosphatase Rac1 (27).
The key responsive transcription factor nuclear factor (NF)-κB has
a crucial role in regulating the expression of various inflammatory
cytokines, and inhibition of NF-κB effectively suppresses the
inflammatory response (28,29). Furthermore, inhibition or
overexpression of Ape-1 may reduce or activate the DNA binding
activity of NF-κB, respectively (30). Therefore, overexpression of Ape-1 may
aggravate the inflammatory response after SBRT, which may induce
the occurrence of RP. APE1 maintains cellular homeostasis (redox
reactions) via the activation of transcription factors that
regulate various physiological processes and that crosstalk with
redox balancing agents (for example, thioredoxin, catalase and
superoxide dismutase) by controlling levels of reactive oxygen and
nitrogen species (31). APE1
expression and/or sub-cellular localization are altered in several
metabolic and proliferative disorders, including in tumors and
aging (32).
In ICAM-1-knockout mice, no inflammatory response
was observed in the lung after exposure to radiation (33). Thoracic irradiation was reported to
significantly increase the expression of ICAM-1, which was
therefore indicated to be associated with lung injury from
radiotherapy (34).
In addition, an animal study has indicated that
IL-17A has an important role in processes of lung injury induced by
radiotherapy. In the process of radiation-induced lung injury,
IL-17A expression exhibited differences in different periods
(35). IL-17A expression was
appreciable at 1 week, peaked at 4 weeks and subsequently declined
at 8 weeks following irradiation. In another study, treatment with
IL-17A antibody alleviated RP and subsequent fibrosis and improved
post-irradiation survival (36).
In conclusion, while the pathogenesis of RP remains
to be fully elucidated at the molecular level, the present study
reported an association between increased serum levels of Ape-1,
ICAM-1 and IL-17A at baseline and the occurrence of RP. Regarding
clinical radiotherapy of NSCLC, high serum levels of the cytokines
Ape-1, ICAM-1 and IL-17A at baseline may indicative of an increased
vulnerability to RP.
Acknowledgements
Not applicable.
Funding
The current research was supported by the Ministry
of Health and Henan Province Health Department (grant no.
201201009), Zhengzhou City Science and Technology Innovation Team
(grant no. 121PCXTD524) and the Scientific and Technological
Project for Henan Health Department (grant no. 201503187).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG and GD made substantial contributions conceiving
and designing the current study. WX, YLu, HG and YJ acquired the
data. XC and YLi analyzed and interpreted the data. GD, WX, YLu, HG
and YJ drafted the manuscript. LG, XC and YLi revised the
manuscript critically for important intellectual content. The final
version of the manuscript was read and approved by all authors, and
each author believes that the manuscript represents honest
work.
Ethics approval and consent to
participate
The present study obtained approval from the
institutional review board of Henan Province Anti-Cancer Hospital.
All patients provided written informed consent.
Patient consent for publication
The patients provided written informed consent for
the publication of associated data and accompanying images..
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slotman BJ, van Tinteren H, Praag JO,
Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C and
Senan S: Use of thoracic radiotherapy for extensive stage
small-cell lung cancer: A phase 3 randomised controlled trial.
Lancet. 385:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zimmermann FB, Geinitz H, Schill S, Grosu
A, Schratzenstaller U, Molls M and Jeremic B: Stereotactic
hypofractionated radiation therapy for stage I non-small cell lung
cancer. Lung Cancer. 48:107–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marks LB, Bentzen SM, Deasy JO, Kong FM,
Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV,
Timmerman RD, et al: Radiation dose-volume effects in the lung. Int
J Radiat Oncol Biol Phys. 76 3 Suppl:S70–S76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogelius IR and Bentzen SM: A
literature-based meta-analysis of clinical risk factors for
development of radiation induced pneumonitis. Acta Oncol.
51:975–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onishi H, Shirato H, Nagata Y, Hiraoka M,
Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y, et al:
Stereotactic body radiotherapy (SBRT) for operable stage I
non-small-cell lung cancer: Can SBRT be comparable to surgery? Int
J Radiat Oncol Biol Phys. 81:1352–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timmerman RD and Kavanagh BD: Stereotactic
body radiation therapy. Curr Probl Cancer. 29:120–157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albain KS, Swann RS, Rusch VW, Turrisi AT
III, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara
DR, et al: Radiotherapy plus chemotherapy with or without surgical
resection for stage III non-small-cell lung cancer: A phase III
randomised controlled trial. Lancet. 374:379–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bradley JD, Hope A, El Naqa I, Apte A,
Lindsay PE, Bosch W, Matthews J, Sause W, Graham MV and Deasy JO:
RTOG: A nomogram to predict radiation pneumonitis, derived from a
combined analysis of RTOG 9311 and institutional data. Int J Radiat
Oncol Biol Phys. 69:985–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu GD, Xia L, Zhu JW, Ou S, Li MX, He Y,
Luo W, Li J, Zhou Q, Yang XQ, et al: Genistein alleviates
radiation-induced pneumonitis by depressing Ape-1/Ref-1 expression
to down-regulate inflammatory cytokines. Cell Biochem Biophys.
69:725–733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Darby SC, McGale P, Taylor CW and Peto R:
Long-term mortality from heart disease and lung cancer after
radiotherapy for early breast cancer: Prospective cohort study of
about 300,000 women in US SEER cancer registries. Lancet Oncol.
6:557–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henson KE, McGale P, Taylor C and Darby
SC: Radiation-related mortality from heart disease and lung cancer
more than 20 years after radiotherapy for breast cancer. Br J
Cancer. 108:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harkenrider MM, Bertke MH, Dunlap NE and
Dragun AE: Stereotactic body radiotherapy (SBRT) for
non-pathologically diagnosed lung cancer patients. J Solid Tumors.
2:2012. View Article : Google Scholar
|
|
16
|
Tucker SL, Liu HH, Liao Z, Wei X, Wang S,
Jin H, Komaki R, Martel MK and Mohan R: Analysis of radiation
pneumonitis risk using a generalized Lyman model. Int J Radiat
Oncol Biol Phys. 72:568–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh D, Ahn YC, Park HC, Lim DH and Han Y:
Prediction of radiation pneumonitis following high-dose thoracic
radiation therapy by 3 Gy/fraction for non-small cell lung cancer:
Analysis of clinical and dosimetric factors. Jpn J Clin Oncol.
39:151–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holly TA, Abbott BG, Al-Mallah M, Calnon
DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain
D, et al: Single photon-emission computed tomography. J Nucl
Cardiol. 17:941–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muehllehner G and Karp JS: Positron
emission tomography. Phys Med Biol. 51:R117–R137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scott CB, Nelson JS, Farnan NC, Curran WJ
Jr, Murray KJ, Fischbach AJ, Gaspar LE and Nelson DF: Central
pathology review in clinical trials for patients with malignant
glioma. A Report of Radiation Therapy Oncology Group 83-02. Cancer.
76:307–313. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crabtree TD, Denlinger CE, Meyers BF, El
Naqa I, Zoole J, Krupnick AS, Kreisel D, Patterson GA and Bradley
JD: Stereotactic body radiation therapy versus surgical resection
for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg.
140:377–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehta V: Radiation pneumonitis and
pulmonary fibrosis in non-small-cell lung cancer: Pulmonary
function, prediction, and prevention. Int J Radiat Oncol Biol Phys.
63:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sura S, Gupta V, Yorke E, Jackson A, Amols
H and Rosenzweig KE: Intensity-modulated radiation therapy (IMRT)
for inoperable non-small cell lung cancer: The Memorial
Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol.
87:17–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang S, Liao Z, Wei X, Liu HH, Tucker SL,
Hu CS, Mohan R, Cox JD and Komaki R: Analysis of clinical and
dosimetric factors associated with treatment-related pneumonitis
(TRP) in patients with non-small-cell lung cancer (NSCLC) treated
with concurrent chemotherapy and three-dimensional conformal
radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 66:1399–1407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Claude L, Pérol D, Ginestet C, Falchero L,
Arpin D, Vincent M, Martel I, Hominal S, Cordier JF and Carrie C: A
prospective study on radiation pneumonitis following conformal
radiation therapy in non-small-cell lung cancer: Clinical and
dosimetric factors analysis. Radiother Oncol. 71:175–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tell G, Damante G, Caldwell D and Kelley
MR: The intracellular localization of APE-1/Ref-1: More than a
passive phenomenon? Antioxid Redox Signal. 7:367–384. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Angkeow P, Deshpande SS, Qi B, Liu YX,
Park YC, Jeon BH, Ozaki M and Irani K: Redox factor-1: An
extra-nuclear role in the regulation of endothelial oxidative
stress and apoptosis. Cell Death Differ. 9:717–725. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linard C, Marquette C, Mathieu J,
Pennequin A, Clarençon D and Mathé D: Acute induction of
inflammatory cytokine expression after gamma-irradiation in the
rat: Effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol
Phys. 58:427–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haase MG, Klawitter A, Geyer P, Alheit H,
Baumann M, Kriegel TM, Kasper M and Baretton GB: Sustained
elevation of NF-kappaB DNA binding activity in radiation-induced
lung damage in rats. Int J Radiat Biol. 79:863–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ando K, Hirao S, Kabe Y, Ogura Y, Sato I,
Yamaguchi Y, Wada T and Handa H: A new APE-1/Ref-1-dependent
pathway leading to reduction of NF-kappaB and AP-1, and activation
of their DNA-binding activity. Nucleic Acids Res. 36:4327–4336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thakur S, Sarkar B, Cholia RP, Gautam N,
Dhiman M and Mantha AK: APE1/Ref-1 as an emerging therapeutic
target for various human diseases: Phytochemical modulation of its
functions. Exp Mol Med. 46:e1062014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tell G, Quadrifoglio F, Tiribelli C and
Kelley MR: The many functions of APE1/Ref-1: Not only a DNA repair
enzyme. Antioxid Redox Signal. 11:601–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koukourakis MI: Radiation damage and
radioprotectants: New concepts in the era of molecular medicine. Br
J Radiol. 85:313–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fox J and Haston CK: CXC receptor 1 and 2
and neutrophil elastase inhibitors alter radiation-induced lung
disease in the mouse. Int J Radiat Oncol Biol Phys. 85:215–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang LP, Wang YW, Wang BZ, Sun GM, Wang XY
and Xu JL: Expression of interleukin-17A in lung tissues of
irradiated mice and the influence of dexamethasone.
ScientificWorldJournal. 2014:2510672014.PubMed/NCBI
|
|
36
|
Wang BZ, Wang LP, Han H, Cao FL, Li GY, Xu
JL, Wang XW and Wang LX: Interleukin-17A antagonist attenuates
radiation-induced lung injuries in mice. Exp Lung Res. 40:77–85.
2014. View Article : Google Scholar : PubMed/NCBI
|