Introduction
Human immunodeficiency virus (HIV) is classified as
a retrovirus, and hepatitis C virus (HCV) is a flavivirus, both of
which are single-stranded RNA viruses and have a chronic process
after infection in human body (1).
At the same time, because the two types of viruses have the same
three routes of infection, mainly blood, sex and mother-to-child
vertical transmission, HIV/HCV coinfection occurs easily in clinic
(2). A study claimed (3) that the infection rate via blood
transmission, especially in intravenous drug abusers and illegal
paid blood donors, is the highest. The study has confirmed that, of
every 1 million HIV-infected people in China, those co-infected
with HCV account for 30–35% (4).
After HCV infection, the proportion of its development to cirrhosis
and even hepatocellular carcinoma is extremely high. After HCV
infection, it will cause similar immune activation mechanism in the
body, leading to significant increases in extrahepatic
complications and mortality rate in patients (5).
Previous studies have confirmed (6) that the main impact of HIV
mono-infection is to reduce the cellular immune function,
especially to significantly reduce the levels and function of
CD4+ T. The immune function of HCV infected patients is
also affected to some extent, and the impact on hepatic function
should not be neglected (7). After
infection of both viruses, especially in the acute infection
period, the body inflammatory response is affected at the same
time. After HCV infection, immune cells will be activated (8) to promote the levels of inflammatory
cytokines, thereby inducing hepatic fibrosis. HIV infection will
directly lead to the activation of hepatic stellate cells, thus
causing hepatic fibrosis (9).
However, there has been no systematic study on the changes of
immune function and the levels of inflammatory cytokines in HIV/HCV
co-infected patients. In this study, we mainly investigated the
changes of immune function and the levels of inflammatory response
cytokines in HIV/HCV co-infected patients.
Materials and methods
General materials
Eighty HIV/HCV infected patients admitted to Qingdao
No. 6 People's Hospital (Qingdao, China) from August 2015 to
December 2017 were selected. All 80 patients were positively
diagnosed with HIV infection. Of these, 40 patients were diagnosed
with HIV/HCV co-infection. The patients were diagnosed by blood
biochemical tests and qualitative analysis of radionuclides, and
they were in accordance with the diagnostic criteria for HIV and
HCV infections set by the Chinese Society of Infectious Diseases,
Chinese Medical Association in 2010, and no anti-HCV or anti-HIV
treatment was given to them before inclusion. Those with malignant
neoplasms, acute infection, severe cardiopulmonary, hepatic and
renal dysfunctions, mental illness, long-term alcohol abuse, HBV
infection, cirrhosis, and those who were pregnant or breastfeeding
were excluded. According to whether the patients were HIV infected
or HIV/HCV co-infected, they were divided into two groups, each
included 40 patients. The observation group consisted of 25 males
and 15 females, aged 18–50 years, with an average of 41.2±2.3
years, whose confirmed duration of HIV infection was from 1 month
to 3 years, with an average of 1.1±0.1 years, and whose confirmed
duration of HCV infection was from 1 month to 1 year, with an
average of 0.8±0.1 months. The control group consisted of 26 males
and 14 females, aged 18–50 years, with an average of 41.3±2.4
years, whose confirmed duration of HIV infection was from 1 month
to 3 years, with an average of 1.2±0.1 months. There were no
statistically significant differences in general data, such as sex,
age and HIV infection duration, between the two groups (P>0.05).
The study was approved by the Ethics Committee of Qingdao No. 6
People's Hospital and informed consents were signed by the patients
or the guardians.
Methods
The changes of the related humoral immune indexes
[immunoglobulin G (IgG), IgA and IgM levels], the related cellular
immune indexes [cluster of differentiation 4+
(CD4+), CD8+ and
CD4+/CD8+ ratio], the related indexes of
hepatic function [alanine aminotransferase (ALT), aspartate
aminotransferase (AST) and total bilirubin levels], the related
indexes of inflammatory response [high-sensitivity C-reactive
protein (hs-CRP), interleukin-1 (IL-1) and tumor necrosis factor-α
(TNF-α)] in the two groups were compared, and the correlation of
hs-CRP level with ALT level, and IgG level and CD4+
level in the observation group was analyzed.
Evaluation criteria
Immune function tests were divided into the test for
humoral immunity and the test for cellular immunity. The main
testing indexes of humoral immunity included IgG (23.6–9.6 mg/l),
IgA (3.14–4.66 mg/l) and IgM (3.05–3.85 mg/l). The main testing
indexes of cellular immunity included CD4+,
CD8+ and CD4+/CD8+ ratio. The main
testing indexes of inflammatory cytokines included hs-CRP (<10
mg/l), TNF-α (1–10 ng/ml) and IL-1 (130–250 ng/ml). The main
testing indexes of hepatic function included ALT (0–40 U/l), AST
(0–40 U/l) and total bilirubin (5.1–19.0 µmol/l).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 (IBM Corp., Armonk, NY, USA) software was used for data
analysis. Measurement data are presented as mean ± standard
deviation, t-test was used for the comparison of the means between
the groups, χ2 test was used for the comparison of the
ratios between the groups, and the Spearman's
correlation-coefficient method was used for correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of the related humoral
immune indexes between the two groups
The levels of related humoral immune indexes (IgG,
IgA and IgM) in the observation group were significantly lower than
those in the control group (P<0.05) (Table I).
 | Table I.Comparison of the related humoral
immune indexes between the two groups (mg/l, mean ± standard
deviation). |
Table I.
Comparison of the related humoral
immune indexes between the two groups (mg/l, mean ± standard
deviation).
| Items | IgG | IgA | IgM |
|---|
| Observation
group | 10.0±0.1 | 2.6±0.1 | 2.3±0.1 |
| Control group | 25.5±0.2 | 4.5±0.2 | 3.5±0.2 |
| t value | 438.406 | 53.740 | 33.941 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of the related cellular
immune indexes between the two groups
The levels of CD4+ and CD8+ in
the observation group were lower than those in the control group
(P<0.05), and the CD4+/CD8+ ratio in the
observation group was lower than that in the control group
(P<0.05) (Table II).
 | Table II.Comparison of the related cellular
immune indexes between the two groups (mean ± standard
deviation). |
Table II.
Comparison of the related cellular
immune indexes between the two groups (mean ± standard
deviation).
| Items | CD4+ | CD8+ |
CD4+/CD8+ |
|---|
| Observation
group | 29.0±0.3 | 25.4±0.3 | 1.62±0.1 |
| Control group | 36.2±0.5 | 31.6±0.4 | 1.94±0.2 |
| t value | 78.095 | 78.424 | 9.051 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of the related indexes of
hepatic function between the two groups
The levels of indexes of hepatic function (ALT, AST
and total bilirubin) in the observation group were significantly
higher than those in the control group (P<0.05) (Table III).
 | Table III.Comparison of the related indexes of
hepatic function between the two groups (mean ± standard
deviation). |
Table III.
Comparison of the related indexes of
hepatic function between the two groups (mean ± standard
deviation).
| Items | ALT (U/l) | AST (U/l) | Total bilirubin
(µmol/l) |
|---|
| Observation
group | 82.3±3.5 | 73.6±3.8 | 32.5±1.3 |
| Control group | 40.1±1.3 | 35.9±1.1 | 16.3±0.9 |
| t value | 71.484 | 60.272 | 64.800 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of the related indexes of
inflammatory response between the two groups
The levels of hs-CRP, IL-1 and TNF-α in the
observation group were significantly higher than those in the
control group (P<0.05) (Table
IV).
 | Table IV.Comparison of the related indexes of
inflammatory response between the two groups (mean ± standard
deviation). |
Table IV.
Comparison of the related indexes of
inflammatory response between the two groups (mean ± standard
deviation).
| Items | hs-CRP (mg/l) | IL-1 (ng/ml) | TNF-α (ng/ml) |
|---|
| Observation
group | 35.1±1.6 | 268.1±19.6 | 17.4±0.6 |
| Control group | 7.9±0.5 | 128.3±10.2 | 8.5±0.3 |
| t value | 102.623 | 40.016 | 83.910 |
| P-value | <0.001 | <0.001 | <0.001 |
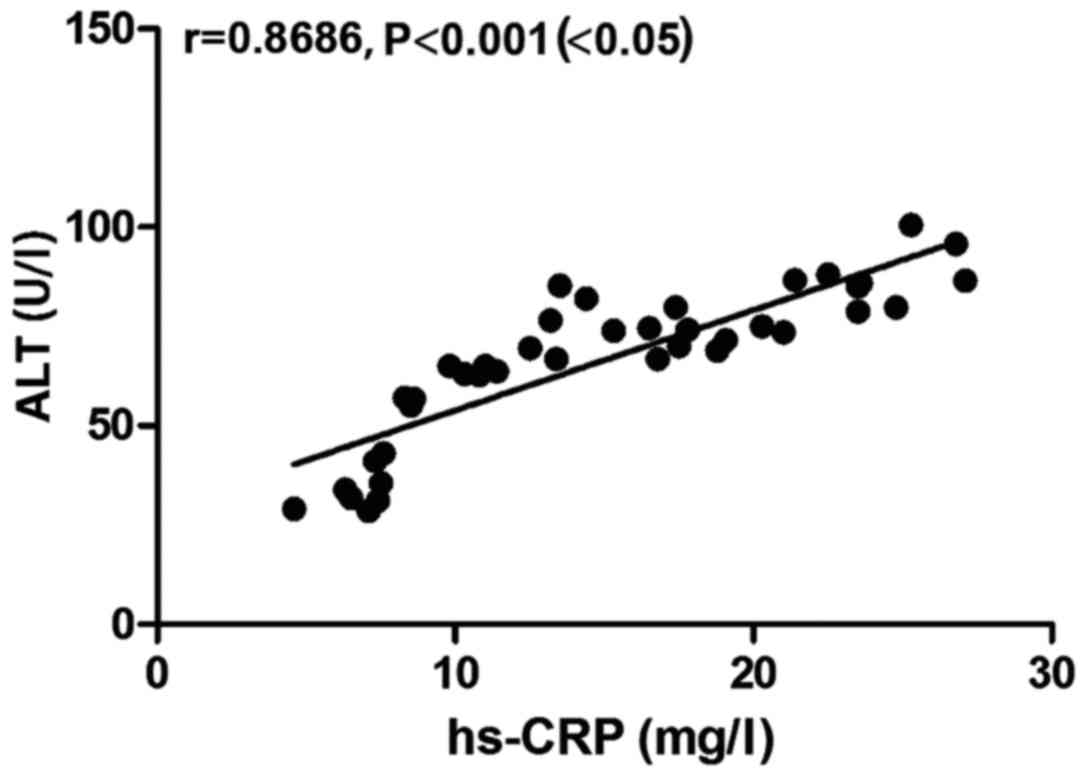
Correlation between hs-CRP level and
ALT level in the observation group
There was a positive correlation between hs-CRP
level and ALT level in the observation group (r=0.8686, P<0.05)
(Fig. 1).
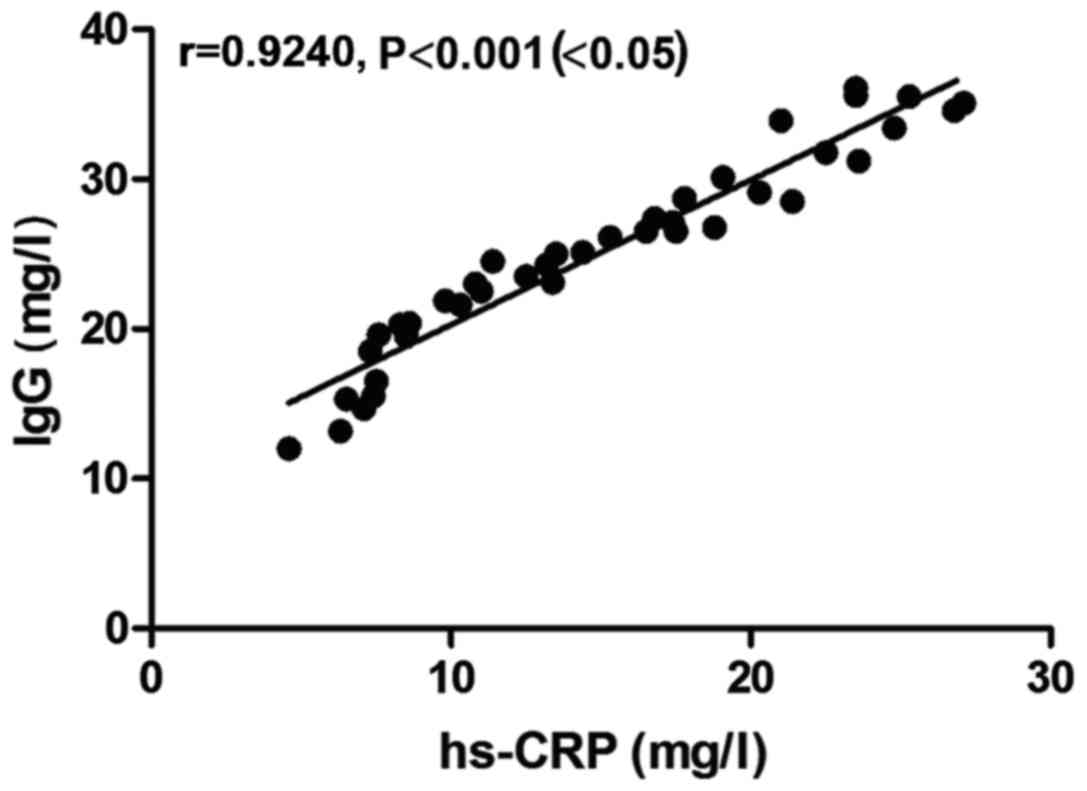
Correlation between hs-CRP level and
IgG level in the observation group
There was a positive correlation between hs-CRP
level and IgG level in the observation group (r=0.9240, P<0.05)
(Fig. 2).
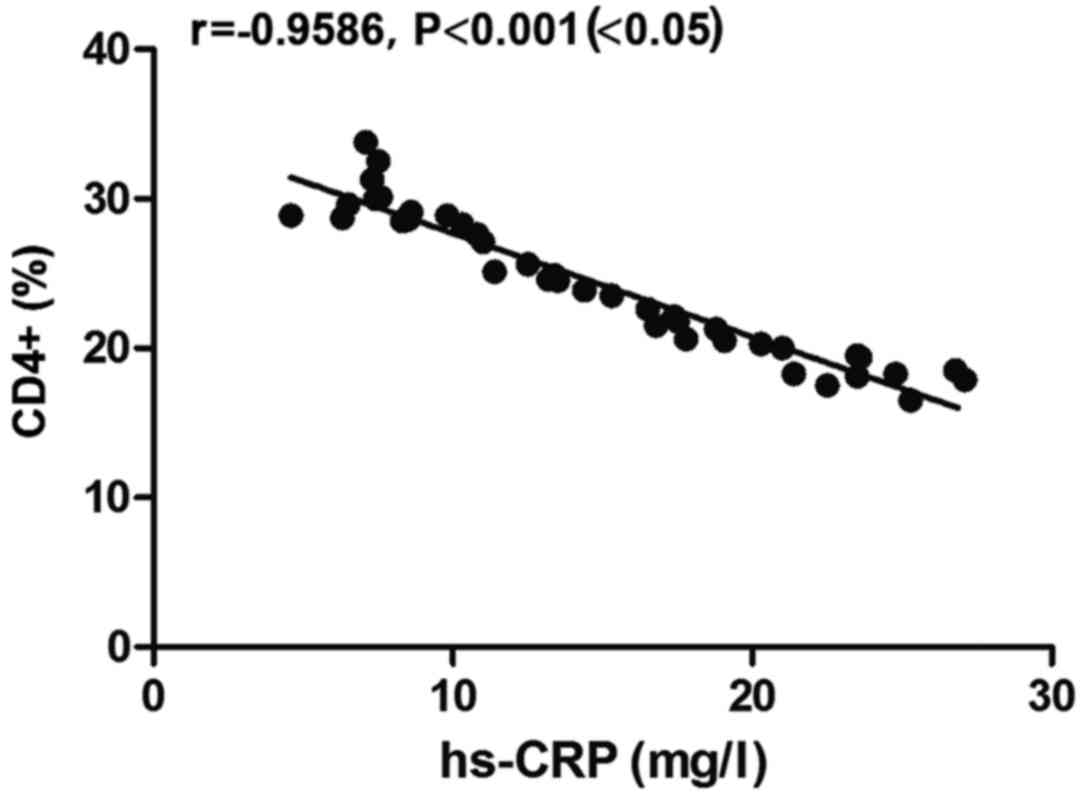
Correlation between hs-CRP level and
CD4+ level in the observation group
There was a negative correlation between hs-CRP
level and CD4+ level in the observation group
(r=−0.9586, P<0.05) (Fig. 3).
Discussion
Previous studies have shown that HIV/HCV
co-infection, compared with HIV mono-infection, causes more serious
damage to immune function, and has a greater impact on hepatic
function (10). hs-CRP is a commonly
used clinical marker of inflammation, and the liver is the main
place for the synthesis of hs-CRP. When the body's inflammatory
response occurs after infection, the hs-CRP level will be
significantly increased (11).
Considering that HIV and HCV have the same routes of transmission
and susceptible populations, the co-infection of HIV with HCV is
common clinically (12). The HIV
patients complicated with HCV infection, compared with HCV
mono-infected patients, have more severe hepatic function
impairment, which may develop into cirrhosis and even hepatoma
sooner and in a higher proportion (13).
In this study, we compared the humoral and cellular
immune functions of HIV/HCV co-infected patients, and investigated
the changes of hs-CRP, IL-1 and TNF-α in the patients. According to
the study of the related humoral and cellular immune indexes, the
levels of related humoral immune indexes (IgG, IgA and IgM) in the
observation group (HIV/HCV co-infected patients) were significantly
lower than those of the control group, and the levels of
CD4+ and CD8+ were lower than those of the
control group, and CD4+/CD8+ ratio was lower
than that of the control group. It suggested that HIV/HCV
co-infection has a greater impact on immune function, and both
humoral immunity and cellular immunity are significantly limited.
In addition, through the comparative study on the related indexes
of hepatic function in the two groups, it was found that the levels
of indexes of hepatic function (ALT, AST and total bilirubin) in
the observation group were significantly higher than those in the
control group. This showed that HIV/HCV co-infected patients are
impaired in hepatic function more severely than HIV mono-infected
patients. At the same time, through the comparative study on the
related indexes of inflammatory response in the two groups, it was
found that the levels of hs-CRP, IL-1 and TNF-α in the observation
group were significantly higher than those in the control group. It
suggested that the inflammatory response of HIV/HCV co-infected
patients is more obvious, and the levels of the related
inflammatory cytokines are significantly increased. Finally, the
correlation study on hs-CRP level with ALT level, IgG level and
CD4+ level in the observation group suggested that the
hs-CRP level was positively correlated with ALT level and IgG
level, and negatively correlated with CD4+ level in the
observation group.
HCV infection will cause significant changes in
immune function, and the hepatocellular inflammatory response
caused by it will lead to hepatocyte fibrosis (14). HIV infection will directly cause the
immune route activation of chemokine CC subclass receptors, which
will cause the activation of hepatic stellate cells and lead to
hepatic fibrosis (15). Among them,
hs-CRP is the most common inflammatory marker in clinic. When the
body has an inflammatory response, the hs-CRP level will be
significantly increased, and the increased level of hs-CRP is
positively correlated with inflammatory response and also
positively correlated with hepatic function impairment (16). HCV infection causes the activation of
immune cells, and leads to the increased secretion of hs-CRP
through positive feedback. In HCV infection, especially in the
acute infection period (17), the
acute inflammatory response induced by hs-CRP will further
aggravate hepatocellular injury and cause cirrhosis, even hepatoma
(18). HIV infection will mainly
inhibit the function of CD4+ T lymphocytes, and lead to
immune tolerance and the lack of effective defensive ability of the
body, then lead to a decrease in the ability of the body to
recognize and eliminate HCV and an increase of the replication of
HCV, and this will further cause an increase in hs-CRP level, while
a decrease in CD4+ level (19). IgG and IgM antibodies are
non-protective antibodies, and HCV infection is mostly presented as
the increase of IgG in the acute infection period, while the
increase of IgM in the chronic infection period. HIV co-infection
with HCV will cause further deterioration of the hepatic function
impairment with a significant increase in IgG, which is associated
with an individualized ability to respond of the immune system, but
after HIV co-infection, it will be significantly decreased
(20).
In conclusion, the humoral and cellular immune
functions of HIV/HCV co-infected patients are significantly
limited, their hepatic function is significantly impaired, and the
levels of inflammatory cytokines are significantly increased. Among
them, the hs-CRP level is positively correlated with hepatic
function and humoral immune function, and negatively correlated
with cellular immune function.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article. The datasets used and/or
analyzed during the present study are available from the
corresponding author on reasonable request.
Authors' contributions
YD and XZ drafted the manuscript, recorded and
analyzed related humoral immune indexes and cellular immune
indexes. GL contributed to the analysis of the hepatic function and
of the indexes of the inflammatory cytokines. All authors read and
approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingdao No. 6 People's Hospital (Qingdao, China) and informed
consents were signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Mínguez C, García-Deltoro M, Flores J,
Galindo MJ, Montero M, Reus S, Carmena J, Masiá M, Amador C and
Ortega E: on the behalf of the COINFECOVA-2 study group:
Interferon-free therapy for treating hepatitis C virus in
difficult-to-treat HIV-coinfected patients. AIDS. 32:337–346.
2018.PubMed/NCBI
|
|
2
|
Collins LF, Chan A, Zheng J, Chow SC,
Wilder JM, Muir AJ and Naggie S: Direct-acting antivirals improve
access to care and cure for patients with HIV and chronic HCV
infection. Open Forum Infect Dis. 5:ofx2642017.PubMed/NCBI
|
|
3
|
Ahmadinejad Z, Abdiliaei Z, Mohamadi R and
Rezahosseini O: Treatment related hematologic changes in a
population of Iranian patients with chronic hepatitis C infection
from 2009 to 2014. Iran J Public Health. 46:1386–1394.
2017.PubMed/NCBI
|
|
4
|
Tavitian-Exley I, Maheu-Giroux M, Platt L,
Heimer R, Uusküla A, Levina O, Vickerman P and Boily MC:
Differences in risk behaviours and HIV status between primary
amphetamines and opioid injectors in Estonia and Russia. Int J Drug
Policy. 53:96–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YC, Ahn JY, Kim JM, Kim YJ, Park DW,
Yoon YK, Song JY, Kim SW, Lee JS, Choi BY, et al: Human
immunodeficiency virus (HIV) and hepatitis virus coinfection among
HIV-infected Korean patients: The Korea HIV/AIDS cohort study.
Infect Chemother. 49:268–274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shmagel KV, Korolevskaya LB, Saidakova EV,
Shmagel NG, Chereshnev VA, Margolis L, Anthony D and Lederman M:
HCV coinfection of the HIV-infected patients with discordant
CD4+ T-cell response to antiretroviral therapy leads to
intense systemic inflammation. Dokl Biol Sci. 477:244–247. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suda G, Ogawa K, Morikawa K and Sakamoto
N: Treatment of hepatitis C in special populations. J
Gastroenterol. 53:591–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charre C, Cotte L, Kramer R, Miailhes P,
Godinot M, Koffi J, Scholtès C and Ramière C: Hepatitis C virus
spread from HIV-positive to HIV-negative men who have sex with men.
PLoS One. 13:e01903402018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramamurthy M, Sankar S, Kannangai R,
Nandagopal B and Sridharan G: Application of viromics: A new
approach to the understanding of viral infections in humans.
Virusdisease. 28:349–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benhammou V, Tubiana R, Matheron S,
Sellier P, Mandelbrot L, Chenadec JL, Marel E, Khoshnood B and
Warszawski J: ANRS CO1/CO11-EPF French Perinatal Cohort study
group: HBV or HCV coinfection in HIV-1-infected pregnant women in
France: Prevalence and pregnancy outcomes. J Acquir Immune Defic
Syndr. 77:439–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohsenizadeh M, Mollaei HR and Ghaziizadeh
M: Seroepidemiological study of hepatitis B, C and HIV among blood
donors in Kerman. Asian Pac J Cancer Prev. 18:3267–3272.
2017.PubMed/NCBI
|
|
12
|
Köse Ş, Ödemiş I, Çelik D, Tatar Gireniz
B, Akbulut I and Çiftdoğan DY: Hepatitis A, B, C and HIV
seroprevalence among Syrian refugee children admitted to outpatient
clinics. Infez Med. 25:339–343. 2017.PubMed/NCBI
|
|
13
|
Raj A, Mittal G and Bahadur H: Factors
affecting the serological testing of cadaveric donor cornea. Indian
J Ophthalmol. 66:61–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Husseinzadeh H, Chiasakul T, Gimotty PA,
Pukenas B, Wolf R, Kelty M, Chiang E, Fogarty PF and Cuker A:
Prevalence of and risk factors for cerebral microbleeds among adult
patients with haemophilia A or B. Haemophilia. 24:1271–277. 2018.
View Article : Google Scholar
|
|
15
|
Fard Najafi S, Schietroma I, Scheri Corano
G, Giustini N, Serafino S, Cavallari EN, Pinacchio C, De Girolamo
G, Ceccarelli G, Scagnolari C, et al: Direct-acting antiviral
therapy enhances total CD4+ and CD8+ T-cells
responses, but does not alter T-cells activation among HCV
mono-infected, and HCV/HIV-1 co-infected patients. Clin Res Hepatol
Gastroenterol. 401:302–362. 2017.
|
|
16
|
Sumbu BMM, Longo-Mbenza B, Ahuka-Mundeke
S, Muwonga JM, Mvumbi-Lelo G, Maphana HM, Kayembe Nzongola-Nkasu D
and Kalumbu FM: Association between the viruses of the acquired
immunodeficiency syndrome and the hepatitis C virus among young
blood donors in Kinshasa: Retrospective analysis of 10 years.
Transfus Clin Biol. 25:26–34. 2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Notari S, Tempestilli M, Fabbri G,
Libertone R, Antinori A, Ammassari A and Agrati C: UPLC-MS/MS
method for the simultaneous quantification of sofosbuvir,
sofosbuvir metabolite (GS-331007) and daclatasvir in plasma of
HIV/HCV co-infected patients. J Chromatogr B Analyt Technol Biomed
Life Sci. 1073:183–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua S, Vigano S, Tse S, Zhengyu O,
Harrington S, Negron J, Garcia-Broncano P, Marchetti G, Genebat M,
Leal M, et al: Pegylated IFN-α-induced NK cell activation is
associated with HIV-1 DNA decline in ART-treated HIV-1/HCV
co-infected patients. Clin Infect Dis. 20:93–96. 2017.
|
|
19
|
D'Aleo F, Ceccarelli M, Rullo Venanzi E,
Facciolà A, Di Rosa M, Pinzone MR, Condorelli F, Visalli G, Picerno
I, Berretta M, et al: Hepatitis C-related hepatocellular carcinoma:
Diagnostic and therapeutic management in HIV-patients. Eur Rev Med
Pharmacol Sci. 21:5859–5867. 2017.PubMed/NCBI
|
|
20
|
Rial-Crestelo D, Rodríguez-Cola M,
González-Gasca FJ, Geijo-Martínez P, Belinchón-Moya O,
Martínez-Alfaro E, Mateos-Rodríguez F, Barberá JR, Yzusqui M,
Casallo S, et al: Effectiveness of direct-acting antiviral therapy
in patients with a HCV/HIV coinfection. A multicenter cohort study.
Rev Esp Enferm Dig. 110:35–43. 2018.PubMed/NCBI
|